Abstract
It remains disputable about perioperative use of renin-angiotensin system inhibitors (RASi) and their outcome effects. This multicenter retrospective cohort study examines association between use of perioperative RASi and outcomes in patients undergoing coronary artery bypass graft and/or valve surgery. After the exclusion, the patients are divided into 2 groups with or without preoperative RASi (PreRASi, n = 8581), or 2 groups with or without postoperative RASi (PostRASi, n = 8130). With using of propensity scores matching to reduce treatment selection bias, the study shows that PreRASi is associated with a significant reduction in postoperative 30-day mortality compared with without one (3.41% vs. 5.02%); PostRASi is associated with reduced long-term mortality rate compared with without one (6.62% vs. 7.70% at 2-year; 17.09% vs. 19.95% at 6-year). The results suggest that perioperative use of RASi has a significant benefit for the postoperative and long-term survival among patients undergoing cardiac surgery.
Subject terms: Therapeutics, Interventional cardiology
Renin-angiotensin system inhibitors (RASi) are beneficial in several classes of cardiovascular patients. However, whether their perioperative use is beneficial and/or safe in cardiac surgery is unclear. Here the authors perform a multicenter retrospective cohort study showing that preoperative and postoperative use of RASi is associated with reduction of mortality in patients undergoing cardiac surgery.
Introduction
In the early 2000s, two large-randomized clinical trials, the Heart Outcomes Prevention Evaluation (HOPE) trial1 and the European Trial on Reduction of Cardiac Events with Perindopril in Stable Coronary Artery Disease2, showed that in patients with risk of cardiovascular disease (CVD) but without heart failure, angiotensin converting enzyme inhibitors (ACE inhibitors) resulted in a relative risk (RR) reduction of about 20% in mortality, MI, and stroke in 4–5 years follow-up. The effects were independent of age, gender, concomitant diseases, or therapy. Thus far, multiple clinical trials and cohort studies have demonstrated that ACE inhibitors are beneficial to patients with hypertension, diabetes, renal disease, heart dysfunction, also to patients with coronary artery disease (CAD), and valve diseases1–8.
Meanwhile, despite accumulating evidence that ACE inhibitors are effective drugs in a broad range of patients with risk of CVD and increasing numbers of patients are treated with ACE inhibitors prior to cardiac surgery, whether or not ACE inhibitors should be continued or given perioperatively remains controversial. Results from previous clinical studies are conflicting and raise more questions than answers9–13. The Guidelines (2011) for coronary artery bypass graft (CABG) from the American Heart Association and American College of Cardiology stated that it is uncertain about the safety of the preoperative administration of ACE inhibitors or angiotensin II receptor blockers (ARBs) in patients on chronic therapy and the safety of initiating ACE inhibitors or ARBs before hospital discharge14. In the updated 2014 ESC/EACTS Guidelines, they state that ACE inhibitors might be stopped 1–2 days before CABG to avoid the potential deleterious consequences of perioperative hypotension15. Thus, there is a need to investigate the safety and effectiveness of preoperative and postoperative RASi therapy in large cardiovascular cohorts.
In this study, we examined both preoperative and postoperative (or perioperative) use of RASi, including ACE inhibitors, ARBs, and direct renin inhibitors, on outcomes of patients undergoing CABG and/or valve surgery. The results show that perioperative use of RASi has a significant benefit for the postoperative and long-term survival among patients undergoing CABG and/or valve surgery.
Results
Characteristics of the study patients
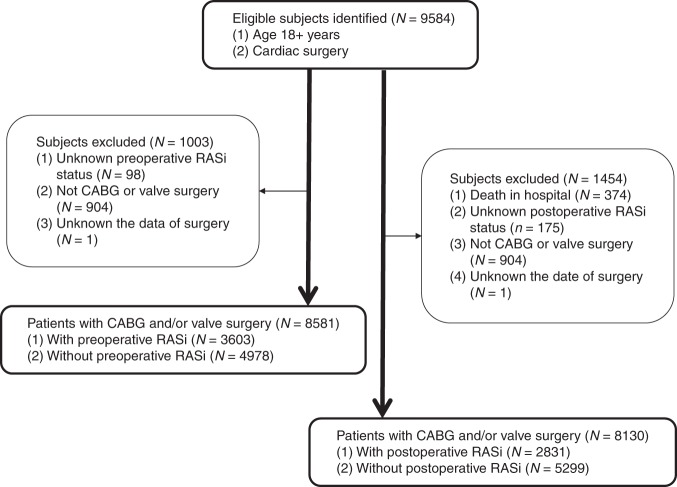
Overall 8581 patients with CABG and/or valve surgery were included in preoperative RASi (PreRASi) groups of this study. Among them, 3603 patients (42.0%) received PreRASi, and 4978 (58.0%) did not (no-PreRASi). Postoperatively (n = 8130), 2831 patients (34.8%) were prescribed RASi at discharge (PostRASi), and 5299 patients (65.2%) did not (no-PostRASi) (Fig. 1). Baseline demographic and clinical data of the patients were presented in Table 1. Before adjustment using propensity scores matching (PSM) or inverse probability weighted (IPW), more patients taking preoperative or postoperative RASi than not taking RASi had diabetes, hypertension, angina, congestive heart failure, and previous myocardial infarction. A greater number of RASi-treated patients were receiving other medications, including beta-blockers, aspirin, and lipid-lowering drugs. Patients taking PreRASi had higher BMI and increased incidence of cerebrovascular disease, peripheral vascular disease, a few of minutes longer of cardiopulmonary bypass time and cross clamp time, a slightly more using artery grafts and off-pump, more using PostRASi as well as decreased incidence of chronic lung disease, and urgent CABG or valve surgery. For patients taking PostRASi, there were similar changes as the patients with PreRASi, but decreased incidence of smoking and more with PreRASi and discharge aspirin (Table 2). After adjustment with PSM, all of clinical covariates were well balanced and no significant differences were found between the two groups except for using RASi.
Fig. 1.
Study population recruitment summary. RASi renin-angiotensin system inhibitors, CABG coronary artery bypass graft
Table 1.
