Abstract
Panax notoginseng is a highly regarded medicinal plant that has been cultivated for more than 400 years in Southwest China. The obstacles associated with the continuous cropping of P. notoginseng are the greatest issues for the development this plant. In the present study, the micro-ecologies of soils differing in the duration of P. notoginseng planting were compared, the results of which could provide important information to aid in solving the problems associated with the continuous cropping of P. notoginseng. Soils in which P. notoginseng had grown for 1, 3 or 5 years, as well as unplanted or fallow soil, which had a P. notoginseng planting interval of 1, 3, 6 or 9 years, were collected in Yunnan Province, China. The numbers and physiological groups of microorganisms, soil enzyme activities and nutrients present in the soil were analyzed to identify the effects of continuous cropping and determine the influence of crop rotation on the soil. After P. notoginseng was planted, the ecological structure of the soil and the balance of soil nutrients changed. These changes in the soil ecosystem prevented the soil from adapting to the continuous cropping of P. notoginseng, which eventually limited the growth of P. notoginseng and increased the incidence of diseases. After rotation of P. notoginseng, some soil indicators were restored, and some indicators with irregular changes may have been caused by crop rotation and field fertilization management practices. Thus, the selection of suitable crop rotations will facilitate the use of continuous cropping for P. notoginseng.
Subject terms: Plant ecology, Soil microbiology
Introduction
Panax notoginseng (Burk.) F.H. Chen is a highly regarded medicinal plant that has been cultivated for more than 400 years in Southwest China, particularly in Yunnan Province. This plant has many important properties, such as antihypertensive, antithrombotic, anti-atherosclerotic and neuroprotective activities1 and is used for the treatment of cardiovascular diseases, inflammation, various body pains, traumas, and internal and external bleeding due to injury2. Continuous cropping, in which the same crop or related crops are continuously planted, decreases the yield and quality of crops3,4. These obstacles associated with continuous cropping have become a major constraint in P. notoginseng cultivation as the land suitable for planting P. notoginseng is limited. Such obstacles have led to an increase in the costs of planting P. notoginseng and decreases in the yield and quality of P. notoginseng5,6. Generally, it should wait many years for P. notoginseng to be planted for a second time after it has been harvested7.
The primary issues associated with continuous cropping include changes in soil microbes, physicochemical properties and allelopathy8–11. Although there are many issues associated with continuous cropping, the most fundamental ones include promoting an imbalance in soil microbiota and diversity, a reduction in beneficial microorganisms, and an enrichment of pathogenic microorganisms and various soil-borne plant diseases12. More importantly, interactions among the various factors associated with to continuous cropping further exacerbate the occurrence of these obstacles13,14. Continuous cropping is closely affected by the types, numbers, and diversity of soil microorganisms14,15. Changes in soil microbial biomass, diversity, and microbiota directly affect crop growth. Previous studies have demonstrated that changes in soil microbiota are the major causes of the obstacles associated with the continuous cropping of P. notoginseng4–7,16,17.
Due to the limited growing area of P. notoginseng, it is imperative to conduct research on the effects of continuous cropping. We hypothesized that soil nutrients, soil microorganisms and soil enzymes would change in response to continuous cropping. Our previous research focused on assessing the yields of volatile oils, the level of saponins, the utilization of water and fertilizer, and the effects of a three-year growth pattern of P. notoginseng; however, the soil microbial and biochemical properties of this system have not been studied18,19. Therefore, in this study, soils currently and previously planted with P. notoginseng were used to study the changes in soil microbes, enzymes, and nutrients during the process of planting P. notoginseng. These changes revealed possible modifications to the soil cause by continuous cropping, providing detailed information on the continuous cropping method used to grow P. notoginseng.
Materials and Methods
Collection of soil samples
The experimental soil samples were collected from Wenshan Autonomous Prefecture, Yunnan Province (103°35~104°45E, 23°18~23°59N). The altitude of the study area is 1539 m, with an average annual temperature of 16.1 °C and annual rainfall of 1008 mm. In addition, the area has 250 to 320 frost-free days and is located within the primary P. notoginseng producing areas. Soils in which P. notoginseng had been grown for 1, 3 or 5 years and those from fields previously planted with P. notoginseng but allowed to fallow for 1, 3, 6 or 9 years were collected. The soils were collected from seven sites in Wenshan and the sampling strategy included collecting soil around P. notoginseng plants, with multipoint sampling also used. The soils for every site were collected three times. All soil samples were placed in sterile bags, and each sample was divided into two subsamples: one portion was air-dried for soil enzyme activity and nutrient analysis, and the others was stored at 4 °C for microbiological experiments20.
