Abstract
Memories are often conceptualized as permanent entities; however, retrieval of memories via stimulus prompts can return them to an active state, which initiates a period of lability before the memories are reconsolidated into long-term storage. Importantly, during this period, memories can be disrupted/altered. A growing body of work has focused on translating animal and experimental science into reconsolidation-based interventions for clinical disorders maintained by maladaptive memories. Interventions targeting reward- and fear-based memories undergirding substance use and anxiety-related disorders, respectively, have shown significant potential. There are several promising pharmacological agents and behavioral approaches that have been used to therapeutically target memory reconsolidation. Here, we discuss the current state of science with special emphasis on the clinical utility of these approaches.
Introduction.
For decades, long term memory (LTM) was assumed to be relatively permanent [1]. More recently, research has examined the feasibility of disrupting or otherwise altering existing memories. This work is grounded in knowledge about processes underlying memory storage. Specifically, as memories are retrieved from LTM they can become destabilized, transitioning through a brief labile state [2] in which they are vulnerable to alteration/disruption before being restabilized (i.e., ‘reconsolidated’) into LTM [3], Clinical scientists have been working towards exploiting this process to modify the maladaptive memories that undergird various psychological disorders.
There is a vast body of literature suggesting that dysregulated learning and memory processes are prominent features of both substance use disorders (SUDs) [4] and anxiety-related disorders (ARDs) [5]. Several recent reviews have sought to characterize the growing body of literature on reconsolidation-based interventions for clinical disorders that aim to reverse this dysregulation [*6–12]. Here we provide a brief primer on reconsolidation interference methods, highlight recent clinical advances and obstacles to intervention development, and discuss areas for future directions.
What is altered?
As early animal studies produced evidence [13,14] that pharmacological disruption of reconsolidation could potentially eradicate the behavior(s) supported by target memories, the notion of memory erasure was forwarded as a realistic endpoint of such interventions [15]. This idea was popularized mostly in media coverage of reconsolidation science (e.g., “A Drug To Cure Fear” [16]) and sparked debate regarding the ethics of altering memories [17]. In reality, there is convincing evidence that reactivated memories are not ‘erased’ per se, but altered such that their emotional/motivational properties are attenuated while the declarative properties of the memory are preserved [18,19]. Thus, an individual with an alcohol use disorder (AUD) does not forget the fact that they crave alcohol; instead, the urge to drink after seeing a vodka advertisement is decreased, thereby decreasing alcohol consumption.
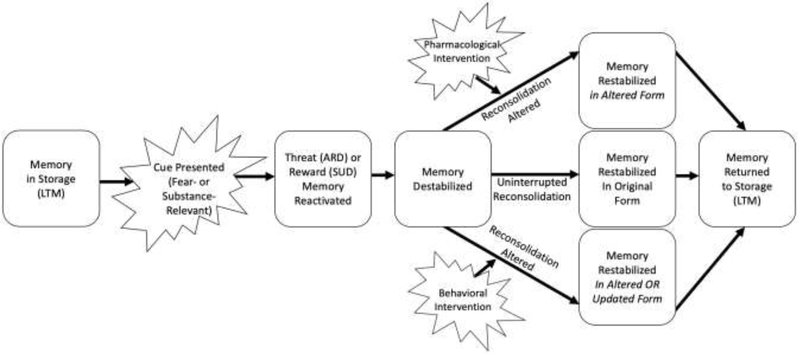
During reconsolidation, target memories are susceptible to significant alteration, either via the strategically timed administration of (a) reconsolidation blocking pharmacological agents, or (b) behavioral ‘updating’ procedures (see Figure 1 and details below). Our recent meta-analysis of such interventions [**20] documented medium-sized effects for pharmacological approaches to ARDs and behavioral approaches to SUDs with small effects for behavioral approaches to ARDs and pharmacological approaches to SUDs. Importantly, this conclusion was based on fewer than twenty methodologically heterogeneous studies and should be replicated when more clinical studies are available. The present discussion focuses on a select set of studies that highlight some of the opportunities and challenges of applying reconsolidation interference, clinically.
