Abstract
Background:
The measurement of liver volume (LV) is considered to be an effective prognosticator for postoperative liver failure in patients undergoing hepatectomy. It is unclear whether LV can be used to predict mortality in cirrhotic patients.
Methods:
We enrolled 584 consecutive cirrhotic patients who underwent computerized topography (CT) of the abdomen for hepatocellular carcinoma surveillance and 50 age, gender, race, and BMI-matched controls without liver disease. Total LV (TLV), functional LV (FLV), and segmental liver volume (in cm3) were measured from CT imaging. Cirrhotic subjects were followed until death, liver transplantation, or study closure date of July 31, 2016. The survival data were assessed with log-rank statistics and independent predictors of survival were performed using Cox hazards model.
Results:
Cirrhotic subjects had significantly lower TLV, FLV, and segmental (all except for segments 1, 6, 7) volume when compared to controls. Subjects presenting with hepatic encephalopathy had significantly lower TLV and FLV than those without HE (p=0.002). During the median follow up of 1,145 days, 112 (19%) subjects were transplanted and 131 (23%) died. TLV and FLV for those who survived were significantly higher than those who were transplanted or dead (TLV:1740 vs 1529 vs 1486, FLV 1691 vs 1487 vs 1444,p <0.0001). In the Cox regression model, age, MELD score, TLV or FLV were independent predictors of mortality.
Conclusion:
Baseline liver volume is an independent predictor of mortality in subjects with cirrhosis. Therefore it may be useful to provide these data while performing routine surveillance CT scan as an important added value. Further studies are needed to validate these findings and to better understand their clinical utility.
Keywords: Liver, diagnostic imaging, portal hypertension
INTRODUCTION
The natural history of cirrhosis is characterized by a compensated stage followed by a decompensated stage[1–3]. Transition to a decompensated stage is manifested by the development of ascites, hepatic encephalopathy, or bleeding varices secondary to portal hypertension[4, 5], The long-term survival of patients in both stages is significantly different with compensated patients having a median survival time of more than 10 years compared to decompensated patients with overall survival less than 2 years[1–3]. Identification of the non-invasive clinical parameters that can accurately predict the clinical progression of liver cirrhosis is of importance as it may lead to early intervention to prevent adverse outcomes before they develop. The measurement of liver stiffness (LS) using transient elastography is commonly used as a method for assessing the degree of fibrosis[6]. The LS-spleen size-to-platelet ratio is found to be a reliable method predicting variceal bleeding among patients with cirrhosis secondary to hepatitis B virus[7]. The ratio can also be used to predict the presence of esophageal varices among those with compensated cirrhosis[8]. Patients with high LS values (≥18 kPa) have significantly higher risks of developing hepatic decompensation compared to those with lower values[9] and LS is useful in screening for liver-related and all-cause mortality, as shown in a recent meta-analysis[10]. In addition to LS, other non-invasive markers such as enhanced liver fibrosis (ELF) score, aspartate to platelet ratio index (APRI), fibrosis-4 (Fib-4) score, as well as MRI imaging derived indices such as ADC maps have been explored as the tools to predict liver-related complications in patients with cirrhosis[11–16].
Abdominal computer tomography (CT) is commonly performed in patients with suspected or known diagnosis of cirrhosis. Several imaging findings suggestive of cirrhosis include an irregular or nodular surface, a blunt hepatic edge, parenchymal abnormalities or changes compatible with portal hypertension[17, 18]. CT is also used as the screening and diagnostic modality for hepatocellular carcinoma (HCC)[19]. CT is associated with increased detection of HCC when compared to ultrasound, despite its higher false positive rate[20].
Cirrhosis is characterized histologically by the presence of fibrosis and regenerative nodules. Liver volume in cirrhosis subjects varies; however, most are much smaller than normal. Imaging-based volumetry has been increasingly utilized in clinical practice to obtain accurate measurements of the liver volume [21–24]. This technique is useful in planning for major hepatic resection and living donor liver transplantation where the size of the remnant liver and liver graft, respectively, affects surgical outcomes[22]. Liver volume (LV), based on MRI imaging was shown to be associated with transplant or death in cirrhotic patients independent of MELD scores during a 5-year follow up[23]. However, the other outcomes related to the development of portal hypertension were not reported.
Due to advances in computation, rapid semi-automatic complete liver segmentation and volumetric analysis may be considered for CT scans of the abdomen in cirrhotic patients.. However, until now it was not clear what would be the added value of by providing this report. The goals of our study are 1) to compare LV stratified by hepatic segments in a large cohort of patients with cirrhosis compared to body-weight matched controls and 2) to determine the association between baseline LV and the presence of portal hypertension complications, and 3) to determine the prognostic significance of providing routine CT derived LV on the long term outcomes of patients with cirrhosis.
METHODS
Study cohort
Subjects were identified retrospectively from consecutive patients with cirrhosis seen in the Liver Transplantation clinic at Indiana University Hospital who underwent CT of the abdomen for HCC surveillance between January 1-June 30, 2009. The diagnosis of cirrhosis was made using radiographic imaging compatible with cirrhosis and/or history of ascites, hepatic encephalopathy and/or the presence of esophageal varices on upper gastrointestinal endoscopy, or biopsy-proven cirrhosis. All patients were at least 18 years old and had baseline laboratory evaluation within 2 weeks from the date of CT evaluation. Patients were excluded if they had liver masses such as HCC, metastatic diseases, or hepatic cysts or had previous history of hepatic resection. During this period, 584 patients met all the inclusion and exclusion criteria. Cirrhosis was determined by Liver biopsy. Baseline demographics, clinical characteristics, as well as laboratory values were recorded. Child-Pugh and MELD scores were calculated as previously described[25]. Medical records, esophagogastroduodenoscopy results, as well as medication lists were reviewed to determine whether patients had any complications from portal hypertension such as hepatic encephalopathy, ascites, or esophageal varices. Another cohort of 50 controls without underlying history of liver diseases who also underwent a CT scan of the abdomen were selected. These controls were age, gender, and body mass index (BMI)-matched to those with cirrhosis. The study design was approved by the Institutional Review Board at the Indiana University Purdue University Indianapolis (IUPUI).
