Abstract
A 36-year-old male was referred to our hospital with left scrotal swelling. Computed tomography revealed a massive tumor in his left scrotum. The tumor extended along the gonadal vein extraperitoneally forming a massive tumor. Pathological examination showed a mixed-type germ cell tumor. Despite several chemotherapeutic treatments, the tumor continued to grow, and the patient died 28 months later after his first presentation at our institution. Autopsy revealed that the tumor comprised rhabdomyosarcoma and mature teratoma. We could not find useful tumor markers to facilitate the diagnosis of rhabdomyosarcoma. However, we recommend rebiopsy or palliative operation as options for re-diagnosis in case of resistant germ cell tumor. Here, we present a case of testicular tumor that exhibited different pathological examination results before and after treatment.
Keywords: Testicular tumor, Rhabdomyosarcoma, Germ cell tumor
Introduction
Testicular tumor affects approximately 1 out of 100,000 males. Non-germ cell malignancy is rare, accounting for 2.9% of all germ cell tumors (GCT) [1]. We describe a case of testicular tumor that was diagnosed as rhabdomyosarcoma (RMS) with mature teratoma at autopsy despite being diagnosed as a mixed-type GCT using the specimen obtained at high orchiectomy.
Case report
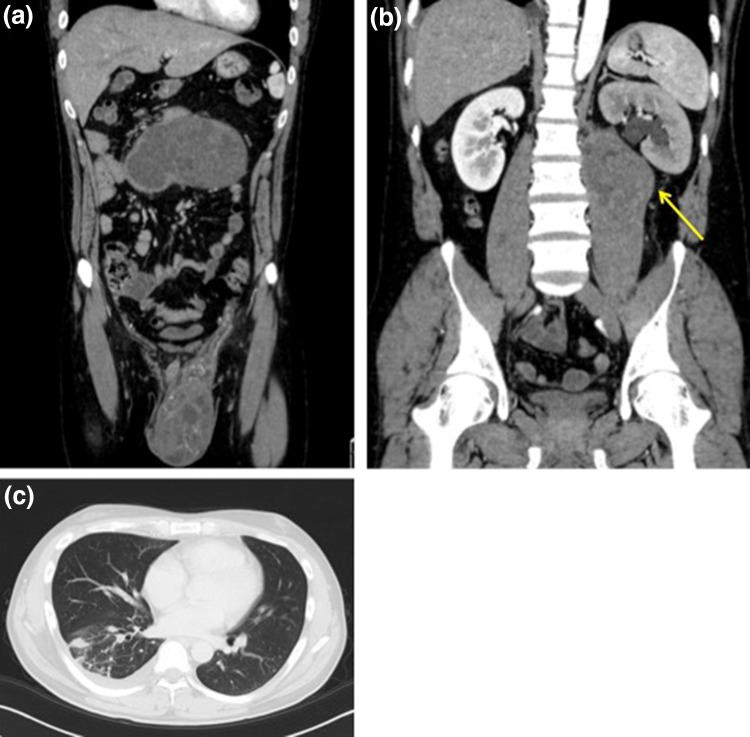
A 36-year-old male was referred to our hospital with left scrotal swelling, which had been persistent for 4 months. In addition, he had experienced abdominal pain and bloody sputum for the past 2 months. An abdominal tumor was found at ultrasound scan at his previous doctor. He had no medical history. Tumor markers were present at high levels, including lactate dehydrogenase (LDH), 586 U/L; alpha-fetoprotein (AFP), 5399.8 ng/mL; and beta human chorionic gonadotropin (βhCG), 14.6 ng/mL. We considered abdominal tumor another primary malignancy different from testicular one (e.g. gastrointestinal tumor) and also examined other tumor markers additionally, including carcinoembryonic antigen (CEA), 25.0 ng/mL; and carbohydrate antigen 19-9 (CA19-9), 25.3 U/mL. Computed tomography (CT) revealed a massive tumor in the left scrotum (8 × 6 × 5 cm). The tumor extended along the gonadal vein forming a massive tumor anterior to the abdominal aorta, and invaded the left iliopsoas muscle and urinary tract (Fig. 1a, b). Furthermore, CT revealed pulmonary metastasis (Fig. 1c), pulmonary embolism, and inferior vena cava embolism.
