Abstract
The ST131 multilocus sequence type (MLST) of Escherichia coli is a globally successful pathogen whose dissemination is increasing rates of antibiotic resistance. Numerous global surveys have demonstrated the pervasiveness of this clone; in some regions ST131 accounts for up to 30% of all E. coli isolates. However, many regions are underrepresented in these published surveys, including Africa, South America, and Asia. We collected consecutive bloodstream E. coli isolates from three countries in Southeast Asia; ST131 was the most common MLST type. As in other studies, the C2/H30Rx clade accounted for the majority of ST131 strains. Clinical risk factors were similar to other reported studies. However, we found that nearly all of the C2 strains in this study were closely related, forming what we denote the SEA-C2 clone. The SEA-C2 clone is enriched for strains from Asia, particularly Southeast Asia and Singapore. The SEA-C2 clone accounts for all of the excess resistance and virulence of ST131 relative to non-ST131 E. coli. The SEA-C2 strains appear to be locally circulating and dominant in Southeast Asia, despite the intuition that high international connectivity and travel would enable frequent opportunities for other strains to establish themselves.
Subject terms: Bacterial genes, Bacterial infection
Introduction
Escherichia coli is a common member of the colonic flora of humans and other animals, where it often appears be a commensal1. However, E. coli also causes a variety of diseases, which are generally classified based on the disease syndromes they cause. Some of these E. coli classifications include EHEC (enterohemmorhagic E. coli), ETEC (enterotoxigenic E. coli), and UPEC (uropathogenic E. coli)1,2. Many authors distinguish between E. coli that are associated with intestinal and extra-intestinal (outside the gastrointestinal tract) diseases; those causing extra-intestinal disease are referred to as a group as ExPEC (extraintestinal pathogenic E. coli). ExPECs commonly cause urinary tract infections, bloodstream infections, meningitis, and soft tissue infections2. These ExPEC infections are typically more medically serious than the intestinal syndromes caused by E. coli, and their treatment relies on effective antibiotic therapy. Unfortunately, as in other bacteria, antibiotic resistance rates in ExPEC strains have been rising in recent years3. In fact, ExPEC strains are generally more antibiotic resistant than other types of E. coli, and the expansion of specific ExPEC clones or multilocus sequence types (MLSTs) have been contributing to the rising rates of E. coli antibiotic resistance, such as ST38, ST405, and ST6484.
Another of these ExPEC sequence types, ST131 E. coli, has been more extensively studied over the last 10 years and found to be rapidly expanding across the globe. ST131 was first described in 20085–7 and has now been found on every continent examined; it accounts for up to 30% of all ExPEC isolates in some regions8. Of particular concern, ST131 strains are frequently resistant to multiple commonly prescribed antibiotics, most prominently fluoroquinolones and beta-lactamases8,9. More specifically, ST131 strains typically carry resistance-conferring mutations in the chromosomal gyrA and parC genes (encoding DNA gyrase and DNA topoisomerase IV, respectively, the target of fluoroquinolones)8–10 as well as a gene encoding a CTX-M-class extended spectrum beta-lactamase (ESBL) (particularly CTX-M-15), either on a plasmid or integrated into the chromosome11. Coupled with the recent spread of ST131, this means that ST131 itself has been responsible for much of the observed rise in antibiotic resistance, particularly ESBL-mediated resistance, in ExPEC globally6,12. In addition to driving resistance, evidence also indicates that ST131 strains may be more virulent, driving higher rates of bacteremia13.
Numerous studies of ST131 have been performed, many of them using whole genome sequencing11,12,14–21. ST131 is often referred to as a clone, or clonal group, as the strains are closely related and appear to have had a single origin5,9,12,20. ST131 strains have been further subclassified into 3 large clades using two closely correlated naming schemes: A/H41, B/H22, and C/H30-R8,12. There are further subdivisions of these clades; of most importance is the subdivision of clade C/H30-R into two subclades, one of which has a high prevalence of the CTX-M-15 ESBL gene (referred to as C2/H30-Rx)8,12,15. Despite the general resistance of C2/H30-Rx strains to more antibiotics, however, all of Clade C/H30-R seems to be participating in the recent global expansion of ST13115.
A Bayesian analysis dated the divergence of clades B and C to 1938–1958 and predicted that they arose in North America15. This same study dated the divergence between clades C1 and C2 to 1980 and noted that, with the exception of a cluster of GI-selC containing strains from the UK, there was no significant geographical clustering. The available data at that time, however, was limited in strains from South America, Africa, and Asia.
In particular, there remains limited data concerning the prevalence and molecular characteristics of E. coli ST131 in Southeast Asian countries, particularly for bacteremia, as previous studies were conducted prior to the identification of ST13122–24 or did not perform the molecular characterization required to identify ST13125–28. From a clinical point of view, due to its association with antibiotic resistance, specific risk factors for acquisition of ST131 are of practical utility; other studies have reported that older age, nursing home residency, urinary tract infections within 30 days, recent hospitalization (i.e. <3 months), and recent exposure to antimicrobial agents29–32 are independent predictors of infection with ST131. A profile of such risk factors, taking into account local microbiology and resistance profiles, can help inform appropriate and timely empirical treatment of infected patients.
To address the paucity of data in Southeast Asia and to discover risk factors for infection with ST131 E. coli, we undertook a multi-national, multi-centre study of E. coli bacteremia cases in Southeast Asia. As in other geographical areas, we found that ST131 E. coli was the most common sequence type of E. coli causing such infections, and we therefore focused on clinical risk factors and phenotypic resistance profiles associated with ST131 infections. We performed genome sequencing on all strains and examined the resistance genes, virulence factors, and phylogenetic relatedness of the ST131 strains. As expected, we found ST131 strains from all three major clades; interestingly, however, in Southeast Asia, C2/H30-Rx strains were all very closely related to each other; we refer to these strains as the SEA-C2 (Southeast Asia-C2) subclone of ST131. Remarkably, we found that the SEA-C2 subclone is solely responsible for the higher observed rates of invasive infection and ESBL-mediated antibiotic resistance associated with ST131 E. coli in Southeast Asia. We conclude that, for Southeast Asia, ST131 is dominated by a locally circulating clone, which may facilitate diagnosis and guide treatment of bacteremia patients in the region.
Methods
Study design and antimicrobial susceptibility testing
Consecutive non-duplicate bacteremic isolates were collected from five hospitals in Southeast Asia (Tan Tock Seng Hospital (TTSH), National University Hospital (NUH), Singapore General Hospital (SGH), Singapore; Thammasat University Hospital (TUH), Thailand; and University Malaya Medical Center (UMMC), Malaysia) and sent to participating clinical microbiological laboratories for antimicrobial susceptibility testing. Strains were collected in July 2015 in the Singapore hospitals and from August to November 2015 in the hospitals in Thailand and Malaysia. Minimum inhibitory concentrations were determined with the VITEK system or E-test for the following antimicrobial agents: amikacin, gentamicin, ampicillin, amoxicilin-clavulanate, piperacillin-tazobactam, cefazolin, ceftriaxone, ceftazidime, cefepime, ciprofloxacin, trimethoprim-sulfamethoxazole, ertapenem, imipenem, and meropenem. The results were interpreted according to either the European Committee on Antimicrobial Susceptibility testing (EUCAST) (http://www.eucast.org) or the Clinical and Laboratory Standards Institute (CLSI) standards33, according to each hospital’s routine practice. Of note, efforts have been made to harmonize guidelines34, and specifically for E. coli, agreement between EUCAST and CLSI guidelines is poor only for amikacin among the antibiotics we tested35. Isolates that demonstrated complete or intermediate resistance to a given antimicrobial agent were considered non-susceptible. E. coli multidrug resistance (MDR) was defined as resistance to one or more agents in three or more classes of tested drugs36. 40 isolates were requested from each participating hospital. Some patients had multiple isolates; in these cases, the first isolate was chosen for inclusion. In total, 185 strains were collected, from which ten were excluded because they were not the first isolate from the patient; one was excluded because it was classified as Klebsiella pneumoniae upon whole genome sequencing; and one was excluded because the isolate did not grow upon receipt. The final set analyzed thus consisted of 173 strains (40 from TTSH, 39 from NUH, 40 from SGH, 18 from TUH, and 36 from UMMC). A flowchart of the samples is shown in Fig. 1.
Figure 1.