CT scan-based liver volume (LV) measurement
LV measurement from CT imaging was performed using the semi-automated interactive software “IntelliSpace Portal Liver Analysis application” (Philips Medical Systems, Best, The Netherland). The majority of scans were acquired on a 64 slice Brilliance CT scanner (Philips Medical Systems, Best, The Netherland). These were acquired with a slice thickness of 4mm ×3 mm, using multi-phase (arterial, portal venous and delayed venous phases) with 100 to 120 cc of iodinated contrast (Isovue 370). The portal venous phase of the scans was chosen for analysis due to the improved liver density. The software utilized a variational approach guided by Hounsfield units and surrounding anatomical structures. The software algorithm first identified the liver contour and vessel segmentation. Subsequently, a semi-automated guided manual placement of 9 key anatomical and vessels landmarks (e.g inferior vena cava, middle and right hepatic veins, left and right portal vein bifurcations) was performed using the software by each reader, providing the Couinaud hepatic segments[26]; in which the volumes were calculated automatically (Supplementary Figure 1). Because the liver and intrahepatic vascular volume were calculated simultaneously, the software provided 2 readouts; total liver volume (liver volume including vascular volume) and functional liver volume (liver volume excluding vascular volume). The measurement was performed independently by MP and MT. The average time per case was 5 minutes, and the learning curve for the software was short[27]. The less experienced reader MP achieved proficiency within a short time frame. The inter-observer agreement using Pearson correlation coefficient for assessment of the total liver volume and each hepatic segments between both MP and MT was 0.97.
Assessment of outcomes and follow up
Only patients with underlying cirrhosis were prospectively followed until death, liver transplantation, or study closure date of July 31,2016. Medical records during the follow up period were reviewed. For compensated patients without complications of portal hypertension (ascites, hepatic encephalopathy, bleeding varices, and HCC) at baseline, the development of these complications during the follow up period was recorded.
Statistical analysis
Basic descriptive statistics, including mean, standard deviations (SD), and frequencies (percentages) were used to characterize the dataset. Appropriate comparison tests including chi-square test, Student’s t-test or analysis of variance (ANOVA) were used. The survival data were univariately assessed with Kaplan-Meier survival estimates, and log-rank statistics were used for comparison of univariate survival curves. Overall survival was estimated as the interval from the date of CT scan imaging to death, transplantation or the end of the study on July 31, 2016. Patients were censored at the time of the transplantation. The evaluation of independent predictors of survival was conducted using the Cox proportional hazards model and reported as hazard ratios (HR) with corresponding 95% confidence intervals (95% CI). A P value less than 0.05 was considered statistically significant. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of patients with cirrhosis and controls
Baseline demographics, clinical characteristics, and laboratory values in patients with cirrhosis and controls are shown in Table 1. There were no differences in age, gender-, race, and BMI between cases and controls.As expected, patients with cirrhosis had lower levels of hemoglobin (12.5 vs. 13.6 g/dl, p=0.002), white blood cells (5.8 vs. 10.3 ×103/mm3, p=0.0001), platelet counts (112.8 vs. 276 ×103/mm3, p=0.001), and albumin (3.0 vs. 4.1 g/dl, p=0.0001), when compared to controls. They had higher levels of AST (77.6 vs. 20.6 U/L, p=0.0001), ALT (53.8 vs. 21.4 U/L, p=0.0001), ALP (135.7 vs. 83.4 U/L, p=0.0001), and MELD scores (11.2 vs. 4.4, p=0.0001).
Table 1:
Baseline characteristics, laboratory values, and liver volume measurements in controls and patients with cirrhosis
| Variables | Controls (N=50) |
Cirrhosis (N=584) |
p-value |
|---|---|---|---|
| Age (Yrs) | 52.3±7.3 | 55.9±10.1 | 0.09 |
| Gender (Men, n %) | 23 (49%) | 360 (61%) | 0.08 |
| Race (Whites, n %) | 41 (87%) | 521 (89%) | 0.77 |
| Body weight (Kg) | 84.9±18.3 | 87.1±21.4 | 0.45 |
| Height (cm) | 170.5±12.1 | 170.8±12.8 | 0.86 |
| Body mass index (kg/m2) | 29.6±6.8 | 30.1±10.9 | 0.63 |
| Diagnosis of cirrhosis (n, %) | |||
| - Hepatitis C - Alcohol - Hepatitis C and alcohol - Non-alcoholic steatohepatitis - Others |
N/A | 143 (24.4%) 85 (14.5%) 64 (10.9%) 71 (12.1%) 221 (38.1%) |
N/A |
| White blood cell counts (×103/mm3) | 10.3±4.5 | 5.8±371.3 | 0.0001 |