Analysis of different species of soil microorganisms
Ten grams of soil was placed into 90 ml of sterile water and shaken well, after which the mixture was diluted in a gradient to different final concentrations. The numbers of bacteria, fungi, and actinomycetes were determined by the serial dilution plate count method. Serial dilutions of 10−4–10−6 for bacteria, 10−1–10−3 for fungi, and 10−3–10−5 for actinomycetes were prepared. Dilutions of different samples were prepared with three replicates, with 0.2 ml of suspension from each serial dilution spread over selective agar medium (Duchefa Biochemie, Holland) for isolation with the medium containing beef extract peptone, rose bengal medium, and Gao I medium. The bacteria, fungi, and actinomycetes were incubated at 28 °C for 2 days, 4 days, and 7 days before counting, respectively21.
Analysis of microbial physiological groups
The study of soil microorganism physiological groups was primarily performed based on the Manual of Soil Microorganism Analysis22 and Soil Microorganism Research Methods23. The differences in microorganisms were measured by the dilution or plate method. The most-probable-number (MPN) procedure was used to count the microorganisms and each sample was counted three times.
Identification of soil fungi
The fungi were identified based on the literature24,25 and the morphological characteristics of fungal colonies grown in PDA medium were observed. Using PDA medium insertion and patches, the mycelial and spore morphologies were observed with a microscope to identify the fungal species.
Analysis of soil enzyme activity
Soil enzyme activities were assayed by the buffer method26,27. First, invertase, amylase and cellulase activity was measured by the salicylate colorimetric method. Phosphatase activity was measured by the colorimetric method using disodium phenyl phosphate. Catalase activity was measured by the titrimetric method using potassium permanganate. Urease activity was measured by the colorimetric method and polyphenol oxidase and peroxidase activities were measured by the colorimetric method using pyrogallic acid28. Enzyme activity was expressed in the following units: invertase, amylase, phosphatase and urease: mg/(g·24 h); cellulase: mg/(10 g·72 h); catalase: ml of 0.1N·KMnO4/(g·20 min); and polyphenol oxidase and peroxidase: mg/(g·2 h). All samples were assayed three times.
Analysis of soil nutrients and organic matter
Soil organic matter was determined using the potassium dichromate volumetric method; available nitrogen was analyzed using an AA3 continuous flow analyzer (SEAL, Germany); available phosphorus was measured by the Mo-Sb colorimetric method; available potassium was determined with the flame photometric method; the water-soluble calcium and magnesium contents were measured by titration using EDTA; and the microelements of soil were determined using the atomic absorption method29–31. All samples were tested three times.
Data analysis
All experimental data were managed using Microsoft Excel 2016. The data were analyzed by one-way ANOVA and Duncan’s multiple comparison test using IBM SPSS Statistics 16.0. Statistical significance was considered at p < 0.05.
Results
Numbers of soil microbes in different cultivated soil
The results showed that the total numbers of bacteria, actinomycetes and microorganisms differed significantly among different cultivation durations, while the numbers of fungi did not show significant differences (Table 1). The numbers of bacteria and actinomycetes in fallow soil were higher than that in soil with different planting durations and first decreased and then increased with years since cultivation. The numbers of fungi in soil with P. notoginseng was lower than that observed in fallow soil.
Table 1.
Numbers of microorganisms in different cultivated soil.
| Planting Year | Bacteria (108 cfu/g) | Fungi (105 cfu/g) | Actinomyces (106 cfu/g) | Total (108 cfu/g) |
|---|---|---|---|---|
| Fallow fields | 5.065a | 4.122a | 7.070a | 5.139a |
| 1 year | 3.845b | 4.061a | 5.616b | 3.905b |
| 3 years | 3.397b | 3.882a | 3.971c | 3.441b |
| 5 years | 4.006b | 3.885a | 6.070b | 4.071b |
Notes: Different lowercase letters indicate significant differences (n = 9, p < 0.05).
Numbers of physiological groups in different cultivated soils
Analysis of physiological groups revealed differences between fallow soiled and planted soil (Fig. 1). The numbers of nitrosobacteria, aromatic- decomposition microbes, Azotobacter, potassium-solubilizing bacteria and phospho-bacteria detected in cultivated soil were lower than those observed in fallow soil. Nitrosobacteria were 4.6–12.2 times more abundant in fallow soil than in the cultivated soil. The numbers of denitrifying bacteria first increased and then decreased with years of cultivation. The numbers of Azotobacter and aromatic-decomposing microbes in the soil decreased with years of cultivation. The numbers of cellulose-decomposing bacteria detected in soil with P. notoginseng for 3 years was much lower than that in fallow and other cultivated soils. Planting of P. notoginseng did not affect the microbial community in the soil, especially with respect to nitrosobacteria, aromatic-decomposing microbes, Azotobacter and phosphate-solubilizing bacteria.
