Abstract
Fatty acid transport protein 4 (FATP4) is an acyl-CoA synthetase that is required for normal permeability barrier in mammalian skin. FATP4 (SLC27A4) mutations cause ichthyosis prematurity syndrome, a nonlethal disorder. In contrast, Fatp4−/− mice die neonatally from a defective barrier. Here we used electron microscopy and lipidomics to characterize defects in Fatp4−/− mice. Mutants showed lamellar body, corneocyte lipid envelope, and cornified envelope abnormalities. Lipidomics identified two lipids previously speculated to be present in mouse epidermis, sphingosine β-hydroxyceramide and monoacylglycerol; mutants displayed decreased proportions of these and the two ceramide classes that carry ultralong-chain, amide-linked fatty acids (FAs) thought to be critical for barrier function, unbound ω-O-acylceramide and bound ω-hydroxyceramide, the latter constituting the major component of the corneocyte lipid envelope. Other abnormalities included elevated amounts of sphingosine α-hydroxyceramide, phytosphingosine non-hydroxyceramide, and 1-O-acylceramide. Acyl chain length alterations in ceramides also suggested roles for FATP4 in esterifying saturated non-hydroxy and β-hydroxy FAs with at least 25 carbons and saturated or unsaturated ω-hydroxy FAs with at least 30 carbons to CoA. Our lipidomic analysis is the most thorough such study of the Fatp4−/− mouse skin barrier to date, providing information about how FATP4 can contribute to barrier function by regulating fatty acyl moieties in various barrier lipids.
Subject terms: Skin diseases, Fatty acids, Sphingolipids, Mass spectrometry, Lipidomics
Introduction
Mammalian skin defends against biological, chemical, and mechanical assaults and acts as a barrier to prevent water loss. Its permeability barrier is established by a series of differentiation events to form the outermost, cornified layer with three components. (1) The cornified envelope of dead, flattened corneocytes1 is a tough, water-insoluble protein sac that envelops keratin fibers. (2) Intercellular lipid lamellae form from secreted and processed lipids from lamellar bodies in granulocytes2. (3) The corneocyte lipid envelope is covalently linked to involucrin in the cornified envelope, functioning as a scaffold for organizing intercellular lipid lamellae, for intercorneocyte cohesion, or as a semipermeable membrane3.
The lipid lamellae are composed of three major types of lipids—free fatty acid (FFA), ceramide, and cholesterol—at an approximately equimolar ratio4. Ceramide is composed of a fatty acid (FA) and a sphingosine as the long-chain base. With many possible modifications, ceramides are a large family of lipids5. Epidermal FAs can contribute to formation of complex lipids or be present in a free form. Several proteins have been implicated in facilitating the uptake of long-chain FAs (LCFA; C12-C20) in mammalian cells6, including a family of fatty acid transport proteins (FATPs), also known as very-long-chain acyl-CoA synthetases7,8. Clinical findings and animal model studies suggest important roles for these candidate transporters in the skin6.
The FATP family comprises six membrane proteins that mediate uptake of LCFA and very-long-chain FAs (VLCFA; ≥ C22)9–12. FATPs show diverse subcellular localizations, including plasma and organellar membranes13,14. FATPs exhibit acyl-CoA synthetase activity and are implicated in facilitating uptake of FAs by vectorial acylation15. The deposition of FATPs in the skin varies substantially16.
We previously identified the wrinkle free mouse that carries a mutation resulting from insertion of a retrotransposon into Slc27a4, the gene encoding FATP417. Mice lacking FATP4 (Fatp4−/− mice) are born with thick, tight, “wrinkle free” skin and a dysfunctional skin barrier, and they die neonatally due to restricted movements and dehydration. Fatp4+/− mice are phenotypically normal. Similar phenotypes have been reported in independent Fatp4 mutants18,19. Expression of either a Fatp4 or a Fatp1 transgene in suprabasal keratinocytes restores the neonatal lethality and rescues the skin phenotype in Fatp4 mutants, demonstrating common substrate preferences, enzymatic activities, and biological functions14,20.
Mutations disrupting human FATP4 are found in patients with ichthyosis prematurity syndrome, which manifests with premature birth, respiratory symptoms, and swollen skin with severe caseosa-like scaling21–23. Surviving patients recover and show a non-scaly ichthyosis during childhood with dry and pruritic skin and often with respiratory and/or food allergies24.
Epidermal lipid analyses of Fatp4−/− newborn mice reveal a decreased level of ceramides carrying fatty acyl chains with 26 or more carbons and an increased level of those with 24 or fewer carbons19,20. Consistent with these results, Fatp4 mutant epidermis displays an increased amount of FFA likely due to failure of mutant keratinocytes to activate longer chain FAs to an acyl-CoA form and accumulation of FFA inside cells14.
To investigate how the defect in FFA activation in Fatp4−/− epidermis affects the incorporation of FAs into ceramides, we performed lipid analyses by thin layer chromatography (TLC) and electrospray ionization-mass spectrometry (ESI-MS) with free, extractable lipids and protein-bound lipids from newborn epidermis. We identified two lipid types previously speculated to be present in normal mice and identified abnormalities in several lipid types. Fatp4−/− lipid abnormalities were remedied by suprabasal keratinocyte expression of either a Fatp4 or a Fatp1 transgene. Consistent with these results, ultrastructural analyses showed that transgene-derived FATP4 or FATP1 could ameliorate most of the defects seen in Fatp4−/− epidermis, including the lamellar body secretory system and corneocyte lipid envelope formation. Our results provide insights into how FATP4 deficiency alter fatty acyl moieties in various barrier lipids and subsequently the permeability barrier function of the skin. Our data also support our previous speculation that FATP4 and FATP1 have overlapping roles in facilitating the usage of ultralong-chain FA (ULCFA; ≥ C25).
Results
Ultrastructural analyses of Fatp4−/− skin
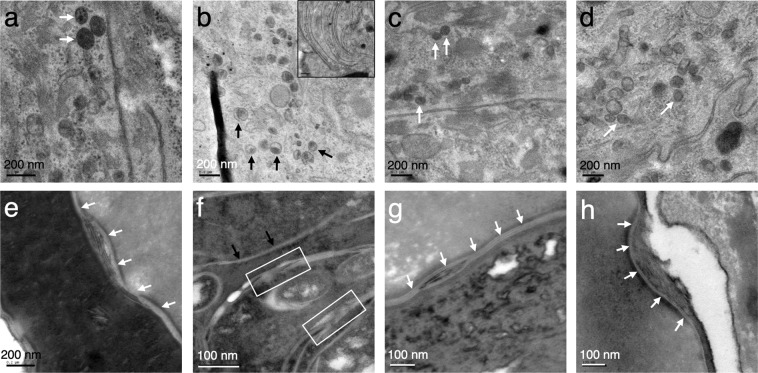
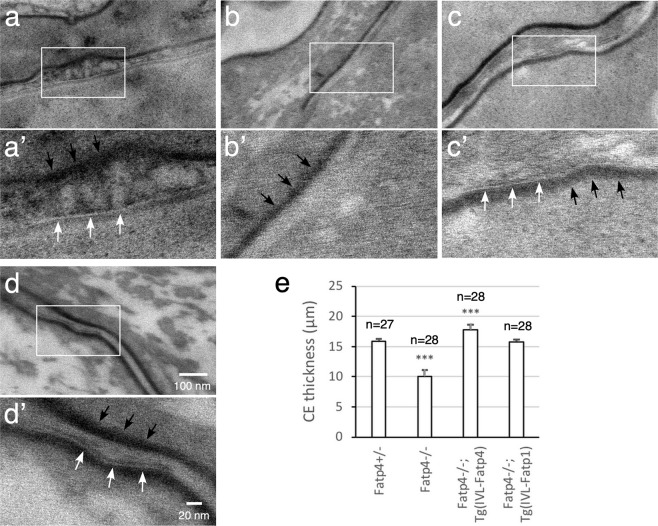
Ultrastructural analysis revealed curved multilamellar membrane-like structures in the granular and cornified layers of Fatp4−/− newborn mice (Fig. 1b, inset) that are also present in ichthyosis prematurity syndrome patients25. Fatp4−/− skin also showed multiple abnormalities in the lamellar body secretory system, including a higher density of lamellar bodies (Fig. 1b) with a decrease in the lamellar contents (black arrows) vs. controls (Fig. 1a, white arrows). In contrast, fusion of lamellar bodies to the plasma membrane, secretion of lipid contents to the extracellular space, and post-secretory processing appeared normal (data not shown). However, the normal-appearing lamellar bilayers were reduced in overall quantity and accompanied by extensive non-lamellar phase separation compared to controls (Fig. 1e,f). Transgenic FATP4 or FATP1 expression in mutant suprabasal keratinocytes rescued most of the ultrastructural abnormalities, with partially corrected lamellar body contents (Fig. 1c,d) and normalization of the quantity and structure of lamellar bilayers (Fig. 1g,h). Fatp4 mutants showed virtually no visible corneocyte lipid envelope compared to controls (Fig. 2a’,b’), which was reverted by transgenic expression of FATP4 or FATP1 (Fig. 2c’,d’).
Figure 1.
Defects in Fatp4−/− lamellar body secretory system ultrastructure. Lamellar body structure ((a–d), reduced osmium tetroxide post-fixation) and extracellular lipid processing ((e–h), ruthenium tetroxide post-fixation) were evaluated by transmission electron microscopy. Typical abnormal curved membrane structures, seen only in Fatp4−/− epidermis, are shown in the inset in (b). Fatp4−/− lamellar bodies (b) were irregularly shaped and incompletely filled (black arrows) compared to control (a) and Fatp4−/− rescued with FATP4 (c) or FATP1 (d) (white arrows show normal lamellar bodies). Secreted lipid in Fatp4−/− was reduced in quantity, as seen by thin sheets of lamellar lipids ((f), black arrows), and exhibited extensive phase separation (white boxes) compared to control (e) and Fatp4−/− rescued with FATP4 (g) or FATP1 (h) (white arrows show normally processed lamellar lipids). Representative images are shown from two mice of each genotype.
Figure 2.
Defects in ultrastructure of Fatp4−/− corneocyte lipid envelope (CLE) and cornified envelope (CE). The CLE and CE were examined by transmission electron microscopy as described in Materials and Methods. The boxed regions in a to d are magnified in a’ to d’, respectively. White arrows indicate typical CLE morphology: a regular, 4 nm thick lucent band on the external face of the CE (black arrows). Fatp4−/− stratum corneum (b’) was characterized by no visible CLE and a thin CE compared to control (a’), FATP4- (c’), and FATP1- (d’) rescued mice. Representative images are shown from two mice of each genotype. CE thickness was quantified (e) as described in Materials and Methods with the n numbers, standard deviation, and statistical significance indicated (***P < 0.001). IVL, involucrin promoter.