| Hemoglobin (g/dl) | 13.6±1.8 | 12.5±5.7 | 0.002 |
| Platelet counts (×103/mm3) | 276.4±101.2 | 112.8±78.4 | 0.001 |
| Blood urea nitrogen (mg/dl) | 13.4±5.8 | 14.8±12.3 | 0.13 |
| Creatinine (mg/dl) | 0.87±0.19 | 1.2±1.5 | 0.0001 |
| Total bilirubin (mg/dl) | 1.5±0.8 | 2.6±3.3 | 0.91 |
| Alanine aminotransferase (ALT, U/L) | 21.4±15.6 | 53.8±86.1 | 0.0001 |
| Aspartate aminotransferase (AST, U/L) | 20.6±10.9 | 77.6±107.5 | 0.0001 |
| Alkaline phosphatase (ALP, U/L) | 83.4±30.8 | 135.7±131.5 | 0.0001 |
| Albumin (g/dl) | 4.1±0.4 | 3.0±0.7 | 0.0001 |
| Total protein (g/dl) | 7.2±0.6 | 7.1±3.2 | 0.30 |
| International normalized ratio (INR) | 1.5±0.4 | 1.7±3.9 | 0.74 |
| MELD scores | 4.4±2.9 | 11.2±7.2 | 0.0001 |
| Liver volume without body weight adjustment | |||
| Total liver volume (TLV, cm3) | 1786.6±421.5 | 1641.7±555.2 | 0.02 |
| Functional liver volume (FLV, cm3) | 1725.8±414.0 | 1595.8±540.1 | 0.04 |
| Portal vein volume (PVV, cm3) | 33.0±18.4 | 29.5±19.6 | 0.23 |
| Segment 1 volume (cm3) | 39.1±20.4 | 44.9±37.1 | 0.10 |
| Segment 2 volume (cm3) | 210.7±82.2 | 257.2±185.7 | 0.001 |
| Segment 3 volume (cm3) | 126.7±82.3 | 182.6±137.3 | 0.001 |
| Segment 4 volume (cm3) | 289.8±103.6 | 248.4±152.1 | 0.01 |
| Segment 5 volume (cm3) | 293.3±118.1 | 229.9±139.1 | 0.0007 |
| Segment 6 volume (cm3) | 175.9±91.5 | 163.7±135.5 | 0.38 |
| Segment 7 volume (cm3) | 275.4±99.4 | 251.2±124.7 | 0.11 |
| Segment 8 volume (cm3) | 314.8±103.3 | 232.2±103.9 | 0.0001 |
| Liver volume with body weight adjustment | |||
| Total volume:body weight (cm3:kg) | 21.5±5.8 | 19.3±6.9 | 0.01 |
| functional volume:body weight (cm3:kg) | 20.8±5.7 | 18.7±6.7 | 0.02 |
| Segment 1:BW (cm3/kg) | 0.5±0.2 | 0.5±0.5 | 0.44 |
| Segment 2:BW (cm3/kg) | 2.5±0.9 | 3.0±1.9 | 0.01 |
| Segment 3:BW (cm3/kg) | 1.5±1.0 | 2.1±1.6 | 0.0002 |
| Segment 4:BW (cm3/kg) | 3.5±1.5 | 2.9±1.8 | 0.0007 |
| Segment 5:BW (cm3/kg) | 3.4±1.3 | 2.7±1.6 | 0.0001 |
| Segment 6:BW (cm3/kg) | 2.1±1.1 | 1.9±1.5 | 0.18 |
| Segment 7:BW (cm3/kg) | 3.3±1.4 | 2.9±1.5 | 0.10 |
| Segment 8:BW (cm3/kg) | 3.7±1.2 | 2.7±1.2 | 0.0001 |
Total liver and segmental volumes in patients with cirrhosis and controls
Patients with cirrhosis had significantly lower total liver volume (TLV, 1641 vs. 1786 cm3, p=0.02) and functional liver volume (FLV, 1595 vs. 1725 cm3, p=0.04) when compared to controls. The results were similar when we adjusted the TLV and FLV with body weight (Table 1). We also observed the differences in the hepatic segmental volumes between two groups (Table 1). The volume of the caudate lobe (segment 1) was higher in patients with cirrhosis than that of controls (44.9 vs. 39.1 cm3), although it did not reach statistical significance (p=0.10). Interestingly, patients with cirrhosis had a higher volume of the left lobes (segment 2 and segment 3), calculated with and without body weight adjustment,when compared to controls (Table 1, p<0.0001). The decrease in total volume in patients with cirrhosis was primarily due to the reduction in the volume of the right lobe (segments 4-8, 1128.7 vs. 1349 cm3, p<0.0001, supplementary Fig 2), notably on segments 4, 5, and 8 (Table 1). There were no differences in TLV, FLV and segmental volumes among different etiologies of cirrhosis (Supplementary Table 1).
Association between liver volumes, baseline MELD score, Child Pugh classification and complications of portal hypertension
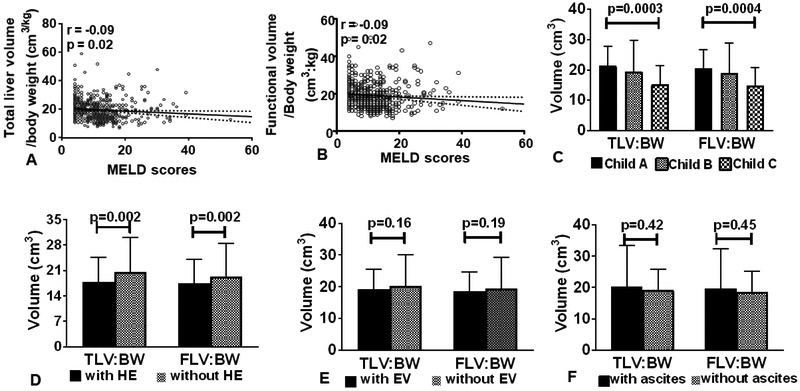
We next determined the association between the liver volumes and severity of underlying liver diseases as indicated by MELD score, Child Pugh classification and the clinical presentations of portal hypertension among patients with cirrhosis at baseline. Due to the association between LV and body weight (supplementary Fig 3), we used the LV to body weight ratio in the analysis. We found an inverse relationship between MELD scores and TLV:BW ratio (r=−0.09, p=0.02, Fig 1A) and FLV:BW ratio (r=−0.09,p=0.02, Fig 1B). Additionally, TLV:BW ratio and FLV:BW ratio were progressively decreased from patients with Child Pugh class A to class C (TLV:BW class A:B:C 21.3:19.5:15.3, p=0.003 and FLV:BW class A:B:C 20.7:18.9:14.8,p=0.004, Fig 1C and Table 2). We found that those with a history of hepatic encephalopathy (HE) at the time of enrollment had a significantly lower TLV:BW (17.9 vs. 20.5,p=0.002) and FLV:BW (17.4 vs. 19.3,p=0.002) compared to those without a history of HE (Fig 1D). No differences in TLV:BW and FLV:BW in patients with and without complications from portal hypertension secondary to esophageal varices (Fig 1E) or ascites (Fig 1F) were observed. Detailed information on LV and segmental volume by the presence of HE, esophageal varices, and ascites is shown in Supplementary Table 2.