Demographic and clinical characteristics: preoperative RAS inhibitors
| Unadjusted | Inverse probability weighted adjusted | Matched data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PreRASi (n = 3603) | No-PreRASiI (n = 4978) | p-value | PreRASi (n = 3603) | No-PreRASi (n = 4978) | p-value | PreRASi (n = 3284) | No-PreRASi (n = 3284) | p-value | |
| Age | 65.9 ± 11.7 | 64.8 ± 13.6 | <0.0001 | 65.6 ± 18.9 | 65.3 ± 17.2 | 0.34 | 65.9 ± 11.8 | 66.0 ± 12.4 | 0.70 |
| Male | 68.9 | 67.4 | 0.14 | 68.2 | 68.0 | 0.83 | 68.4 | 68.7 | 0.83 |
| BMI | 29.2 ± 6.2 | 28.6 ± 6.2 | <0.0001 | 28.9 ± 9.5 | 28.9 ± 8.3 | 0.74 | 29.1 ± 6.1 | 29.1 ± 6.3 | 0.88 |
| Diabetes | 38.7 | 27.6 | <0.0001 | 32.9 | 32.5 | 0.60 | 35.8 | 35.2 | 0.66 |
| Smoker | 40.7 | 39.5 | 0.26 | 40.3 | 40.1 | 0.72 | 40.2 | 40.4 | 0.92 |
| Hypertension | 87.3 | 69.9 | <0.0001 | 78.4 | 77.4 | 0.14 | 86.2 | 87.0 | 0.35 |
| Cerebrovascular disease | 18.1 | 15.4 | 0.001 | 16.8 | 16.7 | 0.83 | 17.5 | 17.1 | 0.70 |
| Peripheral arterial disease | 13.9 | 11.6 | 0.002 | 12.7 | 12.6 | 0.91 | 13.4 | 12.8 | 0.49 |
| Chronic lung disease | 18.8 | 20.1 | 0.04 | 20.3 | 19.8 | 0.49 | 19.6 | 19.7 | 0.90 |
| Family history CAD | 40.5 | 38.3 | 0.04 | 39.7 | 39.3 | 0.58 | 40.6 | 41.3 | 0.58 |
| Angina | 37.2 | 34.6 | 0.02 | 36.1 | 35.9 | 0.73 | 37.8 | 38.3 | 0.51 |
| Congestive heart failure | 32.7 | 28.6 | <0.0001 | 31.4 | 30.7 | 0.30 | 30.7 | 30.5 | 0.89 |
| Previous MI | 36.4 | 30.2 | <0.0001 | 33.5 | 33.0 | 0.47 | 35.0 | 35.1 | 0.94 |
| Beta blockers | 72.6 | 63.3 | <0.0001 | 68.9 | 67.6 | 0.09 | 71.4 | 71.5 | 0.87 |
| Aspirin | 75.9 | 66.6 | <0.0001 | 71.3 | 70.7 | 0.34 | 74.5 | 74.5 | 0.98 |
| Lipid lowering | 82.2 | 74.1 | <0.0001 | 78.3 | 77.7 | 0.35 | 81.1 | 81.5 | 0.73 |
| Urgent | 49.9 | 52.9 | 0.01 | 51.7 | 51.6 | 0.86 | 51.1 | 51.9 | 0.52 |
| CABG | 49.5 | 51.9 | <0.0001 | 55.3 | 55.0 | 0.76 | 58.3 | 58.5 | 0.84 |
| Valve | 24.0 | 29.3 | <0.0001 | 27.0 | 27.1 | 0.90 | 24.8 | 24.9 | 0.93 |
| Post RAS inhibitor | 46.0 | 25.6 | <0.0001 | 45.8 | 26.7 | <0.0001 | 46.0 | 28.5 | <0.0001 |
| Cardiopulmonary bypass time | 130.7 ± 80.5 | 126.3 ± 79.6 | 0.012 | 128.2 ± 121.1 | 128.3 ± 107.0 | 0.96 | 127.5 ± 77.8 | 126.3 ± 79.7 | 0.52 |
| Cross clamp time | 93.8 ± 59.5 | 90.8 ± 58.9 | 0.020 | 92.3 ± 90.2 | 92.2 ± 79.3 | 0.97 | 91.8 ± 58.1 | 90.9 ± 58.9 | 0.53 |
| Number of artery grafts | 0.012 | 0.80 | 0.14 | ||||||
| None | 45.7 | 48.8 | 46.7 | 47.2 | 46.2 | 48.2 | |||
| 1 | 45.5 | 42.4 | 44.4 | 44.0 | 45.3 | 42.8 | |||
| 2 or more | 8.8 | 8.8 | 8.9 | 8.8 | 8.5 | 8.9 | |||
| On-off pump | 0.0021 | 0.80 | 0.39 | ||||||
| Off pump | 6.5 | 6.7 | 6.4 | 6.5 | 6.7 | 6.2 | |||
| On pump (full) | 93.5 | 93.3 | 93.6 | 93.5 | 93.3 | 93.8 | |||
Values are mean ± SD or %. C-index = 0.74. p-values are obtained via analyses using Pearson chi-square test or Wilcoxon rank-sum test (unadjusted) and then IPW or PSM (adjusted)
BMI body mass index, MI myocardial infarction
Table 2.