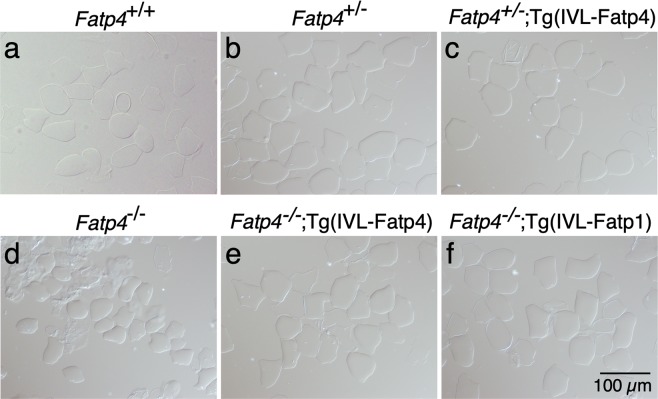
Besides changes in lipid structures, Fatp4 mutant skin showed a significant reduction in thickness of the protein cornified envelope compared to controls (quantification shown in Fig. 2e). Consistent with this, the cornified envelope remaining after boiling the skin with an ionic detergent and a reducing agent was smaller in Fatp4 mutants from embryonic day 16.5 onwards (embryonic day 17.5 shown in Fig. 3d) compared to controls (Fig. 3a–c). Exogenous FATP4 and FATP1 ameliorated the cornified envelope size defect (Fig. 3e,f). Whereas FATP1 expression restored the cornified envelope thickness, FATP4 expression increased the cornified envelope thickness in Fatp4 mutants to a degree that was slightly higher than controls (Fig. 2).
Figure 3.
Defects in Fatp4−/− cornified envelope (CE). The CE was isolated from the dorsal skin of 17.5-day embryos and viewed under a phase contrast microscope. The CE was smaller in Fatp4 mutants (d) compared to controls (a–c) but was normalized by transgene-derived FATP4 (e) and FATP1 (f). Representative images are shown from at least two mice of each genotype. IVL, involucrin promoter.
Alterations of unbound lipids in Fatp4−/− epidermis
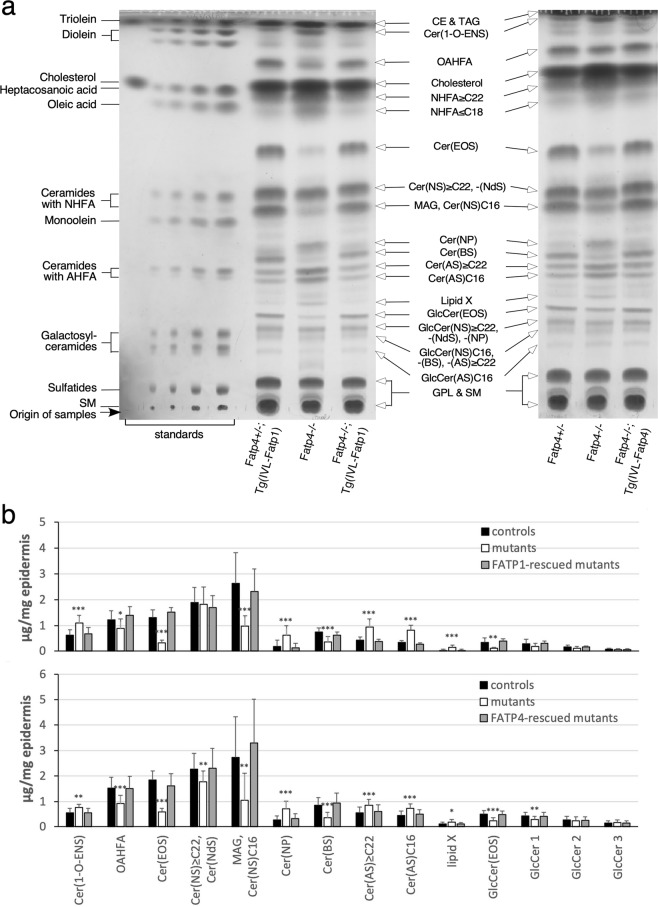
To resolve how the defect in FA activation in Fatp4−/− epidermis affected the incorporation of FAs into ceramides, we performed lipid analyses by TLC and high-resolution ESI-MS with both free, extractable lipids and protein-bound lipids from newborn epidermis. Consistent with previous reports, our TLC analyses of controls showed five major ceramide bands in the free, extractable lipid fraction of the epidermis (Fig. 4a). After recovery from the TLC plate for MS, the five ceramide bands were verified to be: 1) ω-O-acylceramide (Cer(EOS)); 2) a mixture of sphingosine non-hydroxyceramide with a fatty acyl chain of at least 22 carbons (Cer(NS) ≥ C22) and dihydrosphingosine non-hydroxyceramide (Cer(NdS)); 3) sphingosine β-hydroxyceramide (Cer(BS)); 4) sphingosine α-hydroxyceramide with a fatty acyl chain of at least 22 carbons (Cer(AS) ≥ C22); and 5) Cer(AS) with an acyl chain of 16 carbons (Cer(AS)C16). The Cer(BS) band was previously indicated as phytosphingosine (4-hydroxysphinganine) non-hydroxyceramide (Cer(NP))26, but with ESI-MS we verified this band to be Cer(BS) as found in human stratum corneum27, in agreement with original speculation28.
Figure 4.
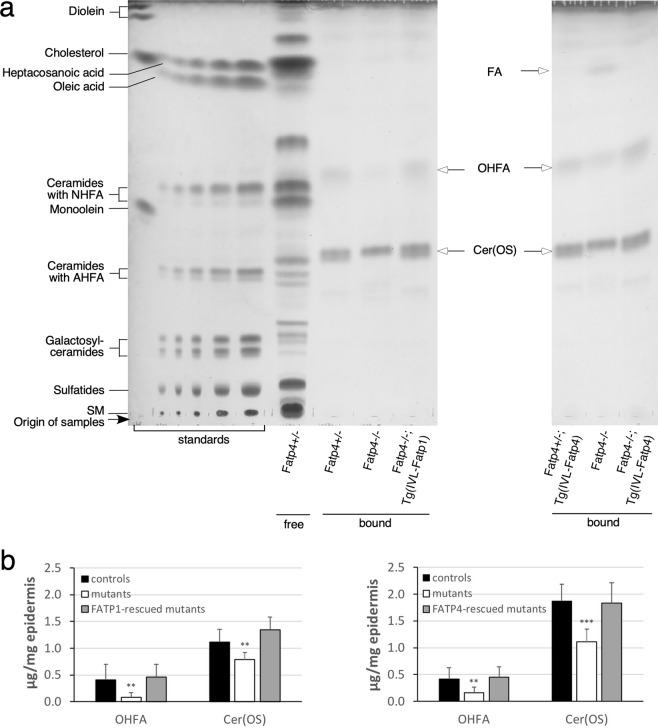
Alterations in the repertoire of unbound epidermal lipids in Fatp4−/− mice. Free, extractable lipids from newborn mouse epidermis were co-resolved with standards by TLC (a) and quantified in µg per mg of dry epidermis (b). The decreased intensities of OAHFA, Cer(EOS), MAG/Cer(NS)C16, Cer(BS), and GlcCer(EOS) bands and the increased levels of Cer(1-O-ENS), Cer(NP), Cer(AS) ≥ C22, Cer(AS)C16, and lipid X observed in Fatp4−/− mice were normalized by expression of Fatp1 (Fatp4−/−; Tg(IVL-Fatp1) in a, left; b, top) and Fatp4 (Fatp4−/−; Tg(IVL-Fatp4) in a, right; b, bottom) transgenes. Lipid standards (a, left) and unbound epidermal lipid types (arrows) are indicated. Quantification data (b) were obtained from two separate studies with standard deviation and statistical significance indicated (*P < 0.05; **P < 0.01; ***P < 0.001). Each study contained 4 each of control, Fatp4−/−, and transgene-rescued mice. Glycerophospholipids (GPL), sphingomyelin (SM), and the previously reported cholesteryl ester (CE), triacylglycerol (TAG), FAs, and cholesterol14 are not included in b. GlcCer 1, 2, and 3 refer to the three bands running immediately behind the GlcCer(EOS) band shown in (a). The TLC results shown in a are from two separate TLC experiments; full vertical lengths are shown. IVL, involucrin promoter.
Compared to controls, Fatp4−/− epidermis displayed abnormal patterns of several ceramide classes including significant reduction in Cer(EOS) and Cer(BS) (Fig. 4a,b). Cer(EOS) contains an amide-linked ULCFA with a terminal ω-hydroxy group that is further esterified with an additional FA, mainly linoleic acid, and is thought to be critical for barrier function29,30. Quantification of Cer(EOS) in two separate studies revealed significant decreases in Fatp4−/− epidermis. The reduction in Cer(EOS) was accompanied by a significantly reduced level of a special FA that migrated immediately ahead of the cholesterol band on TLC with a solvent system for polar lipids (Fig. 4a). This FA band was identified previously to be O-acyl-ω-hydroxy FA (OAHFA)31, a unique type of ULCFA that constitutes the fatty acyl moiety in Cer(EOS). Its identity was verified here by ESI-MS.
Fatp4−/− epidermis showed a discrete band migrating immediately ahead of Cer(BS) that was barely visible in controls (Fig. 4a). ESI-MS identified this to be Cer(NP). Fatp4−/− epidermis also showed significantly increased levels of both Cer(AS) ≥ C22 and Cer(AS)C16 (Fig. 4a,b). In contrast, the amount of the band containing Cer(NS) ≥ C22 and Cer(NdS) was reduced significantly in Fatp4−/− epidermis in one study but unchanged in the other study (Fig. 4b).
Whereas ceramides in control epidermis were resolved into five major bands on TLC, their glycosylated precursors, glucosylceramides (GlcCer), stored in lamellar bodies of granulocytes were separated into four bands (Fig. 4a). With ESI-MS we verified the least and the most polar bands of the four to be GlcCer(EOS) and GlcCer(AS)C16, respectively, as shown previously26,28,32. The other two GlcCer bands were identified to be a mixture of GlcCer(NS) ≥ C22, GlcCer(NdS), and GlcCer(NP), and a mixture of GlcCer(NS)C16, GlcCer(BS), and GlcCer(AS) ≥ C22. In agreement with the reduced Cer(EOS), Fatp4−/− epidermis also showed a reduced amount of GlcCer(EOS) (Fig. 4a,b). Quantification of other GlcCer bands did not reveal significant changes in mutants except reduced GlcCer(NS) ≥ C22, GlcCer(NdS), and GlcCer(NP) mixture (GlcCer 1 in Fig. 4b) in Fatp4−/− epidermis in the same study that showed decreases in Cer(NS) ≥ C22 and Cer(NdS).
