Abstract
BACKGROUND:
Sebaceous carcinoma (SeC) is an uncommon malignancy arising from sebaceous glands of the conjunctiva and skin. Recurrent mutations in the ZNF750 were recently identified in ocular SeC. We assessed whether ZNF750 loss is a specific feature of ocular SeC, or a general feature of sebaceous tumors.
METHODS:
Immunostaining for ZNF750 expression was performed in 54 benign and malignant sebocytic proliferations. Staining for ZNF750 was scored on a 3 tier scale: positive (>75%), partial positive (5–74%), and negative (<5%).
RESULTS:
ZNF750 expression was negative in 4/11 ocular SeC, and partially positive in 4/11 ocular SeC and 6/13 cutaneous SeC. No extraocular tumors were negative. No loss was found in sebaceous adenoma or sebaceous hyperplasia. In 9 previously sequenced ocular SeCs, two lacked detectable somatic mutations in ZNF750, but showed complete loss of staining, indicating non-mutational inactivation of ZNF750.
CONCLUSION:
We show complete loss of the ZNF750 epidermal differentiation regulator in about half of ocular SeC, highlighting the most common genetic defect in this cancer type. Loss of ZNF750 expression is seen even in tumors without truncating mutations and reduced in many of the remaining ocular and cutaneous SeC. In contrast, no ZNF750 loss was detected in benign sebaceous proliferations.
Introduction
SeC occurs in 1–2 per 1,000,000 individuals, with a disease-specific mortality rate of 3%−6.7%2,3. SeC can arise from sebaceous glands at any anatomic site, but 39% of cases occur on the eyelid2. Regardless of anatomic site, SeC has been considered a potential cutaneous marker for Muir-Torre syndrome, a cancer syndrome caused by germline mutations in mismatch repair pathway components MLH1, MSH2, MSH6, and PMS24. However, two recent studies have shown ocular SeC is genetically distinct, lacking DNA mismatch repair defects (either somatic or germline) or UV signature mutations1,5. Instead, ocular SeC appears to harbor a high frequency of truncating mutations in ZNF750, compared to extraocular SeC1.
The ZNF750 transcription factor regulates KLF4 to coordinate terminal keratinocyte differentiation6. While specific alleles are associated with psoriasis and seborrheic dermatitis7, the pathogenesis appears to involve keratinocyte dysmaturation, rather than abnormal sebocyte homeostasis. Although ZNF750 inactivation has been reported in specific subsets of squamous cell carcinoma8, the gene is not considered a major tumor suppressor in skin cancer. While truncating mutations in ZNF750 in ocular SeC were recently detected, the extent and frequency of protein loss remains unknown. To our knowledge, loss of function of ZNF750 has not been assessed in sebaceous carcinoma prior to this. We used immunohistochemistry to query whether ZNF750 protein expression is frequently lost in ocular and cutaneous SeC, what mechanisms might produce such loss, and whether this tumor suppressor is generally inactivated in sebaceous neoplasms.
Methods
With IRB approval from our institutional committee on human research, we searched the pathology archives for prior specimens of ocular SeC, cutaneous SeC, sebaceous adenomas, and sebaceous hyperplasia. 15 consecutive cases of sebaceous hyperplasia, sebaceous adenoma, and 13 cutaneous (i.e. non-ocular) SeC with additional tissue available in the associated paraffin block were collected from our dermatopatholgoy archives. The diagnosis was confirmed by a board certified dermatopathologist. Cases of sebaceous carcinoma in which sebaceous differentiation was not clearly evident in routine sections were additionally stained with adipophilin (Cell Marque, Rocklin, CA; predilute on Leica Bond) to confirm the diagnosis. Eleven cases of ocular sebaceous carcinoma with additional tissue available in the associated paraffin block were obtained from the *** Department of Pathology. The diagnosis was confirmed by three pathologists (JPN, DAS, MB). Formalin-fixed paraffin-embedded (FFPE) sections of 4 μm thickness from each of the tumors were stained with ZNF750 (polyclonal, Millipore-Sigma catalog # HPA021573, Sigma-Aldrich, St. Louis, MO; 1:80 dilution and pH6 antigen retrieval). ZNF immunohistochemical studies were scored as negative (<5% tumor cells positive), partially positive (5–75% tumor cells positive) and positive (>75% tumor cells positive). Only nuclear staining was counted as positive. Both immature basaloid sebocytes and mature lipidized sebocytes were counted in the staining assessment. For ZNF750 staining, keratinocytes in the epidermis and/or conjunctival epithelium served as positive internal controls, and inflammatory and stromal cells served as negative internal controls.