Fig. 1.
Computed tomography showing a massive tumor in the left scrotum, and the tumor continued along the gonadal vein and extraperitoneally formed another tumor (a). The tumor invaded the left iliopsoas muscle and urinary tract (b). Pulmonary metastasis (c)
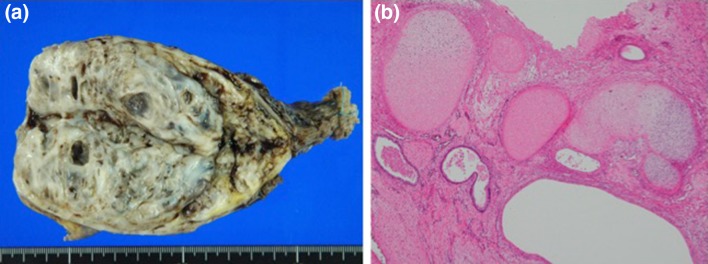
The patient had undergone left high orchiectomy on the following day (Fig. 2a). Pathological examination revealed mixed-type GCT, with immature teratoma (60%) (Fig. 2b), embryonal carcinoma (30%), yolk sac tumor (6%), and seminoma (4%). The TNM classification was pT3N3M1a. The International Germ Cell Consensus Classification risk category suggested a poor risk. The patient underwent antithrombotic drug because of pulmonary and inferior vena cava embolism.
Fig. 2.
Testicular tumor (a). Immature teratoma (b)
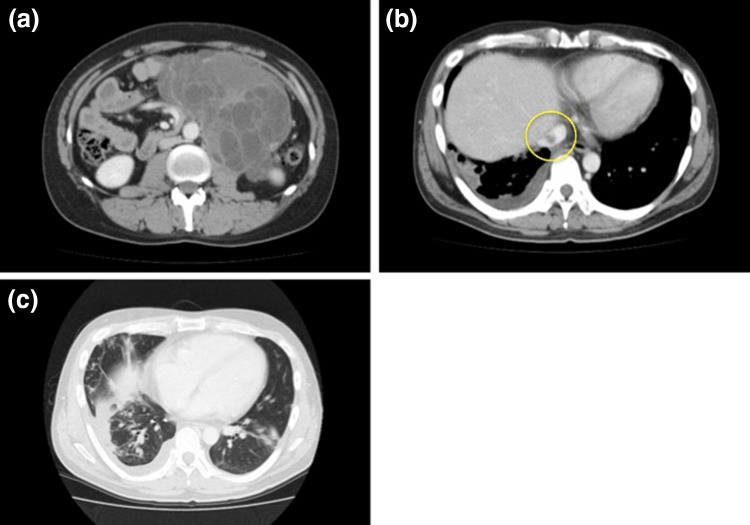
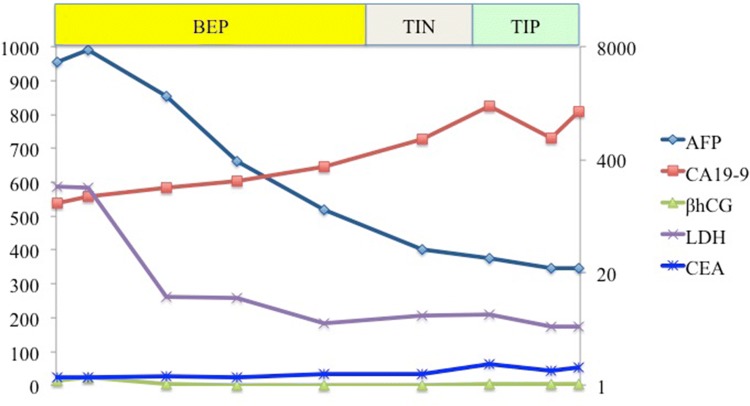
After surgery, the patient underwent chemotherapy and was administered three cycles of BEP (bleomycin at 20 mg/m2 on days 2, 9, and 16, etoposide at 100 mg/m2 on days 1–5, and cisplatin at 20 mg/m2 on days 1–5). After chemotherapy, CT showed enlargement of the tumor and progression of pulmonary metastasis and embolism (Fig. 3). Although we considered palliative operation, we regarded it risky and fatal because of the high possibility of adhesion between abdominal massive tumor and nearby organs and progression of embolism. Thus, he was administered with additional chemotherapy, which comprised one cycle of TIP (paclitaxel at 210 mg/m2 on day 1, ifosfamide at 1200 mg/m2 on days 2–6, and cisplatin at 20 mg/m2 on days 2–6) and one cycle of TIN (paclitaxel at 210 mg/m2 on day 1, ifosfamide at 2000 mg/m2 on days 2–6, and nedaplatin 100 mg/m2 on day 2). Nonetheless, the tumor continued to grow. Figure 4 shows the patterns of the expression of tumor markers during chemotherapy. AFP level markedly declined during treatment, whereas CA19-9 level increased.
Fig. 3.
Abdominal massive tumor continued enlargement and adhesion to nearby organs was suspected (a). Progression of inferior vena cava embolism (b). Progression of pulmonary metastasis (c)
Fig. 4.
