Abstract
Although heart transplantation remains the gold standard for management of heart failure, ventricular assist devices (VAD) have emerged as viable alternatives. VAD implantation improves kidney function. However, whether the improvement is sustained or associated with improved outcomes is unclear.
Herein we assess kidney function improvement, predictors of improvement, and associations with thromboembolism, hemorrhage, and mortality in VAD patients. Kidney function was defined using CKD stages: Stage 1 (glomerular filtration rate (eGFR) ≥ 90 mL/min/1.73m2), Stage 2 (eGFR 60–90 mL/min/1.73m2), Stage 3a (eGFR 45–59 mL/min/1.73m2), Stage 3b (eGFR 30–44 mL/min/1.73m2), Stage 4 (eGFR 15–30 mL/min/1.73m2), and Stage 5 (eGFR<15 mL/min/1.73m2). Improvement in kidney function was defined as an improvement in eGFR that resulted in a CKD stage change to one of lesser severity.
Kidney function improved post implant, and was maintained over 1 year for all patients, except those with baseline Stage-5 CKD. Younger age at implantation (OR 0.93, 95% CI 0.90–0.96, p<0.0001) was associated with sustained improvement in kidney function.
Poor kidney function was associated increased mortality but not with thromboembolism or hemorrhage. Compared to patients with baseline eGFR>45 mL/min/1.73m2; patients with eGFR<45 mL/min/1.73m2 had a higher mortality risk (HR 3.32, 95%CI 1.10–9.98, p=0.03 for Stage 3b; HR 4.07, 95%CI 1.27–13.1, p=0.02 for Stage 4; and HR 4.01, 95%CI 1.17–13.7, p=0.03 for Stage5 CKD).
Kidney function was not associated with thromboembolism or hemorrhage, and sustained improvement was not associated with lower risk of death. However, poor kidney function at implantation was associated with an increased risk of mortality.
Keywords: Ventricular assist device, kidney function, advanced heart failure, mechanical circulatory support
Introduction
The prevalence of chronic kidney disease (CKD) is increasing1 especially in patients with cardiovascular disease. Thirty to forty percent of patients with heart failure (HF) have CKD,2 and the prevalence increases further (up to 63%) among advanced HF patients.3,4 In approximately 25% of advanced HF patients, chronic cardiac dysfunction is responsible for progressive CKD.5,6 CKD is shown to be a stronger predictor of mortality among HF patients compared to cardiac function.7–10
Treatment strategies for HF range from lifestyle changes and medical management to heart transplant or ventricular assist device (VAD) depending on the severity of HF.11,12 Among patients with end-stage HF, VAD implantation is increasingly used as a bridge to transplant (BTT), or as destination therapy (DT).
Patients with VAD implants demonstrate an improvement in kidney function, as measured by any increase in glomerular filtration rate (GFR) following implantation.13 However, the bulk of the evidence supports improvement in the early (7–30 days) post-implant period. Recent studies with longer follow-up also suggest an early improvement in kidney function, but whether this improvement is sustained is unclear. Moreover, these studies defined improvement in kidney function as any increase in GFR, grouped patients using different GFR thresholds (GFR ≥60 vs. <6014,15; GFR ≥40 vs. <4016) or restricted analysis to subgroups, such as those with end-stage renal disease17 or those who survived >3 years post-implantation.18
Herein, we include VAD patients across the kidney function spectrum, use baseline GFR to categorize patients into CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative and define improvement in kidney function as an increase in GFR that results in a CKD stage change to one of lesser severity. Using this framework, we assess improvement in kidney function post-VAD implantation, whether the improvement in kidney function is sustained for a period of one year, identify predictors of kidney function improvement, and examine whether kidney function improvement is associated with a decrease in risk of thromboembolic events, hemorrhagic events, and all-cause mortality.
Materials and Methods
Study Setting
The study enrolled patients who received a ventricular assist device (VAD) from 2002–2012 at the University of Alabama at Birmingham under the approval of the Institutional Review Board.
Inclusion and Exclusion Criteria
Patients aged 19 years and older who had a VAD (continuous-flow or pulsatile flow) placed at UAB from 2002–2012 were included in this study. All patients received post-implant medical care at the implanting center. Most patients received inpatient care for 2–4 weeks post-implantation. After discharge, all patients received care as outpatients at monthly intervals.
Of the 241 VAD patients eligible, 13 patients were implanted at another institution and then received follow-up care at UAB. These individuals were excluded because their baseline kidney function assessment at time of implantation was not available. This resulted in 228 patients in our final study sample.
Data collection
For all patients, information including demographic variables (age at implant, self-reported race, ethnicity etc.), medical history prior to VAD (comorbid conditions, heart failure etiology etc.), and laboratory assessments were collected at baseline (pre-VAD implantation). Clinical data, including medications and laboratory assessments, were collected from each post-VAD clinic visit or hospitalization period during the 1-year follow-up period. All patients were on standard antithrombotic therapy with warfarin (dose adjusted to maintain an international normalized ratioINR goal of 2–3) and aspirin (81 or 325mg /day). Patients were followed for 1-year post-VAD implantation or until explantation of the device, transplant or death prior to 1 year. All adverse events were defined using definitions from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) Registry.19
Definition of Exposure
Kidney function was assessed based on estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration Formula (CKD-EPI)20 which incorporates gender, race, age, and serum creatinine (Scr; mg/dL). We assessed change in kidney function from VAD implantation at the following six time-points: pre-implantation, 14 ± 2 days, 1 month ± 2 days, 3 months ± 7 days, 6 months ± 7 days, 9 months ± 7 days and 12 months ± 7 days post-implantation.