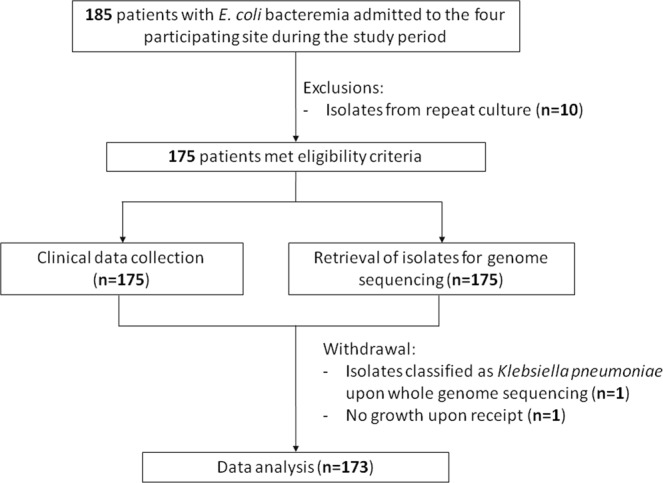
Flow chart of study samples. A flow chart for study enrollment and reasons for sample exclusion is shown.
Data collection and definitions
Data collection included patient demographics (age and gender), underlying comorbidities (Charlson’s comorbidity score37), onset of infection (e.g., community onset, healthcare associated, nosocomial associated), antimicrobial susceptibility profile, source of bacteremia, severity of illness (APACHE II score38), antibiotic treatment (e.g., empiric and definitive antibiotics, duration of regimen), and outcomes (e.g., clinical and microbiological cure, mortality, recurrence). Empiric antibiotics were defined as those given to patients in the first 48 hours before antimicrobial susceptibility data were available, and definitive antibiotics were defined as those guided by the results of antimicrobial susceptibility testing. Clinical cure was defined as resolution of signs and symptoms of infection within seven days. Microbiological cure was defined as documented clearance of E. coli bacteremia within 30 days for a subset of patients with repeat blood cultures. Mortality was defined as all cause mortality at 30 days. Recurrence was defined as the presence of E. coli bacteremia after microbiological cure within 30 days.
The definition of healthcare-associated bacteremia was derived from a study in 200239 with minor amendments. Community-acquired bacteremia was defined by a positive blood culture obtained at the time of hospital admission or within 48 hours after hospital admission for patients who did not fit the criteria for a healthcare-associated infection. Healthcare-associated infections were defined as: (i) a positive blood culture obtained from patients who had been hospitalized for 48 hours or longer; or (ii) a positive blood culture obtained at the time of hospital admission or within 48 hours of admission if the patient fulfilled any of the following criteria: hospitalized within 90 days before culture specimen collection; resident of a nursing home or long-term care facility (LTCF); received intravenous therapy at home within 30 days before the bacteremia; or received wound care, dialysis, and/or chemotherapy within 30 days before the bacteremia.
DNA extraction and sequencing library preparation
Each isolate was streaked to single colonies on LB-agar. A single colony was inoculated into Luria-Bertani broth (Gibco) and cultured overnight at 37 °C with agitation. Cells from 1 ml of this culture were collected by centrifugation at 14,000 × g for 1 minute. Genomic DNA was isolated from the resulting pelleted bacteria using the QIAamp DNA mini kit (Qiagen). DNA samples were quantified using a QUBIT 2.0 fluorometer (Invitrogen). Sequencing libraries were prepared with the Nextera XT Library Prep Kit (Illumina) according to the manufacturer’s instructions. The adapters were indexed using either the Nextera XT Index Kit or the Nextera XT Index Kit v2 (Illumina). Finally, 10 nM of each sample DNA sequencing library were pooled together (giving a final concentration of 10 nM of the aggregate pooled library) and sequenced on a HiSeq 4000 (Illumina) with a 2 × 151 run.
Long read sequencing for ST131-TTSH-ECO-10 was performed as part of the Singapore PoreCamp (http://porecamp.github.io/singapore/). Genomic DNA for ST131-TTSH-ECO-10 was mixed in equal proportions (on a mass basis) with genomic DNA from two other bacterial strains with estimated GC content higher and lower than E. coli (which is approximately 50%). A sequencing library was prepared from this DNA mixture using a Rapid Sequencing Kit (SQK-RAD004) and sequenced on a FLO-MIN107 flow cell using a MinION Mk1 device, with MinKNOW v2.2. Basecalling was performed with Albacore v2.2.7.
Long read sequencing for ST131-TTSH-ECO-16 was performed using a Ligation Sequencing Kit 1D (SQK-LSK108) for library preparation and a FLO-MIN107 flow cell on a GridION running MinKNOW v2.2, with basecalling using Guppy v1.8.5.
Genome sequence analysis
Raw FASTQ reads were used to call resistance genes, virulence factors, and (MLST) using SRST2 (version 0.2.0)40 with default settings. The ARGAnnot database41 supplied with SRST2 was used to identify resistance genes. For virulence factors, we used the VFDB database42, processed as recommended by the SRST2 documentation. The Achtman scheme was used to assign MLSTs43. Publicly available sequence data was downloaded from the Genbank Short Read Archive. A random sample of the Illumina data sets annotated as E. coli as of November 11, 2017 were downloaded and processed identically as described above (only half were used due to data size limitations). Metadata (strain name and country of isolation) were obtained from Genbank using the EDirect utilities (https://ftp.ncbi.nlm.nih.gov/entrez/entrezdirect/). Countries were classified into regions according to the United Nations Geographic Regions scheme (https://unstats.un.org/unsd/methodology/m49/). In total, 10,088 (of 17,262) E. coli short read data sets were downloaded. In addition, 2200 whole genome sequences from the Genbank RefSeq database (all sequences annotated as E. coli as of April 26, 2016) were downloaded. These were processed using the same databases mentioned above for resistance genes, virulence factors, and MLST, but the assemblies were processed using a custom BLASTN-based allele caller instead of SRST2. In total we had 1013 ST131 strains included in this analysis (36 from this study; 140 RefSeq assemblies; 837 public short read data sets).
To create phylogenetic trees, we used a reference-based analysis. The chromosome (excluding plasmids) of the E. coli ST131 strain EC958 genome44 was used as the reference. FASTQ files were mapped using bwa (version 0.7.10)45; indel realignment and SNP (single nucleotide polymorphism) calling was performed using Lofreq* (version 2.1.2) with default parameters46. An overall phylogenetic tree (for all 1013 strains) was made by calculating a dissimilarity matrix using SNPRelate47 and inferring a neighbor-joining tree using APE (version 3.5)48. Approximately maximum likelihood phylogenetic trees were inferred for smaller subsets of strains (<100); these were created using FastTree 2.1.8 with the –gtr and –nt command line options49. All phylogenetic trees were visualised with GGTREE 3.250. All R packages were run in R (3.2.2) (https://www.R-project.org). Delineation of ST131 clades A, B, C1, and C2 were done based on matching strain identifiers with two previous reports12,15; there were no ambiguities in the topology (i.e. all clade A strains from the previous two papers were also phylogenetically closely placed in our trees, and not mixed in with other B, C1, or C2 strains, and similarly for strains from the other clades).
Plasmid analysis
ST131-TTSH-ECO-10 was assembled with canu v1.351 with the genomeSize = 15 m (due to its mixture with two other strains) and -nanopore-raw parameters. Assembled sequences belonging to ST131-TTSH-ECO-10 were identified by mapping the Illumina data to the final assembly, which resulted in only two assembled contigs of 5,160,494 and 181,654 nt. These two contigs were polished with pilon v1.2252 (with default parameters) using the Illumina data, and the 183 kb contig (after polishing) was then used for subsequent analysis.
ST131-TTSH-ECO-16 was assembled using a hybrid strategy with ONT and Illumina reads using Unicycler v0.4.753. This resulted in two large assembled contigs of 5,268,201 and 232,458 nt and four additional small contigs less than 5 kb each. The four small contigs were ignored, and the 232 kb contig was used in subsequent analysis.
The circular plasmid map was created using BRIG54. The default blast parameters for BRIG were used. The bar graph for Fig. 2A and the homology plots for Fig. S1 were generated using custom scripts. In brief, all strains were assembled using velvet v1.2.1055 with the VelvetOptimiser v2.2.4 helper script (https://github.com/tseemann/VelvetOptimiser). The resulting assemblies were analyzed with blastn v2.2.28+56 with default parameters, using the 232 kb pTTSH16 plasmid as a reference database. The bar graph in Fig. 2A represents the number of nucleotides in pTTSH16 that had any blast hit (all hits were >70% identity; >95% of the hits considered were >90% identity; and 89.3% of the hits considered were >99% identity). The plot of conservation in Fig. S1 represents the union of all blast hits reported for each strain to pTTSH16.
Figure 2.