DNA Sequencing
Exome sequencing was performed previously on some of the ocular sebaceous carcinomas in a prior study as previously described.1 Briefly, tissue sections were macrodissected to collect the desired regions. DNA was then extracted using the Qiagen QIAamp DNA FFPE Tissue Kit for DNA. Extracted DNA was quantified using a Qubit fluorometer. DNA-seq libraries were captured to exome regions using xGen Exome Research Panel v1.0 (IDT), and libraries were prepared using the KAPA Hyper prep kit. DNA libraries were sequenced to a target depth of 200x for tumor sample, 100x for normal samples on the Illumina HiSeq platform.
Results
Patient demographic information and tumor location are listed in tables 1 and 2. Sebaceous carcinomas occurred in older adults (mean 75; range 49–96) with no gender discrepancy. The mean age was similar for sebaceous hyperplasia (mean 68, range 38–89) and sebaceous adenomas (mean 71, range 55–88). Sebaceous hyperplasia had a 1.5:1 female to male ratio, and sebaceous adenomas had a 3:1 male to female ratio.
Table 1.
Demographics and ZNF750 staining results for sebaceous hyperplasia and adenomas
| Sebaceous Adenoma | Biopsy site | Age/gender | ZNF750 Stain |
|---|---|---|---|
| 1 | Right posterior neck | 72/M | positive |
| 2 | Left nasal sidewall | 79/M | positive |
| 3 | Left nasal ala | 59/M | positive |
| 4 | Right cheek | 75/M | positive |
| 5 | Left face | 67/F | positive |
| 6 | Left post-auricular | 64/M | positive |
| 7 | Left neck | 70/M | positive |
| 8 | Right cheek | 88/F | positive |
| 9 | Right upper cutaneous lip | 55/F | positive |
| 10 | Left upper arm | 73/F | positive |
| 11 | Right neck | 75/M | positive |
| 12 | Left lower eyelid | 69/M | positive |
| 13 | Right mid upper back | 80/M | positive |
| 14 | Left temple | 64/M | positive |
| 15 | Nasal root | 72/M | positive |
| Sebaceous Hyperplasia | |||
| 1 | Right posterior shoulder | 54/M | positive |
| 2 | Dorsal nose | 58/M | positive |
| 3 | Left forearm | 68/M | positive |
| 4 | Left temple | 73/M | positive |
| 5 | Right cheek | 38/M | positive |
| 6 | Left mid cheek | 55/F | positive |
| 7 | Right eyebrow | 71/F | positive |
| 8 | Left nasal ala | 77/F | positive |
| 9 | Medial forehead | 52/F | positive |
| 10 | Left eyebrow | 68/F | positive |
| 11 | Glabella | 89/F | positive |
| 12 | Left forehead | 78/F | positive |
| 13 | Left clavicle | 73/F | positive |
| 14 | Right malar cheek | 72/M | positive |
| 15 | Right medial cheek | 88/F | positive |
Table 2.