Figure 1.
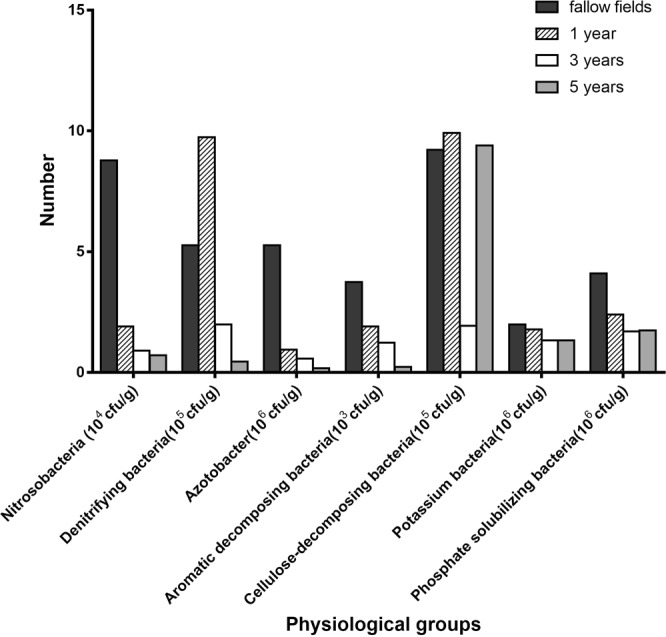
Numbers of physiological groups in different cultivated soils (fallow fields and soils growing P. notoginseng for 1, 3 or 5 years).
Fungi in different cultivated soils
Members of the genera Rhizopus, Mucor, Botrytis, Saccharomyces, Paecilomyces, Aspergillus, and Mortierella were all detected in soils with different planting durations. Trichoderma was only isolated from fallow fields and from soil in which P. notoginseng had been grown for 1 year. Fusarium was only in detected soil with P. notoginseng (Table 2).
Table 2.
The fungal taxa detected in different cultivated soils.
| Species | Fallow fields | 1 year | 3 years | 5 years |
|---|---|---|---|---|
| Rhizopus | √ | √ | √ | √ |
| Mucor | √ | √ | √ | √ |
| Trichoderma | √ | √ | ○ | ○ |
| Botrytis | √ | √ | √ | √ |
| Saccharomyces | √ | √ | √ | √ |
| Penicillium | √ | √ | √ | √ |
| Paecilomyces | √ | √ | √ | √ |
| Aspergillus | √ | √ | √ | √ |
| Mortierella coemans | √ | √ | √ | √ |
| Fusarium | ○ | √ | √ | √ |
Enzyme activity in different cultivated soils
The activities of invertase, amylase, catalase and polyphenol oxidase in fallow soil were higher than those in cultivated soil, but that of cellulase first decreased and then increased with years of cultivation. The urease activity, which was the highest in soil in which P. notoginseng had been growing for 1 year, showed no regular pattern. Phosphatase activity primarily attributable to acid phosphatase, alkaline phosphatase and neutral phosphatase. Table 3 shows that the phosphatase activity trend in the soils with respect to Ph was acidic >neutral >alkaline. The peroxidase activity in soil in which P. notoginseng had been grown for 3 years was much higher than that detected in fallow soil. The activity of polyphenol oxidase first decreased, then increased, and then decreased with years of cultivation. However, the activity of polyphenol oxidase in soil with P. notoginseng was significantly lower than that detected in fallow soil (Table 3).
Table 3.
The enzyme activities detected in different cultivated soils.
| Planting Year | Invertase (mg/(g·24 h)) | Amylase (mg/(g·24 h)) | Cellulase (mg/(10 g·72 h)) | Catalase (0.1 N·KMnO4 ml) | Urease (mg/(g·24 h)) |
|---|---|---|---|---|---|
| unplanted | 362a | 8.72a | 36.6ab | 1.08a | 1.516c |
| 1 year | 263b | 8.30a | 31.9b | 0.855b | 2.494a |
| 3 years | 64.7c | 3.51b | 22.3c | 0.382c | 1.080d |
| 5 years | 68.3c | 1.66c | 41.4a | 0.773b | 1.935b |
| Planting Year | Phosphatase (mg/(g·24 h)) | Peroxidase (mg/(g·2 h)) | Polyphenol Oxidase (mg/(g·2 h)) | ||
| acidic | neutral | alkaline | |||
| unplanted | 294a | 200a | 196a | 3.85b | 2.54a |
| 1 year | 307a | 119b | 82.3b | 4.01b | 1.02b |
| 3 years | 183b | 38.6c | 26.2d | 9.93a | 1.81c |
| 5 years | 273a | 123b | 63.8c | 4.53b | 1.23b |
Notes: Different lowercase letters indicate significant differences (n = 9, p < 0.05).