Figure 1:
Linear regression analysis between MELD score and total liver volume:body weight ratio (A) and functional liver volume:body weight ratio (B). Total liver volume to body weight ratio and functional volume to body weight ratio stratified by baseline Child Pugh Classification (C), hepatic encephalopathy (D), esophageal varices (E), and ascites (F).
Table 2:
Baseline characteristics, laboratory values, and liver volume measurements stratified by Child Pugh Classification.
| Variables | Child Class A (N=122) |
Child Class B (N=189) |
Child Class C (N=45) |
p-value |
|---|---|---|---|---|
| Age (Yrs) | 55.8±10.2 | 55.0±9.7 | 55.8±8.6 | 0.7400 |
| Gender (Men, n %) | 72 (59%) | 117 (62%) | 30 (67%) | 0.6600 |
| Race (Whites, n %) | 104 (85%) | 169 (89%) | 41 (91%) | 0.2600 |
| Body weight (Kg) | 84.1±18.9 | 87.7±21.2* | 93.9±24.9*,$ | 0.0260 |
| Height (cm) | 169.5±15.0 | 171.2±13.9 | 172.2±9.9 | 0.4355 |
| Body mass index (kg/m2) | 29.0±6.3 | 31.1±15.9 | 31.8±8.2 | 0.2749 |
| White blood cell counts (×103/mm3) | 5.7±2.4 | 5.4±2.9 | 6.8±3.0 | 0.0135 |
| Hemoglobin (g/dl) | 13.3±2.1 | 11.8±2.5* | 11.8±2.0* | <0.0001 |
| Platelet counts (×103/mm3) | 136.3±70.9 | 107.5±69.2* | 88.1±44.9*,$ | <0.0001 |
| Blood urea nitrogen (mg/dl) | 13.9±7.9 | 14.0±11.0* | 19.1±22.6*,$ | 0.0305 |
| Creatinine (mg/dl) | 1.2±1.6 | 1.0±1.1 | 1.5±3.2 | 0.1585 |
| Total bilirubin (mg/dl) | 1.1±0.5 | 2.4±2.1* | 6.2±6.1*,$ | <0.0001 |
| Alanine aminotransferase (ALT, U/L) | 54.9±54.2 | 49.7±56.0 | 45.5±32.4 | 0.5341 |
| Aspartate aminotransferase (AST, U/L) | 60.1±55.7 | 76.7±64.9* | 86.9±47.8*,$ | 0.0134 |
| Alkaline phosphatase (ALP, U/L) | 105.6±65.0 | 154.1±164.3* | 144.5±65.7* | 0.0047 |
| Albumin (g/dl) | 3.6±0.6 | 2.8±0.5* | 2.4±0.5*,$ | <0.0001 |
| Total protein (g/dl) | 7.4±0.7 | 6.9±0.9* | 6.5±1.0* | <0.0001 |
| International normalized ratio (INR) | 1.2±0.1 | 1.6±1.5* | 5.5±24.3*,$ | 0.0161 |
| MELD scores | 7.9±5.3 | 11.3±6.0* | 19.2±11.1*,$ | <0.0001 |
| Liver volume without body weight adjustment | ||||
| Total liver volume (TLV, cm3) | 1750.8±505.3 | 1621.4±541.0* | 1381.1±503.0*,$ | 0.0003 |
| Functional liver volume (FLV, cm3) | 1698.4±488.2 | 1576.7±527.6 | 1343.3±479.2*,$ | 0.0004 |
| Segment 1 volume (cm3) | 52.0±51.6 | 49.0±48.6 | 49.2±38.9 | 0.8687 |
| Segment 2 volume (cm3) | 284.8±326.8 | 249.1±112.8 | 219.3±107.4 | 0.1521 |
| Segment 3 volume (cm3) | 205.3±189.3 | 179.0±115.6 | 139.5±97.0*,$ | 0.0279 |
| Segment 4 volume (cm3) | 279.3±139.8 | 251.2±125.9 | 241.1±240.6 | 0.1880 |
| Segment 5 volume (cm3) | 253.3±195.5 | 223.8±123.3 | 187.9±88.1 *,$ | 0.0330 |
| Segment 6 volume (cm3) | 177.8±108.2 | 158.4±119.5 | 130.1+106.3*,$ | 0.0504 |
| Segment 7 volume (cm3) | 257.3±106.3 | 242.2±128.0 | 217.6±109.3 | 0.1518 |
| Segment 8 volume (cm3) | 235.3±107.3 | 227.1±111.1 | 192.0±84.3*,$ | 0.0656 |
| Liver volume with body weight adjustment | ||||
| Total volume:body weight (cm3:kg) | 21.3±6.5 | 19.5±10.3* | 15.3+6.3*,$ | 0.0004 |
| functional volume:body weight (cm3:kg) | 20.7±6.1 | 19.0±10.0* | 14.9+6.0*,$ | 0.0005 |
| Segment 1:BW (cm3/kg) | 0.6±0.7 | 0.6±0.6 | 0.5+0.4 | 0.5290 |
| Segment 2:BW (cm3/kg) | 3.4±3.0 | 3.0±2.0* | 2.4±1.1*,$ | 0.0456 |
| Segment 3:BW (cm3/kg) | 2.5±2.2 | 2.1±1.9* | 1.6+1.1 | 0.0220 |
| Segment 4:BW (cm3/kg) | 3.4±1.8 | 3.0±2.0 | 2.7±2.5 | 0.0671 |
| Segment 5:BW (cm3/kg) | 3.1±2.3 | 2.7±1.8* | 2.1±1.1*,$ | 0.0132 |
| Segment 6:BW (cm3/kg) | 2.1±1.4 | 1.9±1.5* | 1.4±1.1*,$ | 0.0102 |
| Segment 7:BW (cm3/kg) | 3.2±1.4 | 2.9±1.9* | 2.4±1.4 | 0.0438 |
| Segment 8:BW (cm3/kg) | 2.8±1.3 | 2.7±1.5 | 2.1±1.1*,$ | 0.0150 |
significant compared to those in Child Class A,
significant compared to those in Child Class B
Clinical outcomes of patients with cirrhosis
