Abstract
Myelin has traditionally been considered a static structure that is produced and assembled during early developmental stages. While this characterization is accurate in some contexts, recent studies have revealed that oligodendrocyte generation and patterns of myelination are dynamic and potentially modifiable throughout life. Unique structural and biochemical properties of the myelin sheath provide opportunities for the development and implementation of multimodal label-free and fluorescence optical imaging approaches. When combined with genetically encoded fluorescent tags targeted to distinct cells and subcellular structures, these techniques offer a powerful methodological toolbox for uncovering mechanisms of myelin generation and plasticity in the live brain. Here we discuss recent advances in these approaches that have allowed the discovery of several forms of myelin plasticity in developing and adult nervous systems. Using these techniques long-standing questions related to myelin generation, remodeling and degeneration can now be addressed.
Introduction
Neurons communicate via rapid transmission of electrochemical signals. Myelin increases the propagation speed and efficiency of these signals by insulating axons and initiating saltatory conduction (Nave and Trapp, 2008; Salzer and Zalc, 2016). In the central nervous system (CNS) this specialized structure is made by oligodendrocytes which myelinate many neurons by concentrically wrapping multiple layers of cell membrane around each axon. Each myelin segment in a fully myelinated axon is termed an internode as they are bordered by clusters of cytoskeletal proteins and voltage gated channels that constitute the nodes of Ranvier. Failure in oligodendrocyte development or destruction of myelin in disease results in severe defects in axonal signaling, leading to widespread neural circuit dysfunction (Franklin and Ffrench-Constant, 2017). Many questions remain about how oligodendrocytes develop, choose specific axons to myelinate in precise patterns, change their structure over time, and regenerate after their destruction in injury or disease.
Out of all major CNS cell populations, myelinating oligodendrocytes develop last. Peak oligodendrocyte differentiation occurs at different developmental stages for different brain regions. In general, axons in white matter become myelinated first while axons in gray matter regions, such as the cerebral cortex, continue to be myelinated in adulthood. The continued production of oligodendrocytes throughout life is possible due to a population of progenitor cells called NG2 glia (also called oligodendrocyte progenitor cells), often identified by their expression of the NG2 chondroitin sulfate proteoglycan Cspg4 (Nishiyama et al., 1996; Hill and Nishiyama, 2014). NG2 glia are born in the ventricular zones of the developing CNS and migrate throughout the brain to establish permanent residency (Nishiyama et al., 2009; Richardson et al., 2011; Dimou and Götz, 2014). These cells are tiled and maintain a tightly regulated resident population by balancing self-renewal, apoptosis, and oligodendrocyte differentiation (Barres et al., 1992; Trapp et al., 1997; Hughes et al., 2013; Hill et al., 2014). Much is known about the molecular cues that induce oligodendrocyte differentiation from NG2 glia (reviewed in Nishiyama, 2007; Emery, 2010; Liu and Casaccia, 2010; Zuchero and Barres, 2013), however less is known about the mechanisms inducing myelination of some axons and not others and some axonal segments and not others, in specific patterns at discrete time points (Almeida and Lyons, 2017; Osso and Chan, 2017).
Signals from other cell types in the brain are thought to play a major role in myelination including growth factors (ex. platelet derived growth factor, fibroblast growth factor, leukemia inhibitory factor, brain derived neurotrophic factor, etc.) and neurotransmitters (ex. glutamate, GABA, ATP, etc.), among others (Emery, 2010; Zuchero and Barres, 2013). Neuronal activity-dependent signaling to NG2 glia and oligodendrocytes is thought to regulate oligodendrocyte differentiation, axonal selection, and internode stability (Stevens et al., 1998; Gibson et al., 2014; Hines et al., 2015; Mensch et al., 2015; Mitew et al., 2018). Likewise, sensory experience has been shown to modulate oligodendrocyte generation and survival (Barres and Raff, 1999; Liu et al., 2012; Hill et al., 2014; Hughes et al., 2018). Other studies have described a role for axonal biophysical properties, such as axon diameter, in regulating myelination and oligodendrocyte wrapping (Friede, 1972; Voyvodic, 1989; Rosenberg et al., 2008; Lee et al., 2012; Goebbels et al., 2017; Mayoral et al., 2018). Moreover, inhibitory or permissive cues expressed by neurons such as Jam2 (Redmond et al., 2016), ephrin-A1 (Harboe et al., 2018), and Cadm4 (Elazar et al., 2019) and homeostatic control of oligodendrocyte density (Almeida et al., 2018) can influence the specificity of oligodendrocyte membrane wrapping to axons and no other neuronal compartments and to some axons and not others. Finally, cell-intrinsic mechanisms including growth factor sensitivity and electrophysiological properties can impact NG2 glia proliferation, oligodendrocyte generation, and the formation and length of myelin internodes (Hill et al., 2013; Viganò et al., 2013; Hill and Nishiyama, 2014; Bechler et al., 2015). Given these findings it is likely a combination of these, and yet unknown factors, are involved in shaping myelin density and distribution in the brain. New techniques and approaches are needed to determine at what stages of development and in which contexts these different mechanisms prevail.
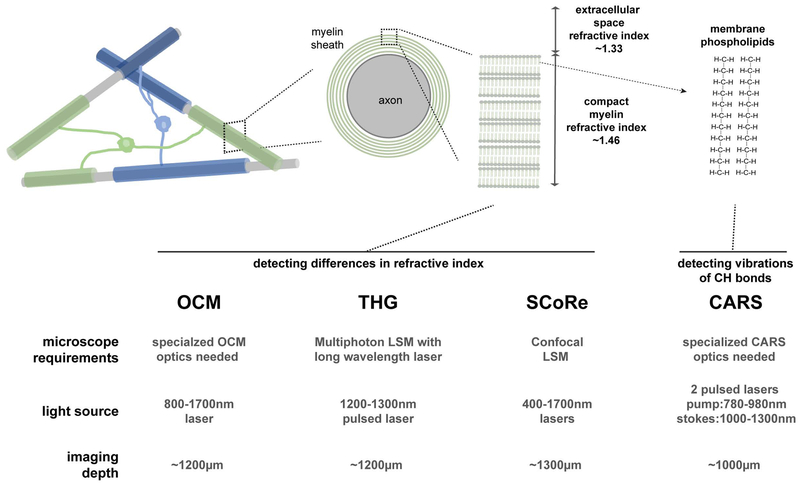
Intravital optical imaging is a powerful approach that can be used to investigate questions related to myelin generation, plasticity and regeneration. The development of genetically encoded fluorescent proteins targeted to different cell types and subcellular compartments has allowed live imaging of NG2 glia behavior, oligodendrocyte differentiation, and myelin plasticity in the spinal cord of the developing zebrafish (Kirby et al., 2006; Almeida et al., 2011; Czopka et al., 2013; Auer et al., 2018) and the cerebral cortex of the mouse (Hughes et al., 2013, 2018; Hill et al., 2014). Additionally, label-free techniques have been developed for live imaging of myelin including coherent anti-Stokes Raman scattering (CARS) (Wang et al., 2005), optical coherence (OCM) (Ben Arous et al., 2011), third harmonic generation (THG) (Farrar et al., 2011) and spectral confocal reflectance (SCoRe) microscopy (Schain et al., 2014; Hill et al., 2018) (Figure 1). A combination of fluorescence-based approaches with intravital label-free imaging provides multifaceted information about myelin structure and compaction, revealing in real-time, details previously accessible only in fixed tissue samples. Here we describe recent developments of these tools and some of the insights gained by imaging myelin in vivo.
