Abstract
Oral intake of regorafenib has been shown to have survival benefits in patients with metastatic colorectal cancer progressing on standard therapies. However, because of adverse effects, the patients sometimes cannot continue treatment with regorafenib. Currently, there is no established supportive therapy that can be performed to aid in continuing regorafenib intake under these problematic conditions. We report the case of a 59-year-old Japanese woman diagnosed with recurrence after curative operation for sigmoid colon cancer (T3N2aM0, Stage IIIC). Despite undergoing multiple lines of standard chemotherapy, disease control could not be maintained. Consequently, regorafenib was started as a late-line treatment. However, after 2 weeks, the patient experienced regorafenib-induced serious erythema multiforme; thus, regorafenib was discontinued and oral prednisolone was started. Regorafenib administration was resumed when the adverse effects resolved and prednisolone was stopped, but skin rash rapidly reappeared. Prednisolone treatment was reintroduced, which cured the rash; thus, after the third attempt to administer regorafenib, prednisolone was continuously administered. There was no relapse of the rash under prednisolone administration, and the patient received a total of 13 courses of regorafenib. Moreover, the metastatic lesions that had started to regrow at the end of the regorafenib therapy showed good response to the rechallenge chemotherapy of folinic acid, fluorouracil, and irinotecan therapy with panitumumab. The sequence of therapies possibly had a positive impact on the patient’s long survival of 30 months after the regorafenib treatment. Systemic administration of steroid is considered as a promising option as a supportive therapy for continuing regorafenib treatment in patients experiencing a severe skin rash.
Keywords: Metastatic colorectal cancer, Regorafenib, Erythema multiforme, Rechallenge chemotherapy, Continuous steroid intake, Supportive therapy
Introduction
Regorafenib is a multikinase inhibitor drug that has shown survival benefits in patients with metastatic colorectal cancer (mCRC) after the failure of early-line chemotherapies. In the correct trial [1], it was demonstrated that regorafenib reduced disease progression and prolonged median survival in patients with mCRC. However, the well-known skin disorders of regorafenib, occurring most likely during the first or second course of treatment, make continuous treatment with this drug difficult [2]. Currently, the standard supportive therapies to address the skin disorders have been moisturizers and topical steroids. Herein, we report the case of a patient with mCRC who experienced erythema multiforme (EM) during treatment with regorafenib, but managed to continue the treatment with simultaneous steroid intake for managing this adverse effect.
Case report
In April 2012, a 59-year-old Japanese woman presented to our institute exhibiting hematochezia. She was diagnosed with sigmoid colon cancer and underwent sigmoid colectomy with regional lymph node dissection. Pathological examination findings indicated moderately differentiated tubular adenocarcinoma with vascular and lymphatic involvements. Surgical margins were cancer-free, and based on the TNM classification of the Union for International Cancer Control, 8th edition [3], the final staging was T3N2aM0, Stage IIIB. Capecitabine and oxaliplatin (CapOX) therapy was given to the patient as an adjuvant chemotherapy [4]. Despite the patient experiencing weight loss and peripheral neuropathy, she completed all eight courses of treatment.