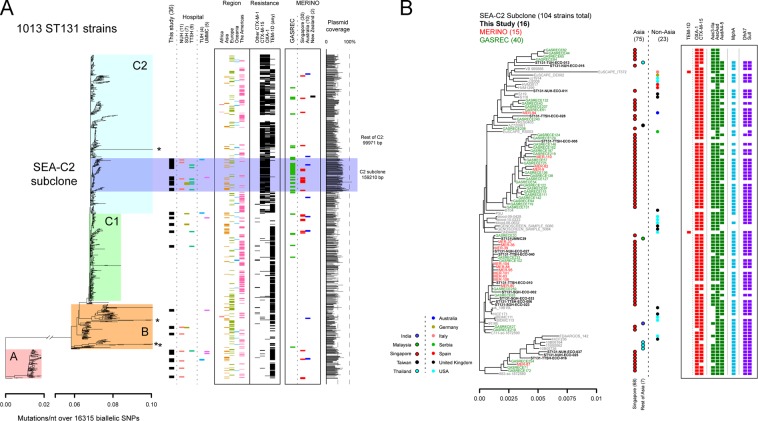
Phylogenomic analysis of ST131 strains (with removal of recombination). (A) Overview of all ST131 strains analyzed in this study. The tree on the left is an approximately maximum-likelihood tree, with mutations indicated on the x-axis at the bottom. Colored boxes indicate different subsets of the ST131 strains. From left to right, bars indicate the new strains contributed by this study (black boxes), the hospital from which the strains in this study were obtained (colored boxes below the “Hospital” label), the WHO region from which the strain was isolated (if available), resistance gene predictions for selected beta-lactamase genes, and the strains included from the GASREC and MERINO studies (with country of origin for MERINO strains). At the far right, the bar graph represents the percentage of the pTTSH16 plasmid that is covered by the assembly for each strain (based on blastn). Average plasmid coverage values for selected subsets of strains are indicated. (B) Expanded view of the SEA-C2 clone. Strains from this study, MERINO, or GASREC are indicated by font size and color; gray labels indicate other public data sets. Country of origin is indicated by the colored circles, with strains from Asia on the left with black outlines and strains from non-Asian areas on the right. Resistance gene predictions for each strain are indicated on the right with colored boxes; each class of resistance gene is in a separate color, with the gene indicated at the top.
Conjugation-related genetic elements were predicted using oriTfinder57. The assemblies above were used in the web application. As a control, oriTfinder was used to predict conjugation-related genes in pEC95858, in which a single relaxase, a single Type IV coupling protein, and one Type IV secretion system locus were found.
Phage sequences were predicted using the PHASTER web tool59. Intact phage sequences were extracted from the summary and matched based on the given phage names and manual BLASTN analysis, which had perfect concordance.
Statistical analysis
Comparisons between ST131 and non-ST131 isolates were evaluated using Chi square and Fisher exact tests. All tests were 2-sided. For comparisons of virulence factor prevalence, P-values were corrected by the Benjamini and Hochberg method60. P values <0.05 were considered statistically significant. The data were analyzed with STATA version 15 (Stata Statistical Software: Release 11, StataCorp LP, College Station, TX) and R (3.4.1).
Ethics approval and consent to participate
The study was approved by the NHG Domain Specific Review Board (ref no: 2015/00777) in Singapore, UMMC Medical Ethics Committee (MED ID No 20158-1545) in Malaysia, and TUH Institutional Review Board in Thailand for the respective the study sites. All patients provided informed consent in accordance with the relevant regulations of the respective approval boards in each country. For patients under the age of 18, informed consent was obtained from a parent and/or legal guardian as per the relevant guidelines/regulations of the respective approval boards in each country. All research described was performed in accordance with relevant guidelines/regulations of the respective approval boards in each country.
Results
Collection of isolates
By collecting all bacteremia cases, regardless of resistance profiles, we were able to assess the association of MLST types with resistance profiles. A total of 5 hospitals in Southeast Asia participated by sending their first 40 consecutive E. coli bacteremia isolates. A total of 185 strains were sequenced using the Illumina platform; after excluding duplicate isolates from the same patient and eliminating mismatches in classification, we had a final data set of 173 sequenced E. coli bacteremia strains, with each strain representing a unique patient.
Clinical features
Of the 173 patients, 77 (44.5%) were male, with a median age of 68 years (range 1–92). The median Charlson’s comorbidity score was 5 (0–13) and the median APACHE II score was 18 (2–58). Onset of bacteremia was community-associated in 81 patients (46.8%), leaving 92 that were healthcare-associated (53.2%). The top three sources of bacteremia were urinary (79 [45.7%]), primary (44 [25.4%]), and intra-abdominal (37 [21.4%]). Clinical cure was documented for 152 patients (87.9%), 30-day recurrence in 6 (3.47%), and 30-day mortality in 27 (15.6%). ST131 infections were similar to non-ST131 infections in terms of demographics, comorbidities, source of bacteremia, and clinical outcomes (Table 1).
Table 1.
Patient clinical parameters, aggregated based on genomic analysis of infecting bacteria.
| Non-ST131 (137 strains, set A) |
ST131 (36 strains, set B) |
SEA-C2 clone (16 strains, set C) |
ST131, not SEA-C2 (20 strains, set D) |
All strains (173 strains) |
P value (A vs B) |
P-value (C vs D) |
P-value (A vs D) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | ||||||
| Demographics | |||||||||||||
| Age (years, median) | 70 | (1–96) | 67.5 | (0.25–91) | 71 | (53–91) | 66.5 | (1–96) | 69 | (0.25–96) | 0.676 | 0.213 | 0.717 |
| Gender (male) | 64 | 46.72% | 13 | 36.11% | 6 | 37.50% | 7 | 35.00% | 77 | 44.51% | 0.266 | 1.000 | 0.349 |
| Comorbidities | |||||||||||||
| Charlson’s comorbidity score | 5 | (0–13) | 6 | (0–12) | 5.5 | (1–10) | 6 | (0–12) | 5 | (0–13) | 0.187 | 0.979 | 0.339 |
| Diabetes | 67 | 48.91% | 15 | 41.67% | 8 | 50.00% | 7 | 35.00% | 82 | 47.40% | 0.460 | 0.500 | 0.338 |
| Renal disease | 30 | 21.90% | 8 | 22.22% | 4 | 25.00% | 4 | 20.00% | 38 | 21.97% | 1.000 | 1.000 | 1.000 |
| Solid tumor | 26 | 18.98% | 9 | 25.00% | 3 | 18.75% | 6 | 30.00% | 35 | 20.23% | 0.485 | 0.700 | 0.247 |
| Cerebral vascular disease | 23 | 16.79% | 5 | 13.89% | 1 | 6.25% | 4 | 20.00% | 28 | 16.18% | 0.803 | 0.355 | 0.752 |
| Acute myocardial infarction | 15 | 10.95% | 5 | 13.89% | 2 | 12.50% | 3 | 15.00% | 20 | 11.56% | 0.571 | 1.000 | 0.705 |
| APACHE 2 (median, range) | 18 | (2–58) | 16 | (6–34) | 15 | (11–33) | 18 | (6–34) | 18 | (2–58) | 0.460 | 0.620 | 0.778 |
| Source of infection | |||||||||||||
| Community | 73 | 53.28% | 8 | 22.22% | 1 | 6.25% | 7 | 35.00% | 81 | 46.82% | 0.001* | 0.053 | 0.154 |
| Healthcare associated | 64 | 46.72% | 28 | 77.78% | 15 | 93.75% | 13 | 65.00% | 92 | 53.18% | |||
| Source of bacteraemia | |||||||||||||
| Urinary | 61 | 44.53% | 18 | 50.00% | 8 | 50.00% | 10 | 50.00% | 79 | 45.66% | 0.578 | 1.000 | 0.811 |
| Unknown (Primary) | 35 | 25.55% | 9 | 25.00% | 4 | 25.00% | 5 | 25.00% | 44 | 25.43% | 1.000 | 1.000 | 1.000 |
| Intraabdominal | 30 | 21.90% | 7 | 19.44% | 3 | 18.75% | 4 | 20.00% | 37 | 21.39% | 0.823 | 1.000 | 1.000 |
| Pulmonary | 9 | 6.57% | 3 | 8.33% | 1 | 6.25% | 2 | 10.00% | 12 | 6.94% | 0.716 | 1.000 | 0.633 |
| Central venous catheter | 2 | 1.46% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 2 | 1.16% | 1.000 | NA | 1.000 |
| Central nervous system | 1 | 0.73% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1 | 0.58% | 1.000 | NA | 1.000 |