During a median follow-up period of 3.1 years (1,145 days), 112 (19.3%) cirrhotic patients were transplanted and 131 (22.6%) patients died. Clinical characteristics of these patients stratified by clinical outcomes are shown in Table 3. Patients who died during the follow up were significantly older (58.4±9.6 yrs) than those who were alive (55.9±10.1 yrs) or transplanted (53.4±9.9, p=0.0004). They also had higher MELD scores at baseline (13.6±8.6). There were several baseline hematological variables which were statistically differences stratified by clinical outcomes during follow up such as baseline while blood cells counts (p=0.03), platelet counts (p=0.0002), and mean corpuscular volume (p=0.003). Interestingly, the baseline serum creatinine levels were comparable among 3 groups (p=0.39). Patients who died (2.7±0.6 g/dl) or were transplanted (3.0±0.7 g/dl) had lower baseline serum albumin compared to those who survived (3.2±0.6 g/dl, p<0.0001).
Table 3:
Baseline characteristics, laboratory values, and liver volume measurements stratified by outcomes during follow up.
| Variables | Alive (N=341) |
Transplanted (N=112) |
Dead (N=131) |
p-value |
|---|---|---|---|---|
| Age (Yrs) | 55.8±10.1 | 53.4±9.9* | 58.4±9.6* | 0.0004 |
| Gender (Men, n %) | 206 (61%) | 74 (66%) | 78 (59%) | 0.53 |
| Race (Whites, n %) | 301 (89%) | 99 (88%) | 119 (90%) | 0.67 |
| Body weight (Kg) | 87.6±20.8 | 88.4±19.9 | 85.2±24.2 | 0.4511 |
| Height (cm) | 170.5±13.5 | 172.5±13.9 | 170.3±10.2 | 0.3052 |
| Body mass index (kg/m2) | 30.6±12.8 | 30.1±8.5 | 29.3±7.4 | 0.4921 |
| White blood cell counts (×103/mm3) | 5.8±3.0 | 5.2±4.1 | 6.4±3.4 | 0.0333 |
| Hemoglobin (g/dl) | 12.6±3.0 | 12.1±2.3 | 12.7±11.0 | 0.6081 |
| Platelet counts (×103/mm3) | 123.0±86.3 | 88.7±44.2 | 107.6±76.0 | 0.0002 |
| Blood urea nitrogen (mg/dl) | 14.1±10.4 | 14.1±7.5 | 17.6±18.5 | 0.0197 |
| Creatinine (mg/dl) | 1.2±1.5 | 1.0±0.9 | 1.3±2.0 | 0.3962 |
| Total bilirubin (mg/dl) | 2.2±2.9 | 2.6±2.0* | 3.7±4.7*,$ | <0.0001 |
| Alanine aminotransferase (ALT, U/L) | 55.6±104.9 | 53.9±63.4 | 48.7±35.7 | 0.7485 |
| Aspartate aminotransferase (AST, U/L) | 76.4±131.9 | 78.8±71.9 | 79.1±49.4 | 0.9608 |
| Alkaline phosphatase (ALP, U/L) | 131.1±138.7 | 142.6±92.3 | 139.1±140.9 | 0.6780 |
| Albumin (g/dl) | 3.2±0.6 | 3.0±0.7* | 2.8±0.6*,$ | <0.0001 |
| Total protein (g/dl) | 7.2±4.1 | 6.9±0.9 | 6.9±1.0 | 0.5521 |
| International normalized ratio (INR) | 1.4±1.0 | 3.0±15.5 | 1.6±0.5 | 0.1272 |
| MELD scores | 10.0±6.3 | 12.0±7.2* | 13.6±8.6*,$ | <0.0001 |
| Liver volume without body weight adjustment | ||||
| Total liver volume (TLV, cm3) | 1740.1±574.4 | 1529.7±506.8* | 1486.6±495.6*,$ | <0.0001 |
| Functional liver volume (FLV, cm3) | 1691.7±558.0 | 1487.2±488.8* | 1444.3±487.5*,$ | <0.0001 |
| Segment 1 volume (cm3) | 44.8±48.0 | 41.6±42.4 | 48.5±49.2 | 0.5375 |
| Segment 2 volume (cm3) | 256.8±147.8 | 277.9±334.9 | 242.8±137.0 | 0.3392 |
| Segment 3 volume (cm3) | 198.5±152.7 | 161.5±104.4* | 161.7±114.6* | 0.0062 |
| Segment 4 volume (cm3) | 259.2±147.8 | 234.6±126.0 | 228.8±177.3 | 0.0911 |
| Segment 5 volume (cm3) | 249.1±154.8 | 206.2±117.5* | 200.7±102.7*,$ | 0.0005 |
| Segment 6 volume (cm3) | 175.7±145.8 | 147.4±97.1 | 149.0±135.0 | 0.0563 |
| Segment 7 volume (cm3) | 269.2±154.9 | 226.7±104.1* | 226.5±106.9* | 0.0003 |
| Segment 8 volume (cm3) | 247.0±105.5 | 216.0±100.7* | 207.6±97.4*,$ | 0.0002 |
| Liver volume with body weight adjustment | ||||
| Total volume:body weight (cm3:kg) | 20.6±8.9 | 17.9±7.4* | 18.4±7.0*,$ | 0.0016 |
| functional volume:body weight (cm3:kg) | 20.0±8.7 | 17.4±7.1* | 17.8±6.8* | 0.0016 |
| Segment 1:BW (cm3/kg) | 0.5±0.6 | 0.5±0.5 | 0.6±0.7 | 0.1459 |
| Segment 2:BW (cm3/kg) | 3.1±1.8 | 3.2±3.0 | 3.0±1.9 | 0.8138 |
| Segment 3:BW (cm3/kg) | 2.4±2.1 | 1.9±1.3* | 2.0±1.4*,$ | 0.0092 |
| Segment 4:BW (cm3/kg) | 3.1±2.1 | 2.7±1.7 | 2.8±2.0 | 0.1347 |
| Segment 5:BW (cm3/kg) | 2.9±1.9 | 2.4±1.5 | 2.4±1.2 | 0.0034 |