Demographics, ZNF750 staining results for sebaceous carcinomas
| Cutaneous SeC | Site | Age/gender | Histopathologic pattern | ZNF750 staining | Mutation analysis |
|---|---|---|---|---|---|
| 1 | Posterior scalp | 80/M | Well-diff | positive | N/A |
| 2 | Right nose | 96/M | Poor-mod diff | partial positive | N/A |
| 3 | Left ear concha | 70/F | Mod-diff | partial positive | N/A |
| 4 | Tip of nose | 75/M | Mod-well diff | Partial positive | N/A |
| 5 | Nose | 64/F | Poor-mod diff | partial positive | N/A |
| 6 | Left neck | 88/F | Mod diff | positive | N/A |
| 7 | Left preauricular area | 66/M | Poor-mod | positive | N/A |
| 8 | Scalp | 81/F | Mod-diff | partial positive | N/A |
| 9 | Left ear | 80/M | Mod-diff | positive | N/A |
| 10 | Glabella | 49/M | Well-diff | Partial positive | N/A |
| 11 | Left lower cutaneous lip | 84/M | Mod-diff | positive | N/A |
| 12 | Right cheek | 79/M | Mod-diff | partial positive | N/A |
| 13 | Right shoulder | 78/F | Poor-mod diff, infiltrative | partial positive | N/A |
| Ocular SeC | |||||
| 1 | eyelid | 73/F | Mod-diff, infiltrative | partial positive | p.E154delinsE |
| 2 | eyelid | 71/F | Mod-diff | partial positive | p.C27R |
| 3 | eyelid | 66/M | Mod-poorly diff | negative | Wild type |
| 4 | eyelid | 78/F | Mod-poorly diff, infiltrative | negative | N/A |
| 5 | eyelid | 69/F | Mod-poorly diff | positive | p.Y205Kfs*163 |
| 6 | eyelid | 80/M | Mod-poorly diff, infiltrative | partial positive | p.Q279* |