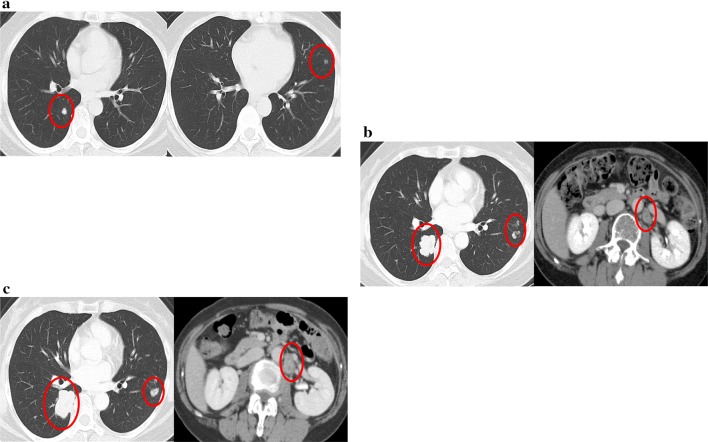
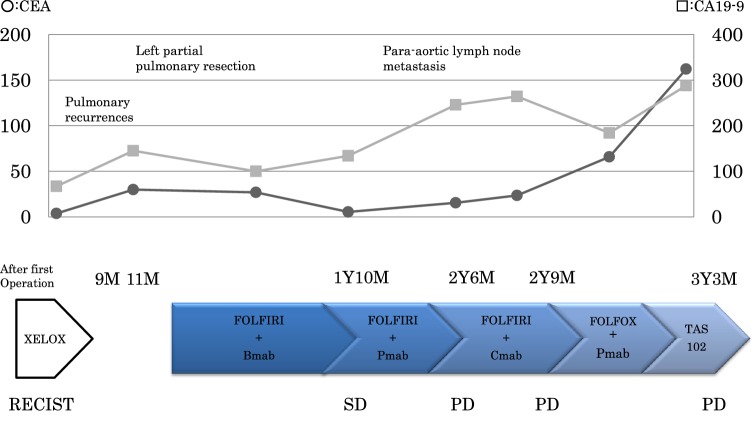
In February 2013, two pulmonary recurrent foci were detected on computed tomography (CT) images 9 months after the first operation. Left partial pulmonary resection was performed, but a new metastatic lesion was immediately found in the left lung (Fig. 1a). Therefore, chemotherapy using folinic acid, fluorouracil, and irinotecan therapy (FOLFIRI) with bevacizumab (B-mab) was started. Due to the diminishing effects of the therapy, B-mab was later changed to anti-epidermal growth factor receptor (EGFR) antibodies, first panitumumab (P-mab) and then cetuximab. Tumor progression of lung metastases and para-aortic lymph node metastases (Fig. 1b) was observed after the patient underwent treatment with 40 courses of FOLFIRI with the above-mentioned molecular target-based drugs. In March 2015, the patient underwent a third-line chemotherapy using folinic acid, fluorouracil, and oxaliplatin therapy with P-mab, but an allergic reaction caused by oxaliplatin resulted in immediate discontinuation of the regimen. Thus, a third-line chemotherapy using a combination of trifluridine–tipiracil (TAS102) was started; however, progression of the metastases (Fig. 1c) was observed as the fourth course was about to be started. Figure 2 shows the treatment courses after the first operation.
Fig. 1.
Computed tomography images of the recurrences during the treatments before regorafenib. The red circles indicate the recurrent lesions. a Two areas of pulmonary recurrences 9 months after primary tumor resection. b Progression of tumor in both lungs and a para-aortic lymph node metastasis 21 months after recurrence. 13 courses of the second-line chemotherapy finished. c Progression of the metastases 30 months after recurrence. TAS102 finished
Fig. 2.