| Others | 6 | 4.38% | 2 | 5.56% | 1 | 6.25% | 1 | 5.00% | 8 | 4.62% | 0.672 | 1.000 | 1.000 |
| Risk factors | |||||||||||||
| Nursing home | 4 | 2.92% | 4 | 11.11% | 3 | 18.75% | 1 | 5.00% | 8 | 4.62% | 0.059 | 0.303 | 0.499 |
| Recent hospitalisation within 90 days | 49 | 35.77% | 25 | 69.44% | 13 | 81.25% | 12 | 60.00% | 74 | 42.77% | 0.001* | 0.277 | 0.049* |
| Antibiotic exposure within 90 days | |||||||||||||
| Carbapenems | 6 | 4.38% | 6 | 16.67% | 5 | 31.25% | 1 | 5.00% | 12 | 6.94% | 0.019* | 0.069 | 1.000 |
| Piperacillin-tazobactam | 14 | 10.22% | 6 | 16.67% | 4 | 25.00% | 2 | 10.00% | 20 | 11.56% | 0.377 | 0.374 | 1.000 |
| Broad-spectrum cephalosporins | 14 | 10.22% | 11 | 10.22% | 7 | 43.75% | 4 | 20.00% | 25 | 14.45% | 0.006* | 0.159 | 0.251 |
| Fluoroquinolones | 13 | 9.49% | 10 | 27.78% | 7 | 43.75% | 3 | 15.00% | 23 | 13.29% | 0.010* | 0.073 | 0.433 |
| Cotrimoxazole | 5 | 3.65% | 1 | 2.78% | 1 | 6.25% | 0 | 0.00% | 6 | 3.47% | 1.000 | 0.444 | 1.000 |
| Aminoglycosides | 4 | 2.92% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 4 | 2.31% | 0.581 | NA | 1.000 |
| ICU admission prior to bacteraemia | 7 | 5.11% | 2 | 5.56% | 2 | 12.50% | 0 | 0.00% | 9 | 5.20% | 1.000 | 0.190 | 0.596 |
| Dialysis within 30 days | 3 | 2.19% | 1 | 2.78% | 0 | 0.00% | 1 | 5.00% | 4 | 2.31% | 1.000 | 1.000 | 0.423 |
| Surgery within 30 days | 7 | 5.11% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 7 | 4.05% | 0.347 | NA | 0.596 |
| Central venous catheter within 30 days | 13 | 9.49% | 2 | 5.56% | 0 | 0.00% | 2 | 10.00% | 15 | 8.67% | 0.740 | 0.492 | 1.000 |
| Urinary catheter within 30 days | 23 | 16.79% | 12 | 33.33% | 4 | 25.00% | 8 | 40.00% | 35 | 20.23% | 0.036* | 0.481 | 0.030* |
| History of urinary infection | 10 | 7.30% | 11 | 30.56% | 6 | 37.50% | 5 | 25.00% | 21 | 12.14% | 0.001* | 0.483 | 0.026* |
| Immunosuppression | 48 | 35.04% | 13 | 36.11% | 5 | 31.25% | 8 | 40.00% | 61 | 35.26% | 1.000 | 0.731 | 0.803 |
| Outcomes | |||||||||||||
| Clinical cure | 120 | 87.59% | 32 | 88.89% | 15 | 93.75% | 17 | 85.00% | 152 | 87.86% | 1.000 | 0.613 | 0.723 |
| Microbiological cure | 107 | 78.10% | 30 | 83.33% | 15 | 93.75% | 15 | 75.00% | 137 | 79.19% | 0.646 | 0.196 | 0.776 |
| 30-day recurrence | 5 | 3.65% | 1 | 2.78% | 0 | 0.00% | 1 | 5.00% | 6 | 3.47% | 1.000 | 1.000 | 0.565 |
| 30-day mortality | 24 | 17.52% | 3 | 8.33% | 1 | 6.25% | 2 | 10.00% | 27 | 15.61% | 0.208 | 0.686 | 0.532 |
*Indicates P < 0.05 (uncorrected); these are also set in bold font.
Prevalence of ST131 in Southeast Asia
We used the sequencing data to infer MLST types. We found 56 distinct STs among the 173 isolates. Six of these STs accounted for 103 (59.5%) of 173 isolates. ST131 was the most common single ST, accounting for 36 (20.8%) of the isolates, followed by ST95 (23 isolates (13.3%)) and ST69 (16 isolates (9.2%)). The rest of the top 6 were ST38 with 10 (5.8%), ST1193 with 10 (5.8%), and ST73 with 8 (4.6%) isolates. We focused our subsequent analyses on the ST131 strains, as they represented the most common sequence type.
One subclone of the C2 clade of ST131 accounts for nearly half of all ST131 in Southeast Asia
We analyzed the 36 ST131 strains in a global context using approximately half of the publicly available E. coli whole genome sequence data present in Genbank as of November 11, 2017. Metadata regarding the geographical location for each strain were available for 698/1013 strains; excluding the strains sequenced as part of this study (leaving 977 strains), isolates from Asia only represented 15.4% of all ST131 isolates (7.1% if the recent published GASREC (Genetic determinants of antimicrobial resistance and its impact on clinical response of bacteremia due to 3rd generation cephalosporin resistant E. coli and K. pneumonia)61,62 and MERINO (Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp.)21,63 studies are excluded, see below), highlighting the generally lower sampling of strains from Asia.
Among the 36 new ST131 strains isolated in this study, all of the major ST131 clades (A, B, C1, and C2) were represented (Fig. 2A). As previously reported, the ESBL phenotype correlated with the presence of the CTX-M-15 gene in clade C2 strains, while CTX-M-9 genes were more common in non-C2 strains. While not an ESBL, the TEM-1D beta-lactamase is also prevalent among ST131 strains. Of further interest, we found that the OXA-1 ESBL was frequently also found in strains carrying CTX-M-15.
Interestingly, 16/36 of our ST131 strains all clustered relatively closely in a subclade of C2 strains. The branch leading to this subclade had a 94.2% bootstrap support, suggesting a monophyletic origin. Closer examination of this subclade (highlighted in dark blue in Fig. 2A; expanded view in Fig. 2B) suggested that many of these strains were isolated from Asia. Strains from this subclade mostly carried CTX-M-15 and OXA-1 ESBLs and were conspicuous for the low prevalence of TEM-1D. Interestingly, it has been noted that the introduction of CTX-M ESBLs into a geographic area tends to supplant existing TEM-class beta-lactamases64.
To verify that this subclade of C2 was truly overrepresented among ST131 strains from Asia, we examined the recently published GASREC data set of E. coli ceftriaxone-resistant bacteremia isolates, all from Singapore61. This data set included 124 total strains, of which 80 were E. coli; of these 80 E. coli, 57/80 (71.3%) were ST131 (including one single locus variant). We also examined the raw sequencing data for E. coli strains collected in the MERINO study, which included ESBL E. coli and K. pneumoniae strains from Singapore, Australia, and New Zealand21. Among the MERINO E. coli strains, 42/66 (63.6%) were ST131. Most of these 42 strains were from Singapore (30/42, 71.4%), with the rest from Australia (10) and New Zealand (2).
Overall, we found strong evidence for an overrepresentation of strains from Asia, and in particular from Singapore, within this subclade of C2 strains. This C2 subclade contained 75/186 (40.3%) of the strains from Asia, compared to 23/512 (4.5%) of the non-Asian strains (p < 2.2e-16, 2-tailed Fisher’s exact test). Even after removing all strains annotated as being from Singapore (including those from this study and in the publicly downloaded data sets), the C2 subclade contained 30 strains total, of which 7 were from Asia (23.3%); in contrast, the rest of the C2 clade contained 225 strains, of which 19 were from Asia (8.4%, p = 0.02033, 2-tailed Fisher’s exact test).
Among the collections with a strong Singaporean representation, the C2 subclade accounted for 16/36 (44.4%) of this study’s ST131 strains; 40/57 (70.1%) of the GASREC ST131 strains; and 14/42 (33.3%) of the MERINO ST131 strains. Only this study included strains from other countries in Southeast Asia, from which we found one strain each from Malaysia and Thailand (only one hospital from each of these countries was included). We therefore hereafter refer to strains within this C2 subclade as belonging to the “SEA-C2 clone”, which represents a previously undefined subset of C2 strains.