In addition to these ceramide and GlcCer classes, mouse epidermal lipids normally contains a less polar ceramide with an ester-linked FA attached to the 1-hydroxyl group of the long-chain base (Fig. 4a)28. By ESI-MS analysis of bands extracted from the TLC plate we recently identified this ceramide ester to be 1-O-acylceramide (Cer(1-O-ENS)33, consistent with a previous report34. Fatp4−/− epidermis showed increased levels of Cer(1-O-ENS) and an unknown ceramide band (Lipid X) that migrated immediately ahead of the GlcCer(EOS) band (Fig. 4a,b). Fatp4−/− epidermis also showed a dramatically reduced amount of a band that migrated immediately behind the Cer(NS) band (Fig. 4a,b). By MS we identified this to be mainly monoacylglycerol (MAG)35, in agreement with original speculation32. This band contained Cer(NS)C16 as a minor component, in contrast to a previous report that it was a major component26. In Fatp4−/− mice expressing either transgenic FATP1 or FATP4 (Fig. 4b), the abnormalities observed in unbound lipids were all remedied.
Alterations of protein-bound lipids in Fatp4−/− epidermis
TLC analysis revealed two major protein-bound lipid bands in epidermal extracts from control newborns (Fig. 5a). By MS they were identified as sphingosine ω-hydroxyceramide (Cer(OS)) and ω-hydroxy FAs (OHFA), the ULCFA that constitutes the fatty acyl moiety in protein-bound Cer(OS), in agreement with other reports26,36. Mutants displayed reduced levels of both protein-bound Cer(OS) and OHFA (Fig. 5b). Another faster migrating FA band reported by others was detected in some of the Fatp4−/− samples examined but not in controls (right panel of Fig. 5a). The abnormalities in Cer(OS) and OHFA were remedied by epidermal FATP1 or FATP4 expression (Fig. 5b).
Figure 5.
Alterations in the repertoire of protein-bound epidermal lipids in Fatp4−/− mice. Protein-bound lipids from newborn mouse epidermis were co-resolved by TLC with standards and free epidermal lipids (a) and quantified in µg per mg of dry epidermis (b). The decreased levels of OHFA and Cer(OS) seen in Fatp4−/− mice were normalized by the Fatp1 (Fatp4−/−; Tg(IVL-Fatp1) in a, left; b, left) and Fatp4 (Fatp4−/−; Tg(IVL-Fatp4) in a, right; b, right) transgenes. A faster migrating FA band was seen in some Fatp4−/− samples. Lipid standards (a, left) and protein-bound epidermal lipids (arrows) are indicated. Quantification data (b) were obtained from two separate studies with standard deviation and statistical significance indicated (**P < 0.01; ***P < 0.001). Each study contained 6 each of control, Fatp4−/−, and transgene-rescued mutant mice. The TLC results shown in a are from two separate TLC experiments; full vertical lengths are shown. IVL, involucrin promoter.
Alterations of FFA and fatty acyl moieties in unbound lipids of Fatp4−/− epidermis
Free fatty acid
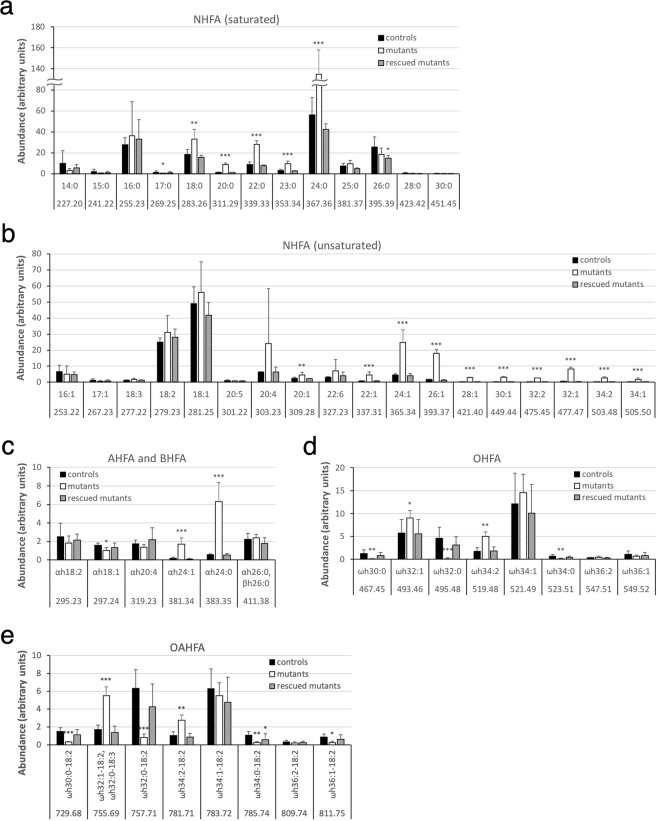
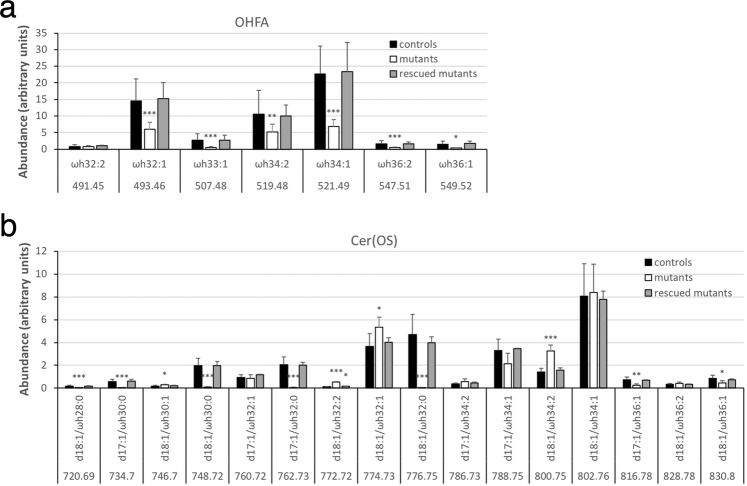
We previously showed that Fatp4−/− epidermis displayed an increased amount of FFA in the unbound lipid fraction likely resulting from Fatp4−/− keratinocytes’ failure to activate ULCFA to an acyl-CoA form, with subsequent accumulation of FFA inside cells14. To investigate the spectrum of the elevated FFA pool and how the incorporation of FAs into unbound lipids was affected in Fatp4−/− epidermis, unbound lipid extract was separated with solid-phase extraction columns into FFA and various lipid fractions and subjected to high-resolution ESI-MS (Figs S1, S2, S3). The results showed that the elevated FFA pool (fractions 4 and 5 in Materials and Methods) in Fatp4−/− epidermis was mainly composed of saturated, non-hydroxy FAs (NHFA) containing 18 to 24 carbons (Fig. 6a) and monounsaturated NHFA containing 20 to 34 carbons (Fig. 6b). The amounts of both saturated and unsaturated α-hydroxy FAs (AHFA) containing 24 carbons were also dramatically increased (Fig. 6c). In contrast, the proportions of saturated OHFA (Fig. 6d) and the OAHFA that carried a saturated fatty acyl backbone (Fig. 6e) were dramatically decreased; examples of OHFA and OAHFA identification are shown in Figs S4, S5.
Figure 6.
Alterations in FFA content in unbound epidermal lipids in Fatp4−/− mice. Pooled fractions 4 and 5 of unbound epidermal lipids of newborns were analyzed by ESI-MS as [M − H]− ions in the negative-ion mode and quantified in arbitrary units. Fatp4−/− mice showed increased levels of saturated NHFA (C18-C24) and monounsaturated NHFA (C20-C34) (a,b), decreased levels of saturated OHFA and the OAHFA with a saturated fatty acyl backbone (d,e), and abnormal levels of AHFA and BHFA (c). Almost all these defects were normalized by the Fatp1 transgene. Data were obtained from 4 each of Fatp4+/− (controls), Fatp4−/−, and FATP1-rescued mutants with m/z ratios, standard deviation, and statistical significance shown (*P < 0.05; **P < 0.01; ***P < 0.001). See Materials and Methods for nomenclature.
Non-hydroxyceramides
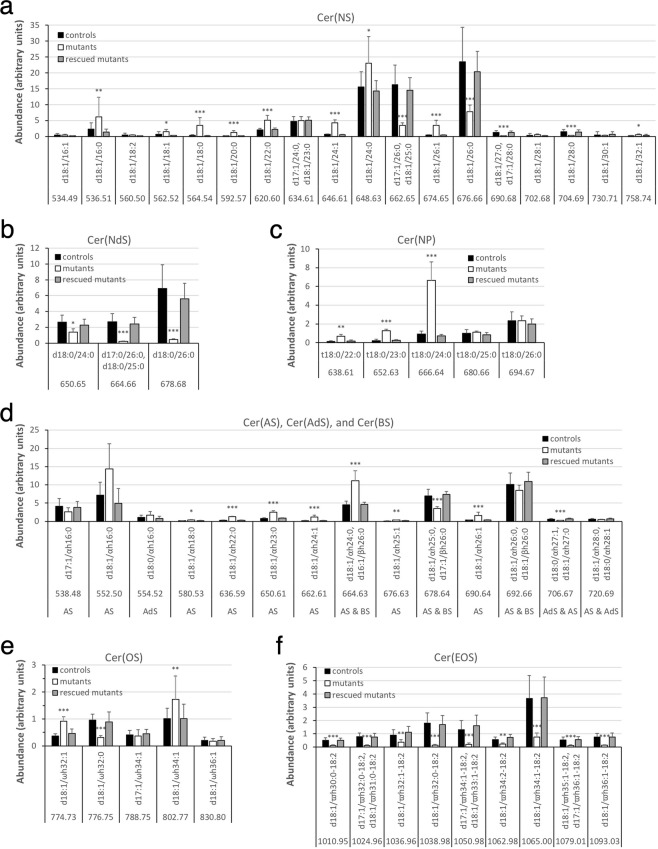
MS of fraction 3 of unbound lipids from control epidermis identified Cer(NS) class carrying an acyl chain of 16 to 32 carbons (Fig. 7a; Fig. S6) and Cer(NdS) class carrying an acyl chain of 24 to 26 carbons (Fig. 7b; Fig. S7). Whereas by TLC the band containing these two ceramide classes in Fatp4−/− epidermis showed an altered level in only one of our two studies, Cer(NS) displayed altered acyl chain length distribution by MS. Fatp4−/− epidermis showed a significantly decreased proportion of Cer(NS) with a saturated acyl moiety containing 25 or more carbons; e.g., two major Cer(NS) species, d17:1/26:0 and d18:1/26:0, were reduced with 4.7- and 3.0-fold changes, respectively. In contrast, Fatp4−/− epidermis showed increased proportions of Cer(NS) species containing either a shorter saturated acyl chain or an unsaturated acyl chain. All Cer(NdS) species identified in control epidermis carried a saturated acyl chain, and their levels were decreased in Fatp4−/− epidermis, especially d18:0/26:0, which showed a 16.0-fold change. The minor lipid Cer(NP) identified in controls comprised a saturated acyl chain carrying 22 to 26 carbons. The increased Cer(NP) in mutants was reflected in species carrying an acyl moiety of 22 to 24 carbons (Fig. 7c; Fig. S8).