Summary of the treatments before regorafenib. The solid line with circles indicates the trend for CEA levels. The solid line with squares indicates the trend for carbohydrate antigen 19–9 levels. CEA carcinoembryonic antigen, CA19–9 carbohydrate antigen 19–9, XELOX capecitabine and oxaliplatin, FOLFIRI folinic acid, fluorouracil, and irinotecan, B-mab bevacizumab, P-mab panitumumab, C-mab cetuximab, FOLFOX folinic acid, fluorouracil and oxaliplatin, TAS-102 trifluridine–tipiracil combination, M month, Y year, SD stable disease, PD progressive disease
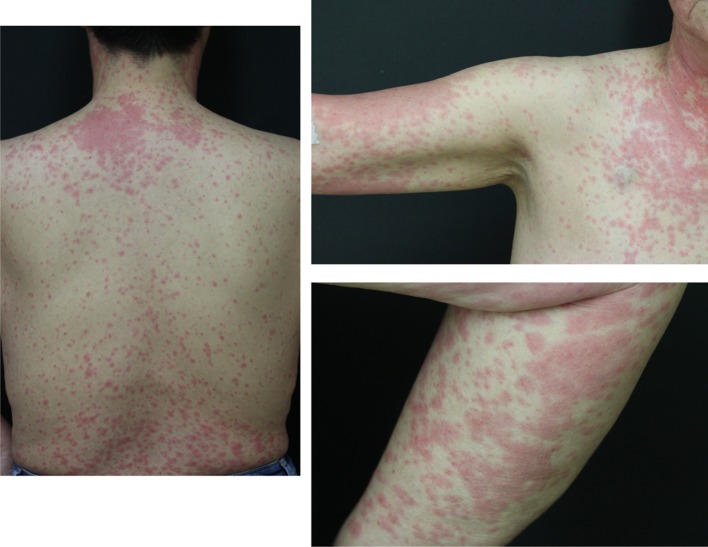
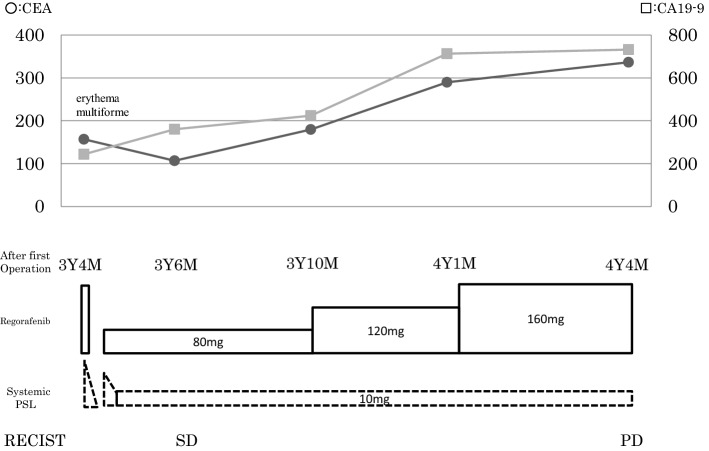
In September 2015, regorafenib at a daily dose of 160 mg was started. After 2 weeks, the patient was urgently hospitalized due to high fever and whole-body rash (Fig. 3). A dermatologist provided a diagnosis of regorafenib-induced EM, which was estimated at grade 3 (common terminology criteria for adverse events: CTCAE v4.0-JCOG), because the percentage of the total body surface area that was affected by EM was more > 30% and conjunctival rash was also observed. Regorafenib treatment was discontinued and prednisolone (PSL) treatment was started at a daily dose of 50 mg orally. After 2 weeks of starting the PSL treatment, the rash disappeared completely. Accordingly, the PSL dose was gradually decreased, and its administration was stopped on day 19 of the treatment. Thirty days after stopping the PSL treatment, regorafenib administration at a daily dose of 80 mg was resumed, but within a few hours of administration, skin rash reappeared. Regorafenib was withdrawn again, and steroid treatment (PSL 30 mg/day) was resumed, which was much effective, and the rash immediately disappeared. Subsequently, treatment with regorafenib at a daily dose of 80 mg, in combination with continuous oral PSL (30 mg/day) was reattempted 7 days after the EM disappeared. Afterward, there were no more occurrences of rash, and the patient was able to tolerate the increase in the regorafenib dose and reached a standard daily dose of 160 mg with PSL (10 mg/day) (Fig. 4). A Proton pump inhibitor was concomitantly used during PSL administration without prophylactic antibiotics. Grade 3 (CTCAE v4.0-JCOG) hand–foot syndrome was found as an adverse event of regorafenib; however, regorafenib therapy was continued with outpatient care by a dermatologist. CT images obtained 3 months after the treatment revealed metastases regression (Fig. 5). Consequently, the patient received 13 courses of regorafenib in total. Although there was a regrowth of the metastases, the patient agreed to receive rechallenge chemotherapy using FOLFIRI with P-mab in October 2016. Subsequent CT examination findings showed that the metastases responded to the rechallenge chemotherapy (Fig. 6). The patient underwent effective administration of a total of 22 courses of FOLFIRI with P-mab. After 11 months, the right iliac bone metastases appeared, after that the best supportive care was done. Until the bone metastasis appears, the quality of life of the patient has been consistently good. Due to the combined therapies and maintenance, the patient survived for of 30 months after the regorafenib treatment.