Demographic and Clinical Characteristics: Post RAS Inhibitors
| Unadjusted | Inverse probability weighted adjusted | Matched | Data | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PostRASi (n = 2831) | No-PostRASiI (n = 5299) | p-value | PostRASi (n = 2831) | No-PostRASi (n = 5299) | p-value | PostRASi (n = 2673) | No-PostRASi (n = 2673) | p-value | |
| Age | 65.8 ± 11.9 | 64.7 ± 13.2 | 0.0004 | 65.2 ± 20.5 | 65.1 ± 15.4 | 0.57 | 65.7 ± 12 | 65.8 ± 12.3 | 0.86 |
| Male | 68.4 | 68.6 | 0.82 | 68.7 | 68.5 | 0.63 | 68.4 | 68.4 | 0.98 |
| BMI | 29.1 ± 6.2 | 28.8 ± 6.2 | 0.09 | 28.9 ± 10.2 | 28.4 ± 7.5 | 0.64 | 29.1 ± 6.2 | 29.0 ± 6.2 | 0.61 |
| Diabetes | 37.8 | 29.5 | <0.0001 | 32.7 | 32.5 | 0.64 | 36.2 | 35.9 | 0.80 |
| Smoker | 38.7 | 41.3 | 0.02 | 39.8 | 40.3 | 0.67 | 39.5 | 39.7 | 0.85 |
| Hypertension | 83.7 | 73.9 | <0.0001 | 78.2 | 78.5 | 0.42 | 83.0 | 83.7 | 0.49 |
| Cerebrovascular disease | 16.8 | 16.0 | 0.34 | 16.1 | 16.7 | 0.53 | 17.0 | 16.3 | 0.53 |
| Peripheral arterial disease | 13.1 | 11.6 | 0.06 | 12.1 | 12.3 | 0.75 | 12.8 | 11.9 | 0.30 |
| Chronic lung disease | 18.5 | 19.5 | 0.30 | 19.40 | 19.0 | 0.82 | 18.8 | 18.7 | 0.94 |
| Family history CAD | 39.1 | 39.5 | 0.68 | 39.0 | 38.8 | 0.61 | 39.3 | 40.3 | 0.78 |
| Angina | 39.3 | 34.1 | <0.0001 | 35.1 | 35.8 | 0.72 | 38.5 | 38.8 | 0.78 |
| Congestive heart failure | 32.3 | 27.7 | 0.0001 | 30.4 | 30.5 | 0.86 | 31.3 | 30.7 | 0.62 |
| Previous MI | 36.5 | 30.2 | <0.0001 | 32.1 | 33.0 | 0.81 | 35.4 | 35.8 | 0.75 |
| Discharge beta blockers | 83.1 | 82.1 | 0.07 | 83.1 | 82.9 | 0.32 | 83.2 | 83.9 | 0.61 |
| Discharge aspirin | 85.7 | 84.2 | 0.03 | 85.7 | 85.8 | 0.69 | 86.8 | 85.1 | 0.25 |
| Discharge lipid lowering | 81.0 | 79.5 | 0.07 | 80.3 | 80.1 | 0.46 | 81.9 | 89.7 | 0.74 |
| Discharge warfarin | 24.5 | 22.8 | 0.07 | 24.0 | 23.5 | 0.35 | 24.1 | 22.0 | 0.15 |
| Urgent | 53.9 | 49.3 | 0.001 | 51.9 | 50.7 | 0.61 | 52.8 | 53.1 | 0.83 |
| CABG | 58.5 | 54.6 | 0.001 | 56.2 | 55.9 | 0.36 | 58.0 | 59.1 | 0.42 |
| Valve | 25.6 | 27.1 | 0.13 | 26.2 | 26.7 | 0.59 | 25.9 | 25.4 | 0.68 |
| Pre RAS inhibitor | 56.9 | 34.7 | <0.0001 | 42.9 | 42.2 | 0.87 | 54.6 | 54.1 | 0.70 |
C-index = 0.66, values are mean ± SD or %. p-values are obtained via analyses using Pearson chi-square test or Wilcoxon rank-sum test (unadjusted) and then IPW or PSM (adjusted)
BMI body mass index, MI myocardial infarction, CAD coronary artery disease
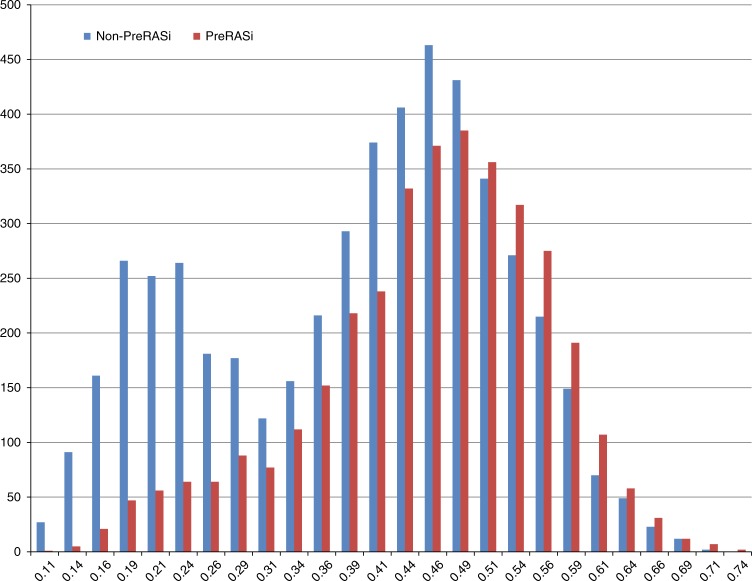
As expected, patients who took PreRASi had a lower probability of being selected as not taking RASi (No-PreRASi, Fig. 2), with the median and interquartile range of the propensity scores for PreRASi reflecting this difference (PreRASi group: median, 0.47; interquartile range, 0.40–0.53; no-PreRASi group: median, 0.42; interquartile range, 0.27–0.49).
Fig. 2.
Distribution of propensity scores for preoperative RASi (PreRASi) in the patient populations (y-axis). The propensity score (x-axis) for PreRASi is the probability given baseline variables that any patient in either group would be selected for PreRASi
Effects of PreRASi on outcomes
Postoperatively, compared with no-PreRASi, PreRASi did not show a significant reduction in heart, brain, and renal complication. MACE, a composite outcome, did show a significant reduction with unadjusted analysis (P = 0.04), which became nonsignificant with the PSM analysis (P = 0.19). In contrast, compared with no-PreRASi, PreRASi was associated with a significant reduction in postoperative 30-day mortality (3.55% vs. 5.04%, OR 0.69, 95% CI 0.54–0.90, P = 0.01 with PSM or unadjusted analysis) (Table 3).
Table 3.