We present CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative guidelines: The groups were Stage 1 (eGFR≥90 ml/min/1.73m2), Stage 2 (eGFR 60–90 ml/min/1.73m2), Stage 3a (eGFR 45–59 ml/min/1.73m2), Stage 3b (eGFR 30–44 ml/min/1.73m2), Stage 4 (eGFR 15–30 ml/min/1.73m2) and Stage 5 (eGFR<15 ml/min/1.73m2).21
Improvement in kidney function was defined as an improvement in eGFR that resulted in a CKD stage change to one of lesser severity. For example, consider two patients with eGFR of 36 at baseline. One patient’s eGFR improves to 52ml/min while the second patient’s eGFR improves to 39ml/min. While eGFR improved in both patients, in the first patient the improvement would reclassify the CKD stage from Stage 3b to Stage 3a.
We also assessed if improvements in eGFR were sustained over the 1-year follow-up period post implantation. Sustained improvement was defined as improvement in CKD stage over baseline and improvement in CKD stage that was sustained over the 1-year follow-up period. Patients with CKD Stage 1 or 2 at baseline who maintained kidney function within the pre-VAD CKD stage were included in the sustained improvement category as they did not have a decrease in kidney function post VAD.
Definition of Outcomes
Outcomes of interest included thromboembolic events, hemorrhagic events, and all-cause mortality. Outcomes were determined as a documented event through review of medical records. Thromboembolic events included ischemic stroke, transient ischemic attack, pulmonary embolus, deep vein thrombosis, pump thrombosis requiring hospitalization, and mediastinal clot requiring surgical removal. Hemorrhagic events included intracranial hemorrhage, pericardial bleeding, intraarticular bleeding, gastrointestinal bleed, greater than 2 units of packed red blood cells administered at one time, and mediastinal bleeding requiring surgical intervention. Mortality was defined as death from any cause.
Statistical Analysis
The χ2 test of independence was used to assess group differences for categorical variables and ANOVA/Student’s t-test or Kruskal-Wallis/Wilcoxon Rank Sum where appropriate for continuous variables. Logistic regression models were used to assess predictors of sustained improvement. Cox proportional hazards models were used to evaluate the association between kidney function and thromboembolism, hemorrhage, and death. Log survival plots were used to assess the proportional hazards assumption. For mortality analysis, follow-up time was censored at time of transplant, explant (removal of the VAD device), or death. For analysis of thromboembolic or hemorrhagic event, the follow-up time was censored at the time of first event; repeated events were not considered. For patients who did not experience a thromboembolic or hemorrhagic event, follow-up time was censored at end of the study (1 year) or explantation (if earlier than 1 year). All tests were performed using SAS version 9.2 (SAS Institute, Cary, NC) at a non-directional alpha level of 0.05.
Results
Overall Population
Of the 241 VAD patients who were treated at UAB between 2002 and 2012, thirteen patients implanted at an outside hospital were excluded because baseline kidney function information was not available. Of the 228 patients (72% men, 71% White) included in the analysis, the mean age at implant was 52 years (14.6 SD), and the majority (57%) received VAD implants as a bridge to transplantation (57 %). At baseline, 35.5% participants had Stage 1 or 2 CKD (eGFR≥60 ml/min/1.73m2), 23% had Stage 3a CKD (eGFR 45–59 ml/min/1.73m2), 23% had Stage 3b CKD (eGFR 30–44 ml/min/1.73m2), 13% had Stage 4 CKD (eGFR 15–29 ml/min/1.73m2) and 5% had Stage 5 CKD (eGFR <15 ml/min/1.73m2). No patients were on dialysis prior to VAD implantation. Baseline characteristics of the cohort, stratified by kidney function, are presented in Table 1. No significant differences were observed between CKD stages and comorbidities except patients with continuous flow devices who had less severe kidney dysfunction (p=0.001), and those with history of diabetes (p=0.02), and history of hypertension (p=0.01) who had more baseline kidney dysfunction.
Table 1.