Fig. 3.
Regorafenib-induced erythema multiforme
Fig. 4.
Summary of the treatments during administration of regorafenib. The solid line with circles indicates the trend for CEA levels. The solid line with squares indicates the trend for carbohydrate antigen 19–9 levels. CEA Carcinoembryonic antigen, CA19–9 carbohydrate antigen 19–9, M month, Y year, PSL prednisolone, SD stable disease, PD progressive disease
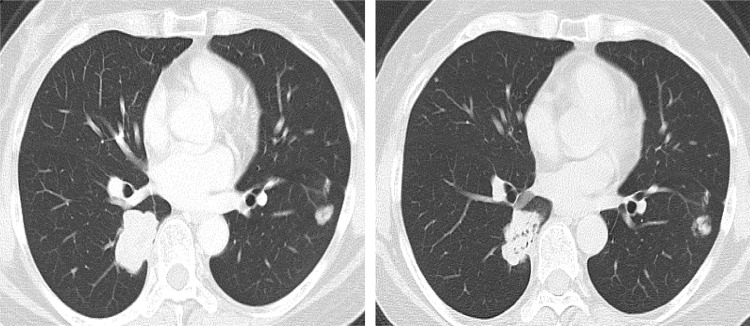
Fig. 5.
Computed tomography images of the pulmonary metastases during regorafenib treatment. The left image is before regorafenib treatment 30 months after recurrence. The right image is after three courses of regorafenib. The metastatic lesions were downsized compared with the status before regorafenib treatment
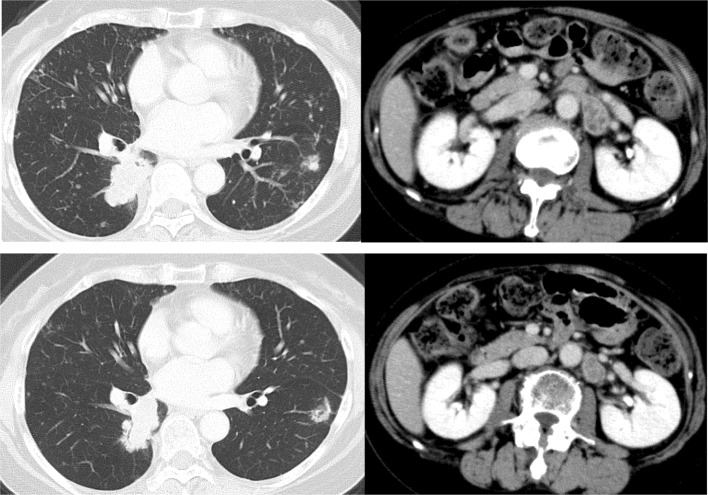
Fig. 6.
Computed tomography images of the pulmonary metastases and a para-aortic lymph node metastasis after the rechallenge chemotherapy. The above image is before the rechallenge chemotherapy 44 months after recurrence. The below image is after six courses of rechallenge chemotherapy. The metastatic lesions were downsized compared with the status before the rechallenge chemotherapy
Discussion
The case reported here suggests that systemic administration of steroids is effective not only in overcoming the adverse effects of regorafenib, but also in maintaining good patient condition for continuation of regorafenib therapy. In addition, owing to the anticancer therapeutic effects of regorafenib and regorafenib-induced cancer susceptibility to rechallenge chemotherapy, steroid therapy might provide a survival benefit.
Although regorafenib reduces disease progression in patients with mCRC, the correct trial [1] has reported the incidence of adverse events to be 93% and that of grade 3 or higher adverse events to be 55%. In addition, it has been known that the adverse effects associated with regorafenib occur in the early phases of the treatment [2]. Therefore, many patients are forced to accept unexpected early termination of the treatment or insufficient administration dose and, thus, fail to benefit from the therapeutic effects of regorafenib.