Soil nutrients in different cultivated soils
The organic matter content first decreased and then increased with planting duration. The organic matter in soil with P. notoginseng was lower than that detected in fallow soil. The change in available N was irregular, and the available N content was highest in soil in which P. notoginseng had been grown for 3 years. Changes in available P, available Mn and water-soluble Mg showed no regular pattern. The available P content in soil in which P. notoginseng had been grown for 1 or 5 years was higher than that detected in soil in which P. notoginseng had been grown for 3 years and in fallow soil. The available K content in soil with P. notoginseng was lower than that detected in fallow soil. With an increase in years of planting, the available K content first decreased, then increased, and then decreased. The available Ni, Cu, Zn and Fe contents in soil with P. notoginseng were lower than those detected in fallow soil, showing a trend of first decreasing and then increasing. In contrast, the water-soluble Ca was higher in soil with P. notoginseng than in fallow soil (Figs 2 and 3).
Figure 2.
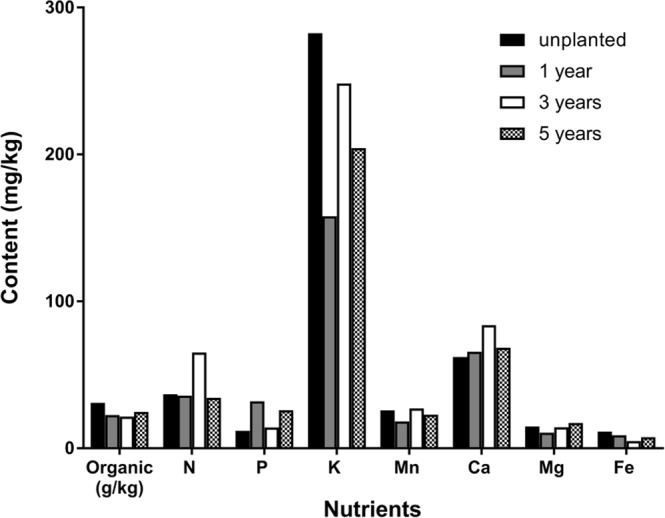
Analysis of soil nutrients (N, P, K, Mn, Ca, Mg, and Fe) in different cultivated soils (fallow fields and soils growing P. notoginseng for 1, 3 or 5 years).
Figure 3.
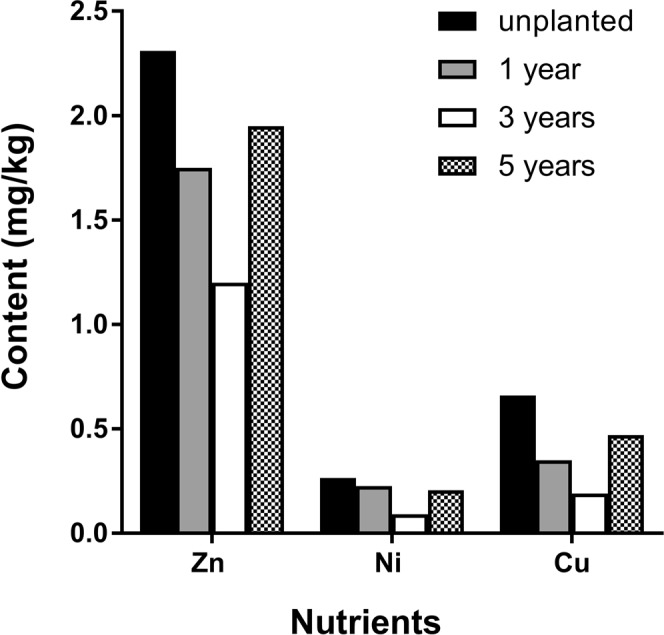
Analysis of soil nutrients (Zn, Ni, and Cu) in different cultivated soils (fallow fields and soils growing P. notoginseng for 1, 3 or 5 years).
Numbers of soil microbes after different planting intervals
There were significant differences in the numbers of microbes in soils with different planting intervals (Table 4). Except for the interval of 1 year, the numbers of fungi did not show significant differences. The numbers of bacteria in the soil with an interval of 9 years were the lowest and increased with shorter planting intervals. However, the opposite trend observed for actinomycetes, the abundance of which decreased with shorter planting intervals, which was the same pattern observed for the total number of microbes. We also observed that the numbers of microbes in unplanted soil and after an interval of 3 years were similar.
Table 4.