| Segment 6:BW (cm3/kg) | 2.0±1.5 | 1.7±1.3 | 1.9±1.9 | 0.2052 |
| Segment 7:BW (cm3/kg) | 3.2±1.7 | 2.7±1.4* | 2.8±1.4* | 0.0050 |
| Segment 8:BW (cm3/kg) | 2.9±1.3 | 2.5±1.3* | 2.5±1.2* | 0.0028 |
significant compared to those who were alive,
significant compared to those who were transplanted
Total liver and segmental volumes in association with clinical outcomes in patients with cirrhosis
Patients who died during the follow up period had significantly lower TLV (1486.6 cm3) and FLV (1444.3 cm3) than those who were alive (TLV: 1740.1 cm3; FLV 1691.1 cm3) or transplanted (TLV: 1529.6 cm3; FLV 1487.2 cm3; p<0.0001). Similar findings were observed when TLV and FLV were normalized by body weight (Table 3).
Factors independently associated with mortality among patients with cirrhosis
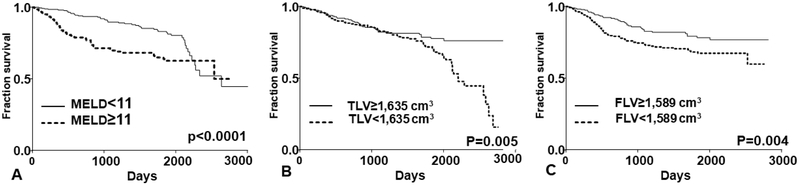
On the univariate analysis, older patients (p=0.002), those with higher MELD scores (p=0.0001), lower TLV (p=0.001) and lower FLV (p=0.001) had significantly higher risk for mortality during the follow up period (Table 4). The effect on TLV and FLV on the mortality was primarily driven by the reduction of right hepatic lobe volume (segments 5-8, Table 4) In the Cox proportional hazard model after controlling for covariates, age p=0.003), MELD score (p=0.001), TLV (model 1, p=0.03, Table 4), and FLV (model 2, p=0.03 Table 4) remained independent predictors of mortality in patients with cirrhosis. Using the log-ranked analysis to determine the effect of each variable on mortality outcome, we found that patients with MELD scores ≥ 11 had significantly higher mortality than those with MELD scores < 11 (Fig 2A). The hazard ratio (HR) for those with a MELD score ≥ 11 compared to those with a MELD score < 11 on mortality was 1.71 (95% CI 1.18-2.47,p=0.005). For TLV, we found that the volume cut-off at 1,635 cm3 was significantly associated with mortality. Those with TLV < 1,635 cm3 had significantly higher mortality than those with TLV ≥ 1,635 cm3 (HR 1.67, 95%CI 1.17-2.43, p=0.005 Fig 2B). For FLV, Those with FLV < 1,589 cm3 had significantly higher mortality than those with FLV ≥ 1,589 cm3 (HR 1.71, 95%CI 1.18-2.47, p=0.005 Fig 2C).
Table 4:
Univariate and multivariate Cox regression analysis on the predictors of mortality in patients with cirrhosis
| Variables | Univariate model | multivariate model 1* | multivariate model 2* | |||
|---|---|---|---|---|---|---|
| Hazard ratio (HR) and 95%CI |
p-value | Hazard ratio (HR) and 95%CI |
p- value |
Hazard ratio (HR) and 95%CI |
p- value |
|
| Age (Yrs) | 1.03 (1.01-1.05) | 0.002 | 1.03 (1.01-1.05) | 0.003 | 1.03 (1.01-1.05) | 0.003 |
| Gender (M vs. F) | 0.89 (0.61-1.30) | 0.55 | - | - | - | - |
| Race (Whites vs. African American) | 0.88 (0.63-2.31) | 0.88 | - | - | - | - |
| Body mass index (kg/m2) | 0.96 (0.98-1.02) | 0.96 | - | - | - | - |
| MELD score | 1.041 (1.025-1.057) | 0.0001 | 1.04 (1.02-1.06) | 0.0001 | 1.04 (1.02-1.06) | 0.0001 |
| Total liver volume (TLV, cm3) | 0.98 (0.98-0.99) | 0.001 | 0.99 (0.99-1.00) | 0.03 | - | - |
| Functional liver volume (FLV, cm3) | 0.98 (0.98-0.99) | 0.001 | - | - | 0.99 (0.99-1.00) | 0.03 |
| Segment 1 volume (cm3) | 1.002 (0.99-1.005) | 0.31 | - | - | - | - |
| Segment 2 volume (cm3) | 0.99 (0.99-1.001) | 0.19 | - | - | - | - |
| Segment 3 volume (cm3) | 0.99 (0.99-1.00) | 0.08 | - | - | - | - |
| Segment 4 volume (cm3) | 0.99 (0.99-1.00) | 0.10 | - | - | - | - |
| Segment 5 volume (cm3)^ | 0.98 (0.96-0.99) | 0.01 | - | - | - | - |
| Segment 6 volume (cm3)^ | 0.99 (0.96-0.99) | 0.04 | - | - | - | - |
| Segment 7 volume (cm3)^ | 0.99 (0.96-0.99) | 0.04 | - | - | - | - |
| Segment 8 volume (cm3)^ | 0.99 (0.96-0.99) | 0.03 | - | - | - | - |
model 1 adjusted for age, MELD, and total liver volume (TLV), model 2 adjusted for age, MELD, and functional liver volume (FLV).