Oral multikinase inhibitors can cause various skin toxicities. The incidence of EM in Japanese patients treated with regorafenib has been reported to be as high as 4.6% (3/65) patients [1]. There have been two reports of patients with mCRC who experienced serious EM [5, 6]. Both these patients developed EM just 2 weeks after the initial intake of regorafenib, after which regorafenib was stopped and steroid therapy was started. Unfortunately, because of the patient’s systemic condition or the choice of best supportive care, they could not accept adequate regorafenib therapy thereafter. Importantly, the occurrence of grade 3 adverse events in late-line therapies is crucial and mostly results in the termination of anticancer therapy. Conversely, regorafenib therapy in the patient in the present report, who experienced regorafenib-induced serious EM, was temporarily stopped; however, using steroids, regorafenib therapy was restarted and maintained using a standard dose and over a long period. To our knowledge, no case reports have introduced the systemic administration of PSL in combination with regorafenib therapy. Notably, our case makes a helpful suggestion that simultaneous regorafenib therapy and oral PSL intake can be a promising treatment for regorafenib-induced EM.
Recent reports have highlighted rechallenge chemotherapy using anti-EGFR drugs for mCRC [7, 8]. The methods that can be used to improve susceptibility to anti-EGFR drugs under another line of therapy remain to be fully clarified. The intratumoral heterogeneity has been mentioned as one hypothesis. It has been reported that 5–10% of mCRC show KRAS molecular heterogeneity between primary, lymph node, and distant metastases [9]. Moreover, a recent study [10] evaluated KRAS gene status into the primary tumor, comparing the tumor center and the invasion fronts. The intratumoral heterogeneity of KRAS mutation was observed in 8% of primary tumors [10]. As a possible underlying mechanism of treatment resistance in patients using anti-EGFR drugs, it is considered that a small population of latent cancer cells with KRAS mutation in a cancer judged as KRAS wild type would relatively proliferate under anti-EGFR treatment, which prompts the cancer to be resistant to anti-EGFR drugs. It is thought that KRAS wild-type cancer cells remaining during the anti-EGFR treatment withdrawal period will proliferate again. Under such situation, other subsequent types of therapies possibly induce restoration of the primary constitution of cancer cells, which might explain the complex phenomenon of successful rechallenge chemotherapy.
Several reports [11, 12] have focused on the effects of rechallenge chemotherapy after regorafenib treatment. Kidd et al. [12] conducted a retrospective review of patients who underwent chemotherapy after regorafenib treatment. They reported that 61% of these patients showed a favorable response to the treatments or had stable conditions. Their review is clinically intriguing, and our present case also demonstrates that rechallenge chemotherapy after regorafenib may be beneficial to patient survival.
Chemotherapies that may cause hypersensitivity reactions can often be continued with desensitization therapy [13, 14]. There are two major types of desensitization therapy: one is to escalate from lower dosage and the other is to use systemic steroids [13, 14]. A representative example of the latter is oxaliplatin. Combining dexamethasone (DEX) as premedication with biweekly or triweekly oxaliplatin infusion can potentially reduce hypersensitivity reactions. Although the amount of DEX premedication is generally 8 mg, several reports recommended high-dose DEX (20 mg) due to the low incidence of hypersensitivity reactions [14]. Pharmacologically, 30 mg of PSL is equal to 4 mg of DEX in the strength of steroids. The overall dose of daily oral intake of 30 mg PSL used in our case was heavier than the standard periodic usage. However, there were notable reports of desensitization therapy combined with sorafenib, which is a molecular target drug like regorafenib [15, 16], where daily oral intake of 25 mg PSL was effectively administered, similar to our case. Although the desensitization therapy for regorafenib has not been established, we conceive that the PSL oral intake could be acceptable to continue regorafenib therapy.