Preoperative RAS inhibitors and postoperative outcomes
| Outcome n (%) | Unadjusted | IPW adjusted | One-to-one matched | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RASi (N = 3603) | Non RASi (N = 4978) | OR 95% CI | p-value | OR 95% CI | p-value | RASi (N = 3284) | Non RASi (N = 3284) | OR 95% CI | p-value | |
| MACE | 417 (11.6) | 650 (13.1) | 0.87 (0.76–0.99) | 0.04 | 0.89 (0.81–0.97) | 0.01 | 377 (11.48) | 419 (12.76) | 0.90 (0.77–1.05) | 0.19 |
| Perioperative MI | 29 (0.80) | 39 (0.78) | 1.03 (0.63–1.67) | 0.91 | 1.07 (0.76–1.48) | 0.69 | 26 (0.79) | 25 (0.76) | 1.02 (0.58–1.78) | 0.95 |
| Heart block | 51 (1.42) | 58 (1.17) | 1.22 (0.83–1.78) | 0.31 | 1.50 (1.16–1.94) | 0.01 | 48 (1.46) | 40 (1.22) | 1.19 (0.77–1.82) | 0.42 |
| Cardiac arrest | 86 (2.39) | 113 (2.27) | 1.05 (0.79–1.40) | 0.72 | 1.11 (0.91–1.41) | 0.29 | 80 (2.44) | 67 (2.04) | 1.28 (0.92–1.79) | 0.47 |
| Permanent stroke | 68 (1.89) | 111 (2.23) | 0.84 (0.62–1.14) | 0.27 | 0.85 (0.69–1.01) | 0.13 | 59 (1.80) | 68 (2.07) | 0.88 (0.62–1.25) | 0.47 |
| TIA | 15 (0.42) | 24 (0.48) | 0.86 (0.45–1.65) | 0.66 | 0.90 (0.57–1.43) | 0.66 | 12 (0.37) | 13 (0.40) | 0.91 (0.41–2.02) | 0.77 |
| Coma | 26 (0.72) | 40 (0.80) | 0.90 (0.55–1.47) | 0.67 | 0.88 (0.62–1.25) | 0.63 | 21 (0.64) | 21 (0.64) | 1.09 (0.59–2.03) | 0.99 |
| Renal failure | 167 (4.64) | 258 (5.18) | 0.89 (0.73–1.09) | 0.25 | 0.86 (0.75–0.97) | 0.03 | 148 (4.51) | 168 (5.12) | 0.91 (0.72–1.15) | 0.42 |
| Total hrs ICU mean ± SD | 112.4 ± 186 | 116.7 ± 179 | 4.51 (−3.27–12.3) | 0.26 | −1.94 (−10.4–6.2) | 0.64 | 112.1 ± 188 | 112.6 ± 167 | 0.55 ± 178 | 0.90 |
| Readmission | 502 (13.93) | 624 (12.54) | 1.13 (0.996–1.28) | 0.06 | 1.10 (1.01–1.21) | 0.02 | 467 (14.22) | 428 (13.03) | 1.10 (0.95–1.27) | 0.20 |
| 30-day mortality | 128 (3.55) | 251 (5.04) | 0.69 (0.54–0.85) | 0.01 | 0.67 (0.58–0.76) | <0.0001 | 112 (3.41) | 165 (5.02) | 0.69 (0.54–0.90) | 0.01 |
Values are mean ± SD or number or %. p-values are obtained via analyses using Pearson chi-square test or Wilcoxon rank-sum test (unadjusted) and then IPW or PSM (adjusted)
MACE major adverse cardiovascular events, MI myocardial infarction, TIA transient ischemic attack
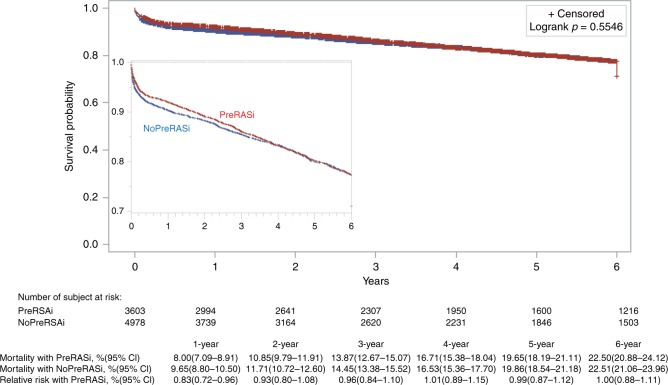
The survival curves with using PreRASi or no-PreRASi are shown in Fig. 3 (unadjusted) and Fig. 4 (adjusted with PSM). The mean, median, and interquartile ranges of the follow-up time were 4.26 years, 3.79 years, and 1.24–6.79 years, respectively. From 1 to 6 year, there was no significant difference in adjusted mortality between PreRASi and no-PreRASi (7.96% vs. 9.79%; RR 0.81, 95% CI 0.74–0.88 and 21.99% vs. 23.00%; RR 0.96, 95% CI 0.89–1.03). Sensitivity analyses performed with the use of the Cox model and the IPW analysis yielded similar results (see Supplementary Information).
Fig. 3.
Rates of survival in patients. Cumulative mortality with and without preoperative RASi (PreRASi) and the relative risk (95% confidence intervals in brackets) of PreRASi as compared with no-PreRASi are shown. Logrank p-value is analyzed with using the Kaplan–Meier method (unadjusted). The inset shows the same data on an enlarged y axis
Fig. 4.