Figure 1: Approaches for label-free myelin imaging.
The myelin sheath is a unique biological structure composed of multiple layers of oligodendrocyte cell membrane wrapped tightly around single axons with very little to no cell cytoplasm between the myelin layers. The differential refractive index of the compact myelin sheath compared to the rest of the brain tissue and the high concentration of phospholipids allows label-free detection of myelin using various optical imaging approaches. These approaches include optical coherence microscopy (OCM), third harmonic generation (THG) microscopy, spectral confocal reflectance (SCoRe) microscopy, coherent anti-Stokes Raman scattering (CARS) microscopy. OCM, THG and CARS require relatively complex instrumentation/light sources while SCoRe can be conducted on conventional confocal laser scanning microscopes (LSMs). Recent advances in each technique using long wavelength light sources (1300–1700nm) have achieved imaging depths above 1mm, potentially allowing intravital imaging of white matter tracts such as the corpus callosum in murine models.
Optical approaches for live myelin imaging
Fluorescence imaging
For most cell types and tissues, fluorescence-based approaches are the standard for live imaging of cellular dynamics (Lichtman and Fraser, 2001; Misgeld and Kerschensteiner, 2006; Liu et al., 2015). This is largely due to the ability to label distinct cell types, subcellular structures, and organelles with genetically encoded fluorescent proteins, indicators, and intravitally applied or injected dyes. These labels can typically be imaged on widely available widefield or laser scanning fluorescence microscopes without complex instrumentation. The options for fluorescent indicators and probes are rapidly expanding and new strategies for delivering these labels to distinct classes of cells are actively being developed.
Live fluorescence imaging has been used in cultures of primary oligodendrocytes, tissue explants, and in organotypic slice cultures (Ohno et al., 2011; Sobottka et al., 2011; Wake et al., 2011; Zhu et al., 2011; Hill et al., 2014). The generation of transgenic mice with specific labeling of oligodendrocyte lineage cells has allowed detailed analyses of NG2 glia proliferation and oligodendrocyte generation (Sobottka et al., 2011; Zhu et al., 2011). Pharmacological and genetic manipulation in these systems allows relatively rapid investigation of the molecular mechanisms involved in initiating different steps in myelin development. Models of demyelination have also been developed in these culture systems which allows detailed cellular and molecular analyses of remyelination (Birgbauer et al., 2004; Zhang et al., 2011). While these approaches can be powerful, limitations for recapitulation of the in vivo environment must be considered.
Zebrafish have become a key model for dynamic analyses of axonal myelination. Optical transparency and genetic tractability are two of the main reasons for this (Lyons and Talbot, 2014; Preston and Macklin, 2015; Ackerman and Monk, 2016; Czopka, 2016). During early developmental periods zebrafish can be anesthetized and immobilized for extended intervals. This permits high resolution cellular imaging for hours to days through their transparent spinal cord. Multiple aspects of myelin development have been discovered using zebrafish as discussed below. Advances in super resolution (Liu et al., 2018) and high frame-rate microscopy techniques (Ahrens and Engert, 2015) combined with the development of genetically encoded sensors for calcium, membrane voltage, and other intracellular ions and neurotransmitters are all likely to provide axonal functional readouts associated with changes in myelination in the coming years.
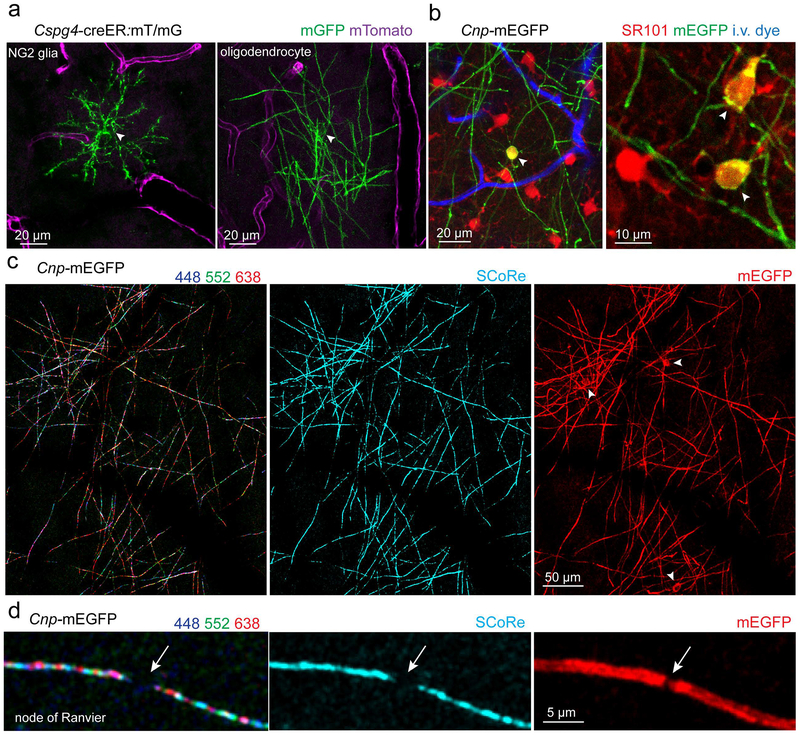
In vivo imaging studies of myelin in mammals has also recently been reported in transgenic mice with genetically encoded fluorescent proteins in oligodendrocyte lineage cells (Figure 2) (Hughes et al., 2013, 2018; Hill et al., 2014, 2018; Williams et al., 2014; Romanelli et al., 2016). These studies have primarily used two-photon fluorescence microscopy in cranial or spinal cord acute or chronic windows. Intravitally applied lipophilic dyes, such as fluoromyelin, can also be used to transiently label the myelin sheath in both the cortex and spinal cord (Romanelli et al., 2013; Schain et al., 2014). Furthermore, myelinating oligodendrocytes can be labeled using sulforhodamine 101 (SR101), a dye that initially gets taken up by astrocytes but spreads to myelinating oligodendrocytes due to gap junction coupling between these two cell populations (Figure 2) (Hill and Grutzendler, 2014). As discussed below, the ability to image mouse cortex and spinal cord over extended periods using fluorescence approaches is likely to answer multiple important questions in a mammalian system.
Figure 2: Intravital imaging of oligodendrocytes and myelin.
a) Fluorescence labeling and in vivo imaging of single oligodendrocyte lineage cells in the mouse cortex. Isolated NG2 glia or oligodendrocytes can be visualized after a low dose injection of tamoxifen to induce cre recombination in single cells in Cspg4-creER transgenic mice. Membrane tethered GFP allows visualization of NG2 glia processes or myelin internodes. Arrowheads indicate cell soma b) The small molecule fluorescent dye sulforhodamine 101 (SR101) allows in vivo visualization of astrocyte and oligodendrocyte gap junction coupling. Astrocytes initially take up the dye which then diffuses to coupled myelinating oligodendrocytes. Arrowheads indicate oligodendrocyte cell soma. c) Label-free spectral confocal reflectance (SCoRe) microscopy in the live mouse cortex. The reflection of multiple wavelengths (left image, 448 nm, 552 nm, and 638 nm laser wavelengths used) is combined to visualize myelination (middle image) which maps precisely with membrane localized EGFP in Cnp-mEGFP transgenic mice (right image). Arrowheads indicate oligodendrocyte cell soma. d) Nodes of Ranvier (arrow) can be visualized in vivo using SCoRe and/or mEGFP labeling.