Figure 1:
Example of reconsolidation-based intervention procedures
Note: LTM=Long-term storage; ARD=anxiety-related disorder; SUD=substance use disorder
Pharmacological Interference.
Theoretically, pharmacological alteration/disruption of memories after their reactivation is achieved by stalling the protein synthesis that is necessary for memories to be restabilized, leading to alterations in affective/motivational properties of the memories [**6] (but see [21] for an alternative conceptualization). As an example, one study [**22] examined the effects of a single dose of propranolol or placebo one hour prior to memory reactivation as well as propranolol six hours after reactivation of smoking memories by nicotine self-administration (cigarette smoking). Propranolol one hour before memory reactivation was associated with reduced craving among smokers whereas there were no significant changes in craving among those given placebo or propranolol six hours after memory reactivation (i.e., after the reconsolidation window theoretically closed). Consistent with theory, propranolol ostensibly exerts effects when active during the lability period following reactivation, but not outside of this window (i.e. after six hours). Propranolol was administered before memory retrieval to theoretically maximize plasma concentration during the reconsolidation window [23]; however, it is unclear whether propranolol acted upon retrieval, reconsolidation, or both.
Similar findings have been reported in the ARD literature. For example among posttraumatic stress disorder (PTSD) patients, one dose of propranolol following a personalized trauma script reactivation was associated with reductions in physiological responses (heart rate, skin conductance) [24]. A more recent study by the same group [**25] demonstrated that six weekly sessions of propranolol + trauma reactivation, where propranolol was administered prior to trauma script memory reactivation, yielded significantly greater reductions in self- and clinician-rated PTSD symptoms relative to placebo + trauma reactivation. Although PTSD symptoms declined in both groups, in line with repeated exposure to trauma cues, the propranolol treated group had significantly greater reductions, theoretically due to its effects on trauma memory reconsolidation. This demonstrates how reconsolidation-based interventions can be integrated with existing evidence-based treatments (e.g., exposure). However, it is unclear whether the observed effects were truly due to reconsolidation interference of propranolol per se; the lack of a propranolol + no reactivation group prevents conclusions regarding the efficacy of propranolol broadly as opposed to propranolol in the specific context of reconsolidation. Further, effects may have been achieved through propranolol’s effects on memory retrieval, memory reconsolidation or both; in the more recent study [**25], propranolol was administered prior to reactivation making it impossible to discern the locus of the effect.
In a report on a series of four cases utilizing 1-2 sessions of post-retrieval propranolol in the treatment of PTSD symptoms, three cases showed symptomatic improvement [26]; in the unsuccessful case, the authors suggest that the intervention may merely have triggered memory retrieval but did not sufficiently induce the labile state necessary for memory interference [cf., 27]. Alternatively, they posited that 1-2 sessions may not have been sufficient, suggesting that more treatment (e.g. [**25]) may be necessary in some cases.
In another study, greater approach behavior towards spiders (i.e., decreased avoidance) was documented in spider phobic individuals who received post-retrieval propranolol [*28]. No such change was found among retrieval + placebo, or propranolol + no reactivation groups, consistent with a reconsolidation-dependent effect. These findings highlight the importance of ensuring that medications exert their effects while memories are in a labile state, as administration of propranolol without initiating a period of lability is not associated with any change in outcomes.