| 7 | eyelid | 75/F | Mod diff | negative | p.S52* |
| 8 | eyelid | 71/M | Mod-poorly diff, infiltrative | negative | Wild type |
| 9 | eyelid | 74/M | Mod-well diff | partial positive | p.K221Lfs*146 |
| 10 | eyelid | 80/F | Mod-diff, infiltrative | positive | p.C34L |
| 11* | eyelid | 80/F | In situ carcinoma only | positive | N/A |
Sebaceous carcinoma in situ; Well-diff= well differentiated; Mod-diff= moderately differentiated; Poor-mod diff= poor to moderately differentiated
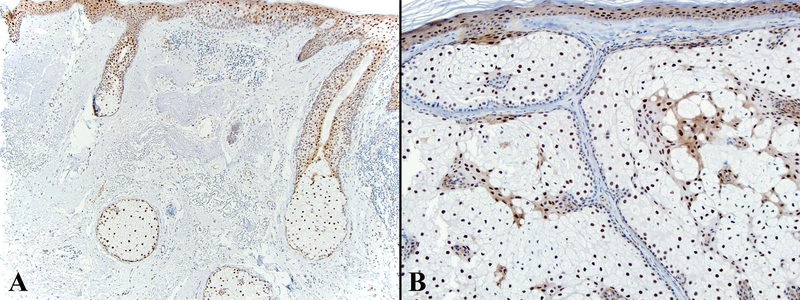
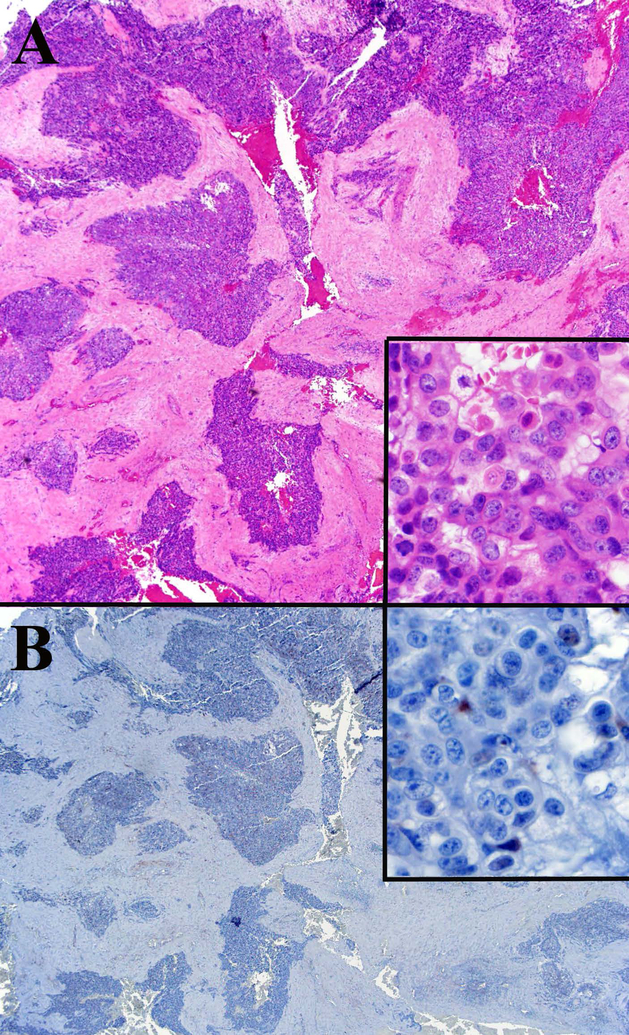
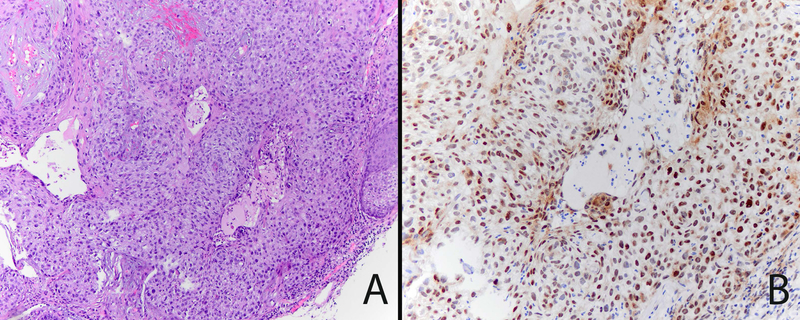
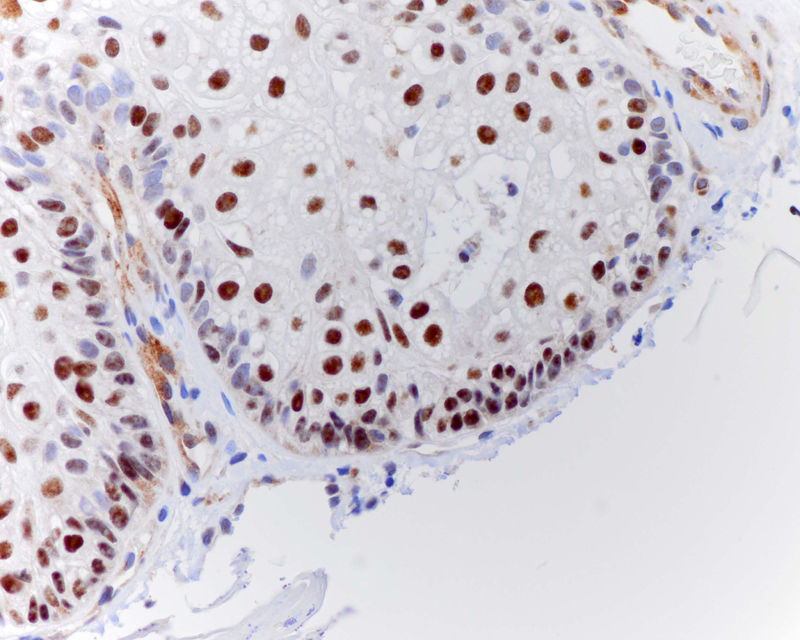
ZNF750 is expressed in normal sebaceous glands and epidermis with a pattern of increasing expression from less differentiated basal epithelial cells to mature sebocytes and keratinocytes (Figure 1A). ZNF750 staining was negative in 36% (4/11) of ocular SeC (Figure 2), partially positive in 36%, and positive in 27%. Negative tumors were frequently poorly differentiated (Table 2). Cutaneous SeC (Figure 3) stained positively for ZNF750 in 54% (7/13), partially positive in 46%, and none were negative. All sebaceous adenomas and sebaceous hyperplasia were uniformly positive with a pattern similar to that seen in normal sebaceous glands with increasing nuclear expression of ZNF750 as germinative sebocytes transition to mature sebocytes (Figures 1,4).