Transition of tumor markers during chemotherapy: AFP level markedly declined during treatment (from 5399.8 to 22.4 ng/mL), whereas that of CA19-9 increased (from 125.3 to 1446.3 U/mL)
After chemotherapy, the patient received the best supportive care (BSC). After the administration of cell-free and concentrated ascites reinfusion therapy for abdominal dropsy, he was evaluated with CT. The tumor continued to enlarge. CA19-9 level also increased after BSC (from 1446.3 to 3391.0 U/mL), but other tumor markers had no remarkable change (LDH, from 174 to 230 U/L; βhCG, from 5.0 to 0.1 ng/mL; AFP, from 22.4 to 47.9 ng/mL; and CEA, from 54.9 to 174.0 ng/mL). After 22 months later after chemotherapy, he was hospitalized at our institution because of poor feeding and difficulty with walking. He complained of abdominal pain and dyspnea. Despite the administration of palliative care, he died 7 days after hospitalization.
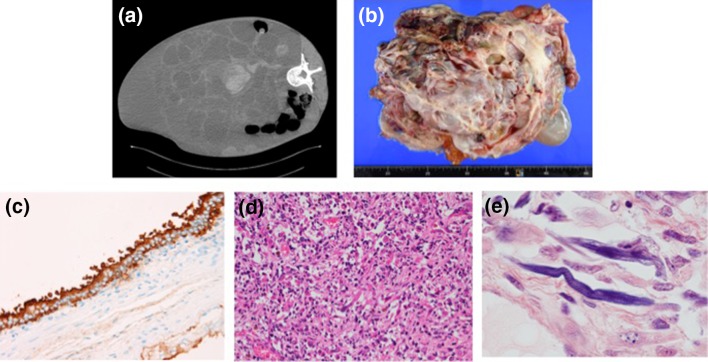
CT images obtained at the time of autopsy revealed a huge tumor in the abdominal cavity and excluded other organs (Fig. 5a). The internal appearance of the tumor suggested bleeding. The pulmonary metastasis and embolism observed previously had worsened. The result of pathologic examination showed a mixed-type tumor, with cystic and solid parts. Total tumor weight was 13 kg (Fig. 5b). Other organs atrophied because of their exclusion by the massive tumor. The cystic portion of the tumor comprised mature teratoma, whereas the solid portion mainly comprised mature teratoma and rhabdomyosarcoma (RMS). In the glandular epithelium of the cyst, immunostaining was positive for CA19-9 (Fig. 5c). In the solid portion of the tumor, undifferentiated neoplastic cells with high nuclear-cytoplasmic ratio and eosinophilic cells differentiated into a striated muscle were mixed (Fig. 5d). Phosphotungstic acid-hematoxylin stain revealed the presence of intracellular striated structures which was characteristic for RMS (Fig. 5e). Immunostaining was positive for myogenin and desmin, and negative for AFP, cluster designation 30, and placental alkaline phosphatase. The tumor no longer appeared to contain yolk sac tumor, embryonal carcinoma, or seminoma.