Although propranolol is the most widely studied, other memory-disrupting agents that interfere with the synaptic plasticity and stabilization processes required for restabilization have been evaluated. For example, the mTOR inhibitor sirolimus (rapamycin) could be used to theoretically disrupt the reconsolidation of trauma memories, given the centrality of mTOR to cellular modification and plasticity [29]. The one study examining sirolimus (relative to placebo) among veterans with PTSD found no overall effect [30]. However, exploratory analyses revealed that sirolimus reduced self- and clinician-rated PTSD symptoms one-month post-intervention among more recently traumatized (post-Vietnam) Veterans but not Veterans with older traumas (e.g., Vietnam-era); these effects were not maintained at three-month follow-up. The authors suggested that the relatively more recent trauma memories were potentially more vulnerable to disruption. However, more work will be needed to evaluate whether there are any such limits (e.g., time) on memory disruption/alteration. In sum, in the only clinical study of sirolimus to date, there were modest findings, which were only evident in a post-hoc analysis of a subset of patients. Additionally, N-methyl-D-aspartate receptor [NMDAR] antagonist memantine has been evaluated among smokers, but did not weaken memory traces [31]. Finally, inhalation of nitrous oxide gas has been examined as a reconsolidation-based agent. Specifically, post-retrieval nitrous oxide gas evidenced some potential to reduce alcohol consumption among hazardous drinkers experiencing accentuated prediction error (i.e., a mismatch between expectation and outcome) at retrieval [32]; these individuals were presented with an alcoholic beverage with the expectation that they would drink it but were ultimately told not to.
Limited conclusions can be drawn from these initial trials and in particular, uncontrolled case reports. Yet, they highlight important considerations for researchers when designing future studies. Specifically, it will be important to determine what number of medicated retrieval sessions are necessary for optimal outcomes. Future work will also need to evaluate what retrieval procedures robustly destabilize memories, which pharmacological agents produce the best therapeutic outcomes and what agents work best for specific disorders (e.g., SUDs vs. ARDs). Lastly, investigators should, where possible, (a) include appropriate controls conditions (e.g., placebo + reactivation and active medication + no reactivation) and, (b) administer disrupting agents after memory retrieval/reactivation to disambiguate reconsolidation-independent drug effects and effects on retrieval vs. reconsolidation processes.
Behavioral Interference.
There are a number of behavioral strategies that putatively impact maladaptive memories in the context of reconsolidation. The most widely studied; retrieval-extinction training (RET), involves repeated exposure to fearful/substance-related stimuli during the reconsolidation window. Theoretically, the repeated presentation of relevant stimuli serves to update the memories (rather than disrupting them, as occurs in pharmacological approaches) with information that is contrary to the original learning (i.e., cue→outcome contingency is updated to cue→no outcome).
In a study of heroin users, RET was associated with reduced heroin cue-reactivity six months post-treatment [33]. Based on these impressive findings, our team examined effects of a similar RET treatment on craving and smoking behavior of nicotine dependent smokers [**34]. The RET group had smoking memories reactivated via a brief ‘smoking video’ whereas the control group viewed a video with no smoking content. Both groups then received 60-minutes of cue exposure. We observed both reduced cue-elicited craving and number of cigarettes per day in the smoking memory reactivation condition. This represents the first and only RET study to demonstrate reductions in substance use behavior and a generalization of these effects to novel smoking cues.
Similar effects with RET have been observed in phobias of spiders [*35,*36] and snakes [*35], with reduced fear levels maintained at six-month follow-up [*36]. The latter studies highlighted the importance of retrieval timing, showing that the period of memory lability is temporary, and that hypothesized effects occur only when treatment falls within this period. Indeed, findings cannot be explained by sheer amount of exposure to phobic material as one study [34] exposed both conditions to matched content (i.e., memory reactivation then exposure vs. exposure then memory reactivation); rather, it was the specific timing (brief activation prompting lability, followed by exposure) that produced enhanced outcomes.
As part of a more comprehensive treatment protocol, RET was implemented into treatment for a subset of patients with flight phobia [37]. Following four sessions of anxiety management, individuals were randomized to receive four virtual reality exposures either preceded by a fear-relevant reactivating cue or neutral cue. Both groups experienced significant and large reductions in fear, which were maintained at one-year follow-up. However, the fear-relevant retrieval group experienced a significantly greater decline in fear cue-induced skin conductance, suggesting that there was added benefit of implementing retrieval-extinction properties into an existing anxiety reduction treatment.