Figure 1.
ZNF750 expression in normal skin (A) and sebaceous hyperplasia (B).ZNF750 is increasingly expressed in the nuclei of keratinocytes in the epidermis and sebocytes in sebaceous glands as cells differentiate into corneocytes and mature sebocytes.A- ZNF750 40x, B- A- ZNF750 100x.
Figure 2.
Ocular sebaceous carcinoma with negative ZNF750 staining. A. Low power view of the tumor shows a large basaloid neoplasm with irregular nodules of cells that have focal sebaceous differentiation (inset).H&E 20x, inset 600x. B. ZNF750 staining is negative in the tumor cells (inset).ZNF750 stain 20x, inset 600x.
Figure 3.
Cutaneous sebaceous carcinoma with positive ZNF750 staining. A. Low power view of the tumor shows large collections of both germinative and mature sebocytes with large pleomorphic nuclei and frequent mitotic figures (inset A-yellow arrows). H&E 20x, inset H&E 600x. Inset B shows ZNF750 staining is weakly positive in the nuclei of the germinative cells and strongly positive in the mature sebocytes in this cutaneous sebaceous carcinoma.ZNF750 stain 100x.
Figure 4.
Sebaceous adenomas all express ZNF750 in a pattern similar to that observed in normal sebaceous glands and sebaceous hyperplasia with increasing expression as germinative sebocytes differentiate to mature sebocytes. A- H&E 200x, B-ZNF750 200x.
Sequencing data for ZNF750 was available for 9 of the 11 ocular SeCs, 7 of which harbored somatic mutations (Table 2). For the two samples in which no ZNF750 mutations were detected, protein expression was completely lost.
Discussion
Cutaneous SeC acquire large mutation burdens, almost exclusively because of defects in DNA mismatch repair or from chronic sun damage. SeC with DNA mismatch repair genotypes are generally more well-differentiated tumors, while the UV signature SeC tend to be more poorly differentiated with infiltrative features1. Interestingly, ocular SeC lack evidence of inactivated DNA mismatch repair proteins, which has been previously regarded as the major contributor to sebaceous carcinoma development. Recent exome sequencing analysis has shown that ocular SeC have frequent mutations in the ZNF750 tumor suppressor gene, as well as frequent TP53 mutations and occasional Notch1/2 mutations.1 Previous studies using targeted next generation sequencing panels on ocular SeC had also shown frequent TP53 mutations and infrequent Notch1 mutations, and additionally found frequent Rb1 mutations.5,9 Ocular SeC which had mutations in both TP53 and Rb1 were more likely to recur.9 Given these new genomic data, we further explored involvement of ZNF750 in sebaceous tumors at the protein expression level and found that dysregulation and loss of ZNF750 is frequent in malignant sebaceous neoplasms, particularly ocular SeC, and retains a normal expression pattern in sebaceous hyperplasia and sebaceous adenomas.
Multiple mechanisms appear to collaborate to attenuate ZNF750 function in ocular SeC. Of the 9 tumors for which sequence data is available, four contain truncating mutations. Two of these (samples 6 and 9) show loss of heterozygosity retaining only the mutated allele, based on the number of sequencing reads of each copy. Both samples demonstrate partial loss of ZNF750 staining. Interestingly, both samples harboring wild-type ZNF750 (3 and 8) show complete loss of staining, suggesting that epigenetic silencing operates in cases where protein function is not compromised by mutation. This observation is similar to that for mismatch repair gene inactivation in MSI-class sebaceous carcinoma, where mismatch repair protein can be lost in the absence of a corresponding truncating mutation. Therefore immunohistochemical staining, as described in this paper, can provide a more comprehensive assessment of whether ZNF750 has been affected through mutation or epigenetic modifications in the pathogenesis of sebaceous tumors. Future studies assessing the role of epigenetic modifications in ZNF750, as well as upstream and downstream proteins in the ZNF750 pathway will be helpful to further understand the molecular pathogenesis of ocular SeC.