Fig. 5.
Massive tumor occupying the abdominal cavity and excluding other organs (a). The tumor comprising cystic and solid ingredients and weighing 13 kg (b). Immunostaining was positive for CA19-9 in the glandular epithelium of the cyst (c). Undifferentiated neoplastic cells with high nuclear–cytoplasmic ratio and eosinophilic cells differentiated into a striated muscle (d). A stained structure in the cells visualized using phosphotungstic acid and hematoxylin staining (e)
We also reevaluated the tumor at high orchiectomy. The immature teratoma was differentiated into a striated muscle, but no malignant findings were found (Fig. 6). In the striated muscle, immunostaining was positive for desmin and myoglobin, but negative for myogenin. Muscle component was accounted less than 10% of teratoma.
Fig. 6.
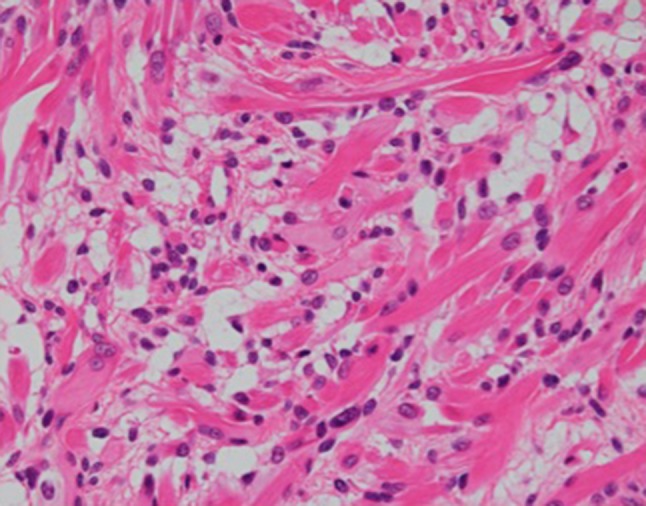
Immature teratoma differentiating into striated muscles
Discussion
In the case presented above, pathological diagnosis using high orchiectomy was a mixed-type GCT, and the patient received chemotherapy for GCT. However, the diagnosis at pathological autopsy was RMS and mature teratoma. Thus, we suspected that chemotherapy was effective against yolk sac tumor, embryonal carcinoma, and seminoma, but not against teratoma and RMS. In addition, there is some possibility of differentiation from teratoma into RMS. Because pathological examination at high orchiectomy revealed differentiation from immature teratoma into a striated muscle, we suspect that striated muscle caused malignancy during chemotherapy. Although immature teratoma was included in GCT at high orchiectomy, the huge tumor consisted of mature teratoma at autopsy. In other words, teratoma matured during chemotherapy. In addition, during chemotherapy, AFP level rapidly decreased from 5339.8 to 22.4 ng/mL. In these points, we suspected growing teratoma syndrome (GTS). GTS is a rare clinical entity, which presents with enlarging teratoma masses of the retroperitoneum or other locations, occurring during or after chemotherapy against GCT, and normalized tumor markers [2]. Gorbatiy et al. reported that the incidence of GTS after chemotherapy was 1.9–7.6% [3]. According to Logothetis criteria, the definition of GTS includes (1) normalization of serum tumor markers, AFP andβhCG; (2) enlarging or new masses despite appropriate chemotherapy for nonseminomatous GCT; and (3) the exclusive presence of mature teratoma in the resected specimen [4]. Although there is a contradiction in terms of exclusive presence of mature teratoma, we suggested that this case had the simultaneous occurrence of GTS and non-germ cell malignancy in GCT.