only segment 5 independently associated with mortality adjusted for age and MELD (HRs 0.99, p=0.05)
Figure 2:
Kaplan Meier analysis of MELD score (A), total liver volume (TLV, B), and functional liver volume (FLV, C) on survival in patients with cirrhosis
DISCUSSION
In our present study, we found that (i) patients with cirrhosis had significantly lower LV when compared to age, gender, and BMI-matched controls, (ii) LV was inversely associated with MELD score and Child Classification at baseline and associated with the presence of HE, and (iii) LV was an independent predictor of mortality in patients with cirrhosis.
Liver volume estimation has been used in pre-operative assessment of patients undergoing liver resection or living donor liver transplantation. In the assessment of surgical eligibility, key considerations include preoperative baseline liver function, liver volume, and remaining liver volume[28]. In fact, the use of CT scan in evaluation of liver volume as part of surgical planning has significantly reduced morbidity and mortality after liver surgery[29]. However, it has also been noted that due to underlying liver diseases, liver volume and weight may differ [21]. In accordance with this observation, it has been hypothesized that liver volume can serve as a non-invasive clinical parameter in predicting long term outcome in patients with cirrhosis. In a small study of 25 cirrhotic patients from viral hepatitis, it was found that liver volume measured from the CT images was progressively decreased from 1,133 cm3 in patients with Child Pugh Class A to 672 cm3 in those with Child Pugh Class C[30]. Patients with volume < 750 cm3 who underwent portocaval shunt procedure had significantly increased risk of hepatic encephalopathy and death at 1-year follow up[30]. In another study, liver volume to ideal body weight ratio trended toward predicting transplant or death in patients with cirrhosis, independent of MELD score[23]. Though previous reports provide important information on the prognostic significance of liver volume in patients with cirrhosis, the studies had a relatively small sample size[30, 31], lack of healthy controls[23], and did not report hepatic segmental volume; which may have influenced long term outcomes.
Our study consisted of a large cohort of patients with cirrhosis who underwent CT imaging as the HCC surveillance protocol. While the liver volume is semi-automatically calculated by the software, its measurement requires the manual tracing of the hepatic contours and localization of intrahepatic vascular structures and biliary anatomy. Such manual methods are operator-dependent; however, we found a high inter-observer agreement between our operators. Further, the average liver volume among controls based on the CT imaging in our study is comparable to the standard liver volume as measured using the automated interactive software to estimate the graft size for living-related liver transplantation[27]; ; suggesting the accuracy of the software which was used in our study.
We found that liver volume of patients with cirrhosis was significantly lower than that of normal healthy controls. According to Couinaud classification, segment I (caudate lobe) receives its supply from both the right and the left portal vein and is drained directly into the inferior vena cava by one or more small hepatic veins[32]. Due to a different blood supply compared to other hepatic segments, this segment is generally enlarged to compensate for the loss of normal liver parenchyma in diseased liver, especially in cirrhosis[33]. While we found the higher segment 1 volume in patients with cirrhosis compared to controls, the difference was not statistical significant. This may perhaps be explained by the limited vector (straight line) segmentation of liver segments compromising segment one analysis. We also found comparable liver volume regardless of the etiologies of cirrhosis. As previously noted, the measurement of liver volume may not directly reflect hepatic function. Child Pugh classification and MELD score are normally used to classify the severity of underlying liver disease in patients with cirrhosis[34, 35]. One interesting observation in our study is the inverse relationship between liver volume and MELD score and the progressive decrease in the liver volume in decompensated stage (Class class B and C) compared to those with compensated stage (Child A); suggesting the significant impairment in hepatic function with lower liver volume. Our assumption may need to be systematically examined in the future studies. Another important finding of our study is the prognostic significance of LV in predicting mortality independent of age and MELD score. This is an intriguing observation given the relative stability of liver volume[23]. From clinical perspectives, it would be of interest to prospectively follow the LV over time and see whether the rate of volume reduction can better predict the long term outcomes in this patient and whether the addition of liver volume to the MELD score will improve the accuracy in predicting mortality.
The strengths of this study are the large sample size and the inclusion of age, gender, race- and BMI-matched controls. We acknowledged the limitations in the retrospective nature of our study design and the lack of hepatic function measurement in correlation with our liver volume data. In future studies, a prospective study to address these shortcomings and compare the prognostic significance between liver volume and another non-invasive parameters (such as liver stiffness, APRI, or Fib-4) in predicting long term outcome in patients with cirrhosis should be explored.
In conclusion, baseline liver volume is an independent predictor of mortality in patients with cirrhosis. Our data suggested that it may be provide an important added value while performing routine CT surveillance in patients with cirrhosis. However further studies are needed to validate these findings and to better understand their clinical utility.