Rates of survival in patients. Cumulative mortality with and without preoperative RASi (PreRASi) and the relative risk (95% confidence intervals in brackets) of PreRASi as compared with no-PreRASi are shown. Logrank p-value is analyzed with using the propensity score matching (adjusted). The inset shows the same data on an enlarged y axis
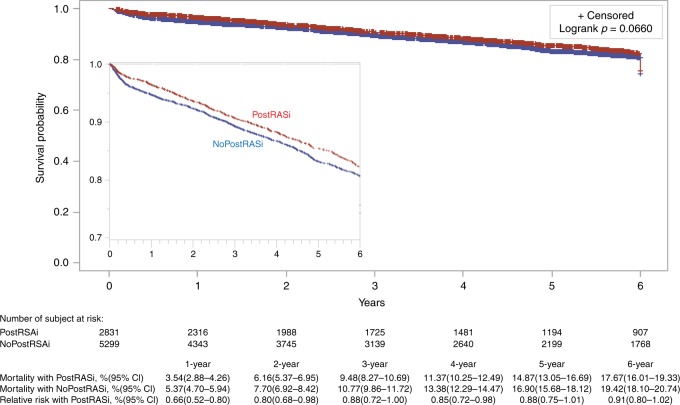
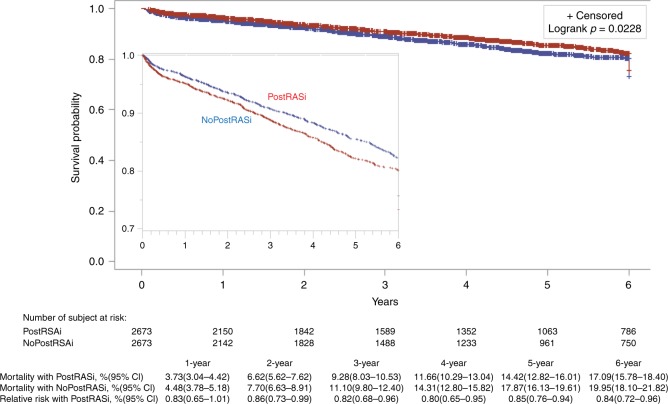
Effects of PostRASi on outcomes
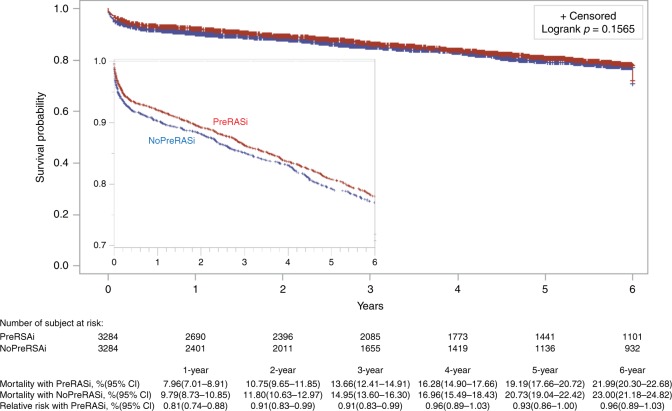
The databases of this cohort of patients provided the discharge prescription of RASi but did not have the records of refill or adherence of RASi, thus this study examined the association between postoperative discharge RASi and long-term mortality. Overall, the discharge prescription of RASi was associated with significant beneficial effects on long-term survival. The mean, median, and interquartile ranges of the follow-up time were 4.43 years, 4.03 years, and 1.55–6.90 years, respectively. The unadjusted survival curves trended to show the survival benefit with RASi (Fig. 5) but it did not reach significant differences between with and without postoperative RASi groups at 3, 5 and 6 year (9.48% vs. 10.77%, RR 0.88, 95% CI 0.72–1.00; 14.87% vs. 16.90%, RR 0.88, 95% CI 0.75–1.01; 17.67% vs. 19.42%, RR 0.91, 95% CI 0.80–1.02, respectively). The survival curves adjusted with the use of the PSM are shown in Fig. 6. At 2, 4, and 6 year, there were significant differences in long-term mortality between with and without postoperative RASi groups (6.62% vs. 7.70%, RR 0.86, 95% CI 0.73–0.99; 11.66% vs. 14.31%, RR 0.80, 95% CI 0.65–0.95; 17.09% vs. 19.95%, RR 0.84, 95% CI 0.72–0.96, respectively).
Fig. 5.
Rates of survival in patients. Cumulative mortality with and without postoperative RASi (PostRASi) and the relative risk (95% confidence intervals in brackets) of PostRASi as compared with no-PostRASi are shown. Logrank p-value is analyzed with using the Kaplan–Meier method (unadjusted). The inset shows the same data on an enlarged y axis
Fig. 6.
Rates of survival in patients. Cumulative mortality with and without postoperative RASi (PostRASi) and the relative risk (95% confidence intervals in brackets) of PostRASi as compared with no-PostRASi are shown. Logrank p-value is analyzed with using the propensity score matching (adjusted). The inset shows the same data on an enlarged y axis
Sensitivity analyses performed with the use of the Cox model and the IPW adjusted analysis yielded similar results (see Supplementary Figs. 1 and 2).
Discussion
The results of this multicenter and cohort study showed that preoperative RASi therapy (vs. no preoperative RASi) was associated with a significant decrease of 30-day mortality but no significant changes in postoperative MACE or long-term survival. Postoperative RASi therapy, i.e., patients with the prescription of discharge RASi, was associated with a significant decrease in long-term mortality, up to 6 years after cardiac surgery (17.09% vs. 19.95%, RR 0.84, 95% CI 0.72–0.96, P = 0.0228), suggesting an association between perioperative RASi and benefits of survival (for both short and long-term survival).
As previous studies showed, ACE inhibitors block the conversion of angiotensin-I to angiotensin-II (a potent vasoconstrictor), and block the breakdown of bradykinin (a potent vasodilator). The effects of angiotensin-II involve all organs and systems in vivo, including cardiovascular, nervous, urinary, endocrine, inflammation, and cell growth16. Blockade of RAS by RASi decreases formation of angiotensin II, increases bradykinin, reduces secretion of aldosterone and vasopressin, with decreased activity of the sympathetic nervous system as well as the trophic effects of angiotensin II. Thus, RASi, by influencing extracellular matrix content, cellular apoptosis, tissue inflammation, and fibrosis, could hypothetically reverse the left ventricular-remodeling process, attenuate aortic valve thickening and prevent thrombosis and plaque rapture6,16,17.
In the early 2000’s, the QUO VAIDS trial9 and APRES trial10 reported that in patients undergoing CABG or percutaneous coronary intervention, quinapril, or ramipril, given before or immediately after coronary revascularization for a long-term (>1 year) can decrease MACE including MI, recurrence of angina pectoris, ischemic stroke, transient ischemic attack, clinical heart failure, or need for revascularization. Those two trials were randomized, although had small sample size (n = 149 or 159, respectively).
In 2008, the IMAGINE trial11(n = 2553) tested whether early initiation (7 days after CABG) of an ACE inhibitor reduced cardiovascular events in stable patients with left ventricular ejection fraction 40%. The trial showed that quinapril (40 mg/day, started ≤ 7 days after surgery) did not improve clinical outcomes including cardiovascular events and death up to 3 years after the surgery in low-risk CABG patients. Instead, quinapril, given postoperatively, increased MACE including cardiovascular death, cardiac arrest, nonfatal MI, coronary revascularization, stroke, and unstable angina or heart failure required hospitalization in the early postoperative period (HR 1.52, 95% CI 1.03–2.26, P = 0.0356). The results of the IMAGINE trial suggested that ACE inhibitors may cause harm to patients undergoing cardiac surgery. The study has its strength of randomization. Nonetheless, this study may have some limitations such as the inclusion of only “low-risk” patients undergoing CABG, starting quinapril acutely in the postoperative period, and possible excessive doses, as a significantly higher incidence of hypotension was found in the patients receiving quinapril (12%) vs. those receiving placebo (5.5%, p < 0.001).