Even with the capabilities of fluorescence microscopy, some limitations include: 1) difficult and time-prohibitive methods for delivering and expressing genetically encoded fluorescent proteins to specific cells; 2) fluorescence bleaching leading to the production of reactive oxygen species and phototoxicity; 3) adverse biological effects of fluorescent protein overexpression in the cell cytoplasm or myelin sheath; 4) variable or insufficient brightness for live imaging in intact systems. Additionally, for myelin imaging there are several other challenges. Cytoplasmic fluorescent proteins do not readily diffuse into compact myelin thus limiting analyses of internode structure using most conventional fluorescent protein labeling strategies. This can be overcome to some extent by using membrane targeted fluorescent proteins (Kirby et al., 2006; Zhu et al., 2011; Chong et al., 2012; Young et al., 2013; Deng et al., 2014). Another limitation of fluorescence labeling is the inability to determine when and where a labeled myelin segment is forming compact myelin. Some of these issues could potentially be overcome with label-free imaging approaches.
Label-free myelin imaging
Myelin has several unique structural and biochemical characteristics that permit the use of different modalities for label-free imaging. The lipid-rich myelin sheath has a distinctive chemical makeup compared to other brain tissue. Additionally, the concentration of lipids within the sheath and membrane compaction means that myelin has a higher refractive index compared to the rest of the more aqueous brain tissue. These characteristics make the development of label-free imaging approaches possible, which can serve as appealing alternatives or supplements to fluorescence imaging (Figure 1).
Coherent anti-Stokes Raman Scattering (CARS) microscopy is a non-linear imaging technique that captures vibrational signatures from defined chemical bonds in order to visualize structures made up of these molecules in a label-free fashion (Evans et al., 2005). CARS microscopy uses two synchronized pulsed lasers that can be tuned for imaging lipids and adipocytes and can thus be used for molecular specific imaging of myelin, based on its high concentration and characteristic composition of lipids and fatty acid chains (Wang et al., 2005). Using CARS, the myelin sheath can be resolved at high resolution revealing structural detail at nodes of Ranvier and cytoplasmic channels running through peripheral myelin called Schmidt Lanterman incisures (Wang et al., 2005). CARS has primarily been used in white matter tracts of the spinal cord and in peripheral nerve with some studies also showing use in the cerebral cortex of anesthetized mice (Evans et al., 2005; Evans and Xie, 2008; Fu et al., 2008). While microscopes with CARS capability are becoming more available, there is currently limited accessibility to this imaging modality for widespread usage.
Optical coherence microscopy (OCM) is a technique that capitalizes on the variation in scattering of near-infrared light between different cellular structures and tissues in order to extract contrast (Huang et al., 1991). This imaging technique has been used extensively for label-free imaging of retinal structure (Hee et al., 1995) but has also been shown to be able to extract images of myelin in the brain (Ben Arous et al., 2011; Henry et al., 2015; Yamanaka et al., 2016). Similar to OCM’s dependence on differential tissue scattering, third harmonic generation (THG) microscopy is a non-linear imaging approach that generates a signal at lipid-water interfaces due to nonlinear coherent scattering (Weigelin et al., 2016). Again based on the unique biophysical properties of myelin, THG can be used for label-free imaging of myelin in vivo (Farrar et al., 2011; Lim et al., 2014). THG imaging requires high intensity long wavelength lasers and high laser power in order to generate bright signals thus photodamage or thermal injury is a possibility. Finally, other structures such as cerebral blood vessels emit THG signals somewhat complicating interpretation of the signal. Both OCT and THG imaging also require somewhat specialized optical components and light sources making their widespread usage currently relatively limited.
As highlighted by the previously discussed approaches, there is significant refractive index mismatch between the myelin sheath and the rest of cellular components found in the brain. Spectral confocal reflectance (SCoRe) microscopy takes advantage of this and uses a standard confocal laser scanning microscope to capture images specific to myelin (Figure 2) (Schain et al., 2014). Multiple wavelengths of reflected laser light are collected and combined into a single image in order to detect compact myelin. This process generates a multicolor reflection pattern that likely arises from heterogeneity in myelin thickness and organization and can be used to trace single axons and detect structural compartments within the myelin sheath such as Schmidt Lanterman incisures (Schain et al., 2014). The signal arises specifically from multilayered myelin which provides additional information about myelin compaction which cannot be determined using fluorescence imaging alone. This technique can be used to visualize myelin in the cerebral cortex, spinal cord, and peripheral nerves deep into tissue using various laser wavelengths (Hill and Grutzendler, 2014; Schain et al., 2014; Hill et al., 2018; Xia et al., 2018). SCoRe requires lower laser intensities compared to confocal or multiphoton fluorescence microscopy. Moreover, out of all of the label-free imaging approaches for myelin, SCoRe is potentially the most widely accessible as it can be carried out on most conventional confocal microscopes without specialized optics or light sources. One consideration for SCoRe microscopy is that myelinated axons traveling in the z-axis cannot be resolved.
Thus far, the application of label-free imaging techniques to investigations of myelin biology has been relatively limited. Clearly, each approach has its advantages and disadvantages and future investigations implementing multimodal fluorescence and label-free imaging can exploit the advantages of each modality while overcoming some of the obstacles inherent to each technique, providing richer information about the composition and structure of myelin sheaths in vivo.
Recent Discoveries
In the following sections we highlight recent discoveries focusing on studies that have used live optical imaging to uncover important mechanisms of myelin biology and pathology.
Dynamics of NG2 glia
Live fluorescence imaging in zebrafish spinal cord and mouse cortex has shown that NG2 glia extend and retract their processes over minutes to hours (Kirby et al., 2006; Hughes et al., 2013). This surveillance behavior is not as rapid or robust as microglia surveillance in the brain (Davalos et al., 2005; Nimmerjahn et al., 2005) but reveals relatively rapid sampling of the surrounding environment by these cells. This process sampling likely has several purposes. First, it could provide one mechanism for NG2 glia to detect adjacent cells in order to maintain a homeostatic tiled population in the brain. There is evidence for contact mediated inhibition between individual NG2 glia processes (Kirby et al., 2006; Hughes et al., 2013) and territory coverage and proliferation by adjacent, remaining cells to fill the void after cell killing via laser ablation or two-photon apoptotic targeted ablation (2Phatal) (Kirby et al., 2006; Hughes et al., 2013; Hill et al., 2017). Second, this behavior could provide a mechanism for detecting unmyelinated, myelin-permissive axons. However, it has not been demonstrated that contact of an unmyelinated axon directly induces oligodendrocyte differentiation from NG2 glia. This axon sampling behavior is abundant in pre-myelinating oligodendrocytes and appears to be involved in axon selection as discussed below. The precise molecular signaling pathways involved in this surveillance behavior have not been identified.