Other putatively therapeutic behavioral methods have been implemented to interfere with reconsolidation. For example, we [*38] reactivated either drinking memories or control (nondrinking) memories in hazardous drinkers prior to counterconditioning (pairing alcohol cues with disgusting outcomes). There was a significant reduction in attentional bias to and liking of alcohol cues in participants who retrieved alcohol memory before counterconditioning compared to the no-reactivation control. However participants in both groups showed similar reductions in drinking. In another study by our group [*39], we used a post-retrieval reappraisal paradigm in an analogue of cognitive therapy, with the aim of altering underlying alcohol-related schemata. Only those who underwent prior alcohol memory reactivation evidenced reduced semantic fluency for positive alcohol-related words. In summary, there are a number of behavioral interventions, adapted from a repertoire of current cognitive-behavioral interventions, which can be administered during the reconsolidation window.
Clinical Potential.
Although reconsolidation-focused interventions are still in early development, existing evidence provides considerable grounds for optimism about their potential. First, it may be highly advantageous to have two distinct therapeutic pathways (behavioral and pharmacological) to maladaptive memory modulation, especially if there are individual differences in responsivity to these modalities; future work is needed to examine such differences. Moreover, within each approach, there are a number of candidate agents (e.g., propranolol, sirolimus) and behavioral methods (e.g., RET, counterconditioning) showing potential. Future research is needed to identify whether utilizing a combination of behavioral and pharmacological methods may confer greater benefits than a monotherapy approach. To date, this has not been examined. Additionally, it is currently unknown to what degree patient characteristics or other contextual variables (e.g., severity of substance involvement) can predict whether behavioral or pharmacological methods may benefit a given individual. Large follow-up studies powered to systematically examine these moderating factors will eventually be needed.
Second, by targeting memory processes underlying both SUDs and ARDs, these methods have transdiagnostic therapeutic potential across a broad range of disorders. Given the high prevalence of SUDs [40], ARDs [41], and their co-occurrence [42], having a treatment approach that can be broadly applied is crucial given the dearth of integrated mood/anxiety-substance abuse treatments currently available.
Third, these approaches are brief. Both behavioral and pharmacological approaches have been successfully implemented in one to six sessions; however, more work is needed to directly study the effect of ‘treatment dose’ on outcomes. Such brevity is in stark contrast to current pharmacological treatments, where clinical impact is predicated on chronic medication use. Likewise, many behavioral treatments require weeks/months to administer. The availability of brief reconsolidation-focused treatments may foster treatment uptake insofar as patients may be more willing to engage in ‘low intensity’ treatment. Moreover, the risks of dropout and potential concerns regarding medication adherence are alleviated because treatments can be as brief as a single therapeutic encounter. Furthermore, the brevity of pharmacological reconsolidation-focused intervention greatly reduces concerns about interactions between the treatment agents and other prescription medications or other substance use.
Fourth, reconsolidation-based interventions are relatively uncomplicated and therefore, easily packaged into existing treatments. These approaches may be employed in fast-paced clinical and medical settings, where clinicians may not have the time or resources to administer (or remain adherent to) lengthy evidence-based behavioral treatments. Furthermore, reconsolidation approaches can be implemented into or alongside other existing treatments [**25,37].
Fifth, the cost associated with these treatments is modest when compared to long-term pharmacotherapy or a full course of behavioral therapy. Reduced time and cost could translate into tremendous reach among typically underserved populations (e.g., low income or rural populations, racial/ethnic minorities).
Finally, there appear to be little to no additional risk associated with the behavioral approaches described here, above and beyond those of existing treatment approaches; the pharmacological approach also has only a modestly more complicated risk profile, especially since the dominant agent to date is the well-tolerated beta-blocker, propranolol.
Future directions.