The results of ZNF750 staining in this study indicate such staining may be useful in the diagnosis of sebaceous tumors. Benign sebaceous tumors exhibit a typical pattern of ZNF750 expression with minimal expression in germinative sebocytes and positive nuclear expression in mature lipidized sebocytes (figure 4). This pattern can be seen in well-differentiated SeC, so it cannot exclude SeC. However, frequently there is abnormal expression of ZNF750 in germinative cells in SeC (figures 5 and 6). When present, this aberrant expression could serve as a clue to malignant transformation in which ZNF750 expression has been upregulated as a tumor suppressor in germinative cells of SeC. Alternatively, loss ZNF750 expression in mature sebocytes can also serve as a clue to malignant transformation, particular in ocular SeC where complete loss of expression can be seen (figure 2).
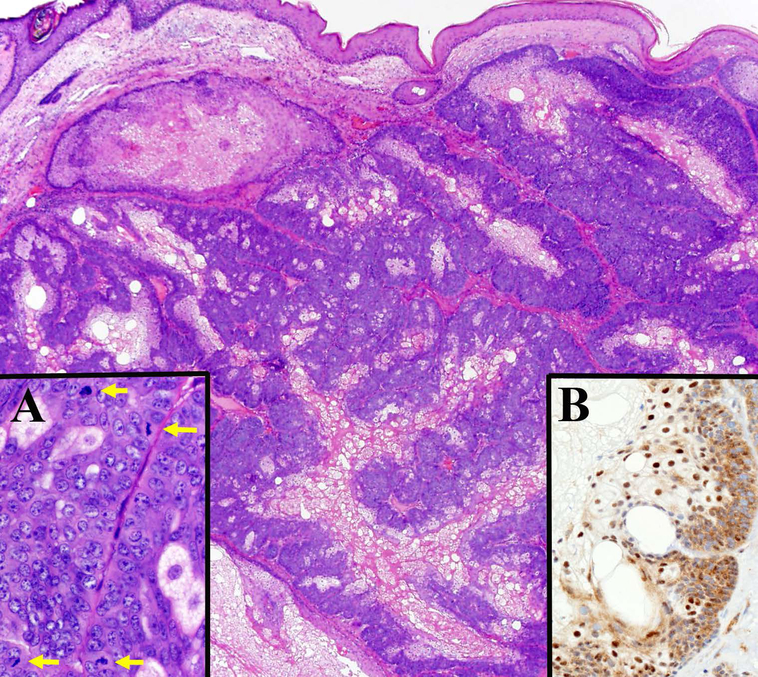
Figure 5.
Poor-moderately differentiated cutaneous SeC with abnormal expression of ZNF750 in immature sebocytes.A- H&E 400x, B- ZNF750 400x.
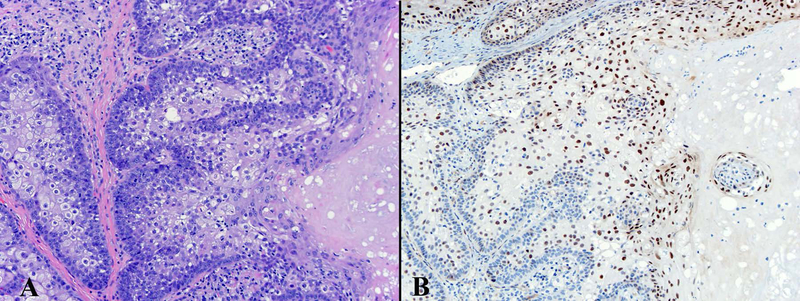
Figure 6.
Abnormal expression of ZNF750 in some of the germinative cells in well differentiated cutaneous SeC. ZNF750 600x.