In addition to process surveillance, NG2 glia also migrate via cell soma translocation over relatively large distances (tens to hundreds of micrometers over hours to weeks) in the developing zebrafish spinal cord (Kirby et al., 2006) and the cerebral cortex of adult mice (Hughes et al., 2013; Hill et al., 2014). This behavior is likely involved in the maintenance of a tiled homeostatic population by balancing self-renewal, apoptosis, and oligodendrocyte differentiation. Signals that induce NG2 glia migration in vitro have been identified but molecular mechanisms involved in directed motility in vivo are not clear. Directed migration can be observed in response to nearby tissue damage (Hughes et al., 2013) and is consistent with accumulation and proliferation of NG2 glia at injury sites (McTigue et al., 2001; Komitova et al., 2011; Dimou and Götz, 2014). Future studies can use these imaging approaches to investigate the spatiotemporal dynamics of this response and the molecules needed to induce directed migration.
NG2 glia receive direct synaptic input from neurons (Bergles et al., 2000, 2010). Evidence suggests that this synaptic input takes place at specialized locations on the NG2 glial cell (Orduz et al., 2015). What is the fate of these synaptic connections when a cell moves its process or migrates to a new location? Do migrating NG2 glia receive synaptic input? It has been shown that this input is maintained during cell division (Kukley et al., 2008) which suggests that it could potentially be maintained during cell movement and process reorganization. Neuronal activity and sensory input could potentially modulate NG2 glia migration behavior and positioning (Hill et al., 2011; Mangin et al., 2012), however more work needs to be done to determine if and when activity-dependent signals are involved in regulating the distribution of NG2 glia throughout the tissue. Studies using live imaging of migratory and surveillance behavior in combination with genetic manipulation of components of the extracellular matrix, cell surface molecules, and diffusible signals released into the microenvironment will help tease apart some of these mechanisms.
Modulation of oligodendrocyte generation
Many molecular signaling pathways have been identified that induce oligodendrocyte differentiation from NG2 glia (reviewed by Levine et al., 2001; Emery, 2010; Liu and Casaccia, 2010; Zuchero and Barres, 2013). Much less is known however, about the discrete morphological stages and the specific signals initiating each step in the transformation from NG2 glia to myelinating oligodendrocyte in the intact brain. Whether or not the decision to differentiate arises cell intrinsically or exclusively from extracellular cues has been a long-standing question. There are multiple molecular signaling pathways activated by extrinsic ligands such as growth factors and neurotransmitters that are known to induce oligodendrocyte differentiation but there is also evidence that cell division events are linked to oligodendrocyte differentiation (Temple and Raff, 1986; Tang et al., 2000). There is also evidence for asymmetric distribution of molecules such as NG2 and GPR17 during NG2 glia division (Sugiarto et al., 2011; Boda et al., 2015) but real-time analyses of these events are lacking. Imaging in slice cultures in combination with transgenic fate mapping in early postnatal development has demonstrated that NG2 glia initially divide symmetrically and then differentiate into oligodendrocytes during a temporal window (Zhu et al., 2011; Hill et al., 2014). After the division event, there is a decision that is made to stay an NG2 glia, differentiate into an oligodendrocyte, or die via apoptosis. Surveillance mechanisms discussed above are likely involved in this process allowing detection of signals from the environment such as changes in sensory input (Hill et al., 2014; Hughes et al., 2018) or damage to myelin. Additional live imaging studies of cell division events will provide more information about these processes.
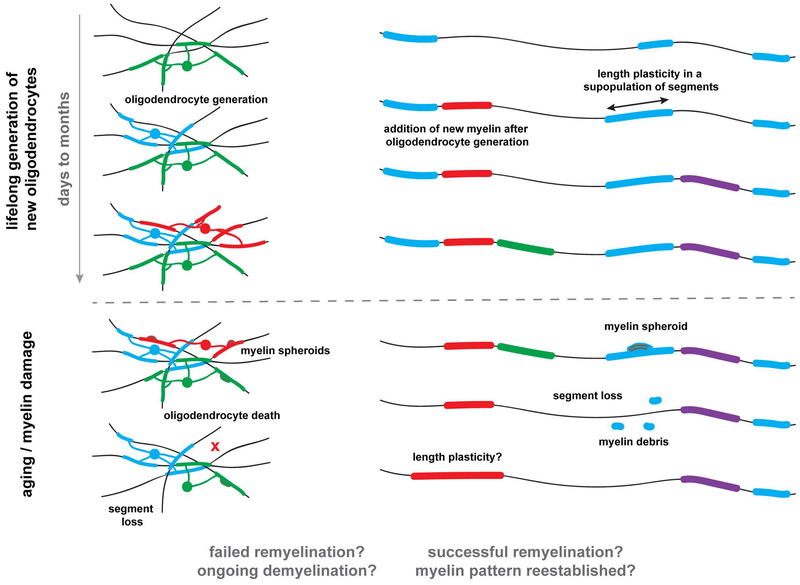
Depending on the brain region, oligodendrocyte generation can extend into late stages of adulthood. Transgenic fate mapping approaches have revealed age and region-dependent differences in NG2 glia differentiation and oligodendrocyte formation (Dimou et al., 2008; Kang et al., 2010; Zhu et al., 2011; Young et al., 2013). Questions remain about how long oligodendrocyte generation extends in different brain regions and if the continual production of oligodendrocytes is for replacement of dying oligodendrocytes or results in the addition of new cells. Recent imaging studies tested some of these questions revealing that oligodendrocyte generation continues into late adulthood in the mouse cerebral cortex. Longitudinal imaging in the adult cortex provided no evidence for oligodendrocyte replacement but instead the continuous addition of new oligodendrocytes (Figure 3) (Hill et al., 2018; Hughes et al., 2018). Functional reasons for ongoing oligodendrocyte production throughout adulthood are not clear.
Figure 3: Forms of myelin plasticity and degeneration in the adult brain.
In vivo imaging studies have discovered protracted ongoing oligodendrocyte generation in the adult brain. The continuous oligodendrocyte production results in progressive myelination of previously unmyelinated and partially myelinated axons over days to months. This form of myelin plasticity is robust in the superficial layers of the mouse cortex and extends until late stages of adulthood. A subpopulation of myelin segments exhibit length plasticity (double arrow) however the majority remain stable after initial formation. In aging or after myelin damage the myelin sheath can display several forms of pathology including the formation of myelin spheroids and blebbing. In some cases, the myelin associated with these spheroids will degenerate. In aging or after myelin damage oligodendrocyte death is also prominent and results in myelin loss and accumulation of myelin debris. Questions remain about the dynamics and patterns of remyelination.
Increasing evidence suggests that oligodendrocyte generation can be modulated by changes in neuronal activity (Fields, 2015) and that forms of motor learning are potentially dependent on the production of new oligodendrocytes (McKenzie et al., 2014; Schneider et al., 2016). Manipulation of neuronal activity via optogenetics, chemogenetics, or changes in sensory input have largely shown increased oligodendrocyte generation in response to increased activity (Gibson et al., 2014; Boda et al., 2015; Romanelli et al., 2016; Hughes et al., 2018; Mitew et al., 2018) and decreased oligodendrocyte generation in response to decreased activity (Liu et al., 2012; Makinodan et al., 2012; Hill et al., 2014). However the role of activity is highly context dependent with other studies showing differing or minor effects (Demerens et al., 1996; Etxeberria et al., 2016; Mayoral et al., 2018). In terms of imaging, recent work found that an enriched whisker sensory environment results in increased survival and incorporation of differentiating oligodendrocytes in the adult cortex (Hughes et al., 2018). This is consistent with fixed tissue work showing decreased survival after whisker sensory deprivation during early postnatal development (Hill et al., 2014). The cellular dynamics and direct signaling mechanisms modulating these effects are unclear. Future work using live imaging will further elucidate the axon specificity of these different forms of activity-dependent myelination and the functional consequences of such cellular changes.