Despite the considerable treatment potential offered by reconsolidation-based interventions, there is a clear need for more well controlled clinical studies. In particular, replication studies should be a priority given that there is some evidence of inconsistent findings [43,44]. Such work could extend current understanding of these interventions by identifying procedural parameters that optimize treatment outcome (e.g., optimal memory reactivation procedures, medication doses, ideal inter-session intervals). Additional longitudinal work is needed to compare the durability of reconsolidation-based treatment outcomes with those achieved by currently available therapies and whether post-treatment booster sessions substantially increase long-term efficacy. Furthermore, the relative benefits of behavioral vs. pharmacological (or combined) approaches to reconsolidation interference need to be clarified for SUDs, ARDs and comorbid presentations. Additionally, the relative benefits of specific pharmacological agents and behavioral paradigms need to be investigated over time. There are limited clinical studies overall, with the majority utilizing propranolol and RET, respectively, and with no direct comparison of approaches.
Conclusions.
Mounting clinical science has focused on translating experimental findings on memory reconsolidation to clinical populations with SUDs and ARDs. These studies show promise in reducing cravings and substance use behaviors for SUDs as well as physiological arousal and symptoms associated with ARDs. Yet, clinical research is still in the nascent stage and key questions remain. More trials are needed comparing and/or combining these approaches to standard-of-care treatments for SUDs and ARDs. If findings are replicated and extended with larger samples and long-term follow-up, these approaches may represent a new era in the behavioral and pharmacological treatment of the most prevalent and disabling behavior disorders.
Highlights.
We provide an introduction to reconsolidation-based clinical interventions
We outline applications in substance use and anxiety-related disorders
We examine recent studies on pharmacological and behavioral approaches
We discuss limitations current work and offer suggestions for next steps
Acknowledgments
Funding
This research was supported by NIDA grants R01 DA035247 (Saladin, PI) and R01 DA043587 (Saladin, PI), NIAAA grant P50 AA010761, NCI grant P30CA138313, and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Numbers UL1 RR029882 and UL1 TR000062.
Footnotes
Conflicts of Interests
There are no other conflicts of interest pertaining to the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].McGaugh JL, Memory–a century of consolidation, Science. 287 (2000) 248–251. [DOI] [PubMed] [Google Scholar]
- [2].Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, Rei D, Bero AW, Phan TX, Wagner F, Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories, Cell. 156 (2014) 261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee JL, Reconsolidation: maintaining memory relevance, Trends Neurosci. 32 (2009) 413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hyman SE, Addiction: a disease of learning and memory, Am. J. Psychiatry. 162 (2005) 1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- [5].Norton PJ, Paulus DJ, Transdiagnostic models of anxiety disorder: Theoretical and empirical underpinnings, Clin. Psychol. Rev. (2017). doi: 10.1016/j.cpr.2017.03.004. [DOI] [PubMed] [Google Scholar]
- **[6].Elsey JW, Van Ast VA, Kindt M, Human memory reconsolidation: A guiding framework and critical review of the evidence., Psychol. Bull. (2018). doi: 10.1037/bul0000152.This comprehensive review describes the translational efforts to advance reconsolidation-based interventions from animal models to human experimental and clinical studies.