Demographic and Clinical Characteristics for the Entire Cohort Stratified by Kidney Function prior to VAD Implantation
| Stage 1 eGFR ≥90 (N=32) |
Stage 2 eGFR 60-89 (N=49) |
Stage 3A eGFR 45-59 (N=53) |
Stage 3B eGFR 30-44 (N=53) |
Stage 4 eGFR 15-30 (N=30) |
Stage 5 eGFR <15 (N=11) |
p-value | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | |||||||
| Age | 43.2 ±15.9 | 51.1 ±15.6 | 51.7 ±13.4 | 56.2±14 | 56.8±11.4 | 52.6±12.6 | 0.002 |
| Body Mass Index | 30.9 ±7.7 | 27.6±8.8 | 27.1±7.8 | 26.7±5.8 | 30.3±8.2 | 25.4±4.6 | 0.11 |
| N (%) | |||||||
| Male | 25 (78) | 39 (80) | 35 (66) | 41 (77) | 20 (67) | 7 (64) | 0.49 |
| Black Race | 14 (44) | 16 (33) | 16 (30) | 10 (19) | 6 (20) | 2 (18) | 0.14 |
| Status within the First Year | 0.06 | ||||||
| Deceased | 4 (12) | 11 (22) | 17 (32) | 19 (36) | 14 (47) | 8 (73) | |
| Transplanted | 10 (31) | 12 (25) | 8 (15) | 4 (8) | 7 (23) | 2 (18) | |
| Recovered | 0 (0) | 1 (2) | 2 (4) | 0 (0) | 0 | 0 | |
| Device Type | 0.001 | ||||||
| Pulsatile Flow | 11 (34) | 18 (37) | 25 (47) | 23 (43) | 19 (63) | 11 (100) | |
| Continuous Flow | 21 (66) | 31 (63) | 28 (53) | 30 (57) | 11 (37) | 0 | |
| Etiology of Heart Failure | 0.17 | ||||||
| Ischemic | 8 (25) | 28 (57) | 25 (47) | 32 (60) | 17 (57) | 6 (55) | |
| Idiopathic | 16 (50) | 14 (28) | 23 (44) | 13 (25) | 10 (33) | 3 (27) | |
| Other | 8 (25) | 7 (14) | 5 (9) | 8 (15) | 3 (10) | 2 (18) | |
| Indication for VAD | 0.07 | ||||||
| Bridge to transplant | 23 (72) | 31 (66) | 31 (58) | 25 (48) | 13 (43) | 8 (73) | |
| Bridge to candidacy | 1 (3) | 3 (6) | 3 (6) | 3 (6) | 5 (17) | 2 (18) | |
| Bridge to recovery | 0 | 0 | 0 | 2 (4) | 1 (3) | 1 (9) | |
| Destination | 8 (25) | 13 (28) | 19 (36) | 22 (42) | 11 (37) | 0 | |
| Comorbid Conditions | |||||||
| Diabetes | 9 (28) | 11 (22) | 24 (45) | 27 (51) | 13 (43) | 2 (18) | 0.019 |
| Chronic Obstructive Pulmonary Disease | 2 (6) | 1 (2) | 2 (4) | 5 (9) | 1 (3) | 0 | 0.51 |
| Congestive Heart Failure | 27 (84) | 44 (90) | 43 (81) | 44 (83) | 24 (80) | 6 (55) | 0.15 |
| Peripheral Vascular Disease | 2 (6) | 2 (4) | 2 (4) | 1 (2) | 1 (3) | 0 | 0.90 |
| Gout | 3 (9) | 9 (18) | 5 (9) | 4 (8) | 5 (17) | 2 (18) | 0.50 |
| Right Ventricular Dysfunction | 6 (19) | 14 (29) | 8 (15) | 14 (26) | 5 (17) | 3 (27) | 0.52 |
| Coronary Artery Disease | 8 (25) | 13 (26) | 22 (41) | 17 (32) | 14 (47) | 2 (18) | 0.21 |
| History of Myocardial Infarction | 5 (16) | 8 (16) | 11 (21) | 14 (26) | 8 (27) | 2 (18) | 0.74 |
| Hypertension | 15 (47) | 28 (57) | 38 (72) | 27 (51) | 10 (33) | 4 (36) | 0.014 |
| Pulmonary Hypertension | 12 (37) | 7 (14) | 13 (24) | 10 (19) | 6 (20) | 1 (9) | 0.16 |
| Hyperlipidemia | 8 (25) | 19 (39) | 22 (41) | 20 (38) | 15 (50) | 1 (9) | 0.14 |
| Atrial Fibrillation | 10 (31) | 16 (33) | 19 (36) | 18 (34) | 10 (33) | 0 | 0.34 |
| Ventricular Tachycardia | 11 (34) | 19 (39) | 28 (53) | 16 (30) | 16 (53) | 3 (27) | 0.11 |
Kidney function is presented as CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative guidelines using estimated glomerular filtration rate (eGFR) from the Chronic Kidney Disease Epidemiology Collaboration Formula (CKD-EPI). The groups are Stage 1 (eGFR≥90 ml/min/1.73m2), Stage 2 (eGFR 60-90 ml/min/1.73m2), Stage 3a (eGRF 45-59 ml/min/1.73m2), Stage 3b (eGFR 30-44 ml/min/1.73m2), Stage 4 (eGFR 15-30 ml/min/1.73m2) and Stage 5 (eGFR<15 ml/min/1.73m2)
Continuous variables were tested using Wilcoxon Rank Sum test and categorical variables were tested using a Chi-squared test
Change in Kidney Function over Time
Within the first 14 days, a majority (56.7%; n=130) of VAD recipients demonstrated meaningful improvement in kidney function compared to baseline kidney function (Figure 1). Regardless of fluctuations in eGFR over time, early improvement in kidney function was sustained for a majority of patients (n=127) for the duration of the 1-year follow up. In patients with baseline GFR≥15, kidney function improved post-VAD implantation with maximal improvement attained at 3 months. Although patients with Stage 5 CKD showed improvement in kidney function over the first 3 months, the improvement was not sustained. There was no difference in improvement in CKD stage after implantation between continuous and pulsatile flow devices.
Figure 1. Change in Kidney Function over 1 year follow-up from VAD Implantation.