Dynamics of myelin formation
Live imaging studies have determined that pre-myelinating oligodendrocytes choose which axons to myelinate over the course of a few hours through a process of axon sampling and internode elongation (Watkins et al., 2008; Almeida et al., 2011; Czopka et al., 2013). Subsequent studies used similar methods in combination with high resolution ultrastructural analyses to characterize the sequence of events for addition of myelin layers during internode formation. This study revealed that new myelin layers form at the interface between the axon and the myelin sheath (Snaidero et al., 2014). Transient formation of myelin cytoplasmic channels was also characterized using these techniques, adding a structural element to CNS myelin that is likely important for the formation, maturation, and pathology of the myelin sheath.
Myelination along stretches of single cortical axons is not always continuous (Tomassy et al., 2014). Since there is protracted generation of new oligodendrocytes in the adult cortex (Young et al., 2013; Hill et al., 2018; Hughes et al., 2018) this raises the question of whether or not unmyelinated regions along partially myelinated axons become filled in over time. Tracing of myelin coverage at single time points in combination with longitudinal in vivo imaging of single labeled axons suggests that this is indeed the case (Hill et al., 2018). Over days to months some non-myelinated regions become myelinated while other stretches along the same axon remain unmyelinated (Figure 3). Signals inducing these myelinating events and the functional consequences of adding one or two internodes along the length of an axon are not known.
Multiple mechanisms are likely involved in the regulation of axon selection for myelination. Exocytosis of neurotransmitters is thought to be one mechanism that modulates internode formation. Genetic manipulation combined with live imaging in zebrafish showed that blocking exocytosis results in decreased internode stability and formation and overall deposition of fewer myelinating segments (Hines et al., 2015; Mensch et al., 2015). Furthermore, imaging in cell cultures has provided evidence for local production of myelin proteins in response to vesicular release (Wake et al., 2015). Interestingly other imaging studies have shown that vesicular release is not important for myelin formation on all types of axons even at the same developmental stage (Koudelka et al., 2016). Separate mechanisms could include axon diameter, axon and oligodendrocyte density, and expression of permissive or inhibitory cell surface cues which may or may not be dependent on neuronal activity or axon molecular identity (Rosenberg et al., 2008; Almeida et al., 2011, 2018; Redmond et al., 2016).
In order to investigate the dynamic intracellular signaling involved in oligodendrocyte myelin segment formation, imaging studies in zebrafish and in slices from mouse cortex have used fluorescent indicators to detect oligodendrocyte calcium fluctuations (Baraban et al., 2018; Krasnow et al., 2018; Battefeld et al., 2019). In zebrafish, calcium transient frequency in developing sheaths was correlated with process elongation while high amplitude long duration calcium fluctuations (on the order of tens of seconds) often preceded process retraction (Baraban et al., 2018; Krasnow et al., 2018). Thus, there appears to be discrete spatiotemporal signals that use different calcium concentration to regulate myelin segment formation. Neuronal activity could be one way to modulate these signals. Consistent with this, electrical stimulation in neurons induced calcium fluctuations in oligodendrocyte processes and TTX mediated inhibition of neuronal activity decreased oligodendrocyte calcium fluctuations (Krasnow et al., 2018). This suggested that dynamic signaling from the neuron is involved in modulating these calcium signals. In contrast, recent work in mouse cortical slices found no significant connection between neuronal activity and calcium transients in oligodendrocyte processes (Battefeld et al., 2019). One important difference between these studies was the developmental stage of the cells being studied as the work in zebrafish was predominantly examining oligodendrocytes just as they were forming myelin segments while work in the mouse was likely analyzing more mature oligodendrocytes. Other considerations when comparing these studies include early zebrafish spinal cord vs. postnatal mouse cortex, genetically encoded vs dye-based calcium indictors, and image acquisition modalities and frame rates. Future work will provide insight into which signaling pathways and stages of internode formation these intracellular calcium signals are involved, and whether or not they are directly modulated by neuronal signaling.
Plasticity of myelin segments
After initial restructuring and selection of axons for myelination, it is not clear if oligodendrocytes maintain the potential for myelin segment length plasticity. Myelin segment length significantly influences action potential conduction (Brill et al., 1977; Waxman, 1997) and can vary widely between different oligodendrocytes in different brain regions and even within the same cell (Chong et al., 2012). Fixed tissue studies have provided evidence that oligodendrocytes generated in adulthood are shorter compared to those generated during development (Young et al., 2013). Additionally, there is evidence that myelin internodes are shorter in the aging brain (Lasiene et al., 2009) and long-term changes in segment length can occur during the remyelination process (Powers et al., 2013). While these studies all suggest the potential for myelin length plasticity, live imaging is the clearest way to determine if and when this type of myelin structural plasticity occurs in vivo.
Longitudinal imaging in zebrafish and mice has now directly investigated this question with the overall conclusion that while it can happen in a subset of myelin segments, the majority do not exhibit significant length plasticity under baseline conditions (Auer et al., 2018; Hill et al., 2018; Hughes et al., 2018). The segments that do exhibit length plasticity are adjacent to non-myelinated axonal regions. As new oligodendrocytes are generated throughout adulthood resulting in gradual filling in of non-myelinated axonal regions, the overall percentage of myelin segments displaying length plasticity declines (Hill et al., 2018; Hughes et al., 2018). Furthermore, established oligodendrocytes have not been observed to sprout entirely new myelinating segments (Hill et al., 2018; Hughes et al., 2018) consistent with the observations that the number of segments that a single oligodendrocyte makes is set in a relatively small time window during initial myelination (Czopka et al., 2013).
Signaling mechanisms regulating myelin segment plasticity are not known. Based on the relationship between neuronal activity and oligodendrocyte formation and survival, one hypothesis is that activity-dependent mechanisms are involved in myelin lengthening or shortening. Interestingly, changes in sensory input do not appear to have a major effect on initiating lengthening or shortening (Hughes et al., 2018). However, oligodendrocyte death can (re)activate plasticity as neighboring myelin segments were observed to exhibit lengthening in response to oligodendrocyte ablation in the zebrafish (Auer et al., 2018). Length changes such as these would theoretically disrupt precise propagation speeds along stretches of single axons. The same study also provided evidence for reestablishment of some of the initial myelin patterns after remyelination (Auer et al., 2018). Hence, axons might possess signaling mechanisms to induce (or reestablish) precise myelin patterns in order to maintain propagation speed in neural circuits, even after myelin damage has occurred. There is evidence that the number and length of myelin segments a single oligodendrocyte forms is dictated by its brain region of origin (Bechler et al., 2015) thus oligodendrocyte cell intrinsic mechanisms are also potentially involved. Clearly intravital imaging approaches are poised to further dissect these intriguing questions.