Numbers of microorganisms present after different interval year.
| Interval Year | Bacteria (108 cfu/g) | Fungi (105 cfu/g) | Actinomyces (106 cfu/g) | Total (108 cfu/g) |
|---|---|---|---|---|
| fallow fields | 3.910b | 2.862a | 0.726c | 3.920b |
| 9 years | 1.012d | 3.117ab | 3.198a | 1.047d |
| 6 years | 2.648c | 2.994ab | 1.267b | 2.654c |
| 3 years | 4.022b | 3.218ab | 0.613cd | 4.031b |
| 1 year | 5.597a | 3.731b | 0.498d | 5.605a |
Notes: Different lowercase letters indicate significant differences (n = 9, p < 0.05).
Numbers of physiological groups after different planting intervals
The abundance of nitrosobacteria detected in previously cultivated soil was lower than that observed in fallow soil, with no significant differences observed between 9, 6, and 1 year intervals. The abundance of Azotobacter after 9 years of planting was similar to that observed in fallow soil but was much higher than that observed in soil with other planting intervals. The number of aromatic-decomposing bacteria was the highest in fallow soil and was 5.4–14.5-fold higher than that observed in planted soil. The abundance of cellulose-decomposing bacteria was highest in the 1-year planting interval and decreased with longer planting intervals. The numbers of potassium- and phosphate-solubilizing bacteria were greater in fallow soil than in soil with different planting intervals (Fig. 4).
Figure 4.
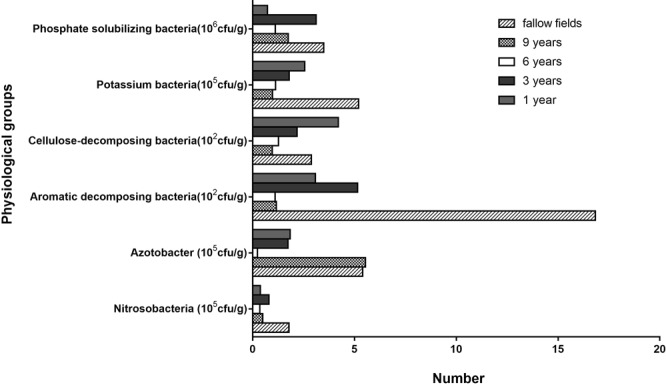
Numbers of physiological groups after different interval years (1, 3, 6 or 9 years).
Enzyme activity after different planting intervals
The variation in soil enzyme activity among planting intervals was different from that observed in unplanted soil (Table 5). The invertase activity in fallow soil and soil with a 1-year planting interval was higher than that detected in soil with other intervals. The invertase activity showed no pattern, as it was highest at an interval of 3 years but lowest at an interval of 6 years. The cellulase activity was highest in fallow soil and the lowest in soil with an interval of 3 years. The catalase activity in fallow soil was higher than that in soil with different planting intervals and decreased with longer planting intervals. The trend in phosphatase activity with respect to pH was acidic >neutral >alkaline. The activity of polyphenol oxidase showed no regular trend, being it was highest at the interval of 3 years and lowest at the interval of 1 year.
Table 5.
The enzyme activities detected after different interval year.
| Interval Year | Invertase (mg/(g·24 h)) | Amylase (mg/(g·24 h)) | Cellulase (mg/(10 g·72 h)) | Catalase (0.1 N·KMnO4 ml) | Peroxidase (mg/(g·2 h)) |
|---|---|---|---|---|---|
| unplanted | 137a | 5.40b | 57.3a | 1.89a | 3.60c |
| 9 years | 114b | 6.97a | 49.4b | 0.547b | 10.9a |
| 6 years | 112b | 3.30c | 47.8b | 0.865c | 10.4a |
| 3 years | 108b | 7.57a | 33.4c | 0.929c | 7.50b |
| 1 year | 125a | 4.75b | 54.1ab | 1.05d | 6.67b |
| Interval Year | Phosphatase (mg/(g·24 h)) | Polyphenol Oxidase (mg/(g·2 h)) | |||
| acidic | neutral | alkaline | |||
| unplanted | 322a | 211a | 39.3a | 1.82b | |
| 9 years | 299c | 85.9c | 23.7b | 1.43bc | |
| 6 years | 235d | 46.7e | 17.0c | 1.84b | |
| 3 years | 209e | 67.4d | 11.9d | 3.36a | |
| 1 year | 325a | 123b | 23.7b | 1.30c | |
Notes: Different lowercase letters indicate significant differences (n = 9, p < 0.05).