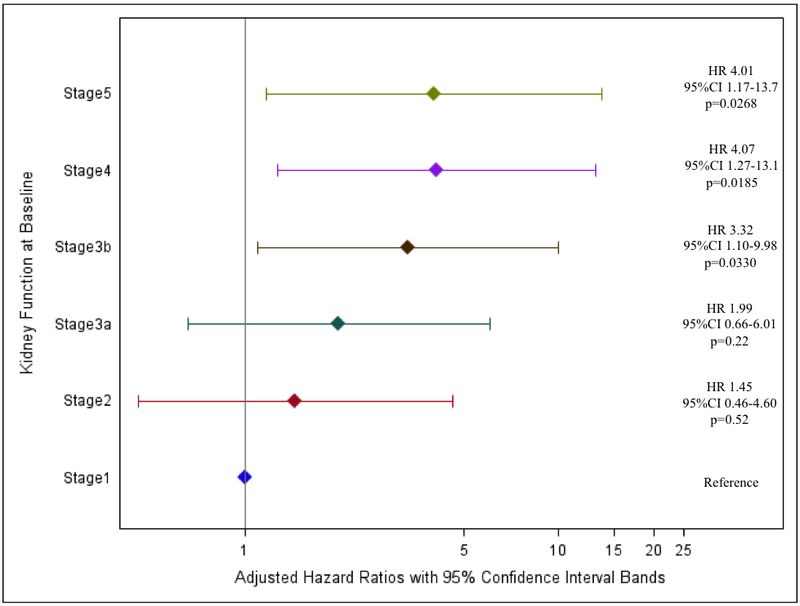
Kidney function is presented as CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative guidelines using estimated glomerular filtration rate (eGFR) from the Chronic Kidney Disease Epidemiology Collaboration Formula (CKD-EPI).
The groups are Stage 1 (eGFR≥90 ml/min/1.73m2), Stage 2 (eGFR 60-90 ml/min/1.73m2), Stage 3a (eGRF 45-59 ml/min/1.73m2), Stage 3b (eGFR 30-44 ml/min/1.73m2), Stage 4 (eGFR 15-30 ml/min/1.73m2) and Stage 5 (eGFR<15 ml/min/1.73m2)
*At 14 days, 200 patients were included and 28 patients were excluded (2 patients received transplants, 1 patient recovered and was explanted, and 25 patients died).
** At 30 days, 188 patients were included and.12 patients were excluded (12 patients died).
§ At 3 months, 158 patients were included and 30 patients were excluded (4 patient withdrawals, 2 patients recovered, 11 patients had a transplant and 13 patients died)
† At 6 months, 136 patients were included and 22 patients were excluded (1 withdrawal, 11 patients received transplants and 10 patients died)
‡At 9 months, 121 patients were included and 15 patients were excluded (10 patients received transplants and 5 patients died)
¥ At 12 months, 104 patients were included and 17 patients were excluded (8 patients received transplants and 9 patients died)
Increasing age at implantation was inversely associated with sustained improvement in kidney function. For each year increase in age at implant, the odds of having sustained improvement in kidney function decreased by 5% (OR 0.95, 95% CI 0.92–0.97, p<0.0001). This association remained after adjusting for baseline CKD stage, gender, race, history of diabetes and history of hypertension (OR 0.93, 95% CI 0.90–0.96, p<0.0001). Patients who sustained kidney function improvement over time were more likely to receive a heart transplant than those who did not sustain kidney function over time (28% vs. 16%, p=0.05).
Kidney Function and Thromboembolism
There were 39 thromboembolic events over a follow-up time of 101 person-years with an incidence rate of 3.9 per 10 person years (95% CI 2.8–5.2). Neither baseline kidney function (Table 2), nor sustained improvement in kidney function at 1 year (data not shown; HR 0.98, 95% CI 0.54–1.78, p=0.95) were associated with the risk of thromboembolic events.
Table 2.
Influence of Baseline Kidney Function on Risk (Hazard Ratio (HR) and 95% Confidence Interval (CI)) of Thromboembolism and Hemorrhage
| Hazard Ratio | 95 % Confidence Interval | p-value | |
|---|---|---|---|
| Thromboembolic Events | |||
| Stage of Kidney Disease at Baseline | |||
| Stage 1 (eGFR≥90) | ref | Ref | ref |
| Stage 2 (eGFR 60-89) | 0.82 | 0.23-2.94 | 0.77 |
| Stage 3a (eGFR 45-59) | 1.47 | 0.47-4.62 | 0.51 |
| Stage 3b (eGFR 30-44) | 1.75 | 0.55-5.58 | 0.35 |
| Stage 4 (eGFR 15-29) | 1.52 | 0.41-5.68 | 0.53 |
| Stage 5 (eGFR <15) | 0.94 | 0.10-8.38 | 0.95 |
| Hemorrhagic Events | |||
| Stage of Kidney Disease at Baseline | |||
| Stage 1 (eGFR≥90) | ref | ref | ref |
| Stage 2 (eGFR 60-89) | 1.55 | 0.56-4.32 | 0.40 |
| Stage 3a (eGFR 45-59) | 1.71 | 0.63-4.67 | 0.29 |
| Stage 3b (eGFR 30-44) | 2.04 | 0.73-5.67 | 0.17 |
| Stage 4 (eGFR 15-29) | 0.99 | 0.26-3.70 | 0.99 |
| Stage 5 (eGFR <15) | n/a | n/a | n/a |
| Death | |||
| Stage of Kidney Disease at Baseline | |||
| Stage 1 (eGFR≥90) | ref | ref | ref |
| Stage 2 (eGFR 60-89) | 1.52 | 0.48-4.77 | 0.47 |
| Stage 3a (eGFR 45-59) | 2.21 | 0.74-6.57 | 0.15 |
| Stage 3b (eGFR 30-44) | 3.12 | 1.16-9.17 | 0.039 |
| Stage 4 (eGFR 15-29) | 3.94 | 1.29-12.0 | 0.02 |
| Stage 5 (eGFR <15) | 7.24 | 2.18-24.1 | 0.0012 |
Kidney function is presented as CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative guidelines using estimated glomerular filtration rate (eGFR) from the Chronic Kidney Disease Epidemiology Collaboration Formula (CKD-EPI).