Supplementary Material
Acknowledgments
Funding sources:
This work was partly supported by VA Merit Award 1I01CX000361 and NIH R01 AA025208 (to S.L)
Abbreviation list
- APRI
aspartate to platelet ratio index
- CT
computer tomography
- ELF
enhanced liver fibrosis
- Fib-4
fibrosis-4
- FLV
functional liver volume
- LS
liver stiffness
- MELD
model for end stage liver disease
- TLV
total liver volume
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Zipprich A, et al. , Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int, 2012. 32(9): p. 1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gines P, et al. , Compensated cirrhosis: natural history and prognostic factors. Hepatology, 1987. 7(1): p. 122–128. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G, Garcia-Tsao G, and Pagliaro L, Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol, 2006. 44(1): p. 217–31. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Current Management of the Complications of Cirrhosis and Portal Hypertension: Variceal Hemorrhage, Ascites, and Spontaneous Bacterial Peritonitis. Dig Dis, 2016. 34(4): p. 382–6. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, et al. , Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology, 2017. 65(1): p. 310–335. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui MS, et al. , Vibration-controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BK, et al. , Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol, 2011. 106(9): p. 1654–62, 1730. [DOI] [PubMed] [Google Scholar]
- 8.Berzigotti A, et al. , Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology, 2013. 144(1): p. 102–111 e1. [DOI] [PubMed] [Google Scholar]
- 9.Kim BK, et al. , Risk assessment of development of hepatic decompensation in histologically proven hepatitis B viral cirrhosis using liver stiffness measurement. Digestion, 2012. 85(3): p. 219–27. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, et al. , Liver stiffness measurement predicted liver-related events and all-cause mortality: A systematic review and nonlinear dose-response meta-analysis. Hepatol Commun, 2018. 2(4): p. 467–476. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Parkes J, et al. , Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut, 2010. 59(9): p. 1245–1251. [DOI] [PubMed] [Google Scholar]
- 12.Sebastiani G, et al. , Prediction of oesophageal varices in hepatic cirrhosis by simple serum non-invasive markers: Results of a multicenter, large-scale study. J Hepatol, 2010. 53(4): p. 630–8. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, et al. , Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology, 2013. 145(4): p. 782–9 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razek A, et al. , Assessment of the liver and spleen in children with Gaucher disease type I with diffusion-weighted MR imaging. Blood Cells Mol Dis, 2018. 68: p. 139–142. [DOI] [PubMed] [Google Scholar]
- 15.Razek A, et al. , Apparent diffusion coefficient value of hepatic fibrosis and inflammation in children with chronic hepatitis. Radiol Med, 2014. 119(12): p. 903–909. [DOI] [PubMed] [Google Scholar]
- 16.Razek AA, et al. , Prediction of esophageal varices in cirrhotic patients with apparent diffusion coefficient of the spleen. Abdom Imaging, 2015. 40(6): p. 1465–9. [DOI] [PubMed] [Google Scholar]
- 17.Huber A, et al. , State-of-the-art imaging of liver fibrosis and cirrhosis: A comprehensive review of current applications and future perspectives. Eur J Radiol Open, 2015. 2: p. 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AD, et al. , Liver Surface Nodularity Quantification from Routine CT Images as a Biomarker for Detection and Evaluation of Cirrhosis. Radiology, 2016. 280(3): p. 771–81. [DOI] [PubMed] [Google Scholar]
- 19.Fitzmorris P and Singal AK, Surveillance and Diagnosis of Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y), 2015. 11(1): p. 38–46. [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi K, et al. , Screening methods for early detection of hepatocellular carcinoma. Hepatology, 1985. 5(6): p. 1100–5. [DOI] [PubMed] [Google Scholar]
- 21.Goumard C, et al. , Is computed tomography volumetric assessment of the liver reliable in patients with cirrhosis? HPB (Oxford), 2014. 16(2): p. 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim MC, et al. , CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol, 2014. 69(9): p. 887–95. [DOI] [PubMed] [Google Scholar]
- 23.Hagan MT, et al. , Liver volume in the cirrhotic patient: does size matter? Dig Dis Sci, 2014. 59(4): p. 886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mokry T, et al. , Accuracy of estimation of graft size for living-related liver transplantation: first results of a semi-automated interactive software for CT-volumetry. PLoS One, 2014. 9(10): p. e110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn W, et al. , MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology, 2005. 41(2): p. 353–358. [DOI] [PubMed] [Google Scholar]
- 26.Couinaud C, The parabiliary venous system. Surg Radiol Anat, 1988. 10(4): p. 311–6. [DOI] [PubMed] [Google Scholar]
- 27.Zahel T, et al. , Rapid assessment of liver volumetry by a novel automated segmentation algorithm. J Comput Assist Tomogr, 2013. 37(4): p. 577–82. [DOI] [PubMed] [Google Scholar]
- 28.Mullin EJ, Metcalfe MS, and Maddern GJ, How much liver resection is too much? Am J Surg, 2005. 190(1): p. 87–97. [DOI] [PubMed] [Google Scholar]
- 29.Kubota K, et al. , Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology, 1997. 26(5): p. 1176–81. [DOI] [PubMed] [Google Scholar]
- 30.Zhu JY, et al. , Measurement of liver volume and its clinical significance in cirrhotic portal hypertensive patients. World J Gastroenterol, 1999. 5(6): p. 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, et al. , Albumin and magnetic resonance imaging-liver volume to identify hepatitis B-related cirrhosis and esophageal varices. World J Gastroenterol, 2015. 21(3): p. 988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann A, et al. , Standard liver volume in the Caucasian population. Liver Transpl Surg, 1999. 5(5): p. 366–8. [DOI] [PubMed] [Google Scholar]
- 33.Choi JH, et al. , Giant hyperplasia of the caudate lobe in a patient with liver cirrhosis: case report and literature review. Gut Liver, 2008. 2(3): p. 205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volk ML, et al. , Hospital readmissions among patients with decompensated cirrhosis. Am. J. Gastroenterol, 2012. 107(2): p. 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Botta F, et al. , MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: a European study. Gut, 2003. 52(1): p. 134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.