Discussion
The most abundant group of soil microorganisms are bacteria, which play important role in the conversion of soil organic and inorganic matter. Bacteria diversity is affected by soil conditions, seasonal plants, and age32. Fungi are some of the primary members involved in the decomposition of soil organic matter and humus, which have an effect on soil fertility. Furthermore, the number and composition of fungi are influenced by the amount of organic present matter in soil33. Members of the phylum Actinomycetes produce nitrogen-containing and nitrogen-free organic compounds in soil and can even decompose soil humus. Actinomycetes are closely associated with soil fertility and plant disease control34. In the present study, the numbers of bacteria and actinomycetes in soil with P. notoginseng were lower than those detected in fallow soil, whereas the numbers of fungi showed no significant difference. Previous studies reported that crop rotation can improve soil microbial structure, which is reflected by an increase by the numbers of bacteria and actinomycetes and a decrease in the numbers of fungi35. In the present study, except for the fallow soil, the numbers of actinomycetes increased with longer planting intervals. Except for the 1-year- interval samples, the abundance of fungi showed no significant difference. Planting P. notoginseng changed the soil microecology, possibly because P. notoginseng releases organic matter into the soil and absorbs inorganic compounds present in soil, which ultimately leads to changes in the structure, ventilation, and biological activity of the soil. Root exudates have inhibitory or promoting effects on microorganisms in the soil. A series of interactions from antagonistic to synergistic occur among the microorganisms and between microbes and plants, resulting in changes in the microbial community structure in the soil. Changes in the community structure of soil microorganisms also results in changes in nitrosobacteria, Azotobacter, aromatic-decomposing bacteria, cellulose-decomposing bacteria phosphate- and potassium-solubilizing bacteria.
Soil enzyme activity is also used as an indicator of changes in the quality and productivity of soil36 and is used to study the short and long-term effects of a disturbance, as the biochemical reactions in soil are mediated by microorganisms37. These reactions are catalyzed by enzymes that play an important role in biogeochemical cycles38. The results of the present study showed that the activities of invertase, amylase, catalase, polyphenol oxidase and phosphatase in soil with P. notoginseng was lower than those detected in fallow soil, whereas the activities of cellulose, urease and peroxidase did not exhibit a regular pattern. The activities of invertase, cellulase, and catalase in fallow soil were higher than those detected in soil with different planting intervals. However, the activity of peroxidase in fallow soil was lower than that detected in soil with different planting intervals. The decrease in invertase, amylase, and cellulase activities associated with the carbon cycle may result from long-term planting of P. notoginseng, which reduces the capacity for soil carbon cycling and the decomposition of specific sugars, starch, and cellulose. A decrease in catalase activity in soil with P. notoginseng is not conducive to the decomposition of hydrogen peroxide in the soil, which may lead to toxic effects of hydrogen peroxide on plants. Soil polyphenol oxidases play a role in the decomposition of phenolic acids in soil. With an increasing duration of cultivation, a decrease in polyphenol oxidase activity leads to a decrease in the capacity for phenolic acid decomposition. Phenolic acids are allelochemicals, an increase in which may be one of the reasons for the development of continuous cropping as the number of years of planting increases.
Soil fertility refers to the ability of the soil to support plant growth and coordinate nutrient conditions and environmental conditions, including effective and potential fertility39. In the present study, the amounts of organic matter, available N, Ni, Cu, Zn and Fe in soil with P. notoginseng were lower than those detected in fallow soil. However, the amount of water-soluble Ca was higher than that observed in fallow soil, whereas the amount of available N, available P, available Mn and water-soluble Mg showed no regular pattern. The results showed that the amount of organic matter in soil with P. notoginseng was lower than that present in fallow soil. On the one hand, this pattern may be due to decomposition by microorganisms in the soil, as this process converts soil organic matter into inorganic components that can be used by plants. Alternatively, this pattern may have an anthropogenic cause, as the removal of debris is another reason for reduced organic matter in soil, and this process eventually leads to a decrease in the soil organic matter content.
Conclusion
The effects of continuous cropping were complex, resulting from the combined effects of three complex systems: plants, soil, and microorganisms. By studying different soils with P. notoginseng, we observed that the ecological structure of microbes and the balance of soil nutrients changed. These changes in the soil ecosystem prevented the soil from adapting to the continuous cropping of P. notoginseng, which eventually led to the inhibition of P. notoginseng growth and increased the incidence of diseases. Soil organic fertilizers, microbial fertilizers, and essential trace elements could be used to supplement the missing elements in the soil, to restore the balance of the soil and alleviate the obstacles associated with continuous cropping of P. notoginseng.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81703641), the China Postdoctoral Science Foundation (2019M652146), the Department of Education Scientific Research Project of Zhejiang province (Y201738462) and the National Project for Standardization of Chinese Materia Medical (ZYBZH-C-TJ-55). The authors gratefully acknowledge the Yunnan TASLY Notoginseng Planting Co., Ltd. The authors would like to thank Prof. David Popovich from Massey University of New Zealand for revising this manuscript.