The groups are Stage 1 (eGFR≥90 ml/min/1.73m2), Stage 2 (eGFR 60-90 ml/min/1.73m2), Stage 3a (eGRF 45-59 ml/min/1.73m2), Stage 3b (eGFR 30-44 ml/min/1.73m2), Stage 4 (eGFR 15-30 ml/min/1.73m2) and Stage 5 (eGFR<15 ml/min/1.73m2)
Follow-up is calculated as time from VAD to first event or end of study period (1 year or time until explantation/transplantation) for thromboembolic events and hemorrhagic events. Follow-up time for incidence of death is calculated as time to death or end of study period (1 year or explantation/transplantation
Cox proportional hazards models were used to assess the risk of thromboembolism and hemorrhage, and death. The proportional hazards assumption was met.
Kidney Function and Hemorrhage
There were 58 hemorrhagic events over a follow-up time of 101 person-years with an incidence rate of 5.7 per 10 person years (95% CI 4.4–7.4). There was no statistically significant association with baseline kidney function and risk of hemorrhagic events (Table 2). There was no significant reduction in the risk of hemorrhage for those who sustained kidney function improvement over the follow-up as compared to those who did not sustain improvement (HR 0.75, 95% CI 0.46–1.22, p=0.24).
Kidney Function and Death
A total of 74 deaths occurred over a follow-up time of 153.6 person-years with an incidence rate of 4.8 per 10 person-years (95% CI 3.8–6.0). The primary etiology of death was multi-organ failure (85%; 63 of 74 deaths). The risk of death was higher amongst patients with compromised kidney function at baseline (Table 2). Patients with CKD stage 3b (HR 3.12, 95%CI 1.16–9.17, p=0.039), CKD stage 4(HR 3.94, 95%CI 1.29–12.0, p=0.02) and CKD stage 5 (HR 7.24, 95%CI 2.18–24.1, p=0.001) had a significantly higher risk of mortality compared to those with CKD stage 1. This association persisted, even after adjustment for device type, age at implant, history of diabetes, and history of hypertension (Figure 2). The risk of death was 3 times higher for CKD stage 3b (HR 3.32, 95%CI 1.10–9.98, p=0.03), and 4 times higher for CKD stage 4 (HR 4.07, 95%CI 1.27–13.1, p=0.02) and Stage 5 (HR 4.01, 95%CI 1.17–13.7, p=0.03) compared to those with CKD stage 1. Improvement in kidney function that was sustained for 1-year was not associated with a lower risk of death (HR 1.03, 95%CI 0.58–1.84, p=0.91).
Figure 2. Adjusted Relative Risk Estimates for Mortality Stratified by baseline CKD Stage.
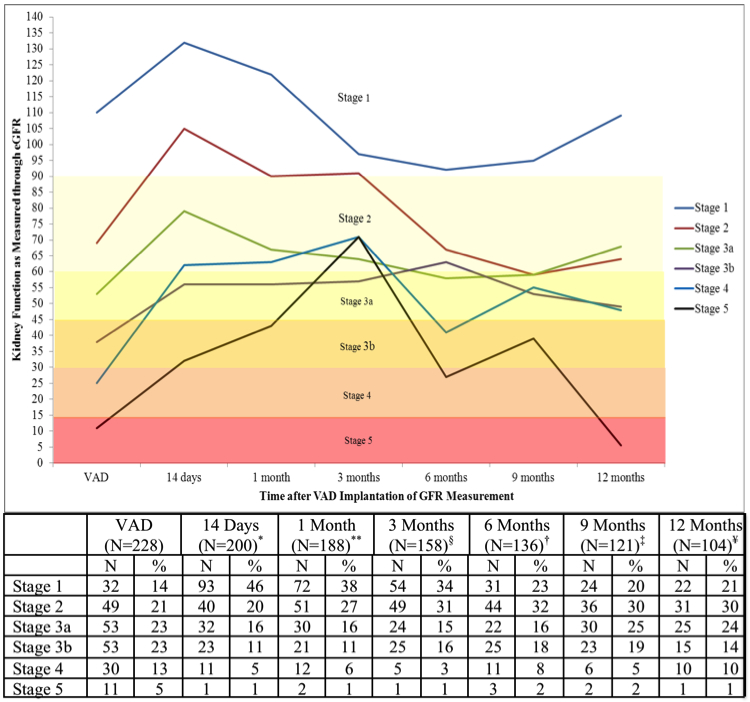
Kidney function is presented as CKD stages as recommended by the Kidney Disease Outcomes Quality Initiative guidelines using estimated glomerular filtration rate (eGFR) from the Chronic Kidney Disease Epidemiology Collaboration Formula (CKD-EPI).
The groups are Stage 1 (eGFR≥90 ml/min/1.73m2), Stage 2 (eGFR 60-90 ml/min/1.73m2), Stage 3a (eGRF 45-59 ml/min/1.73m2), Stage 3b (eGFR 30-44 ml/min/1.73m2), Stage 4 (eGFR 15-30 ml/min/1.73m2) and Stage 5 (eGFR<15 ml/min/1.73m2)
Cox proportional hazards models were used to assess the adjusted risk of death. The proportional hazards assumption was met.
Discussion
Our study demonstrates that VAD implantation is associated with clinically meaningful improvement in kidney function that is sustained over 1- year for most patients. However, improvement in kidney function did not influence the risk of thromboembolism, hemorrhage or mortality. Although patients with Stage 5 CKD (baseline GFR<15) showed improvement in kidney function over the first 3 months, the improvement was not sustained. We found that kidney function at the time of implantation increased the risk of mortality, with patients in the more advanced stages of kidney dysfunction having the highest risk of death.