Figure 7.
Alterations in ceramide content in unbound epidermal lipids in Fatp4−/− mice. Fraction 3 of unbound epidermal lipids of newborn mice was analyzed by ESI-MS as [M − H]− ions in the negative-ion mode and quantified in arbitrary units. Fatp4−/− mice showed a shift to the Cer(NS) species with a shorter saturated acyl chain (a), decreased levels of the Cer(NdS) and Cer(OS) species that contained a saturated acyl chain (b,e) and of all Cer(EOS) species (f), and increased levels of several species in Cer(NP) and Cer(AS) (c,d). These defects were all normalized by the Fatp1 transgene. Data were obtained from 4 each of Fatp4+/− (controls), Fatp4−/−, and FATP1-rescued mutants with m/z ratios, standard deviation, and statistical significance shown (*P < 0.05; **P < 0.01; ***P < 0.001). See Materials and Methods for nomenclature.
Hydroxyceramides
By TLC Fatp4−/− epidermis showed decreased Cer(BS) and increased levels of two relatively polar ceramide classes, Cer(AS) ≥ C22 and Cer(AS)C16, in the unbound lipid fraction. MS identified three Cer(BS) species in fraction 3 from controls, all carrying the same ß-hydroxy FA (BHFA) 26:0 but each with a different long-chain base d16:1, d17:1, or d18:1 (Fig. 7d). Cer(AS) in controls contained an AHFA chain carrying 16 to 28 carbons (Fig. 7d). All three Cer(BS) species identified shared the same m/z ratios with their corresponding Cer(AS) isomers, e.g., d18:1/βh26:0 & d18:1/αh26:0 both at an m/z ratio of 692.66 when detected as [M - H]− ions, making it difficult to assess the exact changes in these ceramides individually in mutants. Of the other Cer(AS) species that did not have m/z ratios the same as Cer(BS), several were increased in mutants, such as d18:1/αh22:0 and d18:1/αh26:1. By TLC Fatp4−/− epidermis displayed increased Cer(AS)C16; however, by MS the changes in amounts of the two individual Cer(AS)C16 species identified were not statistically significant. MS of fraction 3 also revealed Cer(AdS) and Cer(OS) as minor ceramide classes in control (Fig. 7d,e; Fig. S9). The only Cer(OS) species that carried a saturated acyl chain, d18:1/ωh32:0, was decreased in abundance in Fatp4−/− epidermis.
ω-O-acylceramide
From fraction 3 of unbound lipids from control, MS also identified the Cer(EOS) class carrying 30 to 36 carbons in the OHFA backbone (Fig. 7f). As reported by others, the OHFA moiety of Cer(EOS) was esterified mainly with linoleic acid (18:2). The reduced Cer(EOS) in Fatp4−/− epidermis detected by TLC was attributed to reduced Cer(EOS) species carrying a saturated OHFA backbone and those carrying an unsaturated OHFA backbone. For example, the two most abundant species in control mice, d18:1/ϖh34:1-18:2 and d18:1/ϖh32:0-18:2, showed decreased proportions in Fatp4−/− epidermis with 4.8- and 11.9-fold changes, respectively.
Monoacylglyerol
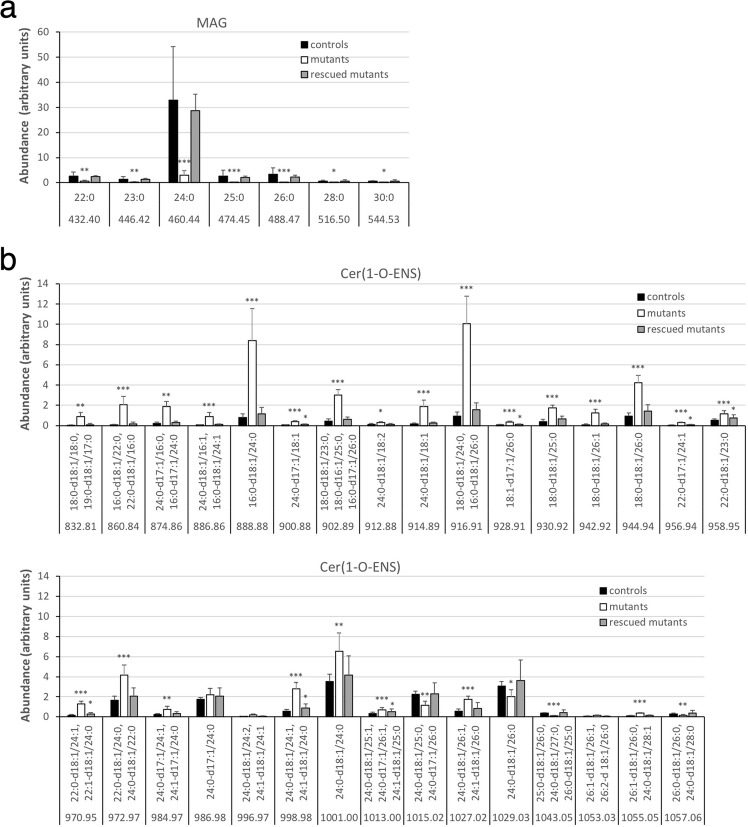
In addition to ceramide classes, MS identified several MAG species in fraction 3 of unbound lipids from control epidermis. They carried a saturated fatty acyl chain ranging from 22 to 30 carbons with 24:0 being the dominant one (Fig. 8a), consistent with a previous report32. Fatp4−/− epidermis showed dramatic reductions in levels of all MAG species.
Figure 8.
Alterations in MAG and 1-O-acylceramide content in unbound epidermal lipids in Fatp4−/− mice. Fractions 3 and 2 of unbound epidermal lipids of newborn mice were analyzed by ESI-MS in the positive-ion mode as [M + NH4]+ ions (a) and as [M + H]+ ions (b), respectively, and quantified in arbitrary units. The decreased levels of MAG species seen in Fatp4−/− mice were all normalized by the Fatp1 transgene (a). Fatp4−/− mice showed increased levels of many Cer(1-O-ENS) species that carried 48 or fewer total carbons in their two acyl chains, almost all of which were normalized by the Fatp1 transgene (b). Data were obtained from 4 each of Fatp4+/− (controls), Fatp4−/−, and FATP1-rescued mutants with m/z ratios, standard deviation, and statistical significance shown (*P < 0.05; **P < 0.01; ***P < 0.001). See Materials and Methods for nomenclature. MAG species are listed by their acyl groups. Cer(1-O-ENS) species at the same m/z ratio are listed in descending order in amount found in controls.
1-O-acylceramide
Cer(1-O-ENS), the least polar ceramide in our study, was identified by MS in fraction 2 from control epidermis (Fig. 8b). The increase in Cer(1-O-ENS) level in Fatp4−/− epidermis by TLC was reflected by the drastically increased levels of many of those carrying 48 or fewer total carbons in their two acyl chains. In contrast, Fatp4−/− epidermis displayed significantly decreased levels of those with two saturated, longer acyl chains, e.g., 25:0-d18:1/26:0.
Glucosylceramide
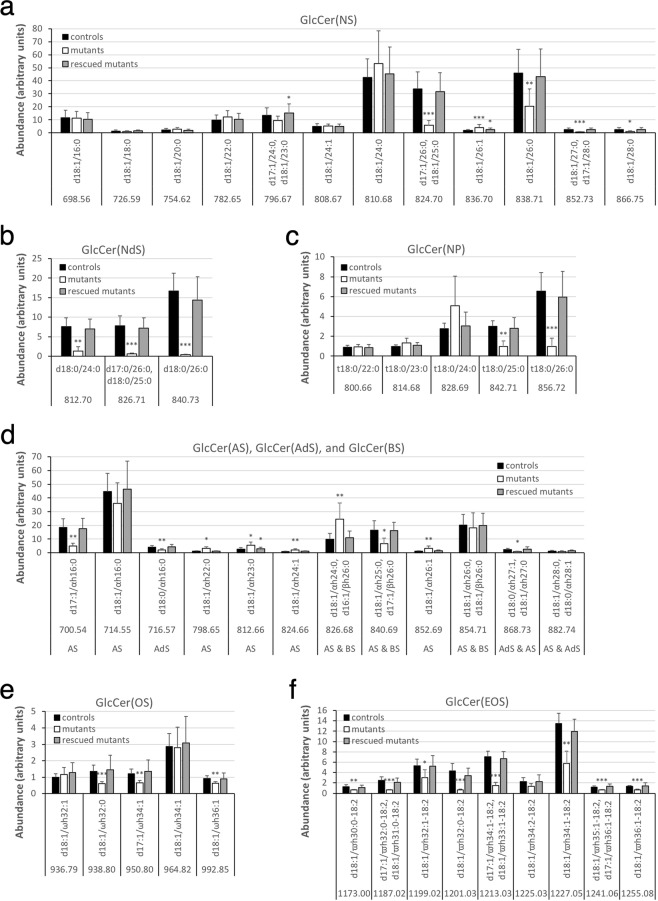
MS on fraction 6 of unbound lipids from control showed glycosylated precursors of most ceramide species identified in our study (Fig. S10). All three GlcCer(NdS) species (Fig. 9b) and nearly all GlcCer(EOS) species (Fig. 9f) were reduced in Fatp4−/− epidermis, comparable to the reductions seen in the corresponding Cer(EOS) species. For example, as the GlcCer(EOS) species d18:1/ϖh32:0-18:2 showed the most dramatic reduction (6.6-fold changes) in mutants, its corresponding processed Cer(EOS) species also showed the most dramatic reduction (11.9-fold changes). The proportions of the several GlcCer(AS) species with a distinct m/z ratio showed similar changes to their corresponding Cer(AS) species except those carrying an acyl chain of 16 carbons (Fig. 9d). In contrast, the changes in abundance of other GlcCer classes did not show good correlation with those of the corresponding processed forms of Cer. For example, whereas data on GlcCer(NS) in mutants reflected the significantly decreased proportions of Cer(NS) species with a saturated fatty acyl moiety containing 25 or more carbons, they did not reveal the corresponding increased proportions of Cer(NS) species containing an unsaturated acyl chain or a shorter saturated acyl chain (Fig. 9a). Also Fatp4−/− epidermis showed decreased GlcCer(NP) species carrying a saturated acyl moiety with 25 or more carbons, in contrast to unchanged abundance of their corresponding Cer(NP) species (Fig. 9c).
Figure 9.