Myelin damage and repair
Myelin damage is associated with many forms of CNS injury and disease. The development of intravital imaging approaches has provided new tools to further understand how myelin degenerates, is cleared from the tissue, and is repaired via remyelination. Recent work in zebrafish and xenopus has described both laser and genetic tools for oligodendrocyte ablation in order to investigate the regenerative response by remaining cells (Kirby et al., 2006; Kaya et al., 2012; Auer et al., 2018). As discussed above, these studies have described important steps involved in internode degeneration and patterns of myelin regeneration (Auer et al., 2018).
Intravital imaging in mouse spinal cord using the multiple sclerosis-like animal model experimental autoimmune encephalomyelitis (EAE), has also revealed several fundamental steps in myelin degeneration. These include the formation of myelin out-foldings during early stages of tissue damage called myelinosomes (Romanelli et al., 2016), phenotypic transitions of different classes of immune cells at distinct stages of disease (Locatelli et al., 2018), and cellular and molecular mechanisms of axonal damage and degeneration (Williams et al., 2014; Witte et al., 2019). Overall these studies provide compelling examples of how intravital imaging reveals previously unrecognized discrete transition points associated with neuropathology that are potentially targetable via therapeutics.
In addition to primary demyelinating diseases there is also evidence for significant age-related myelin degeneration in both humans and other mammals which is thought to contribute to age-related cognitive slowing (Bartzokis et al., 2010). This degeneration is characterized by myelin swelling and ballooning, spheroid formation, internode loss, oligodendrocyte death and overall decrease in myelin density (Lintl and Braak, 1983; Sturrock, 1987; Peters, 2002; Bartzokis, 2004; Peters and Kemper, 2012). Recently, label-free SCoRe imaging in aged mice allowed detection of myelin degeneration in vivo. This approach revealed long-term formation of myelin spheroids, accumulation of myelin debris, internode degeneration and significant myelin and oligodendrocyte loss over extended periods (Hill et al., 2018). Moreover, these experiments established these techniques for intravital investigations of myelin degeneration.
Finally, clearance of myelin debris is a crucial step that must occur to permit tissue repair and successful remyelination (Franklin and Ffrench-Constant, 2017). Microglia are intimately involved in this process and there is evidence from fixed tissue and intravital imaging that age-related myelin degeneration results in long-term accumulation of myelin debris within microglia (Safaiyan et al., 2016; Cantuti-Castelvetri et al., 2018; Hill et al., 2018). It does not appear that microglia are actively phagocytosing intact or damaged myelin internodes but instead pick up shed debris or dying cells (Hill et al., 2018). Eventually they become overwhelmed with excess debris which coincides with decreased functionality in aging (Safaiyan et al., 2016; Cantuti-Castelvetri et al., 2018). Similar mechanisms could be involved in diseases associated with delayed or disrupted clearance of cell debris and highlights important multicellular interactions that should be further studied using live multicellular imaging.
Concluding remarks
Increasing evidence suggests that myelination is more than just axonal insulation produced during development but is instead a dynamic structure that is continually generated and modified throughout life. Intravital imaging of myelin in animal models has provided multiple insights into the cellular and molecular mechanisms involved in oligodendrocyte differentiation, axon selection, myelin remodeling, and cellular degeneration in both injury and disease. These studies have predominantly implemented fluorescence-based methods for detection and visualization of these events, however the recent development of imaging techniques that permit specific label-free imaging of myelin provide powerful complementary approaches for imaging in intact, live tissue. Recent advances using longer wavelength laser light has allowed increased penetration into tissue with both OCM and SCoRe microscopy demonstrating acute imaging of white matter tracts in the corpus callosum in animal models (Chong et al., 2015; Yamanaka et al., 2016; Xia et al., 2018). Further refinement of these approaches and exploration of the differences in refractive index and molecular organization of developing and damaged myelin are clear examples of avenues worth pursuing with label-free techniques. Overall, future work applying a combination of fluorescence and label-free approaches in parallel with genetic manipulation and optical probes of cell physiology will uncover how this elegant structure develops, changes throughout life, and is disrupted in disease.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: R00-NS099469 and P20-GM113132 to R.A.H. and R01-NS089734, R21-NS087511 and R21-NS088411to J.G. This work was also supported by a New Vision Award through the Donors Cure Foundation to R.A.H. and a National Multiple Sclerosis Society grant to J.G.
References
- Ackerman SD, Monk KR. 2016. The scales and tales of myelination: using zebrafish and mouse to study myelinating glia. Brain Res 1641:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens MB, Engert F. 2015. Large-scale imaging in small brains. Curr Opin Neurobiol 32:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Czopka T, Ffrench-Constant C, Lyons DA. 2011. Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development 138:4443–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Lyons DA. 2017. On myelinated axon plasticity and neuronal circuit formation and function. J Neurosci 37:10023–10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RG, Pan S, Cole KLH, Williamson JM, Early JJ, Czopka T, Klingseisen A, Chan JR, Lyons DA. 2018. Myelination of Neuronal Cell Bodies when Myelin Supply Exceeds Axonal Demand. Curr Biol 28:1296–1305.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer F, Vagionitis S, Czopka T. 2018. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr Biol 28:549–559.e3. [DOI] [PubMed] [Google Scholar]
- Baraban M, Koudelka S, Lyons DA. 2018. Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat Neurosci 21:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. 1992. Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70:31–46. [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. 1999. Axonal control of oligodendrocyte development. J Cell Biol 147:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G 2004. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging 25:5–18. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. 2010. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging 31:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A, Popovic MA, Vries SI de, Kole MHP. 2019. High-Frequency Microdomain Ca2+ Transients and Waves during Early Myelin Internode Remodeling. Cell Reports 26:182–191.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechler ME, Byrne L, Ffrench-Constant C. 2015. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr Biol 25:2411–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Arous J, Binding J, Léger J-F, Casado M, Topilko P, Gigan S, Boccara AC, Bourdieu L. 2011. Single myelin fiber imaging in living rodents without labeling by deep optical coherence microscopy. J Biomed Opt 16:116012. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jabs R, Steinhäuser C. 2010. Neuron-glia synapses in the brain. Brain Res Rev 63:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. 2000. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405:187–191. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Rao TS, Webb M. 2004. Lysolecithin induces demyelination in vitro in a cerebellar slice culture system. J Neurosci Res 78:157–166. [DOI] [PubMed] [Google Scholar]
- Boda E, Di Maria S, Rosa P, Taylor V, Abbracchio MP, Buffo A. 2015. Early phenotypic asymmetry of sister oligodendrocyte progenitor cells after mitosis and its modulation by aging and extrinsic factors. Glia 63:271–286. [DOI] [PubMed] [Google Scholar]
- Brill MH, Waxman SG, Moore JW, Joyner RW. 1977. Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. J Neurol Neurosurg Psychiatry 40:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil M-T, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M. 2018. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359:684–688. [DOI] [PubMed] [Google Scholar]
- Chong SP, Merkle CW, Cooke DF, Zhang T, Radhakrishnan H, Krubitzer L, Srinivasan VJ. 2015. Noninvasive, in vivo imaging of subcortical mouse brain regions with 1.7 μm optical coherence tomography. Opt Lett 40:4911–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SYC, Rosenberg SS, Fancy SPJ, Zhao C, Shen Y-AA, Hahn AT, McGee AW, Xu X, Zheng B, Zhang LI, Rowitch DH, Franklin RJM, Lu QR, Chan JR. 2012. Neurite outgrowth inhibitor Nogo-A establishes spatial segregation and extent of oligodendrocyte myelination. Proc Natl Acad Sci USA 109:1299–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czopka T 2016. Insights into mechanisms of central nervous system myelination using zebrafish. Glia 64:333–349. [DOI] [PubMed] [Google Scholar]