Kidney function has been shown to improve after VAD implantation, but clinically significant improvement and duration of improvement has not been well characterized. Our study fills this gap by investigating long-term kidney function with detailed information on the change in CKD stage and eGFR after VAD implantation. The limited follow-up in prior reports has enabled demonstration of short-term kidney function improvement. Only two studies have explored kidney function over a longer time period (Table 3). Hasin et al illustrated improvement in eGFR post implant to 6 months in 83 VAD patients, and Kirklin et al illustrated improvement in serum creatinine and BUN up to 2 years post implant in 4917 VAD patients.15,20–23 However, both of these studies assessed kidney function over time as continuous measures. Presenting changes in kidney function as a continuous measure (eGFR) and categorical measure (CKD stage) in Figure 1 provides another facet and timeline of changing kidney function post VAD implantation. Our findings confirm previous observations that kidney function improves after VAD implantation and demonstrates that the improvements are clinically meaningful and sustained. However, among patients with Stage 5 CKD at baseline, the improvements are only temporal and not as sustained as previously hypothesized.
Table III.
Prior VAD Studies that Investigate Kidney Function post VAD Implantation and the Relationship between Kidney Function and Mortality
| Authors | Sample Size |
Definition of Kidney Function |
Assessment of Kidney Function |
Outcome of Interest |
Key Findings |
|---|---|---|---|---|---|
| Kirklin (2013)22 | 4,917 | eGFR <30 for severe kidney disease and eGFR 30-60 for moderate kidney disease | Assessed at baseline, BUN and creatinine were assessed up to 4 years after implantation | Death, change in serum creatinine and BUN | Pre-implant renal dysfunction is related to higher mortality post VAD implant. |
| Borgi (2013)31 | 100 | Acute Renal Failure (ARF) | Assessed at baseline and 7 days | Change in KF and its influence on 1-year mortality | Postoperative ARF is associated with mortality |
| Iwashima et al (2012)32 | 110 | Change in estimated glomerular filtration rate (eGFR) from baseline to 2 weeks | Assessed at baseline and 2 weeks | Mortality at 2 years | Impaired renal function as well as renal function that does not improve with VAD placement are predictors of mortality |
| Hasin et al (2012)33 | 83 | eGFR | Assessed before VAD, and 1,3 and 6 months after VAD | eGFR | Renal function improves after VAD implantation |
| Butler et al (2006)34 | 220 | Renal function as defined by creatinine clearance | Assessed 1 month post VAD | All-cause mortality, and disease specific mortality at 1 year | Poor renal function at baseline is associated with worse outcomes. Patients with improving renal function after VAD placement experience improved outcomes |
| Genovese et al (2010)35 | 163 | Acute Renal Failure (ARF) | Assessed 60 days post implantation | One year mortality | The presence of some adverse events increased the risk of mortality, namely renal events, respiratory events, bleeding events and reoperation |
| Ma et al (2008)36 | 28 | Renal function as measured through creatinine | Assessed at baseline and 1 month post implant | Length of recovery | Pre-VAD renal function is predictive of post-VAD renal function and length of ICU stay |
| Russell et al (2009)37 | 309 | Renal function as defined by BUN and creatinine | Assessed at baseline and 6 months post VAD | Renal function as defined by BUN and creatinine | Implantation of a VAD improves renal function |
| Sandner et al (2009)38 | 86 | Pre-VAD eGFR | Assessed prior to VAD | Mortality post-VAD to 6 months | Patients with pre-VAD renal dysfunction have poorer outcomes than patients without pre-VAD renal dysfunction. Renal function improves regardless of pre-VAD renal status. |
| Sandner et al (2008)39 | 92 | Renal function as defined by creatinine and eGFR | Assessed at baseline and 3 months | Renal function, and mortality at 3 months | There is no difference between continuous flow and pulsatile flow devices on renal function |
| Singh et al (2011)40 | 116 | Creatinine clearance prior to VAD | Assessed prior to VAD | Creatinine clearance 1 month after VAD implant | VAD use improves renal function after implantation |
| Raichlin et al (2016)16 | 165 | Baseline glomerular filtration rate (bGFR) ≤40 and bGFR >40 | Assessed at baseline, 1, 3, 6, and 12 months after implantation | Length of hospitalization, death, dialysis, and time on dialysis at 1 year | GFR increased significantly at 1 month. At 1 year, patients with bGFR >40 had a return to pre-LVAD GFR, whereas those with bGFR ≤40 had a sustained increase in GFR. |
| Mohamedali et al (2017)14 | 213 | Pre-implant GFR <60 (moderate/severely reduced renal function) or GFR ≥ 60 (normal/mildly impaired renal function) | Assessed at baseline and at discharge | All-cause mortality and hospitalizations | Higher all-cause mortality in patients with pre-implant GFR<60. Those who do not improve GFR to ≥ 60 after implantation, have highest risk of cardiovascular morbidity and mortality. |
| Daimee et al (2017)29 | 184 | GFR<45 (low renal function), GFR 45-59 (intermediate renal function), and GFR ≥ 60 (normal renal function) | Assessed at baseline, day 1, day 7, 1 month, 3 months, 6 months, 1 year, and 2 years post-LVAD | All-cause mortality, first hospital readmission due to any cause within 2 years | Those with pre-LVAD GFR<45 experienced sustained improvement in renal function after 2 years follow-up. Patients with no improvement in renal function after 1 month had increased risk of mortality and readmission. |
Bansal et al (2018)17: Not included due to primarily looking at patients with ESRD
The greatest improvements in kidney function are realized early, in the first 14 days post implant, as organ perfusion is increased. Although improvements in kidney function are sustained, early gains in eGFR are attenuated after 14 days. Therefore gain in kidney function after 14 days may more closely represent the organ capacity that was previously masked due to poor kidney perfusion in these patients with poor cardiac function.