In addition, two large cohort studies reported conflicting results. Based on 3052 patients on ACE inhibitors treatment matched to a control group at single center, Miceli et al.12 reported (in 2009) that preoperative therapy with ACE inhibitors was associated with an increased risk of mortality (OR 2.00, 95% CI 1.17–3.42, P < 0.03) in patients undergoing CABG. In contrast, based on a prospective cohort study of 4224 patients undergoing CABG from multicenters (72 medical institutions), Drenger et al.13 reported (in 2012) that continuation of ACE inhibitors or de novo ACE inhibitors therapy early after CABG was associated with improved inhospital outcomes, including death, cardiac, cerebral, and renal complication. Of note, in the study by Miceli et al.12 the dose of ACE inhibitors given in this cohort of patients was likely too high, as evidenced by increased inotropic use in these patients. In the study by Drenger et al.13, however, no clinically significant differences in blood pressure and cardiac output were noted among the groups.
Recently RIAS trial (2015) showed that ACE inhibition leads to a modest, but progressive reduction in left ventricular mass in asymptomatic patients with moderate to severe aortic stenosis compared with placebo, with trends towards improvements in myocardial physiology and slower progression of valvular stenosis18. In a cohort study (2014), the prescription RASi after surgical aortic valve replacement was found to be associated with improved long-term survival (up to 10 yeas) in patients with severe aortic stenosis (594 matched pairs of treated and untreated patients)8. The latest clinical study (2018) showed that among patients who underwent transcatheter aortic valve replacement, receiving a prescription of RASi at hospital discharge compared with no prescription was significantly associated with a lower risk of mortality and heart failure readmission19.
With regard to those conflicting results as above, large clinical trials have established beyond reasonable doubt the value of ACE inhibitors for patients with risk of CVD1–4. While researchers have known for some time that when a trial demonstrates an overall effect, whether beneficial or harmful, the direction of this effect is usually consistent within subgroups, as described by DeMets and Califf20. Thus, questions arise on whether ACE inhibitors are harmful or beneficial to a subgroup of CVD patients: cardiac surgery patients.
As shown in previous studies, hypotension or “vasoplegia” is still a potential side effect of perioperative use of ACE inhibitors11,21–25. The history of ACE inhibitors development in patients with acute MI (AMI) is very interesting and may repeat itself in other subsets of patients with CVD1,2. In the early 1990s investigators started examining the effects of ACE inhibitor application in patients suffering AMI. An early study, CONSENSUS II trial (in 1992)26 showed that enalapril therapy did not improve survival in patients with AMI, instead caused more early hypotension. This disappointing result, nonetheless, was soon overcome by a series of clinical trials that demonstrated the remarkable survival benefits of ACE inhibitors in patients with AMI. A key is that ACE inhibitors were titrated carefully in the immediate postinfarct period in these trials to avoid the development of hypotension27–30. In the HOPE trial1, a low dose of ramipril (starting with 2.5 mg per day to a full dose of 10 mg per day) or placebo were given to patients for a mean of 5 years. As a result, the risk reduction was largely independent of lowering blood pressure in the ramipril group (only 2–3 mmHg reduction), suggesting that the inhibition of tissue ACE-mediated processes by ramipril therapy without significant hypotensive effect was responsible for the benefits of ramipril.
Overall, careful titration of low-dose RASi, to avoid excessive hypotension, in patients undergoing cardiac surgery may unmask the outcome benefits provided by preoperative RAS inhibitor therapy. Based on the findings from the present study and previous studies, postoperative RASi should be prescribed indefinitely to patients undergoing CABG and/or valve surgery (if no contraindication), particularly due to its long-term survival benefits1,2,8. An observational study design, as shown in this study, from a large clinical databases can be more representative to real patient population, may better reflect real-world practice, relatively inexpensive, the ethical issues appear less complex (no patients are randomized). In addition, the studies are in progress to use the big data and/or large observational study for causal analyses, besides correlation analyses31–33.
Concerning the limitations of this study, first, although the PSM and other sensitivity analyses were used in this study to reduce overt biases, the potential flaws of a nonrandomized study may remain. Second, clinically it would be appropriate to classify the patients into two groups: taking RASi during both the pre- and postoperative period (continuation) and not taking RASi at any point during the perioperative period (no RASi). However, it would introduce an “immortal” time bias since exposed patients who received their first prescription had to survive until their second prescription34. Third, we lacked data on the dose and adherence of RASi in this cohort of patients. The reported rates of patient RASi adherence for cardiovascular protection are high, range from 68 to 92% in the literature35,36. Finally, both CABG and valve surgery patients were included in this study since RASi have been shown to be beneficial in both groups of patients1,2,6–10,12,18,19. The association we found in this study suggests that perioperative RASi may provide cardiovascular protection with potential long-term benefits for survival. The results of this study also indicate that further investigations, including both RCTs and pragmatic studies are still needed to assess the safety and effectiveness of RASi in patients undergoing cardiac surgery.
Methods
Study design and oversight
This study was a multicenter, retrospective, and cohort study involving consecutive patients (n = 9584) receiving cardiac surgery including CABG and/or valve surgery at three tertiary medical centers (Thomas Jefferson University Hospital, Abington Memorial Hospital and UC Davis Medical Center, dated from 2001 to 2015). The study was in compliance with the Declaration of Helsinki37, approved by Jefferson Institutional Review Boards (IRBs, it covers both Thomas Jefferson University hospital and Abington Memorial Hospital-Jefferson Health) and University California Davis IRB. The individual consent was waived in compliance with the HIPAA regulations and the waiver criteria. After the exclusion, the patients were divided into two groups with or without PreRASi (n = 8581), or two groups with or without PostRASi (n = 8130), respectively. Preoperative or postoperative use of RASi is defined as any patient who received RASi within 48 h preceding surgery or was prescribed RASi at discharge.