The rate of non-germ cell malignancy in GCT is 2.9%; the most commonly reported tissue-type tumor is sarcoma [1]. Comiter et al. described that an overall survival rate with a follow-up of 4 years for teratomas with secondary malignancy was 52%. However, those with RMS had a poorer outcome; only 25% with a mean follow-up of 3.5 years. They suggested that the differentiation of RMS from GCT predicts poor prognosis [5]. Giannatempo et al. described that teratoma with malignant transformation was found in the primary tumor in 167/320 patients (52.9%), and the distribution of transformed histologies was rhabdomyosarcoma in 13.5% [6]. Furthermore, complete resection predicts good prognosis in patients with RMS [7, 8], and standard chemotherapy against RMS is different from that against GCT [vincristine and actinomycin (VA) or vincristine, actinomycin, and cyclophosphamide (VAC)] [9]. Moreover, surgical complete resection is gold standard treatment for GTS, since teratomas are resistant to chemotherapy [2]. Expeditious resection is important, so it can develop unresectable disease. If the surgery delayed, fatal complication may be caused by local compression, including obstructive renal function degeneracy or bowel or vessel obstruction [10]. In this case, we could not select palliative operation because of the risk of adhesion and embolism. If complete resection would be performed, the compression other organ could be relieved and those function could be recovered. Although the problems would remain like pulmonary and inferior vena cava embolism and possibility of recurrence, it might prolong survival time and improve quality of life of the patient not a little. We believe that the treatment plan could have been changed if the presence of RMS had been known when the patient was undergoing chemotherapy.
During chemotherapy, the results of laboratory blood work showed that AFP level rapidly decreased (from 5339.8 to 22.4 ng/mL), whereas CA19-9 level increased (from 125.3 to 1446.3 U/mL). We considered the levels of these tumor markers in our differential diagnosis of RMS. No previous studies have described a significant increase in CA19-9 level in RMS. Miyachi et al. reported that microRNA 206 was useful [11], but the tumor marker is not available for use in routine clinical practice. Conversely, there are several reports, suggesting that CA19-9 has a possibility of serum marker for GCT. Minamide et al. reported an increase in CA19-9 level was associated with testicular GCT [12]. Tsuruta et al. described that five of eight patients with an embryonal carcinoma had an elevated serum CA19-9 [13]. In the present case, most of the teratoma was cystic. We suspect that the glandular epithelium of the cyst may have produced CA19-9 during chemotherapy. In the glandular epithelium of the cyst, immunostaining was positive for CA19-9. This result may support the hypothesis. Indraneel reported that serum and voided urine CA19-9 level in adult patients with giant hydronephrosis were significantly greater than those in control group [14]. Meyer described that increasing CA19-9 was caused by proliferation and inflammation of renal tubular epithelium and renal pelvis [15]. In the present case, left hydronephrosis was revealed at CT at the initial diagnosis. However, during chemotherapy, as the abdominal massive tumor enlarged kidneys were atrophied due to compression, leading to renal dysfunction. Although hydronephrosis was improved by renal function degeneracy, CA19-9 was increasing continuously. We considered that hydronephrosis was not only reason for increasing CA19-9 level. It is difficult to suspect RMS based on the evidence provided by tumor markers alone.