Decreased kidney function is the most common contraindication to heart transplantation.24 Previous studies have shown that among patients with HF receiving heart transplants, those maintained on VADs (vs. inotrope support) have better kidney function at time of transplantation.25 Therefore our results, wherein 55.8% of VAD patients demonstrated improvement in kidney function are of particular importance for two reasons. First, kidney function improvement (or lack thereof) post-VAD implantation facilitates assessment of organ capacity that was previously masked due to poor kidney perfusion in these patients with poor cardiac function. However as we did not have assessment of right heart function, we cannot determine whether the improvement in kidney function is due to better renal perfusion or due to reduced pressure in the venous return. Second, sustained improvement in kidney function improves the candidacy for receiving a heart transplant. The latter is supported by our findings wherein patients who demonstrated sustained kidney function improvement over time were more likely to than those who did not sustain kidney function over time.
In our study, neither baseline kidney function nor improvement in kidney function was associated with thromboembolism or hemorrhage. This is contrary to reports from VAD14 and non-VAD patient populations that have demonstrated that kidney function improvement is associated with a decrease in risk of thromboembolism and hemorrhage.23,26–28 The lack of association in our study could be due to two reasons. First, patients who demonstrated sustained improved kidney function on VAD support were more likely to be transplanted, and therefore censored from further analysis. Second, unlike non-VAD patient populations, patients with VADs have a different risk profile for these events due to the complex nature of the device itself and its effects on the coagulation system and the vasculature. Given these considerations, along with the baseline risk in patients with cardiovascular disease in general, improvement in kidney function alone, even if sustained over a one year period, may not be enough to outweigh the risk associated with the VAD device itself or mitigate the risk associated with longstanding cardiovascular disease. Moreover, the one-year follow-up time may have limited our ability to detect associations between kidney function and thromboembolism or hemorrhage.
Decreased kidney function prior to VAD implant was associated with higher mortality consistent with recent reports.14,29 Although improvement in kidney function decreases mortality in non-VAD patients, our findings did not demonstrate decrease in mortality among VAD recipients. This may be explained by the higher and long-standing disease burden in patients with advanced heart failure. Although VAD implantation improves kidney function and reverses the cardiorenal syndrome, the improvements do not attenuate the risk of mortality.
While previous studies have illustrated the influence of pre-VAD kidney function on post-VAD mortality, these studies have primarily assessed eGFR, creatinine and BUN as continuous measures, and their subsequent association with mortality. Consistent with previous studies, poor kidney function pre-VAD is associated with a higher mortality rate.14,22,29 We investigated CKD stage pre-VAD implantation and the association with mortality post-VAD implantation. Furthermore, we categorized CKD stage 3 into stage 3a (eGFR 45–59 ml/min) and stage 3b (eGFR 30–44 ml/min) as Levey et al have demonstrated clinically meaningful differences in mortality among the two groups.30 To our knowledge, this report is the first to report that VAD patients stage 3b CKD have a higher mortality, compared to those with stage 3a CKD.
The study has several strengths including a large (n=228) sample size, a racially diverse population, uniformity of care at a single institution, detailed clinical documentation, and ascertainment of clinically relevant events. Moreover, only four patients transferred care out-of-state. Therefore, we could not ascertain their status for this analysis. Kidney function was assessed at multiple time points, and improvement in kidney function is defined using a clinically relevant rubric. However we recognize its limitations such as lack of ascertainment of biomarkers. We also recognize our findings from this retrospective and single center study may not be generalizable to larger VAD populations. Using CKD staging to characterize baseline kidney dysfunction may misclassify patients as having kidney disease when the kidney dysfunction could be due to acute kidney injury instead of chronic kidney disease. We did not assess whether there is an association between right ventricular dysfunction or infections and kidney dysfunction. We recognize that this is a single center study. Therefore independent validation of our findings in larger datasets is needed to confirm our findings.
Regardless of baseline kidney function, most patients experience an improvement in kidney function after VAD implantation. Regardless of the improvement in kidney function post VAD implant, risk of mortality is driven by baseline kidney function. Patients with CKD stage 3b, 4 or 5 prior to VAD are ay an increased risk of mortality post-VAD implantation. This has important implications on patient selection during evaluation for VAD therapy. Further research is needed to establish whether patients with decreased kidney function due to advancing heart failure would benefit from earlier implantation of a VAD to reduce the mortality associated with advancing kidney dysfunction.