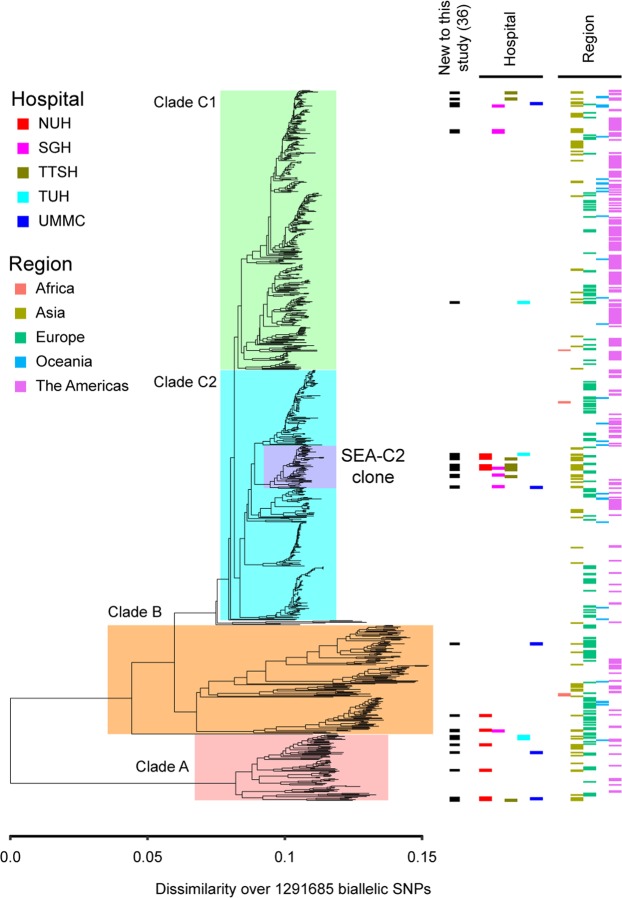
The above analysis, in which we found the SEA-C2 strains to be closely related and forming an apparently monophyletic subclone of the C2 clade, was performed after removing the recombination regions identified by Petty, et al.12; this reduced the phylogenetic analysis to 16,315 SNPs. Including all genomic regions, even when they may have undergone recombination, has the potential to alter the phylogenetic relationships as recombinant regions confound the “true” phylogenetic signal derived from vertically inherited sequence differences. It may also enhance the appearance of relatedness among strains if they share the same recombinant sequences. A neighbor-joining tree constructed from SNP distances (including all genomic regions) agreed with the previous result: the SEA-C2 clone strains were very closely related to each other, and they appeared to have a monophyletic origin (Fig. 3).
Figure 3.
Phylogenomic analysis of ST131 strains (without removing recombination). A neighbor joining tree with all SNP positions is shown. Major clades of ST131 are highlighted by colored boxes. The SEA-C2 clone is highlighted in purple. From left to right, bars indicate the new strains contributed by this study (black boxes), the hospital from which the strains in this study were obtained (colored boxes below the “Hospital” label), and the WHO region from which the strain was isolated (if available). The SEA-C2 clone is still monophyletic in this analysis.
The SEA-C2 clone is solely responsible for the higher beta-lactam resistance associated with ST131 in Southeast Asia
As expected, the ST131 strains isolated in this study were, overall, more antibiotic resistant than the non-ST131 strains. (Of note, a very low rate of carbapenem resistance (one non-ST131 strain) was found in this study).
ST131 isolates exhibited significantly higher prevalence of resistance (based on antibiograms) to beta-lactam, aminoglycoside, and fluoroquinolone antibiotics than non-ST131 strains (Table 2, compare sets A and B). Strains from the SEA-C2 clone were also highly resistant to these same antibiotics (Table 2, set C). Surprisingly, when SEA-C2 strains were excluded, the remaining ST131 strains were not significantly different in resistance to non-ST131 strains (Table 2, sets A and D).
Table 2.
Antibiotic resistance phenotypes and resistance gene presence, aggregated based on genomic analysis of infecting bacteria.
| Antibiotic | Non-ST131 (137 strains, set A) |
ST131 (36 strains, set B) |
SEA-C2 clone (16 strains, set C) |
ST131, not SEA-C2 (20 strains, set D) |
P value (A vs B) | P-value (C vs D) | P-value (A vs D) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | Number | Percentage | Number | Percentage | ||||
| Ampicillin | 89 | 65.0% | 29 | 80.56% | 15 | 93.75% | 14 | 70.00% | 0.107 | 0.104 | 0.803 |
| Amoxicilin-clavulanate | 28 | 20.4% | 16 | 44.40% | 13 | 81.25% | 3 | 15.00% | 0.005* | 0.000* | 0.766 |
| Piperacillin-tazobactam | 9 | 6.6% | 6 | 16.67% | 5 | 31.25% | 1 | 5.00% | 0.089 | 0.069 | 1.000 |
| Cefazolin/Cefalexin | 48 | 35.0% | 22 | 61.11% | 13 | 81.25% | 9 | 45.00% | 0.007* | 0.041* | 0.457 |
| Ceftriaxone | 29 | 21.2% | 21 | 58.33% | 13 | 81.25% | 8 | 40.00% | 0.000* | 0.019* | 0.088 |
| Ceftazidime | 24 | 17.5% | 18 | 50.00% | 11 | 68.75% | 7 | 35.00% | 0.000* | 0.078 | 0.077 |
| Cefepime | 22 | 16.1% | 20 | 55.56% | 12 | 75.00% | 8 | 40.00% | 0.000* | 0.049* | 0.028* |
| Imipenem | 1 | 0.7% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1.000 | 1.000 | 1.000 |
| Meropenem | 1 | 0.7% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1.000 | 1.000 | 1.000 |
| Ertapenem | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 0.000* | 1.000 | 1.000 |
| Gentamicin | 17 | 12.4% | 15 | 41.67% | 11 | 68.75% | 4 | 20.00% | 0.000* | 0.006* | 0.313 |
| Amikacin | 4 | 2.9% | 4 | 11.11% | 4 | 25.00% | 0 | 0.00% | 0.059 | 0.031* | 1.000 |
| Ciprofloxacin | 34 | 24.8% | 25 | 69.44% | 15 | 93.75% | 10 | 50.00% | 0.000* | 0.009* | 0.031* |
| Co-trimoxazole | 61 | 44.5% | 20 | 55.6% | 12 | 75.00% | 8 | 40.00% | 0.264 | 0.049* | 0.811 |
| Broad-spectrum cephalosporins | 30 | 21.9% | 21 | 58.3% | 13 | 81.25% | 8 | 40.00% | 0.000* | 0.019* | 0.095 |
| MDR resistance | 48 | 35.0% | 24 | 66.7% | 14 | 87.50% | 10 | 50.00% | 0.000* | 0.032* | 0.220 |
| Resistance Gene | |||||||||||
| CTX-M-15 | 4 | 2.92% | 14 | 38.89% | 12 | 75.00% | 2 | 10.00% | 0.000* | 0.000* | 0.169 |
| Other CTX-M | 14 | 10.22% | 6 | 16.67% | 0 | 0.00% | 6 | 30.00% | 0.377 | 0.024* | 0.024* |
| OXA-1 | 8 | 5.84% | 14 | 38.89% | 14 | 87.50% | 0 | 0.00% | 0.000* | 0.000* | 0.393 |
| OXA-48 | 1 | 0.73% | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 1.000 | 1.000 | 1.000 |
| TEM-1D | 71 | 51.82% | 11 | 30.56% | 0 | 0.00% | 11 | 55.00% | 0.000* | 0.001* | 0.816 |
| Other beta-lactamase^ | 9 | 6.57% | 2 | 5.56% | 1 | 6.25% | 1 | 5.00% | 1.000 | 1.000 | 1.000 |
* indicates P < 0.05 (uncorrected); these are also set in bold font.
^Includes AmpH, CMY, DHA.
These results for phenotypic antibiotic resistance were mirrored in our genomic analysis. Among the 173 E. coli isolates, 41 (24.7%) of E. coli isolates carried a gene encoding a CTX-M enzyme, most commonly CTX-M-15 (18/173, 10.4%) followed by CTX-M-27 (13/173, 7.5%). The gene encoding CTX-M-15 was significantly more prevalent among ST131 isolates (14/36, 38.9%) than non-ST131 isolates (4/137, 2.9%; p = 4.626e-08, 2-tailed Fisher’s exact test).
Numerous studies have associated CTX-M-15 with the C2 clade of ST131. Among the 36 ST131 strains in this study, CTX-M-15 was common and also mostly accounted for by C2 strains, all but one of which was in the SEA-C2 clone. We also found a higher prevalence of the OXA-1 gene among our ST131 strains. Interestingly, when we excluded the SEA-C2 strains, the remaining ST131 strains no longer had a significant enrichment of either CTX-M-15 or OXA-1 genes compared with non-ST131 strains (Table 2).
The TEM-1D beta-lactamase is neither an ESBL nor a carbapenemase, and therefore has not been a major focus of attention in previous ST131 studies. Intriguingly, we found that SEA-C2 strains generally do not carry the TEM-1D gene; the only exceptions are two strains that were isolated from the US and Europe. Similar patterns of antibiotic resistance gene presence/absence were observed in the GASREC (39/39 with CTX-M-15; 0/39 with TEM-1D) and MERINO (15/15 with CTX-M-15; 0/15 with TEM-1D) SEA-C2 strains.