Alterations in GlcCer content in unbound epidermal lipids in Fatp4−/− mice. Fraction 6 of unbound epidermal lipids of newborn mice was analyzed by ESI-MS as [M − H]− ions in the negative-ion mode and quantified in arbitrary units. Fatp4−/− mice showed decreased levels of the GlcCer(NS), GlcCer(NdS), and GlcCer(NP) species that contained a longer saturated acyl chain (a,b,c), of several GlcCer(OS) species (e), and of nearly all GlcCer(EOS) species (f), and increased levels of several GlcCer(AS) species (d). Almost all these defects were normalized by the Fatp1 transgene. Data were obtained from 5 each of Fatp4+/− (controls), Fatp4−/−, and FATP1-rescued mutants with m/z ratios, standard deviation, and statistical significance shown (*P < 0.05; **P < 0.01; ***P < 0.001). See Materials and Methods for nomenclature.
Transgenic FATP1 expression in granulocytes ameliorated nearly all the compositional abnormalities detected in unbound epidermal lipids of Fatp4−/− mice (Figs 6–9).
Alterations of OHFA and fatty acyl moieties in protein-bound lipids in Fatp4−/− epidermis
MS of pooled fractions 4 and 5 of protein-bound lipids from control epidermis identified OHFA containing 32 to 36 carbons, all carrying one or two unsaturated bonds with ωh34:1 and ωh32:1 being the most abundant (Fig. 10a). Fatp4−/− epidermis displayed significantly decreased proportions of nearly all OHFA species. MS of fraction 3 of protein-bound lipids from control epidermis revealed Cer(OS) species carrying a saturated or unsaturated OHFA chain ranging from 28 to 36 carbons (Fig. 10b), in contrast to the protein-bound OHFA that all carried one or two unsaturated bonds. Fatp4−/− epidermis displayed diminished levels of all saturated Cer(OS) species, especially d18:1/ωh32:0, which showed an over 170-fold decrease in abundance, and increased or unchanged levels of unsaturated species. Transgenic FATP1 expression in granulocytes normalized nearly all the compositional irregularities observed in protein-bound epidermal lipids of Fatp4−/− mice (Fig. 10).
Figure 10.
Alterations in FA and ceramide content in protein-bound epidermal lipids in Fatp4−/− mice. Pooled fractions 4 and 5 (a) and fraction 3 (b) of protein-bound epidermal lipids of newborn mice were analyzed by ESI-MS as [M − H]− ions in the negative-ion mode and quantified in arbitrary units. The decreased levels of nearly all OHFA species (a) and all saturated Cer(OS) species (b) seen in Fatp4−/− mice were mostly normalized by the Fatp1 transgene. Data were obtained from 4 each of Fatp4+/− (controls), Fatp4−/−, and FATP1-rescued mutants with m/z ratios, standard deviation, and statistical significance shown (*P < 0.05; **P < 0.01; ***P < 0.001). See Materials and Methods for nomenclature.
Discussion
Fatp4−/− epidermis displayed multiple ultrastructural anomalies in the lamellar body secretory system, a defective corneocyte lipid envelope, and a thinned cornified envelope. These anomalies reflect the lipid composition changes observed in both the unbound and protein-bound epidermal lipids of Fatp4−/− mice, which normally constitute the intercellular lipid lamellae and the corneocyte lipid envelope, respectively.
Our TLC and ESI-MS analyses of the unbound fraction of epidermal lipids from normal newborn mice identified five major ceramide classes, Cer(EOS), Cer(NS), Cer(NdS), Cer(BS), Cer(AS), and three minor classes, Cer(1-O-ENS), Cer(NP), and Cer(OS). Our study is unique in that we identified Cer(BS) and the non-ceramide lipid MAG, compared to what has been reported previously26,28,32,34. Epidermal Cer(BS) and MAG were first reported in newborn mice nearly three decades ago based on TLC and chemical reactivities28 but were not verified by MS until now. Our quantification results for unbound lipids showed that Fatp4−/− newborn epidermis displayed dramatically decreased proportions of OAHFA, Cer(EOS), MAG/Cer(NS)C16, Cer(BS), and GlcCer(EOS), and increased proportions of Cer(1-O-ENS), Cer(NP), Cer(AS) ≥ C22, Cer(AS)C16, and the unknown lipid X. On the other hand, protein-bound lipids from Fatp4−/− epidermis displayed dramatically decreased proportions in both OHFA and Cer(OS), two major components of the corneocyte lipid envelope.
Our analysis of the unbound lipid fraction also revealed alterations in ceramide composition in Fatp4−/− epidermis (See Summary in Table 1). Fatp4−/− epidermis showed profound reductions in production of all Cer(EOS) species that carried a saturated or unsaturated OHFA backbone of at least 30 carbons. Second, changes in several other ceramide classes displayed a switch to a composition with decreased abundance of ceramides containing a saturated acyl chain of at least 25 carbons and with increased abundance of ceramides containing a shorter, saturated acyl chain. Taken together, our results suggest a less hydrophobic composition of the permeability barrier structure in Fatp4−/− epidermis. Our data also demonstrate crucial roles for FATP4 in the uptake and/or activation of saturated NHFA and BHFA with at least 25 carbons and of saturated or unsaturated OHFA with at least 30 carbons for subsequent synthesis of their corresponding classes of ceramides. For example, FATP4 activates OHFA into a CoA form following the cytochrome P450 CYP4F22-catalyzed ω-hydroxylation of FAs that have been lengthened by elongation of VLCFA protein 4 (ELOVL4), as hypothesized for the production of Cer(EOS)25,37,38.
Table 1.
Summarized alterations in fatty acyl chain length and saturation in unbound and protein-bound epidermal lipids in Fatp4−/− mice.
| Fraction number | Lipid class | Acyl chain lengtha | Changes in Fatp4−/− | ||||
|---|---|---|---|---|---|---|---|
| Saturated | Unsaturated | ||||||
| ≥C25 | <C25 | ≥C25 | <C25 | ||||
| Unbound FAs | 4 & 5 | NHFA | C14-C34 | ≅ | ↑e | ↑ | ↑e |
| AHFA | C18-C26 | ? | ↑ | n.a. | varied | ||
| BHFA | C26 | ? | n.a. | n.a. | n.a. | ||
| OHFA | C30-C36 | ↓ | n.a. | ↑e | n.a. | ||
| OAHFA | C30-C36 | ↓ | n.a. | ↑e | n.a. | ||
| Unbound lipids | 3 | Cer(NS) | C16-C32 | ↓ | ↑ | ↑e | ↑e |
| Cer(NdS) | C24-C26 | ↓ | ↓ | n.a. | n.a. | ||
| Cer(NP) | C22-C26 | ≅ | ↑ | n.a. | n.a. | ||
| Cer(AS) | C16-C28 | ? | ↑d | ↑ | ↑ | ||
| Cer(AdS) | C16, C27, C28 | n.a. | ≅ | ? | n.a. | ||
| Cer(BS) | C26 | ↓c | n.a. | n.a. | n.a. | ||
| Cer(OS) | C32-C36 | ↓ | n.a. | ↑e | n.a. | ||
| Cer(EOS) | C30-C36 | ↓ | n.a. | ↓ | n.a. | ||
| 6 | GlcCer(NS) | C16-C28 | ↓ | ≅ | ↑ | ≅ | |
| GlcCer(NdS) | C24-C26 | ↓ | ↓ | n.a. | n.a. | ||
| GlcCer(NP) | C22-C26 | ↓ | ≅ | n.a. | n.a. | ||
| GlcCer(AS) | C16-C28 | ? | ↑d | ↑ | ↑ | ||
| GlcCer(AdS) | C16, C27, C28 | n.a. | ↓ | ? | n.a. | ||
| GlcCer(BS) | C26 | ? | n.a. | n.a. | n.a. | ||
| GlcCer(OS) | C32-C36 | ↓ | n.a. | ↓e | n.a. | ||
| GlcCer(EOS) | C30-C36 | ↓ | n.a. | ↓ | n.a. | ||
| 2 | 1-O-Cer(ENS) |
C16-C28; C16-C26b |
↓ | ↑e | ↑e | ↑ | |
| 3 | MAG | C22-C30 | ↓ | ↓ | n.a. | n.a. | |
| Protein-bound FAs | 4 & 5 | OHFA | C32-C36 | n.a | n.a | ↓ | n.a |
| Protein-bound lipids | 3 | Cer(OS) | C28-C36 | ↓ | n.a. | varied | n.a. |
Fractionated unbound and protein-bound epidermal lipids of newborn mice were analyzed by ESI-MS and quantified as described in Materials and Methods. Changes in lipid abundance in Fatp4−/− mice compared to controls were summarized in 4 categories based on the chain length (≥C25 or <C25) and saturation (saturated or unsaturated) of the fatty acyl chains. 1-O-Cer(ENS) species that carry two saturated acyl chains are categorized as saturated whereas those that carry one or two unsaturated acyl chains are categorized as unsaturated. aamide-linked acyl chain or acyl backbone; b1-O-linked acyl chain; cassessed by TLC; dnot for C16; enot for all species; ↑ and ↓, abundance significantly increased or decreased, respectively, compared to controls; ≅, abundance unchanged compared to controls; ?, abundance unknown; n.a., non-applicable.
That Fatp4−/− newborn epidermis revealed a switch to a less hydrophobic lipid composition is in agreement with the defective barrier that we previously reported39. The correlation between lipid composition and skin barrier is reminiscent of our previous findings that type II diester wax synthesized by sebaceous glands expressing a subnormal level of FATP4 bore fatty acyl moieties of a shorter carbon chain length with a higher degree of unsaturation. Those changes resulted in an increased fluidity of the sebum and less waterproofing of the fur40.
Data from several other animal models also support the importance of Cer(EOS) in permeability barrier function and perinatal survival, including mice deficient in ELOVL431,41–43, ceramide synthase 344, patatin-like phospholipase domain-containing 1 (PNPLA1)45–48, acyl CoA:diacylglycerol acyltransferase 249, α/β hydrolase domain containing protein 5 (ABHD5)36, stearoyl CoA desaturase 250, and sphingolipid activator protein precursor/prosaposin26. These proteins are all involved in pathways contributing to synthesis or availability of Cer(EOS). In contrast, animal models that display non-lethal phenotypes and relatively mild barrier defects do not show changed levels of epidermal Cer(EOS). For example, whereas Fatp4−/− mice showed at least 200% increased transepidermal water loss vs. controls14, Acbp−/− mice51 deficient in acyl-CoA binding protein and Elovl3−/− mice52 lacking the enzyme in elongating fatty acyl chains up to 24 carbons53 showed only ~50% and ~70% increased transepidermal water loss, respectively.