Author Contributions
P.X. and Z.L. conceived and designed the research. Y.Z.1, Y.Z.2 and P.X. analyzed the results. L.X. and Y.Z.1 collected the plant samples. L.X., Y.Z. and P.X. performed the experiments. Y.Z.2 and P.X. wrote the manuscript.
Data Availability
The data used to support the findings of our study are presented in the article and readers can assess it through the article content. The raw data can be requested from the corresponding author via e-mail.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Pengguo Xia, Email: xpg_xpg@zstu.edu.cn.
Zongsuo Liang, Email: liangzs@ms.iswc.ac.cn.
References
- 1.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J of Pharm & Pharmacol. 2010;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 2.Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng. Vaccine. 2005;23:5533–5542. doi: 10.1016/j.vaccine.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 3.Ge LC, Ming LX, Guo WJ. Effect of soybean continuous cropping on bulk and rhizosphere soil microbial community function. Acta Ecologica Sinica. 2006;26:1144–1150. doi: 10.1016/S1872-2032(06)60004-8. [DOI] [Google Scholar]
- 4.Yang JZ, et al. The Effect on Growth of Panax notoginseng in Soil under Rotation for the Different Years. Research & Practice on Chinese Medicines. 2012;2:6–8. [Google Scholar]
- 5.Qian P, et al. Phylogenetic and Metabolic Responses of Rhizosphere Microbes to the Cultivation of Panax notoginseng. Journal of Biobased Materials & Bioenergy. 2016;10(5):370–377. doi: 10.1166/jbmb.2016.1616. [DOI] [Google Scholar]
- 6.Tan Y, et al. Rhizospheric soil and root endogenous fungal diversity and composition in response to continuous Panax notoginseng cropping practices. Microbiological Research. 2016;194:10–19. doi: 10.1016/j.micres.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Dong L, et al. Soil bacterial and fungal community dynamics in relation to Panax notoginseng death rate in a continuous cropping system[J] Scientific Reports. 2016;6:31802. doi: 10.1038/srep31802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin XG, et al. Changes of soil microbiological properties caused by land use changing from rice-wheat rotation to vegetable cultivation. Environ. Geochem. Health. 2004;26:119–128. doi: 10.1023/B:EGAH.0000039574.99651.65. [DOI] [PubMed] [Google Scholar]
- 9.Liu SH, et al. Influence of garlic continuous cropping on rhizosphere soil microorganisms and enzyme activities. Scientia Agricultura Sinica. 2010;43:1000–1006. [Google Scholar]
- 10.Machado S, Petrie S, Rhinhart K, Qu A. Long-term continuous cropping in the Pacific Northwest: Tillage and fertilizer effects on winter wheat, spring wheat, and spring barley production. Soil Tillage Res. 2007;94:473–481. doi: 10.1016/j.still.2006.09.007. [DOI] [Google Scholar]
- 11.Chen H, et al. Effects of successive cropping Rehmannia glutinosa on rhizosphere soil microbial flora and enzyme activities. Chinese Journal of Applied Ecology. 2007;18:2755. [PubMed] [Google Scholar]
- 12.Hiddink GA, Termorshuizen AJ, Bruggen AHCV. Mixed Cropping and Suppression of Soilborne Diseases. Springer Netherlands. 2010;4:119–146. [Google Scholar]
- 13.Ma HY, Xu J, Zheng CS, Sun X, Shu HR. Effects of Continuous Cropping System on the Soil Physical-Chemical Properties and Biological Properties of Gerbera jamesonii. Scientia Agricultura Sinica. 2011;44:3733–3740. [Google Scholar]
- 14.Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Bron PA, Van BP, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 16.Ma L, et al. Phylogenetic diversity of bacterial endophytes of Panax notoginseng with antagonistic characteristics towards pathogens of root-rot disease complex. Antonie Van Leeuwenhoek. 2013;103:299–312. doi: 10.1007/s10482-012-9810-3. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Liu XL, Li S, Miao Z. In vitro inhibition of fungal root-rot pathogens of Panax notoginseng by Rhizobacteria. Plant Pathol. J. 2009;5:70–76. doi: 10.5423/PPJ.2009.25.1.070. [DOI] [Google Scholar]
- 18.Xia P, et al. Optimal fertilizer application for Panax notoginseng and effect of soil water on root rot disease and saponin contents. J. Ginseng Res. 2016;40(1):38–46. doi: 10.1016/j.jgr.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia P, et al. Accumulation of saponins in Panax notoginseng during its growing seasons. Ind. Crops Prod. 2017;104:287–292. doi: 10.1016/j.indcrop.2017.04.045. [DOI] [Google Scholar]
- 20.Huang L, et al. Correlation among soil microorganisms, soil enzyme activities, and removal rates of pollutants in three constructed wetlands purifying micro-polluted river water. Ecol. Eng. 2012;46:98–106. doi: 10.1016/j.ecoleng.2012.06.004. [DOI] [Google Scholar]
- 21.Isaac, S., Jennings, D. Microbial culture. Microbial Culture (1995).