Strains in the SEA-C2 clone have a conserved plasmid
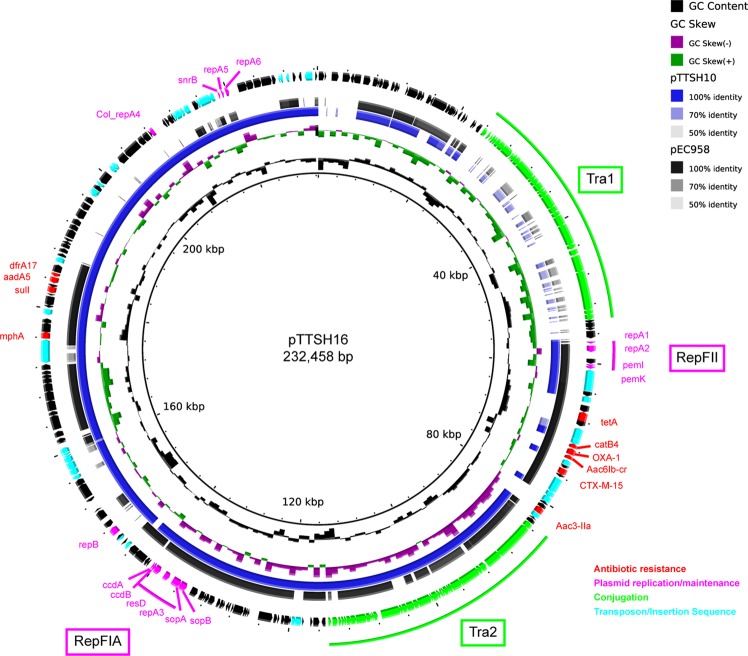
We used long-read sequencing on two representative strains, ST131-TTSH-ECO-10 and ST131-TTSH-ECO-16, to definitively identify plasmid sequences. These strains differed in their beta-lactamase gene content; ST131-TTSH-ECO-10 lacked the CTX-M-15 and OXA-1 genes, while ST131-TTSH-ECO-16 had them (most of the SEA-C2 strains were similar to ST131-TTSH-ECO-16 in this respect) (Fig. 2B). We assembled 183,189 bp and 232,458 bp circular plasmids, respectively, designating them pTTSH10 and pTTSH16. Both plasmids appeared to have two copies of IncF replication sequences (denoted RepFIA and RepFII based on >97% blastn identity to the corresponding sequences in pEC958)58. They also carried four resistance genes in common: aadA5 (aminoglycoside), drfA17 (trimethoprim), mphA (macrolides), and sulI (sulfa). In addition, the pTTSH16 plasmid carried 6 additional resistance genes: aac3-IIa and aac6Ib-cr (aminoglycosides); catB4 (chloramphenicol); CTX-M-15 and OXA-1 (ESBLs); and tetA (tetracycline). The online prediction tool oriTfinder (which predicts conjugation-related genes) identified a relaxase and Type IV conjugation protein in both pTTSH10 and pTTSH16. There was one type IV secretion system (T4SS) cluster in pTTSH10 but two T4SS clusters in pTTSH16, as seen in Fig. 4. Finally, an oriT transfer origin was only identified in pTTSH16.
Figure 4.
Similarity of SEA-C2 plasmids to the pEC958 ST131 plasmid. From inner to outer circle, the following are indicated: GC content, GC skew, pTTSH10, pEC958, gene annotation, and labels of selected genes/loci. The rings representing pTTSH10 and pEC958 consist of a colored bar indicating the sequence identity to pTTSH16 (used as the reference). Areas with less than 50% identity are considered not present and indicated by the absence of a colored bar in that section of the ring, as indicated by the legend at the top right. Genes with certain functional annotations are indicated in color, as indicated by the legend at the bottom right. Gene names for resistance genes and plasmid replication genes are indicated; loci containing conjugation-related genes and clusters of replication genes are annotated with a colored line and a boxed label.
Using the larger pTTSH16 plasmid as a reference, pTTSH10 shared 72.7% of the same sequence (using a cutoff of >90% nucleotide identity), with the major difference being the absence (in pTTSH10) of ~40 kb containing genes encoding a second set of conjugation-related proteins. Both of these plasmids are also similar to another well-characterized large pEC958 plasmid from the ST131 C2 strain EC958 (Fig. 4). The pEC958 plasmid showed the most difference in the two ~40 kb regions encoding conjugation machinery in pTTSH16.
Given the overlap in resistances identified in SEA-C2 strains with genes encoded on pTTSH16 (namely, CTX-M-15, OXA-1, aadA5, aac3-IIa, aac6Ib-cr, dfrA17, and sulI), we expected that most of the SEA-C2 strains would carry a similar plasmid. Using blastn to assess the coverage of pTTSH16 in assemblies of all the ST131 strains, we indeed found that the SEA-C2 strains had a significantly higher coverage of pTTSH16 (median 159,210 bp) compared with the rest of the C2 clade (99,971 bp) or all ST131 strains (98,912 bp) (Figs 2A and S1). We therefore conclude that the similar resistance profile among the SEA-C2 strains, particularly for ESBL genes, is driven by this conserved plasmid.
The SEA-C2 clone is responsible for the higher virulence of ST131
ST131 strains have been reported to be proficient at causing UTI and bacteremia9,65. Our results are also consistent with generally higher virulence of ST131 strains. Similar to previous studies29–32, using a univariate analysis, we found the following clinical risk factors were associated with infection by an ST131 strain: healthcare-associated bacteremia; prior hospitalization within 90 days; urinary catheterization within 30 days; a history of urinary tract infections within 30 days before culture specimen collection; and recent exposure to carbapenems, piperacillin-tazobactam, or fluoroquinolones (Table 1). As seen with antibiotic resistances, when we removed strains from the SEA-C2 clone, we found no differences in any clinical parameters between patients infected by ST131 and non-ST131 strains.
SEA-C2 strains have a similar virulence factor profile to other clade C2 strains
Given that SEA-C2 strains are driving the association between ST131 and virulence, we examined these strains for any associated virulence factor differences. We found several genes that are found together on a common ExPEC pathogenicity island, PAI-II66–68: pap genes encoding P pili; hlyA encoding a hemolysin; cnf encoding the cytotoxic necrotizing factor toxin; and the tia/hek outer membrane protein. These genes might be involved in the pathogenesis of the SEA-C2 clone; alternatively, the close relatedness of the SEA-C2 strains may mean this enrichment is due to a shared phylogenetic history. Indeed, overall, the pattern of virulence factor presence in the SEA-C2 clone was generally quite similar to that for other C2 ST131 strains (Fig. S2).
Looking within the SEA-C2 strains, we found that the hlyABCD operon, cnf1, and tia/hek genes were also unevenly distributed, and enabled differentiation of even further subdivisions of the SEA-C2 clone. One additional virulence factor, the cdiA/B contact-dependent inhibition system, was also significantly different among the subgroups of the SEA-C2 clone. These virulence factors were not uniformly conserved among the strains from Southeast Asia newly reported here. Therefore, while these genes are potential contributors to the expansion of the SEA-C2 strains in the region, the other possibility that their nonuniform distribution is the result of gene gain/loss events amplified by clonal expansion must be considered.
We also examined whether a unique phage profile might be associated with SEA-C2 strains. Using 4 complete or nearly complete SEA-C2 genomes (ST131-TTSH-ECO-10, ST131-TTSH-ECO-16, BIDMC111 (GCF_001030625.1), and BIDMC113 (GCF_001030675.1)) as well as a closely related non-SEA-C2 genome (BWH55 (GCF_001030365.1)), we found a largely conserved phage repertoire. Five intact phage loci were identified in common in all of these strains. Among the SEA-C2 strains, one additional phage was in common (the closest match was Enterobacteria phage mEp460, Genbank accession NC_019716, length 44.5 Kb). The SEA-C2 phage averaged 97.4% nucleotide identity over 29.6 Kb of the mEp460 sequence and contained intact genes encoding tail, protease, portal, terminase, lysin, and integrase proteins. The non-phage genes, which may have a role in virulence or fitness, encoded 6 hypothetical genes; methlyase and restriction nuclease proteins for the EcoRII restriction modification system; a DNA adenine methyltransferase; an arginyl-tRNA synthetase; a ClpP protease subunit; and a Lom outer membrane protein. All of these functions are also commonly found in other annotated Enterobacterial phages.
Discussion
The recent global spread of the ST131 clone of E. coli is a remarkable example of the rapid expansion of a successful pathogen3,8,9. ST131 is made up of four predominant clades or sublineages: A, B, C1, and C28,12,15. These can be differentiated by multiple genetic and some phenotypic methods, most accurately with whole genome phylogeny, specific gene allele differences (such as in fimH), and resistance gene profiles9,15,69. To date, the global success of ST131 has been largely due to the C1 and C2 clades, a pattern that has been verified by multiple investigators examining strains across multiple continents (see, for example9,70).
The bulk of data on ST131, however, remains confined to the Americas (dominated by the USA), Europe, and Oceania (mostly represented by Australia). Based on this broad international spread, it has been presumed that other geographical regions would show a similar pattern of ST131 prevalence23; this hypothesis has largely been validated as data has become available in Asia, Africa, the Middle East, and South America9. However, these other regions remain relatively undersampled, and the possibility remains for distinct patterns in the local microbiology. Indeed, hints of locally prevalent clones, possibly indicating local variation in selection pressures or transmission dynamics, have been reported for E. coli ST13171 as well as other bacteria (such as Shigella sonnei72, Streptococcus pneumoniae73, and Salmonella Typhi74).