The identification of Cer(BS) and MAG in this study and of Cer(BS) in our recent report on human stratum corneum lipids27 is unique. To our knowledge, Cer(BS) and MAG have not been identified in human or other mammalian skin. Fatp4−/− epidermis showed dramatically decreased proportions of all MAG species carrying a saturated acyl chain ranging from 22 to 30 carbons. In an in vitro study, a mixture of MAG species containing a saturated acyl chain ranging from 22 to 26 carbons displayed better occlusive properties than petroleum jelly, which contains a mixture of hydrocarbons, suggesting a role for MAG in the skin barrier54. It is unlikely that the epidermal MAG was mainly produced from enzymatic or non-enzymatic lipolysis of triacylglycerols and diacylglycerols. First, our MS analyses revealed that epidermal triacylglycerols in control mice comprised only 46 to 56 carbons in total in the three fatty acyl chains esterified to the glycerol backbone (data not shown), with those carrying 52 and 54 carbons being the most abundant species, indicating shorter acyl chains in triacylglycerols than in MAG. Second, the reduced level of MAG in Fatp4−/− epidermis was accompanied by an unaltered level of triacylglycerols, as shown in our previous study14, implicating no correlation in production between these two lipids. From where epidermal MAG is derived remains to be investigated.
With a stratum corneum lipid model, it has been reported that the absence of Cer(EOS) or substituting FFA with shorter chain length ones results in altered lipid lamellae organization and increased permeability55–57. Effects of perturbed lipid composition on intercellular lipid lamellae organization and/or barrier function have been revealed in human skin disorders such as atopic eczema and Netherton syndrome58–60, two inflammatory diseases that manifest with skin barrier defects and allergy features. Some of the lipid composition defects shared by these disorders were also observed in Fatp4−/− epidermis, including a reduced level of long-chain ceramides and increased levels of short-chain ceramides, Cer(AS), and monounsaturated FFA.
Changes in abundance of Cer(EOS) and several Cer(AS) ≥ C22 species in mutants were well reflected in their corresponding GlcCer precursors, but changes in Cer(NS), Cer(NP) and Cer(AS)C16 were not. This suggests that GlcCer is not the only precursor for these ceramides, consistent with the finding in mouse and human epidermal lipids that ceramides 2 (Cer(NS)) and 5 (Cer(AS)C16) could also be derived from sphingomyelin packaged in the lamellar bodies61.
Our data showed that the bound Cer(OS) in Fatp4−/− epidermis was significantly reduced and switched from a more to a less hydrophobic composition. Our recent data from patients and animal models with mutations in epidermal lipid synthesis indicate that the corneocyte lipid envelope originates from the limiting membrane of lamellar bodies and functions as a bidirectional scaffold for the formation of both intercellular lipid lamellae and cornified envelope25. Thus the abnormalities in bound Cer(OS) likely contribute to the defective barrier in Fatp4−/− mice. Several other animal models showing defective skin barrier also displayed alterations in ultrastructure of corneocyte lipid envelope or depletion or reduction in bound Cer(OS) levels26,36,41,44,45,62,63. In 12R-lipoxygenase- and ABHD5-deficient epidermis, both saturated and unsaturated bound Cer(OS) species were dramatically reduced. In contrast, FATP4-deficient epidermis showed depletion in all saturated bound Cer(OS) species but various changes in unsaturated Cer(OS) species.
With the substrate preference of FATP4 towards saturated NHFA and BHFA (≥C25) and saturated or unsaturated OHFA (≥C30), it is expected that these FFA molecules would accumulate inside Fatp4 mutant keratinocytes due to an inability of cells to drive the formation and usage of their acyl-CoA derivatives. However, by MS the FFA that showed the most dramatic elevation in abundance in Fatp4−/− epidermis was unsaturated NHFA (≥C26). It is possible that this resulted from conversion of saturated NHFAs into unsaturated ones to prevent lipotoxicity (see below). Alternatively, the accumulation of FFA in Fatp4−/− epidermis could also result from faster delivery of FAs into cells by an unknown mechanism, increased synthesis of FAs, or increased degradation of other lipids into FFA. Accumulation of FFA in the skin has also been reported in other animal models. For example, a similar compensatory response to impaired Cer(EOS) synthesis and skin barrier was observed in PNPLA1-deficient mice46.
The observed abnormalities in FFA could change the stratum corneum pH or cause cytotoxicity6. For example, the decreased level of FFA in Acbp−/− mice is accompanied by an increased pH in the stratum corneum, which may affect the ionization of FAs, leading to instability of the lamellar membrane and a defective barrier51. In cultured cells it was reported that saturated LCFA like palmitic acid leads to cell death through lipid remodeling, oxidative stress, or endoplasmic reticulum stress, whereas unsaturated FFA like oleic acid protects from lipotoxicity64,65. Lipotoxicity can also result from the detergent effect of excess FFA with calcium66.
In summary, we obtained epidermal lipid data by high resolution MS for accurate results, verified the presence of epidermal Cer(BS) and MAG by MS, and verified the lipid band of Cer(NP) on TLC plates. Our lipidomic analysis also represents the most thorough such study of the permeability barrier in Fatp4−/− mice to date, providing information about how FATP4 can contribute to barrier function by regulating fatty acyl moieties in various barrier lipids including ceramides and MAG. Understanding how FATP4 regulates lipid metabolism is important for elucidating the pathogenesis and potential therapies for ichthyosis prematurity syndrome and related disorders.
Materials and Methods
Mice
Fatp4 mutant (Slc27a4wrfr) and human involucrin promoter-driven Fatp4 and Fatp1 transgenic mice have been previously described14. All animal experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Washington University Institutional Animal Care and Use Committee.
Electron microscopy
The ultrastructural analysis by EM was performed as described25. To visualize the corneocyte lipid envelope and quantify cornified envelope dimensions, skin samples were flash frozen and thawed in absolute pyridine for 2 h at RT. Following standard fixation, samples were post-fixed in reduced osmium tetroxide or ruthenium tetroxide before epoxy embedding. The samples were cut on a Leica Ultracut E microtome (Leica microsystems, Wetzlar, Germany) and imaged on a JEOL 100CX transmission electron microscope (JEOL, Tokyo, Japan) using a Gatan digital camera. For quantification, the thickness of the cornified envelope was measured in at least 25 randomly selected positions in 5 random high-powered electron micrographs of the mid stratum corneum from two mice of each genotype. The observer recording these measurements was blinded to the groups.
Isolation of cornified envelope
Dorsal skin (5 × 5 mm) of embryonic day 16.5 or older mouse embryos was minced using a razor blade and boiled for 5 min in 200 µl of extraction buffer containing 0.1 M Tris, pH8.5, 2% SDS, 20 mM DTT, 5 mM EDTA, pH8.0. After 10 min of centrifugation, the pellet was resuspended in 100 µl of extraction buffer, put onto slides, and photographed in phase contrast under a Nikon Eclipse E800 microscope67.
Extraction of epidermal lipids
To obtain free extractable lipids, a lyophilized 10 mg epidermis sample prepared as described14 was soaked in 0.8 ml water in a centrifuge tube for 5 min, and 3 ml chloroform/methanol (1:2, v:v) were added. Following 30 sec of vortexing and 2 h of shaking at room temperature, an additional 1 ml chloroform and 1 ml water were added. The extraction tube was vortexed for another 30 sec, centrifuged at 2,000 rpm for 5 min, and the lower, organic layer and the epidermis was each transferred to new tubes. The organic layer was re-extracted in 1 ml chloroform, 1 ml methanol, and 0.9 ml water with vortexing and centrifugation. The organic phase was transferred to a vial, dried with a nitrogen evaporator (Organomation Associates, Inc., Berlin, MA), resuspended in 200 µl chloroform/methanol (2:1), and stored at −20 °C.
To extract protein-bound lipids, the epidermis obtained from the extraction above was depleted of residual free lipids by shaking for 1 h sequentially in 2 ml chloroform/methanol mix at a 1:1, 1:2, and 0:2 ratio. After discarding the extract, 1 ml 50 mM NaOH in methanol was added to the epidermis followed by incubation at 56 °C for 2 h with occasional mixing. The reaction was then neutralized with 30 µl of 2 N HCl and extracted in 2 ml chloroform and 2 ml water. The organic layer was collected in a vial. The upper phase and the epidermis were reextracted with 2 ml chloroform, and the organic phase was collected and pooled with the previous extract. The pooled extract was dried, resuspended, and stored as described above.
For quantification of free extractable lipids by MS, lipid standards were added to samples at the beginning of extraction as follows: 1 µg 1-oleoyl-N-heptadecanoyl-D-erythro-sphingosine (18:1-d18:1/17:0-Cer(1-O-ENS); 860526 from Avanti Polar Lipids, Alabaster, AL) for Cer(1-O-ENS); 3 µg tridecanoic acid (13:0-FA; 1161 from Matreya LLC, Pleasant Gap, PA) for FAs; 2 µg N-decanoyl-D-erythro-sphingosine (d18:1/10:0-Cer(NS); 1333 from Matreya) for Cer(NS), Cer(NdS), and Cer(EOS); 11 µg monoheptadecanoin (17:0-MAG; M-159 from Nu-Chek Prep, Elysian, MN) for MAG; 1 µg N-alpha-hydroxydodecanoyl-D-erythro-sphingosine (d18:1/αh12:0-Cer(AS); 2042 from Matreya) for Cer(AS), Cer(AdS), Cer(BS), Cer(OS), and Cer(NP); and 0.2 µg D-glucosyl-β1-1’-N-dodecanoyl-D-erythro-sphingosine (d18:1/12:0-GlcCer(NS); 860543 P from Avanti) for all GlcCer.
For quantification of protein-bound lipids by MS, lipid standards were added to samples at the beginning of incubation in alkaline solution as the following: 1 µg 17-hydroxyheptadecanoic acid (ωh17:0-OHFA; 1760 from Matreya) for OHFA; and 1 µg N-alpha-hydroxydodecanoyl-D-erythro-sphingosine for Cer(OS).
Lipid analysis and quantification by TLC
Flexible silica gel 60 plates (Sigma, St. Louis, MO) were baked at 100 °C for 30 min in an oven prior to use. Free extractable or protein-bound lipids, along with lipid standards, were applied to plates and resolved in a solvent mixture of chloroform/methanol/water (40:10:1) in a pre-equilibrated tank until the solvent front moved 7 cm from the bottom of the plate. The plates were air-dried for 5 min and re-resolved twice in a mixture of chloroform/methanol/glacial acetic acid (95:4.5:0.5) until the solvent front reached the top of the plate, with 5-min air-dry between the two developments. Resolved plates were dried, and lipid spots were visualized by spraying with 3% copper acetate in 8% phosphoric acid followed by charring at 180 °C for 15 min68.