ZNF750 has also been reported as recurrently mutated in esophageal SCC8–11, but its inactivation is not common in SCC arising at other anatomic sites. The discovery that ZNF750 inactivation is a general feature of ocular SeC is particularly intriguing because of this SeC subtype’s low mutation rates, often less than 100 mutations per exome. Therefore a combinatorial loss of TP53 and/or Rb1 in combination with ZNF750 may be sufficient to initiate tumor formation in periocular sebocytes, a low threshold that could explain the predilection of SeC for eyelid skin (~39% overall).
In contrast to the patterns detected with ZNF750, NOTCH1 mutations are infrequent in ocular SeC, but common in extraocular SeC1. Like ZNF750, Notch receptors play a tumor suppressive role in keratinocytes12. Therefore, we speculate it is possible that inactivation of one of these genes and its downstream targets is required for tumorigenesis in sebocytes, depending on the cell of origin and its anatomic site. As a tumor suppressor, ZNF750 cannot be directly inactivated for therapeutic purposes13. However downstream targets of ZNF750 have been recently discovered14,15, elucidating possible alternative targeting strategies for this pathway. Reduced ZNF750 expression also predicts more aggressive esophageal SCC16, making it possible that detecting its loss in SeC may help identify the small proportion of tumors that recur and metastasize. Additional study correlating ZNF750 expression with clinical follow up data is necessary to assess if ZNF750 truly correlates with clinical outcomes.
Acknowledgments
Funding/Support: This work was supported by UCSF Department of Dermatology. D.A.S. is supported by the NIH Director’s Early Independence Award (DP5 OD021403) and the UCSF Physician-Scientist Scholar Program.
Footnotes
Financial Disclosure of the Authors: There are no conflicts to disclose.
The authors declare no conflict of interest.
References
- 1.North JP, Golovato J, Vaske C, et al. Cell of origin and mutation pattern define three clinically distinct classes of sebaceous carcinoma. Nat. Commun 9, 1894(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta T, Wilson LD & Yu JB A retrospective review of 1349 cases of sebaceous carcinoma. Cancer 115, 158–165 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Kyllo RL, Brady KL & Hurst EA Sebaceous carcinoma: review of the literature. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al 41, 1–15 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Ponti G & Longo C Microsatellite instability and mismatch repair protein expression in sebaceous tumors, keratocanthoma, and basal cell carcinomas with sebaceous differentiation in Muir-Torre syndrome. J. Am. Acad. Dermatol 68, 509–510 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Tetzlaff MT, Singh RR, Seviour EG, et al. Next‐generation sequencing identifies high frequency of mutations in potentially clinically actionable genes in sebaceous carcinoma. J. Pathol 240, 84–95 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Sen GL, Boxer LD, Webster DE, et al. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev. Cell 22, 669–677 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnbaum RY, Zvulunov A, Hallel-Halevy D, et al. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nat. Genet 38, 749–751 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Hazawa M, Lin DC, Handral H, et al. ZNF750 is a lineage-specific tumour suppressor in squamous cell carcinoma. Oncogene 36, 2243–2254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tetzlaff MT, Curry JL, Ning J, et al. Distinct Biological Types of Ocular Adnexal Sebaceous Carcinoma: HPV-Driven and Virus-Negative Tumors Arise through Nonoverlapping Molecular-Genetic Alterations. Clin Cancer Res. 2019. February 15;25(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 10.Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat. Genet 46, 467–473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada G, Niidam A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology 150, 1171–1182 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Wang NJ, Sanborn Z, Arnett KL, et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. U. S. A 108, 17761–17766 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin D-C, Wang M-R & Koeffler HP Targeting genetic lesions in esophageal cancer. Cell Cycle 13, 2013–2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boxer LD, Barajas B, Tao S, et al. ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes. Genes Dev. 28, 2013–2026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan L, Yang H, Tang W, et al. Pathway-focused PCR array profiling of CAL-27 cell with over-expressed ZNF750. Oncotarget 9, 566–575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otsuka R, Akutsu Y, Sakata H, et al. ZNF750 Expression Is a Potential Prognostic Biomarker in Esophageal Squamous Cell Carcinoma. Oncology (2017). doi: 10.1159/000484932 [DOI] [PMC free article] [PubMed] [Google Scholar]