- Czopka T, Ffrench-Constant C, Lyons DA. 2013. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev Cell 25:599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. 2005. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. 1996. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA 93:9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Kim B, He X, Kim S, Lu C, Wang H, Cho S-G, Hou Y, Li J, Zhao X, Lu QR. 2014. Direct visualization of membrane architecture of myelinating cells in transgenic mice expressing membrane-anchored EGFP. Genesis 52:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Götz M. 2014. Glial cells as progenitors and stem cells: new roles in the healthy and diseased brain. Physiol Rev 94:709–737. [DOI] [PubMed] [Google Scholar]
- Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. 2008. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci 28:10434–10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar N, Vainshtein A, Golan N, Vijayaragavan B, Schaeren-Wiemers N, Eshed-Eisenbach Y, Peles E. 2019. Axoglial Adhesion by Cadm4 Regulates CNS Myelination. Neuron 101:224–231.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B 2010. Regulation of oligodendrocyte differentiation and myelination. Science 330:779–782. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR. 2016. Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J Neurosci 36:6937–6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CL, Potma EO, Puoris’haag M, Côté D, Lin CP, Xie XS. 2005. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. PNAS 102:16807–16812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CL, Xie XS. 2008. Coherent anti-stokes Raman scattering microscopy: chemical imaging for biology and medicine. Annu Rev Anal Chem (Palo Alto Calif) 1:883–909. [DOI] [PubMed] [Google Scholar]
- Farrar MJ, Wise FW, Fetcho JR, Schaffer CB. 2011. In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys J 100:1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. 2015. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJM, Ffrench-Constant C. 2017. Regenerating CNS myelin - from mechanisms to experimental medicines. Nat Rev Neurosci 18:753–769. [DOI] [PubMed] [Google Scholar]
- Friede RL. 1972. Control of myelin formation by axon caliber (with a model of the control mechanism). J Comp Neurol 144:233–252. [DOI] [PubMed] [Google Scholar]
- Fu Y, Huff TB, Wang H-W, Wang H, Cheng J-X. 2008. Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy. Opt Express 16:19396–19409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. 2014. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Káradóttir RT, Nave K-A. 2017. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci 20:10–15. [DOI] [PubMed] [Google Scholar]
- Harboe M, Torvund‐Jensen J, Kjaer‐Sorensen K, Laursen LS. 2018. Ephrin-A1-EphA4 signaling negatively regulates myelination in the central nervous system. Glia 66:934–950. [DOI] [PubMed] [Google Scholar]
- Hee MR, Izatt JA, Swanson EA, Huang D, Schuman JS, Lin CP, Puliafito CA, Fujimoto JG. 1995. Optical Coherence Tomography of the Human Retina. Arch Ophthalmol 113:325–332. [DOI] [PubMed] [Google Scholar]
- Henry FP, Wang Y, Rodriguez CLR, Randolph MA, Rust EAZ, Winograd JM, de Boer JF, Park BH. 2015. In vivo optical microscopy of peripheral nerve myelination with polarization sensitive-optical coherence tomography. J Biomed Opt 20:046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Damisah EC, Chen F, Kwan AC, Grutzendler J. 2017. Targeted two-photon chemical apoptotic ablation of defined cell types in vivo. Nat Commun 8:15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Grutzendler J. 2014. In vivo imaging of oligodendrocytes with sulforhodamine 101. Nat Methods 11:1081–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Li AM, Grutzendler J. 2018. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci 21:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Natsume R, Sakimura K, Nishiyama A. 2011. NG2 cells are uniformly distributed and NG2 is not required for barrel formation in the somatosensory cortex. Mol Cell Neurosci 46:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Nishiyama A. 2014. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia 62:1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A. 2014. Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci 17:1518–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RA, Patel KD, Medved J, Reiss AM, Nishiyama A. 2013. NG2 cells in white matter but not gray matter proliferate in response to PDGF. J Neurosci 33:14558–14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. 2015. Neuronal activity biases axon selection for myelination in vivo. Nat Neurosci 18:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Et A. 1991. Optical coherence tomography. Science 254:1178–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Kang SH, Fukaya M, Bergles DE. 2013. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci 16:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE. 2018. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat Neurosci 21:696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. 2010. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya F, Mannioui A, Chesneau A, Sekizar S, Maillard E, Ballagny C, Houel-Renault L, Dupasquier D, Bronchain O, Holtzmann I, Desmazieres A, Thomas J-L, Demeneix BA, Brophy PJ, Zalc B, Mazabraud A. 2012. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J Neurosci 32:12885–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B. 2006. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat Neurosci 9:1506–1511. [DOI] [PubMed] [Google Scholar]
- Komitova M, Serwanski DR, Lu QR, Nishiyama A. 2011. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia 59:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA. 2016. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr Biol 26:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow AM, Ford MC, Valdivia LE, Wilson SW, Attwell D. 2018. Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo. Nat Neurosci 21:24–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. 2008. Glial cells are born with synapses. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. [DOI] [PubMed] [Google Scholar]
- Lasiene J, Matsui A, Sawa Y, Wong F, Horner PJ. 2009. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 8:201–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng Z-Q, Corey JM, Chan JR. 2012. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods 9:917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Reynolds R, Fawcett JW. 2001. The oligodendrocyte precursor cell in health and disease. Trends in Neurosciences 24:39–47. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Fraser SE. 2001. The neuronal naturalist: watching neurons in their native habitat. Nat Neurosci 4 Suppl:1215–1220. [DOI] [PubMed] [Google Scholar]
- Lim H, Sharoukhov D, Kassim I, Zhang Y, Salzer JL, Melendez-Vasquez CV. 2014. Label-free imaging of Schwann cell myelination by third harmonic generation microscopy. Proc Natl Acad Sci USA 111:18025–18030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintl P, Braak H. 1983. Loss of intracortical myelinated fibers: a distinctive age-related alteration in the human striate area. Acta Neuropathol 61:178–182. [DOI] [PubMed] [Google Scholar]
- Liu J, Casaccia P. 2010. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci 33:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. 2012. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T-L, Upadhyayula S, Milkie DE, Singh V, Wang K, Swinburne IA, Mosaliganti KR, Collins ZM, Hiscock TW, Shea J, Kohrman AQ, Medwig TN, Dambournet D, Forster R, Cunniff B, Ruan Y, Yashiro H, Scholpp S, Meyerowitz EM, Hockemeyer D, Drubin DG, Martin BL, Matus DQ, Koyama M, Megason SG, Kirchhausen T, Betzig E. 2018. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Lavis LD, Betzig E. 2015. Imaging Live-Cell Dynamics and Structure at the Single-Molecule Level. Molecular Cell 58:644–659. [DOI] [PubMed] [Google Scholar]
- Locatelli G, Theodorou D, Kendirli A, Jordão MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, Meissner F, Prinz M, Kerschensteiner M. 2018. Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci 21:1196–1208. [DOI] [PubMed] [Google Scholar]