For quantification of lipid types, lipid spots on charred TLC plates were scanned and quantified in ImageJ (http://rsbweb.nih.gov/ij/) with co-chromatographed standards as follows: Oleic acid (18:1-FA; U-46A from Nu-Chek) in the range of 0.5 to 4 µg for quantifying OAHFA, and in the range of 0.5 to 8 µg for OHFA; monoolein (18:1-MAG; 1787-1AMP from Sigma) in the range of 0.5 to 4 µg for MAG; bovine ceramides with NHFA (1056 from Matreya) in the range of 0.265 to 2.12 µg for Cer(1-O-ENS), Cer(EOS), and Cer(NS); bovine ceramides with AHFA (1056 from Matreya) in the range of 0.235 to 1.88 µg for Cer(AS), Cer(BS), Cer(NP), and lipid X, and in the range of 0.235 to 3.76 µg for Cer(OS); and bovine galactosylceramides (1128 from Matreya) in the range of 0.5 to 4 µg for all GlcCer bands. The amount of each lipid band was indicated in µg/mg of dry epidermal weight.
Fractionation of lipids
Fractionation of lipids was performed as previously described with some modifications69. Crude lipids were dried, re-dissolved in 500 µl chloroform, and loaded onto a 3 mL/500 mg Macherey-Nagel (Duren, Germany) amino Chromabond Sep-Pak column that was pre-washed with 2 × 3 mL hexane. The column was first eluted with 3 mL hexane:diethyl ether (90:10) (Fraction 1), followed by 3 mL hexane:ethyl acetate (75:25) (Fraction 2), 3 mL chloroform:methanol (15:1) (Fraction 3), 2 × 3 mL diisopropyl ether:acetic acid (98:5) (Fractions 4 and 5), 3 mL acetone:methanol (9:1.4) (Fraction 6), 3 mL chloroform:methanol (2:1) (Fraction 7), and finally eluted with 3 mL chloroform:methanol (1:2) (Fraction 8) by gravity. The eluants were dried in nitrogen, re-dissolved in chloroform (for fractions 1 to 5) or chloroform/methanol (2:1) (for fractions 6 to 8), and stored at −20 °C.
Recovery of lipids from TLC plates
To recover lipid bands from TLC pates, crude or fractionated lipids were resolved on TLC plates that had been blank-developed in chloroform/methanol (1:1) overnight to remove impurities. The resolution condition was as described above except that the preparative plate of fraction 2 lipids was resolved in a mixture of chloroform/methanol/glacial acetic acid (95:4.5:0.5) until the solvent front moved 16 cm from the bottom of the plate and then in a mixture of hexane/diethyl ether/acetic acid (75:25:1) until the solvent front reached the top of the plate. One of the resolved sample lanes was cut out from the plate and charred as fiduciary markers. With alignment to the charred lane, lipid bands of interest on the uncharred plate were marked with a pencil, scraped out with a razor blade, and extracted by vortexing in chloroform:methanol (2:1; 1.5 ml/cm2 gel scraped) for 30 sec followed by centrifugation. The extract was then collected, dried, resuspended in chloroform or chloroform/methanol (2:1), and stored at −20 °C.
Lipid analysis and quantification by MS
Lipid analysis was performed on a Thermo Scientific (San Jose, CA) LTQ Orbitrap Velos mass spectrometer with Xcalibur operating system as described previously27. Fractionated lipids or lipids recovered from TLC plates were diluted in methanol with 1% NH4OH and loop injected into the ESI source using a built-in syringe pump that delivered a constant flow of methanol with 1% NH4OH at a flow rate of 15 µl/min. The ESI needle was set at 4.0 kV, and temperature of the heated capillary was 300 °C. The automatic gain control of the ion trap was set to 5 × 104, with a maximum injection time of 50 ms. Helium was used as the buffer and collision gas at a pressure of 1 × 10−3 mbar (0.75 mTorr). The MSn (n = 2, 3) spectra were acquired for structural identification27, and the experiments were carried out with an optimized relative collision energy ranging from 30–45% and with an activation q value at 0.25, and an activation time at 10 ms to leave a minimal residual abundance of precursor ion (around 20%). The mass selection window for the precursor ions was set at 1 Da wide to admit the monoisotopic ion to the ion-trap for CID for unit resolution detection in the ion-trap or high-resolution accurate mass detection in the Orbitrap mass analyzer. Mass spectra were accumulated in the profile mode, typically for 2–10 min for MSn spectra (n = 2–4). Lipid quantitation was achieved by high resolution ESI-MS measurement on the lipid fractions with internal standards that were added prior to extraction. All ceramides were analyzed as the [M − H]− ions in the negative-ion mode except for 1-O-acylceramides which were analyzed as the [M + H]+ ions in the positive-ion mode. MAG was detected as the [M + NH4]+ ions in the positive ion mode. The intensity ratio of individual lipid species to the internal standard was calculated, normalized to the dry epidermal weight, and the abundance of individual lipid species was plotted in arbitrary units.
Nomenclature
The following designations and abbreviations recommended by IUPAC (https://www.qmul.ac.uk/sbcs/iupac/lipid/) are used. The designation of ceramide is in the form of d(or t) long-chain base /FA, with d denoting a dihydroxy and t denoting a trihydroxy long-chain base. Thus, for example, the sphingosine (sphing-4-enine) and sphinganine long-chain bases are designated as d18:1, and d18:0, respectively. Fatty acyl moieties with or without hydroxyl substituent are denoted as hFA or nFA, respectively, and sphingosine ceramides with α-, β-, or ω-hydroxyl fatty acyl substituent are designated as d18:1/αhFA-Cer, d18:1/βhFA-Cer, d18:1/ωhFA-Cer, respectively. Therefore, N-α-, N-β-, and N-ω-hydroxy-palmitoyl-sphingosine, for example, are designated as d18:1/αh16:0-Cer, d18:1/βh16:0-Cer, d18:1/ωh16:0-Cer, respectively. To categorize the ceramide subfamilies, the nomenclature of Motta et al.70, expanded by Robson et al.71 and Masukawa et al.72, was adopted. The initial letter of the sphingoid bases S, dS, P, and H are used to represent sphingosine, dihydrosphingosine, phytosphingosine, and 6-hydroxysphingosine, respectively, and the FA residues N, A, B, and O represent nonhydroxylated acyl, α-hydroxyacyl, β-hydroxyacyl, and ω-hydroxyacyl, respectively. Thus, for example, the d18:0/16:0-Cer, d18:1/βh16:0-Cer, and d18:1/ωh16:0-Cer belong to the Cer(NdS), Cer(BS), and Cer(OS) subfamilies, respectively.
Statistical analysis
Two-tailed, unpaired Student’s t-tests were used to determine statistical differences in cornified envelope measurements. Two-way mixed model ANOVA tests were used in quantification of lipids by TLC and ESI-MS. Differences were considered significant when P < 0.05.
Supplementary information
Acknowledgements
This work was supported by National Institutes of Health grants R01AR049269 (to JHM) and R01AR061106 (to PME). We thank Jennifer Richardson for mouse genotyping and the Mouse Genetics Core for generating transgenic mice and for husbandry. We thank the Digestive Diseases Research Core Center Murine Models Core (P30DK052574) for supporting production of transgenic mice. MS was performed with support from the Diabetes Research Center’s Metabolomics Core (P30DK020579), the Nutrition Obesity Research Center’s Biomolecular Analysis Core (P30DK056341), and the Washington University Mass Spectrometry Resource (P41GM103422). Mice were housed in a facility supported by NCRR grant C06RR015502.
Author Contributions
This study was conceived by M.-H.L. and J.H.M.; experimental work was carried out by M.-H.L., F.-F.H., and D.C.; data were analyzed by M.-H.L., F.-F.H., P.M.E. and J.M.; original draft was written by M.-H.L. and J.H.M.; all authors reviewed the manuscript.
Data Availability
Lipidomics data have been deposited into the EMBL-EBI MetaboLights database73 with the identifier MTBLS1138. The dataset can be accessed at https://www.ebi.ac.uk/metabolights/MTBLS1138.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49684-y.