In this work, the first international, prospective sampling of E. coli bloodstream isolates in Southeast Asia, we found that ST131, as in many other areas of the world, is the most prevalent sequence type of E. coli. We also found that the C2 clade of ST131 was responsible for most of the ST131 infections. Remarkably, in our study, all but one of the ST131 C2 strains were very closely related, forming a monophyletic subclone within C2 that we refer to as the SEA-C2 clone. As has been reported in other studies, the ST131 C2 strains were driving both higher virulence and higher antibiotic resistance rates among ST131 strains. Interestingly, removal of the SEA-C2 clone strains from our data set resulted in the other ST131 strains having no higher virulence or resistance than the non-ST131 E. coli strains captured in this survey.
The majority of our patients and strains were from Singapore, and therefore the large majority of SEA-C2 strains were also from Singapore. Furthermore, in two other surveys of E. coli isolates that have included Southeast Asia (the GASREC61 and MERINO21 trials), only Singaporean strains were included. In these two studies, the SEA-C2 clone again represented the great majority of the Singaporean C2 ST131 strains, but not of the Oceania strains from the MERINO study. In addition, by design, our study captured consecutive E. coli bloodstream isolates regardless of resistance pattern; in contrast, both the GASREC and MERINO trials included only bloodstream isolates that were resistant to 3rd-generation cephalosporins, and thus could not assess whether exclusion of the SEA-C2 clone strains also led to a similar rate of resistance between other ST131 strains and non-ST131 strains. Given previously published data on the overall resistance of H30-Rx/C2 strains in general8,9, however, we speculate that only within Singapore will the SEA-C2 clone be responsible for the higher resistance of ST131 strains overall. Finally, given the high representation of Singapore in this study, GASREC, and MERINO, the SEA-C2 clone is dominated by Singaporean isolates. Removing these from the data set, however, still leads to the result that strains isolated from Asia are overrepresented in the SEA-C2 clone.
While strains in the SEA-C2 clone were responsible for the association of ST131 with higher virulence and antibiotic resistance, the SEA-C2 clone itself seemed to be similar to other C2 strains overall in terms of these characteristics; indeed, virulence factor presence has been presumed to partially underlie the success of ST131, but no convincing examples have yet been described9. Clinically, ST131 infections in this study were associated with similar patterns of disease and risk factors to previous reports29–32: a history of urinary infections, healthcare-associated infections, and recent exposure to fluoroquinolones and third-generation cephalosporins. ST131 infections were three times more likely among healthcare-associated infections compared with community acquired infections; this contrasts with some early studies in which community acquired ST131 was more common13,31,75–78, but it agrees with other reports29,79,80. Healthcare-associated infection and a high Charlson’s score were independent risk factors for mortality in our study, similar to previous studies with mortality analysis32,81.
With respect to other genetic features of the SEA-C2 clone, we noted very similar antibiotic resistance and virulence factor profiles within the SEA-C2 clone, as would be expected for a closely related subclone of bacteria. The similarity in antibiotic resistance profiles was largely driven by a common plasmid found in SEA-C2 subclone strains, which is similar (with the exception of two conjugation-associated regions) to the well-described pEC958 multidrug resistance plasmid from the EC958 ST131 strain (itself a C2, but not SEA-C2, strain)44,58. Interestingly, nearly all strains in the SEA-C2 clone lacked a TEM-1D gene, which is common but not universally present in other C2 ST131 strains (of note, the TEM-1D gene is present on the pEC958 plasmid).
With respect to virulence factors, again there was high similarity among the SEA-C2 clone strains, which probably accounts for some of the genes being significantly overrepresented (supported by the close clustering of strains by genome-wide SNPs including potential recombination regions). Several of these genes are commonly found on a large pathogenicity island, called PAI-II, present in many ExPEC, particularly UPEC67,68. Of note, the pap gene locus is known to be important for strains to cause pyelonephritis, although the asymptomatic bacteriuria strain E. coli 83972 carries the pap operon and is used as a pre-emptive probiotic colonization strain in patients prone to urinary tract infection82. The hlyA hemolysin, in the context of urinary tract infection, is regulated by the Cpx stress response system and capable of inducing Caspase-1/Caspase-4 dependent inflammatory cell death in vitro; in vivo, overexpression of this toxin leads to rapid exfoliation of bladder epithelial cells, with the net effect of reducing bacterial burdens83. Expression of the Cnf1 toxin leads to a cytopathic effect in infected epithelial cells in cell culture, though there is conflicting data for its role during urinary tract infection in animal models84,85. Finally, the similar tia and hek adhesins are known to be important for invasion in intestinal pathogenic E. coli86,87 and NMEC88, respectively, but data for these has been limited to in vitro cell culture studies. Overall, while these virulence factors likely contribute to the virulence of strains that carry them, we suspect that their overrepresentation in the SEA-C2 clone (relative to the C2 strains in general) is probably due to the close phylogenetic relatedness of these strains. The fact that the SEA-C2 strains are similar to each other is consistent with a recent analysis of the accessory and regulatory genome regions of a diverse set of ST131 strains, which found multiple subtypes of C2 ST131 strains that share similar core and accessory genome features89. The SEA-C2 subclone differs, however, in that it appears to be more strongly geographically localized to SEA than the other C2 ST131 subtypes (which were noted to all be found in multiple continents and multiple host species)89.
Conclusions
Our study is notable for examining strains in an undersampled geographical region (Southeast Asia) and regardless of resistance profile. This allowed us to characterize both the local microbiology in the region and the relative resistance rates among bacteremia strains. We have found that, like in other regions, the C2 clade of ST131 is contributing to the prevalence of ST131 infections and their higher antibiotic resistance rates, particularly for ESBL phenotypes. However, our study highlights a unique and previously undefined subclone of C2 strains, the SEA-C2 clone, which is highly prevalent in Singapore and significantly enriched in Asia in general. Many studies have provided strong evidence for an overall paradigm of ST131 being a globally disseminated clone8,9,70,89; however, we now show that Asia, and Singapore in particular, has a strong local bias in the C2 ST131 strains present. Given that Singapore is a highly connected transport hub, a prominent tourist destination, and a leading center for medical tourism, we speculate that there is ample opportunity for globally disseminated C2 ST131 strains to enter Singapore. (Of note, in Singapore, the NDM-1 carbapenemase is also being driven by local transmission of a single plasmid among multiple Enterobacteriaceae90, despite a similar opportunity for import of international NDM-1 carrying strains.) Therefore, further studies of the dynamics of strain importation and local transmission will be required to understand the reasons behind the prevalence of the SEA-C2 clone within Singapore and the region.
Supplementary information
Acknowledgements
We thank Sarah C. Geiger for helpful comments and discussions on the manuscript; Balamurugan Periaswamy and the GERMS Platform at GIS for help with DNA purification and whole genome sequencing; and Varnica Khetrapal and the Singapore NanoPore Camp organizers and participants for help with long read sequencing. This work was supported by the National Medical Research Council, Ministry of Health, Singapore grant nos. NMRC/CIRG/1357/2013, NMRC/CIRG/1467/2017, and NMRC/OFIRG/0009/2016; the Genome Institute of Singapore (GIS)/Agency for Science, Technology, and Research (A*STAR); A*STAR grant no. IAF311018; and the Singapore Infectious Disease Clinical Research Network. The funding bodies had no role in the design of the study, the collection, analysis, and interpretation of data, and the writing of the manuscript.
Author Contributions
S.L.C., A.A. and D.C.L. designed the study. S.L.C., S.K., S.A., S.F.S.O., P.P.D., T.H.K., K.L.C., N.A., N.S., R.D.V., J.G.X.W. and D.C.L. collected data. S.L.C., D.Y., A.A. and D.C.L. analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Data Availability
All raw sequencing data described in this manuscript is available in the Genbank repository, under BioProject PJRNA488345.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Swaine L. Chen and Ying Ding contributed equally.
Contributor Information
Swaine L. Chen, Email: slchen@gis.a-star.edu.sg
David C. Lye, Email: david_lye@ttsh.com.sg
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49467-5.