- [7].Elsey JW, Kindt M, Tackling maladaptive memories through reconsolidation: From neural To clinical science, Neurobiol. Learn. Mem. 142 (2017) 108–117. doi: 10.1016/j.nlm.2017.03.007. [DOI] [PubMed] [Google Scholar]
- [8].Elsey JW, Kindt M, Breaking boundaries: optimizing reconsolidation-based interventions For strong and old memories, Learn. Mem. 24 (2017) 472–479. doi: 10.1101/lm.044156.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beckers T, Kindt M, Memory Reconsolidation Interference as an Emerging Treatment for Emotional Disorders: Strengths, Limitations, Challenges and Opportunities, Annu. Rev. Clin. Psychol. 13 (2017) 99–121. doi: 10.1146/annurev-clinpsy-032816-045209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee JL, Nader K, Schiller D, An update on memory reconsolidation updating, Trends Cogn. Sci. 21 (2017) 531–545. doi: 10.1016/j.tics.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kindt M, The surprising subtleties of changing fear memory: a challenge for translational science, Phil Trans R Soc B. 373 (2018) 20170033. doi: 10.1098/rstb.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Faliagkas L, Rao-Ruiz P, Kindt M, Emotional memory expression is misleading: delineating transitions between memory processes, Curr. Opin. Behav. Sci. 19 (2018) 116–122. doi: 10.1016/j.cobeha.2017.12.018. [DOI] [Google Scholar]
- [13].Bernardi RE, Lattal KM, Berger SP, Postretrieval propranolol disrupts a cocaine conditioned place preference, Neuroreport. 17 (2006) 1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- [14].Debiec J, LeDoux JE, Nader K, Cellular and systems reconsolidation in the hippocampus, Neuron. 36 (2002) 527–538. doi: 10.1016/S0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- [15].Giustino TF, Fitzgerald PJ, Maren S, Revisiting propranolol and PTSD: Memory erasure or extinction enhancement?, Neurobiol. Learn. Mem. 130 (2016) 26–33. doi: 10.1016/j.nlm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Friedman RA, A drug to cure fear., N. Y. Times; (2016). Retrievehttp://www.nytimes.com/2016/01/24/opinion/sunday/a-drug-to-cure-fear.htm. [Google Scholar]
- [17].Kleinfeld JS, Beyond therapy: biotechnology and the pursuit of happiness, Washington (DC), 2003. [Google Scholar]
- [18].Soeter M, Kindt M, Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience, Front. Behav. Neurosci. 9 (2015) 122. doi: 10.3389/fnbeh.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kindt M, Soeter M, Vervliet B, Beyond extinction: erasing human fear responses and preventing the return of fear, Nat. Neurosci. 12 (2009) 256. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- **[20].Walsh KH, Das RK, Saladin ME, Kamboj SK, Modulation of naturalistic maladaptive memories using behavioural and pharmacological reconsolidation-interfering strategies: a systematic review and meta-analysis of clinical and ‘sub-clinical’studies, Psychopharmacology (Berl.). (2018) 1–21. doi: 10.1007/s0021.This meta-analysis examines the efficacy of reconsolidation-based interventions for substance and fear/anxiety disorders. Analyses compare the strength of effects of behavioral vs. pharmacological approaches for substance and fear/anxiety disorders, respectively.
- [21].Gisquet-Verrier P, Riccio DC, Memory integration: An alternative to the consolidation/reconsolidation hypothesis, Prog. Neurobiol. (2018). doi: 10.1016/j.pneurobio.2018.10.002. [DOI] [PubMed] [Google Scholar]
- **[22].Xue Y-X, Deng J-H, Chen Y-Y, Zhang L-B, Wu P, Huang G-D, Luo Y-X, Bao Y-P, Wang Y-M, Shaham Y, Shi J, Lu L, Effect of selective inhibition of reactivated nicotine-associated memories with propranolol on nicotine craving, JAMA Psychiatry. 74 (2017) 224–232. doi: 10.1001/jamapsychiatry.2016.3907.This randomized controlled trial demonstrated the efficacy of a single dose of pre-activation propranolol in smokers relative to (1) propranolol after the reconsolidation window theoretically closes, and (2) pre-activation placebo among smokers. Notably, this study used cigarette smoking to prompt retrieval rather than conditioned stimuli (e.g., images).