- 22.Wollum, A. G. Cultural Methods for Soil Microorganisms. Methods of Soil Analysis. part. chemical & Microbiological Properties (1982).
- 23.Zhao, G., Wang, H. Y. Soil microorganism bio-diversity molecule ecology research approach. Forest By-Product and Speciality in China (2006).
- 24.Seth RK, Alam S, Shukla DN. Isolation and identification of Soil Fungi from Wheat Cultivated Area of Uttar Pradesh. J. Plant Pathol. Microbiol. 2016;7:384–386. [Google Scholar]
- 25.Nagamani, A., Kunwar, I. K., Manoharachary, C. Hand book of soil fungi (2006).
- 26.Nannipieri P, et al. Soil enzymology: classical and molecular approaches. Biol. Fertil. Soils. 2012;48:743–762. doi: 10.1007/s00374-012-0723-0. [DOI] [Google Scholar]
- 27.Shukla, G., Varma, A. Soil Enzymology (2011).
- 28.Kucharski J, Tomkiel M, BacMaga M, Borowik A, Wyszkowska J. Enzyme activity and microorganisms diversity in soil contaminated with the Boreal 58 WG herbicide. J. Environ. Sci. Health, Part B. 2016;51:446–454. doi: 10.1080/03601234.2016.1159456. [DOI] [PubMed] [Google Scholar]
- 29.Zdenko, L., Domagoj, R., Brigita, P. Sampling of soil and plant for agrochemical and pedological analysis (2014).
- 30.Singh V, Agrawal HM. Qualitative soil mineral analysis by EDXRF, XRD and AAS probes. Radiat. Phys. Chem. 2012;81:1796–1803. doi: 10.1016/j.radphyschem.2012.07.002. [DOI] [Google Scholar]
- 31.Ji TW. Comparison on determining the organic matter contents in the soils by different heating methods in the potassium dichromate-volumetric method. Acta Agriculturae Zhejiangensis. 2005;17(5):311–313. [Google Scholar]
- 32.Hrynkiewicz K, Baum C, Leinweber P. Density, metabolic activity and identity of cultivable rhizosphere bacteria on Salix viminalis in disturbed arable and landfill soils. J Plant Nutr Soil Sci. 2010;173:747–756. doi: 10.1002/jpln.200900286. [DOI] [Google Scholar]
- 33.Hua J, Liu G, Huang J. Effect of continuous cropping of sesame on rhizospheric microbial communities. Acta Ecologica Sinica. 2012;32:2936–2942. doi: 10.5846/stxb201104010422. [DOI] [Google Scholar]
- 34.Huang, Y. Q. Studies on effects of continuous cropping obstacle on growth of Peanut and its mechanism (2011).
- 35.Yang FJ, Wu HT, Wei M, Wang XF, Shi QH. Effects of rotation and fallowing on the microbial communities and enzyme activities in a solar greenhouse soil under continuous cucumber cropping. Chinese Journal of Applied Ecology. 2009;20:2983–2988. [PubMed] [Google Scholar]
- 36.Blanca EG, Gerardo VM, Luc D, Víctor OP. Enzyme activities and metabolic profiles of soil microorganisms at KILN sites in Quercus, spp. temperate forests of central Mexico. Applied Soil Ecology. 2012;52:48–55. doi: 10.1016/j.apsoil.2011.10.010. [DOI] [Google Scholar]
- 37.Eivazi F, Bayan MR. Effects of long-term prescribed burning on the activity of select soil enzymes in an oak-hickory forest. Can. J. For. Res. 1996;26:1799–1804. doi: 10.1139/x26-204. [DOI] [Google Scholar]
- 38.Wittmann C, Kähkönen MA, Ilevesniemi H, Kurola J, Salkinoja-Salonen MS. Areal activities and stratification of hydrolytic enzymes involved in the biochemical cycles of carbon, nitrogen, sulphur and phosphorus in podzolized boreal forest soils. Soil Biol. Biochem. 2004;36:425–433. doi: 10.1016/j.soilbio.2003.10.019. [DOI] [Google Scholar]
- 39.Tiessen H, Cuevas E, Chacon P. The role of soil organic matter in sustaining soil fertility. Nature. 1994;371:783–785. doi: 10.1038/371783a0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of our study are presented in the article and readers can assess it through the article content. The raw data can be requested from the corresponding author via e-mail.