- Lyons DA, Talbot WS. 2014. Glial cell development and function in zebrafish. Cold Spring Harb Perspect Biol 7:a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. 2012. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin J-M, Li P, Scafidi J, Gallo V. 2012. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci 15:1192–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral SR, Etxeberria A, Shen Y-AA, Chan JR. 2018. Initiation of CNS myelination in the optic nerve is dependent on axon caliber. Cell Rep 25:544–550.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. 2014. Motor skill learning requires active central myelination. Science 346:318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. 2001. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci 21:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. 2015. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 18:628–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kerschensteiner M. 2006. In vivo imaging of the diseased nervous system. Nat Rev Neurosci 7:449–463. [DOI] [PubMed] [Google Scholar]
- Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B. 2018. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nature Communications 9:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave K-A, Trapp BD. 2008. Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci 31:535–561. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318. [DOI] [PubMed] [Google Scholar]
- Nishiyama A 2007. Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Komitova M, Suzuki R, Zhu X. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. 1996. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43:299–314. [DOI] [PubMed] [Google Scholar]
- Ohno N, Kidd GJ, Mahad D, Kiryu-Seo S, Avishai A, Komuro H, Trapp BD. 2011. Myelination and Axonal Electrical Activity Modulate the Distribution and Motility of Mitochondria at CNS Nodes of Ranvier. J Neurosci 31:7249–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orduz D, Maldonado PP, Balia M, Vélez-Fort M, de Sars V, Yanagawa Y, Emiliani V, Angulo MC. 2015. Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex. eLife 4:e06953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osso LA, Chan JR. 2017. Architecting the myelin landscape. Curr Opin Neurobiol 47:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A 2002. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 31:581–593. [DOI] [PubMed] [Google Scholar]
- Peters A, Kemper T. 2012. A review of the structural alterations in the cerebral hemispheres of the aging rhesus monkey. Neurobiol Aging 33:2357–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers BE, Sellers DL, Lovelett EA, Cheung W, Aalami SP, Zapertov N, Maris DO, Horner PJ. 2013. Remyelination reporter reveals prolonged refinement of spontaneously regenerated myelin. Proc Natl Acad Sci USA 110:4075–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston MA, Macklin WB. 2015. Zebrafish as a model to investigate CNS myelination. Glia 63:177–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen Y-AA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, Chan JR. 2016. Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination. Neuron 91:824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. 2011. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron 70:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli E, Merkler D, Mezydlo A, Weil M-T, Weber MS, Nikić I, Potz S, Meinl E, Matznick FEH, Kreutzfeldt M, Ghanem A, Conzelmann K-K, Metz I, Brück W, Routh M, Simons M, Bishop D, Misgeld T, Kerschensteiner M. 2016. Myelinosome formation represents an early stage of oligodendrocyte damage in multiple sclerosis and its animal model. Nat Commun 7:13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli E, Sorbara CD, Nikić I, Dagkalis A, Misgeld T, Kerschensteiner M. 2013. Cellular, subcellular and functional in vivo labeling of the spinal cord using vital dyes. Nature Protocols 8:481–490. [DOI] [PubMed] [Google Scholar]
- Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. 2008. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci USA 105:14662–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M. 2016. Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL, Zalc B. 2016. Myelination. Curr Biol 26:R971–R975. [DOI] [PubMed] [Google Scholar]
- Schain AJ, Hill RA, Grutzendler J. 2014. Label-free in vivo imaging of myelinated axons in health and disease with spectral confocal reflectance microscopy. Nat Med 20:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Gruart A, Grade S, Zhang Y, Kröger S, Kirchhoff F, Eichele G, Delgado García JM, Dimou L. 2016. Decrease in newly generated oligodendrocytes leads to motor dysfunctions and changed myelin structures that can be rescued by transplanted cells. Glia 64:2201–2218. [DOI] [PubMed] [Google Scholar]
- Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave K-A, Simons M. 2014. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell 156:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N. 2011. CNS live imaging reveals a new mechanism of myelination: The liquid croissant model. Glia 59:1841–1849. [DOI] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. 1998. Control of myelination by specific patterns of neural impulses. J Neurosci 18:9303–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock RR. 1987. Age-related changes in the number of myelinated axons and glial cells in the anterior and posterior limbs of the mouse anterior commissure. J Anat 150:111–127. [PMC free article] [PubMed] [Google Scholar]
- Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J, Phillips J, Siu J, Lim DA, Vandenberg S, Stallcup W, Berger MS, Bergers G, Weiss WA, Petritsch C. 2011. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 20:328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DG, Tokumoto YM, Raff MC. 2000. Long-Term Culture of Purified Postnatal Oligodendrocyte Precursor Cells: Evidence for an Intrinsic Maturation Program That Plays Out over Months. The Journal of Cell Biology 148:971–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S, Raff MC. 1986. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell 44:773–779. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. 2014. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. 1997. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol 137:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viganò F, Möbius W, Götz M, Dimou L. 2013. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16:1370–1372. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. 1989. Target size regulates calibre and myelination of sympathetic axons. Nature 342:430–433. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. 2011. Control of local protein synthesis and initial events in myelination by action potentials. Science (New York, NY). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. 2015. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nature Communications 6:7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Fu Y, Zickmund P, Shi R, Cheng J-X. 2005. Coherent anti-stokes Raman scattering imaging of axonal myelin in live spinal tissues. Biophys J 89:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, Emery B, Mulinyawe S, Barres BA. 2008. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron 60:555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. 1997. Axon-glia interactions: building a smart nerve fiber. Curr Biol 7:R406–10. [DOI] [PubMed] [Google Scholar]
- Weigelin B, Bakker G-J, Friedl P. 2016. Third harmonic generation microscopy of cells and tissue organization. J Cell Sci 129:245–255. [DOI] [PubMed] [Google Scholar]
- Williams PR, Marincu B-N, Sorbara CD, Mahler CF, Schumacher A-M, Griesbeck O, Kerschensteiner M, Misgeld T. 2014. A recoverable state of axon injury persists for hours after spinal cord contusion in vivo. Nat Commun 5:5683. [DOI] [PubMed] [Google Scholar]
- Witte ME, Schumacher A-M, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, Sánchez P, Williams PR, Griesbeck O, Naumann R, Misgeld T, Kerschensteiner M. 2019. Calcium Influx through Plasma-Membrane Nanoruptures Drives Axon Degeneration in a Model of Multiple Sclerosis. Neuron 101:615–624.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia F, Wu C, Sinefeld D, Li B, Qin Y, Xu C. 2018. In vivo label-free confocal imaging of the deep mouse brain with long-wavelength illumination. Biomed Opt Express, BOE 9:6545–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka M, Teranishi T, Kawagoe H, Nishizawa N. 2016. Optical coherence microscopy in 1700 nm spectral band for high-resolution label-free deep-tissue imaging. Sci Rep 6:31715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Psachoulia K, Tripathi RB, Dunn S-J, Cossell L, Attwell D, Tohyama K, Richardson WD. 2013. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77:873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jarjour AA, Boyd A, Williams A. 2011. Central nervous system remyelination in culture — A tool for multiple sclerosis research. Experimental Neurology 230:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. 2011. Age-dependent fate and lineage restriction of single NG2 cells. Development 138:745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA. 2013. Intrinsic and extrinsic control of oligodendrocyte development. Curr Opin Neurobiol 23:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]