This is the first report to indicate that long-term using regorafenib has become possible by systemic administration of steroid, which has highlighted the efficacy of rechallenge chemotherapy and contributed to the long-term prognosis. The aggressive supportive care during regorafenib treatment may be necessary and the systemic administration of steroids may be considered depending on the case.
Funding
This report was not funded.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The patient has already died, but informed consent was obtained from the bereaved in this case report. There is no identifying information about patient in the article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicenter, randomized, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamoorthy SK, Relias V, Sebastian S, et al. Management of regorafenib-related toxicities: a review. Therap Adv Gastroenterol. 2015;8:285–297. doi: 10.1177/1756283X15580743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brierley JD, Gospodarowicz MK, Wittekind C, editors. The TNM classification of malignant tumours. 8. United States of America: Wiley-Blackwell; 2017. [Google Scholar]
- 4.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1–34. doi: 10.1007/s10147-017-1101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsunaga M, Ushijima T, Fukahori M, et al. Erythema multiforme induced by regorafenib. J Gen Fam Med. 2017;18:90–91. doi: 10.1002/jgf2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mii Y, Fukuoka E, Murata K, et al. A case of erythema multiforme induced by regorafenib therapy for metastatic colon cancer. Gan To Kagaku Ryoho. 2014;41:1841–1843. [PubMed] [Google Scholar]
- 7.Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: How to come away from acquired resistance? Ann Oncol. 2012;23:2313–2318. doi: 10.1093/annonc/mdr623. [DOI] [PubMed] [Google Scholar]
- 8.Tonini G, Imperatori M, Vincenzi B, et al. Rechallenge therapy and treatment holiday: different strategies in management of metastatic colorectal cancer. J Exp Clin Cancer Res. 2013;32:92. doi: 10.1186/1756-9966-32-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2006;26(25):4217–4219. doi: 10.1200/JCO.2008.18.7286. [DOI] [PubMed] [Google Scholar]
- 10.Baldus SE, Schaefer KL, Engers R, et al. Prevalence and heterogeneity of KRAS, BRAF, and PIK3CA mutations in primary colorectal adenocarcinomas and their corresponding metastases. Clin Cancer Res. 2010;16(3):790–799. doi: 10.1158/1078-0432.CCR-09-2446. [DOI] [PubMed] [Google Scholar]
- 11.Bertocchi P, Aroldi F, Prochilo T, et al. Chemotherapy rechallenge after reforafenib treatment in metastatic colorectal cancer: still hope after the last hope? J Chemother. 2017;29:102–105. doi: 10.1080/1120009X.2016.1247205. [DOI] [PubMed] [Google Scholar]
- 12.Kidd MT, Wilcox RE, Rogers J, et al. Efficacy of chemotherapy after treatment with regorafenib in metastaic colorectal cancer (mCRC) J Clin Oncol. 2015;33:678. doi: 10.1200/jco.2015.33.3_suppl.678. [DOI] [Google Scholar]
- 13.Naoto T, Koji M, Takuma O, et al. 4-step 4-h carboplatin desensitization protocol for patients with gynecological malignancies showing platinum hypersensitivity: a retrospective study. Int J Clin Oncol. 2015;20(3):566–573. doi: 10.1007/s10147-014-0731-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoichiro Y, Keiji H, Hiroshi M, et al. A single-arm Phase II validation study of preventing oxaliplatin-induced hypersensitivity reactions by dexamethasone: the AVOID trial. Drug Des Devel Ther. 2015;9:6067–6073. doi: 10.2147/DDDT.S94901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisashi N, Etsuro H, Koji T, et al. A case of desensitization of sorafenib after tumor lysis syndrome and erythema multiform in the patient with advanced hepatocellular carcinoma. Kanzo. 2014;55:221–227. doi: 10.2957/kanzo.55.221. [DOI] [Google Scholar]
- 16.Bauer C, Przybilla B, Rueff F. Severe cutaneous reaction to sorafenib: induction of tolerance. Acta Derm Venereol. 2008;88:627–628. doi: 10.2340/00015555-0517. [DOI] [PubMed] [Google Scholar]