- [23].Johnsson G, Regardh CG, Clinical pharmacokinetics of β-adrenoceptor blocking drugs, Clin. Pharmacokinet. 1 (1976) 233–263. doi: 10.2165/00003088-197601040-00001. [DOI] [PubMed] [Google Scholar]
- [24].Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK, Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress, J. Psychiatr. Res. 42 (2008) 503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- **[25].Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK, Reduction of PTSD Symptoms With Pre-Reactivation Propranolol Therapy: A Randomized Controlled Trial, Am. J. Psychiatry. 175 (2018) 427–33. doi: 10.1176/appi.ajp.2017.17050481.This randomized controlled trial demonstrates the benefits of a multi-session protocol administering propranolol (vs. placebo) along with exposure to trauma cues among individuals with PTSD. Although exposure to trauma was associated with improved outcomes, the addition of propranolol was associated with superior outcomes.
- [26].Kindt M, van Emmerik A, New avenues for treating emotional memory disorders: towards a reconsolidation intervention for posttraumatic stress disorder, Ther. Adv. Psychopharmacol. 6 (2016) 283–295. doi: 10.1177/2045125316644541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Finnie PS, Nader K, The role of metaplasticity mechanisms in regulating memory destabilization and reconsolidation, Neurosci. Biobehav. Rev. 36 (2012) 1667–1707. doi: 10.1016/j.neubiorev.2012.03.008. [DOI] [PubMed] [Google Scholar]
- *[28].Soeter M, Kindt M, An abrupt transformation of phobic behavior after a post-retrieval amnesic agent, Biol. Psychiatry. 78 (2015) 880–886. doi: 10.1016/j.biopsych.2015.04.006.This three-group (post-retrieval propranolol, post-retrieval placebo, propranolol without retrieval) randomized controlled trial documents the benefit of post-retrieval propranolol in the reduction of spider fear Post-retrieval placebo and propranolol without memory retrieval did not impact fear ratings.
- [29].Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, Clish CB, Kaminski J, Xiao S, Zu Horste GM, Pawlak M, CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity, Cell. 163 (2015) 1413–1427. doi: 10.1016/j.cell.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Surfs A, Smith J, Powell C, North CS, Interfering with the reconsolidation of traumatic memory: sirolimus as a novel agent for treating veterans with posttraumatic stress disorder, Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 25 (2013) 33. [PMC free article] [PubMed] [Google Scholar]
- [31].Das RK, Hindocha C, Freeman TP, Lazzarino AI, Curran HV, Kamboj SK, Assessing the translational feasibility of pharmacological drug memory reconsolidation blockade with memantine in quitting smokers, Psychopharmacology (Berl.). 232 (2015) 3363–3374. doi: 10.1007/s00213-015-3990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Das RK, Walsh K, Hannaford J, Lazzarino AI, Kamboj SK, Nitrous oxide may interfere with the reconsolidation of drinking memories in hazardous drinkers in a prediction-error-Dependent manner, Eur. Neuropsychopharmacol. (2018). doi: 10.1016/j.euroneuro.2018.05.001. [DOI] [PubMed] [Google Scholar]
- [33].Xue Y-X, Luo Y-X, Wu P, Shi H-S, Xue L-F, Chen C, Zhu W-L, Ding Z-B, Bao Y, Shi J, A memory retrieval-extinction procedure to prevent drug craving and relapse, Science. 336 (2012) 241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **[34].Germeroth LJ, Carpenter MJ, Baker NL, Froeliger B, LaRowe SD, Saladin ME, Effect of a brief memory updating intervention on smoking behavior: a randomized clinical trial, JAMA Psychiatry. 74 (2017) 214–223. doi: 10.1001/jamapsychiatry.2016.3148.Smokers were shown videos of smoking cues after reactivation of smoking memories or no reactivation. There were reductions in both cigarettes per day and cravings in the group shown videos after reactivation. This randomized controlled trial represents the first and only retrieval-extinction study to demonstrate reductions in substance use behavior (cigarettes per day) as well as cravings.