Acknowledgments
Conflicts of Interest and Sources of Funding
This work is supported in part by American Heart Association (Award number 13PRE1383003) and the National Institute of Health (RO1HL092173; K24HL133373, and T32HG008961). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHA and NIH.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. : Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–47, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Adams KF Jr., Fonarow GC, Emerman CL, et al. : Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149: 209–16, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Smith GL, Lichtman JH, Bracken MB, et al. : Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 47: 1987–96, 2006. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chornic Kidney Disease and End-Stage Renal Disease in the United States. 2013. [Google Scholar]
- 5.Ronco C, Haapio M, House AA, Anavekar N and Bellomo R: Cardiorenal syndrome. J Am Coll Cardiol 52: 1527–39, 2008. [DOI] [PubMed] [Google Scholar]
- 6.McAlister FA, Ezekowitz J, Tarantini L, et al. : Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circ Heart Fail 5: 309–14, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Hillege HL, Girbes AR, de Kam PJ, et al. : Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203–10, 2000. [DOI] [PubMed] [Google Scholar]
- 8.McClellan WM, Flanders WD, Langston RD, Jurkovitz C and Presley R: Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol 13: 1928–36, 2002. [DOI] [PubMed] [Google Scholar]
- 9.McAlister FA, Ezekowitz J, Tonelli M and Armstrong PW: Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 109: 1004–9, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Hillege HL, Nitsch D, Pfeffer MA, et al. : Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671–8, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Jessup M, Albert NM, Lanfear DE, et al. : ACCF/AHA/HFSA 2011 survey results: current staffing profile of heart failure programs, including programs that perform heart transplant and mechanical circulatory support device implantation. J Card Fail 17: 349–58, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, Go AS, Lloyd-Jones DM, et al. : Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation 123: e18–e209, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demirozu ZT, Etheridge WB, Radovancevic R and Frazier OH: Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant 30: 182–7, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Mohamedali B, Bhat G: The Influence of Pre-Left Ventricular Assist Device (LVAD) Implantation Glomerular Filtration Rate on Long-Term LVAD Outcomes. Heart Lung Circ 26: 1216–1223, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Hasin T, Topilsky Y, Schirger JA, et al. : Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol 59: 26–36, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Raichlin E, Baibhav B, Lowes BD, et al. : Outcomes in Patients with Severe Preexisting Renal Dysfunction After Continuous-Flow Left Ventricular Assist Device Implantation. ASAIO J 62: 261–7, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Bansal N, Hailpern SM, Katz R, et al. : Outcomes Associated With Left Ventricular Assist Devices Among Recipients With and Without End-stage Renal Disease. JAMA Intern Med 178: 204–209, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia Y, Forest S, Friedmann P, et al. : Factors Associated with Prolonged Survival in Left Ventricular Assist Device Recipients. Ann Thorac Surg, 2018. [DOI] [PubMed] [Google Scholar]
- 19.De Schryver EL, van Gijn J, Kappelle LJ, Koudstaal PJ and Algra A: Non-adherence to aspirin or oral anticoagulants in secondary prevention after ischaemic stroke. J Neurol 252: 1316–21, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Valente MA, Hillege HL, Navis G, et al. : The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail 16: 86–94, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Levin A and Stevens PE: Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kirklin JK, Naftel DC, Kormos RL, et al. : Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant 32: 1205–13, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Lutsey PL, Astor BC, Wattanakit K, Heckbert SR, Cushman M: Chronic kidney disease and venous thromboembolism: a prospective study. Nephrol Dial Transplant 25: 3296–301, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirklin JK, Naftel DC, Kormos RL, et al. : Third INTERMACS Annual Report: the evolution of destination therapy in the United States. J Heart Lung Transplant 30: 115–23, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Bank AJ, Mir SH, Nguyen DQ, et al. : Effects of left ventricular assist devices on outcomes in patients undergoing heart transplantation. Ann Thorac Surg 69: 1369–74; discussion 1375, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR and Folsom AR: Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol 19: 135–40, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attallah N, Yassine L, Fisher K and Yee J: Risk of bleeding and restenosis among chronic kidney disease patients undergoing percutaneous coronary intervention. Clin Nephrol 64: 412–8, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Limdi NA, Beasley TM, Baird MF, et al. : Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol 20: 912–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daimee UA, Wang M, Papernov A, et al. : Renal Function Changes Following Left Ventricular Assist Device Implantation. Am J Cardiol 120 (12): 2213–2220, 2017. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, de Jong PE, Coresh J, El Nahas M, et al. : The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Borgi J, Tsiouris A, Hodari A, Cogan CM, Paone G and Morgan JA: Significance of postoperative acute renal failure after continuous-flow left ventricular assist device implantation. Ann Thorac Surg 95: 163–9, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Iwashima Y, Yanase M, Horio T, et al. : Serial changes in renal function as a prognostic indicator in advanced heart failure patients with left ventricular assist system. Ann Thorac Surg 93: 816–23, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Hasin T, Marmor Y, Kremers W, et al. : Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol 61: 153–63, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Butler J, Geisberg C, Howser R, et al. : Relationship between renal function and left ventricular assist device use. Ann Thorac Surg 81: 1745–51, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Genovese EA, Dew MA, Teuteberg JJ, et al. : Early adverse events as predictors of 1-year mortality during mechanical circulatory support. J Heart Lung Transplant 29: 981–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma L, Fujino Y, Matsumiya G, Sawa Y and Mashimo T: Renal function with left ventricular assist devices: the poorer the preoperative renal function, the longer the recovery. Med Sci Monit 14: CR621–7, 2008. [PubMed] [Google Scholar]
- 37.Russell SD, Rogers JG, Milano CA, et al. : Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation 120: 2352–7, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Sandner SE, Zimpfer D, Zrunek P, et al. : Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg 87: 1072–8, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Sandner SE, Zimpfer D, Zrunek P, et al. : Renal function after implantation of continuous versus pulsatile flow left ventricular assist devices. J Heart Lung Transplant 27: 469–73, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Singh M, Shullo M, Kormos RL, et al. : Impact of renal function before mechanical circulatory support on posttransplant renal outcomes. Ann Thorac Surg 91: 1348–54, 2011. [DOI] [PubMed] [Google Scholar]