In the present case, there were no clues indicating RMS. Based on the possibility that non-germ cell malignancy can differentiate, we recommend rebiopsy or palliative surgery in case of resistant GCT during treatment if possible. We could not perform palliative operation, but we should have taken rebiopsy at the timing of tumorous progression and decreasing of tumor marker for GCT after early chemotherapy in view of non-germ cell malignancy and GTS. Giannatempo et al. showed that malignant transformation found after treatment for GCT was associated with inferior survival [6]. The effectiveness of chemotherapy for testicular mixed GCT with RMS has not yet been established. Korfel et al. reported remarkable efficacy for high-dose chemotherapy with epirubicin, etoposide, ifosfamide, and cisplatin in the treatment of patients with testicular GCT with RMS [16]. Further investigation is required to optimize a regimen for use against GCT and RMS.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ishikawa Prefectural Central Hospital Institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Motzer Robert J, Amsterdam Alison, Prieto Victor, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol. 1998;159:133–138. doi: 10.1016/S0022-5347(01)64035-7. [DOI] [PubMed] [Google Scholar]
- 2.Scavuzzo A, Santana Rios ZA, Reynoso Noveron N, et al. Growing teratoma syndrome. Case Rep Urol. 2014;2014:139425. doi: 10.1155/2014/139425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbatiy V, Spiess P, Pisters S, et al. The growing teratoma syndrome: current review of the literature. Indian J Urol. 2009;25:186–189. doi: 10.4103/0970-1591.52910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logothetis CJ, Samuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer. 1982;50:1629–1635. doi: 10.1002/1097-0142(19821015)50:8<1629::AID-CNCR2820500828>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Comiter CV, Kibel AS, Richie JP, et al. Prognostic features of teratomas with malignant transformation: a clinicopathological study of 21 cases. J Urol. 1998;159:859–863. doi: 10.1016/S0022-5347(01)63754-6. [DOI] [PubMed] [Google Scholar]
- 6.Giannatempo P, Pond GR, Sonpavde G, et al. Treatment and clinical outcomes of patients with teratoma with somatic-type malignant transformation: an international collaboration. J Urol. 2016;96:95–100. doi: 10.1016/j.juro.2015.12.082. [DOI] [PubMed] [Google Scholar]
- 7.Oberlin O, Rey A, Lyden E, et al. Prognostic factors in metastatic rhabdomyosarcomas: results of a pooled analysis from United States and European cooperative groups. J Clin Oncol. 2008;26:2384–2389. doi: 10.1200/JCO.2007.14.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 9.Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma—a report from the intergroup rhabdomyosarcoma study IV. J Clin Oncol. 2003;1:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 10.Spiess PE, Kassouf W, Brown GA, et al. Surgical management of growing teratoma syndrome. The J Urol. 2007;177:1330–1334. doi: 10.1016/j.juro.2006.11.086. [DOI] [PubMed] [Google Scholar]
- 11.Mitsuru Miyachi, Kunihiko Tsuchiya, Hideki Yoshida, et al. Circulating muscle-specific microRNA, miR-206, as a potential diagnostic marker for rhabdomyosarcoma. Biochem Biophys Res Commun. 2010;10:89–93. doi: 10.1016/j.bbrc.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Masahiro M, Ikuyoshi H, Shigeyuki Y. CA19-9 producing testicular tumor: a case report. Acta Urol Jpn. 2000;46:45–47. [PubMed] [Google Scholar]
- 13.Tsuruta T, Ogawa A, Ikado S, et al. CA19-9: a possible serum marker for embryonal carcinoma. Urol Int. 1997;58:20–24. doi: 10.1159/000282939. [DOI] [PubMed] [Google Scholar]
- 14.Indraneel B, Vinay T, Sher S, et al. Role of urinary and serum carbohydrate antigen 19–9 as a biomarker in diagnosis of adult giant hydronephrosis. J Clin Diagn Res. 2016;10:8–11. doi: 10.7860/JCDR/2016/21400.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer A, Kausch I, Kruger S, et al. Elevation of CA19-9 in giant hydronephrosis induced by a renal calculus. Urology. 2004;63:381–382. doi: 10.1016/j.urology.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 16.Korfel A, Fischer L, Foss H-D, et al. Testicular germ cell tumor with rhabdomyosarcoma successfully treated by disease-adapted chemotherapy including high-dose chemotherapy: case report and review of the literature. Bone Marrow Transplant. 2001;28:787–789. doi: 10.1038/sj.bmt.1703212. [DOI] [PubMed] [Google Scholar]