References
- 1.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 2.Breiden B, Sandhoff K. The role of sphingolipid metabolism in cutaneous permeability barrier formation. Biochim Biophys Acta. 2014;1841:441–452. doi: 10.1016/j.bbalip.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Elias PM, et al. Formation and functions of the corneocyte lipid envelope (CLE) Biochim. Biophys. Acta. 2014;1841:314–318. doi: 10.1016/j.bbalip.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias PM, Menon GK. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991;24:1–26. doi: 10.1016/B978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 5.Rabionet M, Gorgas K, Sandhoff R. Ceramide synthesis in the epidermis. Biochim. Biophys. Acta. 2014;1841:422–434. doi: 10.1016/j.bbalip.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Lin MH, Khnykin D. Fatty acid transporters in skin development, function and disease. Biochim. Biophys. Acta. 2014;1841:362–368. doi: 10.1016/j.bbalip.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson CM, Stahl A. SLC27 fatty acid transport proteins. Mol. Aspects Med. 2013;34:516–528. doi: 10.1016/j.mam.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimeno RE. Fatty acid transport proteins. Curr. Opin. Lipidol. 2007;18:271–276. doi: 10.1097/MOL.0b013e3281338558. [DOI] [PubMed] [Google Scholar]
- 9.Coe NR, Smith AJ, Frohnert BI, Watkins PA, Bernlohr DA. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J. Biol. Chem. 1999;274:36300–36304. doi: 10.1074/jbc.274.51.36300. [DOI] [PubMed] [Google Scholar]
- 10.Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J. Biol. Chem. 2005;280:11948–11954. doi: 10.1074/jbc.M412629200. [DOI] [PubMed] [Google Scholar]
- 11.Mihalik SJ, et al. Participation of two members of the very long-chain acyl-CoA synthetase family in bile acid synthesis and recycling. J. Biol. Chem. 2002;277:24771–24779. doi: 10.1074/jbc.M203295200. [DOI] [PubMed] [Google Scholar]
- 12.Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J. Biol. Chem. 2007;282:20573–20583. doi: 10.1074/jbc.M700568200. [DOI] [PubMed] [Google Scholar]
- 13.Kazantzis M, Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim. Biophys. Acta. 2012;1821:852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin MH, Miner JH. Fatty acid transport protein 1 can compensate for fatty acid transport protein 4 in the developing mouse epidermis. J. Invest. Dermatol. 2015;135:462–470. doi: 10.1038/jid.2014.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol. Mol. Biol. Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmuth M, et al. Differential expression of fatty acid transport proteins in epidermis and skin appendages. J. Invest. Dermatol. 2005;125:1174–1181. doi: 10.1111/j.0022-202X.2005.23934.x. [DOI] [PubMed] [Google Scholar]
- 17.Moulson CL, et al. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc. Natl. Acad. Sci. USA. 2003;100:5274–5279. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao J, et al. A spontaneous Fatp4/Scl27a4 splice site mutation in a new murine model for congenital ichthyosis. PLoS One. 2012;7:e50634. doi: 10.1371/journal.pone.0050634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann T, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J. Cell Biol. 2003;161:1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moulson CL, et al. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J. Biol. Chem. 2007;282:15912–15920. doi: 10.1074/jbc.M701779200. [DOI] [PubMed] [Google Scholar]
- 21.Bygum A, Westermark P, Brandrup F. Ichthyosis prematurity syndrome: a well-defined congenital ichthyosis subtype. J. Am. Acad. Dermatol. 2008;59:S71–74. doi: 10.1016/j.jaad.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Klar J, et al. Mutations in the fatty acid transport protein 4 gene cause the ichthyosis prematurity syndrome. Am. J. Hum. Genet. 2009;85:248–253. doi: 10.1016/j.ajhg.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobol M, Dahl N, Klar J. FATP4 missense and nonsense mutations cause similar features in Ichthyosis Prematurity Syndrome. BMC Res. Notes. 2011;4:90. doi: 10.1186/1756-0500-4-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khnykin D, et al. Ichthyosis prematurity syndrome: clinical evaluation of 17 families with a rare disorder of lipid metabolism. J. Am. Acad. Dermatol. 2012;66:606–616. doi: 10.1016/j.jaad.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Crumrine D, et al. Mutations in Recessive Congenital Ichthyoses Illuminate the Origin and Functions of the Corneocyte Lipid Envelope. J. Invest. Dermatol. 2019;139:760–768. doi: 10.1016/j.jid.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doering T, et al. Sphingolipid activator proteins are required for epidermal permeability barrier formation. J. Biol. Chem. 1999;274:11038–11045. doi: 10.1074/jbc.274.16.11038. [DOI] [PubMed] [Google Scholar]
- 27.Hsu FF. Complete structural characterization of ceramides as [M−H](−) ions by multiple-stage linear ion trap mass spectrometry. Biochimie. 2016;130:63–75. doi: 10.1016/j.biochi.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madison KC, Swartzendruber DC, Wertz PW, Downing DT. Sphingolipid metabolism in organotypic mouse keratinocyte cultures. J. Invest. Dermatol. 1990;95:657–664. doi: 10.1111/1523-1747.ep12514333. [DOI] [PubMed] [Google Scholar]
- 29.Holleran WM, Takagi Y, Uchida Y. Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett. 2006;580:5456–5466. doi: 10.1016/j.febslet.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Uchida Y, Holleran WM. Omega-O-acylceramide, a lipid essential for mammalian survival. J. Dermatol. Sci. 2008;51:77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.McMahon A, Butovich IA, Kedzierski W. Epidermal expression of an Elovl4 transgene rescues neonatal lethality of homozygous Stargardt disease-3 mice. J. Lipid Res. 2011;52:1128–1138. doi: 10.1194/jlr.M014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madison KC, Swartzendruber DC, Wertz PW, Downing DT. Murine keratinocyte cultures grown at the air/medium interface synthesize stratum corneum lipids and “recycle” linoleate during differentiation. J. Invest. Dermatol. 1989;93:10–17. doi: 10.1111/1523-1747.ep12277335. [DOI] [PubMed] [Google Scholar]
- 33.Lin MH, Miner JH, Turk J, Hsu FF. Linear ion-trap MS(n) with high-resolution MS reveals structural diversity of 1-O-acylceramide family in mouse epidermis. J. Lipid Res. 2017;58:772–782. doi: 10.1194/jlr.D071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabionet M, et al. 1-O-acylceramides are natural components of human and mouse epidermis. J. Lipid Res. 2013;54:3312–3321. doi: 10.1194/jlr.M040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu FF, Turk J. Electrospray ionization multiple-stage linear ion-trap mass spectrometry for structural elucidation of triacylglycerols: assignment of fatty acyl groups on the glycerol backbone and location of double bonds. J. Am. Soc. Mass Spectrom. 2010;21:657–669. doi: 10.1016/j.jasms.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radner FP, et al. Growth retardation, impaired triacylglycerol catabolism, hepatic steatosis, and lethal skin barrier defect in mice lacking comparative gene identification-58 (CGI-58) J. Biol. Chem. 2010;285:7300–7311. doi: 10.1074/jbc.M109.081877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyama M. Corneocyte lipid envelope (CLE), the key structure for skin barrier function and ichthyosis pathogenesis. J. Dermatol. Sci. 2017;88:3–9. doi: 10.1016/j.jdermsci.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Ohno Y, et al. Essential role of the cytochrome P450 CYP4F22 in the production of acylceramide, the key lipid for skin permeability barrier formation. Proc. Natl. Acad. Sci. USA. 2015;112:7707–7712. doi: 10.1073/pnas.1503491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin MH, Chang KW, Lin SC, Miner JH. Epidermal hyperproliferation in mice lacking fatty acid transport protein 4 (FATP4) involves ectopic EGF receptor and STAT3 signaling. Dev. Biol. 2010;344:707–719. doi: 10.1016/j.ydbio.2010.05.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin MH, Hsu FF, Miner JH. Requirement of fatty acid transport protein 4 for development, maturation, and function of sebaceous glands in a mouse model of ichthyosis prematurity syndrome. J. Biol. Chem. 2013;288:3964–3976. doi: 10.1074/jbc.M112.416990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol. Sci. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasireddy V, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (>=C28) and the unique {omega}-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon A, et al. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol Vis. 2007;13:258–272. [PMC free article] [PubMed] [Google Scholar]
- 44.Jennemann R, et al. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- 45.Grond S, et al. PNPLA1 Deficiency in Mice and Humans Leads to a Defect in the Synthesis of Omega-O-Acylceramides. J. Invest. Dermatol. 2017;137:394–402. doi: 10.1016/j.jid.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirabayashi T, et al. PNPLA1 has a crucial role in skin barrier function by directing acylceramide biosynthesis. Nat. Commun. 2017;8:14609. doi: 10.1038/ncomms14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pichery M, et al. PNPLA1 defects in patients with autosomal recessive congenital ichthyosis and KO mice sustain PNPLA1 irreplaceable function in epidermal omega-O-acylceramide synthesis and skin permeability barrier. Hum. Mol. Genet. 2017;26:1787–1800. doi: 10.1093/hmg/ddx079. [DOI] [PubMed] [Google Scholar]
- 48.Hirabayashi T, Murakami M, Kihara A. The role of PNPLA1 in omega-O-acylceramide synthesis and skin barrier function. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2018;1864:869–879. doi: 10.1016/j.bbalip.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 49.Stone SJ, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 50.Miyazaki M, Dobrzyn A, Elias PM, Ntambi JM. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc. Natl. Acad. Sci. USA. 2005;102:12501–12506. doi: 10.1073/pnas.0503132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bloksgaard M, et al. The acyl-CoA binding protein is required for normal epidermal barrier function in mice. J. Lipid Res. 2012;53:2162–2174. doi: 10.1194/jlr.M029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westerberg R, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J. Biol. Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- 53.Ohno Y, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 2010;107:18439–18444. doi: 10.1073/pnas.1005572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perez B, et al. Fractionated aliphatic alcohols as synthetic precursors of ultra long-chain monoacylglycerols for cosmetic applications. Int. J. Cosmet. Sci. 2017;39:511–517. doi: 10.1111/ics.12404. [DOI] [PubMed] [Google Scholar]
- 55.de Jager M, et al. A novel in vitro percutaneous penetration model: Evaluation of barrier properties with P-aminobenzoic acid and two of its derivatives. Pharmaceutical Research. 2006;23:951–960. doi: 10.1007/s11095-006-9909-1. [DOI] [PubMed] [Google Scholar]
- 56.Groen D, Poole DS, Gooris GS, Bouwstra JA. Is an orthorhombic lateral packing and a proper lamellar organization important for the skin barrier function? Biochim. Biophys. Acta. 2011;1808:1529–1537. doi: 10.1016/j.bbamem.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Bouwstra JA, Gooris GS, Dubbelaar FE, Ponec M. Phase behavior of lipid mixtures based on human ceramides: coexistence of crystalline and liquid phases. J. Lipid Res. 2001;42:1759–1770. [PubMed] [Google Scholar]
- 58.Janssens M, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012;53:2755–2766. doi: 10.1194/jlr.P030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Smeden J, et al. Intercellular skin barrier lipid composition and organization in Netherton syndrome patients. J. Invest. Dermatol. 2014;134:1238–1245. doi: 10.1038/jid.2013.517. [DOI] [PubMed] [Google Scholar]
- 60.van Smeden J, et al. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014;23:45–52. doi: 10.1111/exd.12293. [DOI] [PubMed] [Google Scholar]
- 61.Uchida Y, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J. Lipid Res. 2000;41:2071–2082. [PubMed] [Google Scholar]
- 62.Epp N, et al. 12R-lipoxygenase deficiency disrupts epidermal barrier function. J. Cell Biol. 2007;177:173–182. doi: 10.1083/jcb.200612116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krieg P, et al. Aloxe3 knockout mice reveal a function of epidermal lipoxygenase-3 as hepoxilin synthase and its pivotal role in barrier formation. J. Invest. Dermatol. 2013;133:172–180. doi: 10.1038/jid.2012.250. [DOI] [PubMed] [Google Scholar]
- 64.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab. 2009;10:9–12. doi: 10.1016/j.cmet.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Listenberger LL, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mauldin EA, et al. Cellular and Metabolic Basis for the Ichthyotic Phenotype in NIPAL4 (Ichthyin)-Deficient Canines. Am. J. Pathol. 2018;188:1419–1429. doi: 10.1016/j.ajpath.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaubert J, Cheng J, Segre JA. Ectopic expression of kruppel like factor 4 (Klf4) accelerates formation of the epidermal permeability barrier. Development. 2003;130:2767–2777. doi: 10.1242/dev.00477. [DOI] [PubMed] [Google Scholar]
- 68.Macala LJ, Yu RK, Ando S. Analysis of brain lipids by high performance thin-layer chromatography and densitometry. J. Lipid Res. 1983;24:1243–1250. [PubMed] [Google Scholar]
- 69.Bodennec J, et al. A procedure for fractionation of sphingolipid classes by solid-phase extraction on aminopropyl cartridges. J Lipid Res. 2000;41:1524–1531. [PubMed] [Google Scholar]
- 70.Motta S, et al. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta. 1993;1182:147–151. doi: 10.1016/0925-4439(93)90135-N. [DOI] [PubMed] [Google Scholar]
- 71.Robson KJ, Stewart ME, Michelsen S, Lazo ND, Downing DT. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J. Lipid Res. 1994;35:2060–2068. [PubMed] [Google Scholar]
- 72.Masukawa Y, et al. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 2008;49:1466–1476. doi: 10.1194/jlr.M800014-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Haug K, et al. MetaboLights–an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 2013;41:D781–786. doi: 10.1093/nar/gks1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lipidomics data have been deposited into the EMBL-EBI MetaboLights database73 with the identifier MTBLS1138. The dataset can be accessed at https://www.ebi.ac.uk/metabolights/MTBLS1138.