Data collection
The patient data collected included demographics, patient history, medical record information, preoperative risk factors, preoperative medications, intraoperative, and postoperative data. For missing variables at low-missing rate, the values were imputed to the median for continuous variables (after stratifying on relevant variables to enhance prediction of the missing value), and the most frequent value for binary and polytomous variables. For missing at random, multiple imputation procedures were applied to replace each missing value with a set of plausible values that represent the uncertainty about the right value to impute38,39.
Measurement of outcomes
The primary endpoint was the mortality. The 30 day and long-term all-cause mortality was determined from the Society of Thoracic Surgeons (STS) Registry of the study sites and the Social Security Death Index40. The survival time (time-to-event) of the subject began when the subject had cardiac surgery, and ended when the end-point (the death) was reached or the subject was censored from the study41. Other outcomes, as defined by the STS national criteria, of this study include postoperative renal failure, readmission, intensive care unit (ICU) length of stay, and a composite outcome—major adverse cardiovascular events (MACE), the latter included permanent or transient stroke, coma, perioperative MI, heart block, and cardiac arrest42.
Adjustment for differences between groups
It was anticipated that patients with or without preoperative or postoperative RASi would differ significantly with respect to baseline (before surgery) characteristics. The propensity scores, reflecting the probability that a patient would receive preoperative RASi, were developed with the use of logistic regression to adjust for between-group differences in baseline characteristics of the patients. The individual variables included in the propensity model are listed in Tables 1 and 3. The PSM was used for estimating treatment effects for specific groups. We matched patients based on the propensity score using a caliper width of 0.2 of the pooled standard deviation of the propensity score, and compare with the results on the basis of greedy matching with 3 or 4 decimal places43–45.
Statistical analysis
Continuous and categorical variables are reported as mean ± SD or percentages, and analyzed by Pearson chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. MACEs were analyzed with using Pearson chi-square test or Wilcoxon rank-sum test (unadjusted) and then PSM (adjusted). For long-term survival, survival curves were estimated with using the Kaplan–Meier method (unadjusted)46, then reestimated with using the PSM approach (adjusted). For each group with or without preoperative or postoperative RASi, the survival curves adjusted with the use of the PSM represented the expected rate of survival if the treatment of interest (with or without preoperative and postoperative RASi) were applied to all study patients. Using estimated rates of survival among patients undergoing cardiac surgery with or without preoperative and postoperative RASi, we calculated risk ratios at specific time points and used bootstrap methods to obtain 95% confidence intervals (CI), adjusting for multiplicity and false discovery to assess the effects of RASi for survival for 1–6 year.
To conduct sensitivity analysis, survival curves were reestimated separately for patients with or without preoperative and postoperative RASi with the use of Cox proportional-hazard models without propensity scores47. Covariates for each model were identical to those in the propensity model described above. Further, the IPW approach was also used to examine the average treatment effect among the study population48–50. In order to limit the influence of extreme IPW, we used truncation of weights by setting the propensity score to the standard range. Percentages, RR, odds ratio (OR), 95% CI, and P values (two-sided) < 0.05 were given in the results. SAS version 9.4 (SAS Institute, Inc., Cary, NC) and SPSS 17.0 software for Windows (SPSS Inc., Chicago, IL) were used for the statistical analysis.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank Margaret Lusardi, RN and Kristan Devine, CRNP for their contribution to the data collection and entry. Q.D. and H.N. were supported by Tangdu and Xijing hospital, respectively, for the visiting scholarship in Thomas Jefferson University. Q.D. also supported by the National Natural Science Foundation of China (81671195) and Science and Technology Development Fund of Shaanxi Province (2016SF-185), China. Z.Z., H.L., and J.S. were supported by their institution, respectively.
Author contributions
Q.D., Z.Z. and J.S. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; Study concept and design: H.L. and J.S.; Acquisition, analysis, or interpretation of data: Q.D., Z.Z., H.L., H.N., M.B., J.E.G., N.Y., D. B., R.M. and J.S; Drafting of the paper: Q.D., Z.Z., H.L., and J.S.; Critical revision of the paper for important intellectual content: Q.D., Z.Z., H.L., H.N., M.B., J.E.G., N.Y., D. B., R.M. and J.S; Statistical analysis: Q.D. and Z.Z.; Obtained funding: H.L. and J.S.; Administrative, technical, or material support: Z.Z., H.L., N.Y., D.B., R.M., and J.S.
Data availability
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Massimo Caputo, John Pepper and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Qian Ding, Zugui Zhang.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-11678-9.
References
- 1.Yusuf S, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 2.Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study) Lancet. 2003;362:782–788. doi: 10.1016/S0140-6736(03)14974-4. [DOI] [PubMed] [Google Scholar]
- 3.Danchin N, et al. Angiotensin-converting enzyme inhibitors in patients with coronary artery disease and absence of heart failure or left ventricular systolic dysfunction: an overview of long-term randomized controlled trials. Arch. Intern. Med. 2006;166:787–796. doi: 10.1001/archinte.166.7.787. [DOI] [PubMed] [Google Scholar]
- 4.Dagenais GR, Pogue J, Fox K, Simoons ML, Yusuf S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–588. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the european society of cardiology. Eur. Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 6.Davin L, Dulgheru R, Lancellotti P. ACE inhibitors in aortic stenosis: no fear just hope. Eur. Heart J. Cardiovasc. Imaging. 2015;16:828–830. doi: 10.1093/ehjci/jev082. [DOI] [PubMed] [Google Scholar]
- 7.Shi P, et al. The effects of preoperative renin-angiotensin system inhibitors on outcomes in patients undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2013;27:703–709. doi: 10.1053/j.jvca.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Goel SS, et al. Renin-angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis: a cohort study. Ann. Intern. Med. 2014;161:699–710. doi: 10.7326/M13-1505. [DOI] [PubMed] [Google Scholar]
- 9.Oosterga M, et al. Effects of quinapril on clinical outcome after coronary artery bypass grafting (The QUO VADIS Study). QUinapril on Vascular Ace and Determinants of Ischemia. Am. J. Cardiol. 2001;87:542–546. doi: 10.1016/S0002-9149(00)01428-4. [DOI] [PubMed] [Google Scholar]