- *[35].Telch MJ, York J, Lancaster CL, Monfils MH, Use of a brief fear memory reactivation procedure for enhancing exposure therapy, Clin. Psychol. Sci. 5 (2017) 367–378. doi: 10.1177/2167702617690151.This randomized controlled trial demonstrated the benefit of exposure therapy in a retrieval-extinction training (RET) framework relative to traditional exposure therapy for phobias. Conducting exposures after the reconsolidation window was opened (with a brief memory retrieval) was associated with better outcomes than traditional exposures (followed by a brief memory retrieval).
- *[36].Björkstrand J, Agren T, Ahs F, Frick A, Larsson E-M, Hjorth O, Furmark T, Fredrikson M, Think twice, it’s all right: long lasting effects of disrupted reconsolidation on brain and behavior in human long-term fear, Behav. Brain Res. 324 (2017) 125–129. doi: 10.1016/j.bbr.2017.02.016.This randomized controlled trial demonstrated that repeated exposure to feared stimuli during the reconsolidation window (10 minutes following retrieval) was associated with attenuated amygdala activity, which was maintained at six-month follow-up. Changes in amygdala activity were not evident in the group where the reconsolidation window was opened and subsequently closed prior to exposure (i.e., 6 hours between retrieval and exposure).
- [37].Maples-Keller JL, Price M, Jovanovic T, Norrholm SD, Odenat L, Post L, Zwiebach L, Breazeale K, Gross R, Kim S, Targeting memory reconsolidation to prevent the return of fear in patients with fear of flying, Depress. Anxiety. 34 (2017) 610–620. doi: 10.1002/da.22626. [DOI] [PubMed] [Google Scholar]
- *[38].Das RK, Lawn W, Kamboj SK, Rewriting the valuation and salience of alcohol-related stimuli via memory reconsolidation, Transl. Psychiatry. 5 (2015) e645. doi: 10.1038/tp.2015.132.This randomized controlled trial documents the use of counterconditioning (pairing of disgusting stimuli with alcohol cues) during the reconsolidation window. Counterconditioning during the reconsolidation window was associated with reduced attentional bias to alcohol cues.
- *[39].Hon T, Das RK, Kamboj SK, The effects of cognitive reappraisal following retrieval-procedures designed to destabilize alcohol memories in high-risk drinkers, Psychopharmacology (Berl.). 233 (2016) 851–861. doi: 10.1007/s00213-015-4164-y.This randomized controlled trial provides preliminary support for the use of cognitive reappraisal during the reconsolidation window. Reappraisal of alcohol-related memories following alcohol memory reactivation was associated with reduced semantic fluency for alcohol-related words, perhaps impacting alcohol-related schemata.
- [40].Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS, Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions–III, JAMA Psychiatry. 73 (2016) 39–47. doi: 10.1001/jamapsychiatry.2015.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Remes O, Brayne C, van der Linde R, Lafortune L, A systematic review of reviews on the prevalence of anxiety disorders in adult populations, Brain Behav. 6 (2016) e00497. doi: 10.1002/brb3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lai HMX, Cleary M, Sitharthan T, Hunt GE, Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: A systematic review and meta-analysis, Drug Alcohol Depend. 154 (2015) 1–13. doi: 10.1016/j.drugalcdep.2015.05.031. [DOI] [PubMed] [Google Scholar]
- [43].Shiban Y, Brüiting J, Pauli P, Mühlberger A, Fear reactivation prior to exposure therapy: does it facilitate the effects of VR exposure in a randomized clinical sample?, J. Behav. Ther. Exp. Psychiatry. 46 (2015) 133–140. doi: 10.1016/j.jbtep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- [44].Wood NE, Rosasco ML, Suris AM, Spring JD, Marin M-F, Lasko NB, Goetz JM, Fischer AM, Orr SP, Pitman RK, Pharmacological blockade of memory reconsolidation in posttraumatic stress disorder: Three negative psychophysiological studies, Psychiatry Res. 225 (2015) 31–39. doi: 10.1016/j.psychres.2014.09.005. [DOI] [PubMed] [Google Scholar]