- 10.Kjoller-Hansen L, Steffensen R, Grande P. The Angiotensin-converting Enzyme Inhibition Post Revascularization Study (APRES) J. Am. Coll. Cardiol. 2000;35:881–888. doi: 10.1016/S0735-1097(99)00634-8. [DOI] [PubMed] [Google Scholar]
- 11.Rouleau JL, et al. IMAGINE (Ischemia Management with Accupril post-bypass Graft via Inhibition of the coNverting Enzyme) Investigators. Effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;117:24–31. doi: 10.1161/CIRCULATIONAHA.106.685073. [DOI] [PubMed] [Google Scholar]
- 12.Miceli A, et al. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2009;54:1778–1784. doi: 10.1016/j.jacc.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Drenger B1, et al. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypass: effects on in-hospital morbidity and mortality. Circulation. 2012;126:261–269. doi: 10.1161/CIRCULATIONAHA.111.059527. [DOI] [PubMed] [Google Scholar]
- 14.Hillis LD, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. 2011;124:e652–e735. doi: 10.1161/CIR.0b013e31823c074e. [DOI] [PubMed] [Google Scholar]
- 15.Authors/Task Force members. Windecker S, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 16.Weir MR. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin. Ther. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Sun JZ, Cao LH, Liu H. ACE inhibitors in cardiac surgery: current studies and controversies. Hypertens. Res. 2011;34:15–22. doi: 10.1038/hr.2010.188. [DOI] [PubMed] [Google Scholar]
- 18.Bull Sacha, et al. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor Ramipril In Aortic Stenosis (RIAS trial) Eur. Heart J. Cardiovasc. Imaging. 2015;16:834–841. doi: 10.1093/ehjci/jev043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inohara T, et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA. 2018;320:2231–2241. doi: 10.1001/jama.2018.18077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMets DL, Califf RM. Lessons learned from recent cardiovascular clinical trials: Part I. Circulation. 2002;106:746–751. doi: 10.1161/01.CIR.0000023219.51483.66. [DOI] [PubMed] [Google Scholar]
- 21.Licker M, et al. Long-term angiotensin-converting enzyme inhibitor treatment attenuates adrenergic responsiveness without altering hemodynamic control in patients undergoing cardiac surgery. Anesthesiology. 1996;84:789–800. doi: 10.1097/00000542-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Boldt J, Schindler E, Harter K, Gorlach G, Hempelmann G. Influence of intravenous administration of angiotensin-converting enzyme inhibitor enalaprilat on cardiovascular mediators in cardiac surgery patients. Anesth. Analg. 1995;80:480–485. doi: 10.1097/00000539-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Tamayo E, et al. Evaluation of the withdrawal of ACE inhibitors in coronary artery surgery. Clin. Ter. 2004;155:171–174. [PubMed] [Google Scholar]
- 24.Raja SG, Fida N. Should angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists be omitted before cardiac surgery to avoid postoperative vasodilation? Interact. Cardiovasc. Thorac. Surg. 2008;7:470–475. doi: 10.1510/icvts.2007.174698. [DOI] [PubMed] [Google Scholar]
- 25.Ryckwaert F, Colson P. Hemodynamic effects of anesthesia in patients with ischemic heart failure chronically treated with angiotensin-converting enzyme inhibitors. Anesth. Analg. 1997;84:945–949. doi: 10.1213/00000539-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Swedberg K, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II) N. Engl. J. Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 27.GISSI-3: effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell’infarto Miocardico. Lancet 343, 1115–1122 (1994). [PubMed]
- 28.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet 345, 669–685 (1995). [PubMed]
- 29.Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) study investigators. N. Engl. J. Med. 1995;332:80–85. doi: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer MA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE investigators. N. Engl. J. Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 31.Shiffrin RM. Drawing causal inference from Big Data. Proc. Natl Acad. Sci. USA. 2016;113:7308–7309. doi: 10.1073/pnas.1608845113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernán MA. How to estimate the effect of treatment duration on survival outcomes using observational data. BMJ. 2018;360:k182. doi: 10.1136/bmj.k182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH, Yoon HJ. Medical big data: promise and challenges. Kidney Res. Clin. Pract. 2017;36:3–11. doi: 10.23876/j.krcp.2017.36.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suissa S. Immortal time bias in pharmaco-epidemiology. Am. J. Epidemiol. 2008;15:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 35.Vegter S1, et al. Compliance, persistence, and switching patterns for ACE inhibitors and ARBs. Am. J. Manag. Care. 2011;17:609–616. [PubMed] [Google Scholar]
- 36.Pucci M, Martin U. Detecting non-adherence by urine analysis in patients with uncontrolled hypertension: rates, reasons and reactions. J. Hum. Hypertens. 2017;31:253–257. doi: 10.1038/jhh.2016.69. [DOI] [PubMed] [Google Scholar]
- 37.World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 38.Weintraub WS, et al. Comparative effectiveness of revascularization strategies. N. Engl. J. Med. 2012;366:1467–1476. doi: 10.1056/NEJMoa1110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shahian DM, et al. Predictors of long-term survival after coronary artery bypass grafting bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study) Circulation. 2012;125:1491–1500. doi: 10.1161/CIRCULATIONAHA.111.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huntington J, Butterfield M, Fisher J, Torrent D, Bloomston M. The Social Security Death Index (SSDI) most accurately reflects true survival for older oncology patients. Am. J. Cancer Res. 2013;3:518–522. [PMC free article] [PubMed] [Google Scholar]
- 41.Rich JT, et al. A practical guide to understanding Kaplan–Meier curves. Otolaryngol. Head Neck Surg. 2010;143:331–336. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.STS. Adult cardiac data specifications. Version 2.61 (August 24, 2007). https://www.sts.org/sites/default/files/documents/AdultCVDataSpecifications2.61.pdf
- 43.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 44.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_Part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 45.Kurth T, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am. J. Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 47.Therneau, T. M. & Grambsch, P. M. Modeling survival data: extending the Cox mode (Springer, New York, 2000).
- 48.Robins JM, Mark SD, Newey WK. Estimating exposure effects by modelling the expectation of exposure conditional on confounders. Biometrics. 1992;48:479–495. doi: 10.2307/2532304. [DOI] [PubMed] [Google Scholar]
- 49.Rotnitzky A, Li L, Li X. A note on overadjustment in inverse probability weighted estimation. Biometrika. 2010;97:997–1001. doi: 10.1093/biomet/asq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med. Care. 2007;45(Suppl. 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed in this study are available from the corresponding author on reasonable request.