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 3.Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect. Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 4.Pitout JDD. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front. Microbiol. 2012;3:9. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolas-Chanoine M-H, et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2008;61:273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 6.Coque TM, et al. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 2008;14:195–200. doi: 10.3201/eid1402.070350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau SH, et al. Major uropathogenic Escherichia coli strain isolated in the northwest of England identified by multilocus sequence typing. J. Clin. Microbiol. 2008;46:1076–1080. doi: 10.1128/JCM.02065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee R, Johnson JR. A new clone sweeps clean: the enigmatic emergence of Escherichia coli sequence type 131. Antimicrob. Agents Chemother. 2014;58:4997–5004. doi: 10.1128/AAC.02824-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicolas-Chanoine M-H, Bertrand X, Madec J-Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014;27:543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J. Infect. Dis. 2013;207:919–928. doi: 10.1093/infdis/jis933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price LB, et al. The epidemic of extended-spectrum-β-lactamase-producing Escherichia coli ST131 is driven by a single highly pathogenic subclone, H30-Rx. MBio. 2013;4:e00377–13. doi: 10.1128/mBio.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petty NK, et al. Global dissemination of a multidrug resistant Escherichia coli clone. Proc. Natl. Acad. Sci. USA. 2014;111:5694–5699. doi: 10.1073/pnas.1322678111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitout, J. D. D., Gregson, D. B. & Campbell, L. Molecular characteristics of extended-spectrum-β-lactamase-producing Escherichia coli isolates causing bacteremia in the Calgary Health Region from 2000 to 2007 …. Antimicrob. Agents Chemother (2009). [DOI] [PMC free article] [PubMed]
- 14.Stoesser N, et al. Evolutionary History of the Global Emergence of the Escherichia coli Epidemic Clone ST131. MBio. 2016;7:e02162. doi: 10.1128/mBio.02162-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben Zakour NL, et al. Sequential Acquisition of Virulence and Fluoroquinolone Resistance Has Shaped the Evolution of Escherichia coli ST131. MBio. 2016;7:e00347–16. doi: 10.1128/mBio.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyoshi-Akiyama, T. et al. Comparative Genome Analysis of Extended-Spectrum-β-Lactamase-Producing Escherichia coli Sequence Type 131 Strains from Nepal and Japan. mSphere1 (2016). [DOI] [PMC free article] [PubMed]
- 17.Kanamori, H. et al. Genomic Analysis of Multidrug-Resistant Escherichia coli from North Carolina Community Hospitals: Ongoing Circulation of CTX-M-Producing ST131-H30Rx and ST131-H30R1 Strains. Antimicrob. Agents Chemother. 61 (2017). [DOI] [PMC free article] [PubMed]
- 18.Runcharoen C, et al. Whole genome sequencing of ESBL-producing Escherichia coli isolated from patients, farm waste and canals in Thailand. Genome Med. 2017;9:81. doi: 10.1186/s13073-017-0471-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranjan, A. et al. Comparative Genomics of Escherichia coli Isolated from Skin and Soft Tissue and Other Extraintestinal Infections. MBio8 (2017). [DOI] [PMC free article] [PubMed]
- 20.Shaik, S. et al. Comparative Genomic Analysis of Globally Dominant ST131 Clone with Other Epidemiologically Successful Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. MBio8 (2017). [DOI] [PMC free article] [PubMed]
- 21.Harris Patrick N A, Ben Zakour Nouri L, Roberts Leah W, Wailan Alexander M, Zowawi Hosam M, Tambyah Paul A, Lye David C, Jureen Roland, Lee Tau H, Yin Mo, Izharuddin Ezlyn, Looke David, Runnegar Naomi, Rogers Benjamin, Bhally Hasan, Crowe Amy, Schembri Mark A, Beatson Scott A, Paterson David L, Harris-Brown Tiffany, Lorenc Penelope, McNamara John, Underwood Neil, Eisenmann Jared, Stewart James, Henderson Andrew, Ali Jaminah, Chiang Donald, Hwa Soh Siew, Kang Yvonne, Pei Ong Siew, Ying Ding, Holland Umit, Korman Tony. Whole genome analysis of cephalosporin-resistant Escherichia coli from bloodstream infections in Australia, New Zealand and Singapore: high prevalence of CMY-2 producers and ST131 carrying blaCTX-M-15 and blaCTX-M-27. Journal of Antimicrobial Chemotherapy. 2017;73(3):634–642. doi: 10.1093/jac/dkx466. [DOI] [PubMed] [Google Scholar]
- 22.Deen J, et al. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect. Dis. 2012;12:480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 23.Rogers BA, Sidjabat HE, Paterson DL. Escherichia coli O25b-ST131: a pandemic, multiresistant, community-associated strain. J. Antimicrob. Chemother. 2011;66:1–14. doi: 10.1093/jac/dkq415. [DOI] [PubMed] [Google Scholar]
- 24.Sheng W-H, Badal RE, Hsueh P-R, SMART Program Distribution of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in the Asia-Pacific region: results of the study for Monitoring Antimicrobial Resistance Trends (SMART) Antimicrob. Agents Chemother. 2013;57:2981–2988. doi: 10.1128/AAC.00971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nivesvivat T, Piyaraj P, Thunyaharn S, Watanaveeradej V, Suwanpakdee D. Clinical epidemiology, risk factors and treatment outcomes of extended-spectrum beta-lactamase producing Enterobacteriaceae bacteremia among children in a Tertiary Care Hospital, Bangkok, Thailand. BMC Res. Notes. 2018;11:624. doi: 10.1186/s13104-018-3729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dat VQ, et al. Bacterial bloodstream infections in a tertiary infectious diseases hospital in Northern Vietnam: aetiology, drug resistance, and treatment outcome. BMC Infect. Dis. 2017;17:493. doi: 10.1186/s12879-017-2582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myat TO, et al. ESBL- and Carbapenemase-Producing Enterobacteriaceae in Patients with Bacteremia, Yangon, Myanmar, 2014. Emerg. Infect. Dis. 2017;23:857–859. doi: 10.3201/eid2305.161100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nor Azizah A, et al. Community-acquired bacteremia in Paediatrics: Epidemiology, aetiology and patterns of antimicrobial resistance in a tertiary care centre, Malaysia. Med. J. Malaysia. 2016;71:117–121. [PubMed] [Google Scholar]
- 29.Banerjee R, et al. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect. Control Hosp. Epidemiol. 2013;34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croxall G, et al. Molecular epidemiology of extraintestinal pathogenic Escherichia coli isolates from a regional cohort of elderly patients highlights the prevalence of ST131 strains with increased antimicrobial resistance in both community and hospital care settings. J. Antimicrob. Chemother. 2011;66:2501–2508. doi: 10.1093/jac/dkr349. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee R, et al. Predictors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli infection in a Midwestern community. Infect. Control Hosp. Epidemiol. 2013;34:947–953. doi: 10.1086/671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Cerero L, et al. Escherichia coli belonging to the worldwide emerging epidemic clonal group O25b/ST131: risk factors and clinical implications. J. Antimicrob. Chemother. 2014;69:809–814. doi: 10.1093/jac/dkt405. [DOI] [PubMed] [Google Scholar]
- 33.Cockerill, F. R. Performance Standards for Antimicrobial Susceptibility Testing: Twentieth Informational Supplement. (Clinical and Laboratory Standards Institute (CLSI), 2010).
- 34.Kahlmeter G. The 2014 Garrod Lecture: EUCAST - are we heading towards international agreement? J. Antimicrob. Chemother. 2015;70:2427–2439. doi: 10.1093/jac/dkv145. [DOI] [PubMed] [Google Scholar]
- 35.Kassim A, Omuse G, Premji Z, Revathi G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a University teaching hospital in Nairobi, Kenya: a cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2016;15:21. doi: 10.1186/s12941-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magiorakos, A. P., Srinivasan, A. & Carey, R. B. Multidrug‐resistant, extensively drug‐resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology (2012). [DOI] [PubMed]
- 37.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 38.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit. Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Friedman ND. Health Care–Associated Bloodstream Infections in Adults: A Reason To Change the Accepted Definition of Community-Acquired Infections. Ann. Intern. Med. 2002;137:791. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 40.Inouye M, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta SK, et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob. Agents Chemother. 2014;58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Zheng D, Liu B, Yang J, Jin Q. VFDB 2016: hierarchical and refined dataset for big data analysis–10 years on. Nucleic Acids Res. 2016;44:D694–7. doi: 10.1093/nar/gkv1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wirth T, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forde BM, et al. The complete genome sequence of Escherichia coli EC958: a high quality reference sequence for the globally disseminated multidrug resistant E. coli O25b:H4-ST131 clone. PLoS One. 2014;9:e104400. doi: 10.1371/journal.pone.0104400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilm A, et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng X, et al. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics. 2012;28:3326–3328. doi: 10.1093/bioinformatics/bts606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popescu A-A, Huber KT, Paradis E. ape 3.0: New tools for distance-based phylogenetics and evolutionary analysis in R. Bioinformatics. 2012;28:1536–1537. doi: 10.1093/bioinformatics/bts184. [DOI] [PubMed] [Google Scholar]
- 49.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu G, Smith DK, Zhu H, Guan Y, Lam TT-Y. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 51.Koren S, et al. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alikhan N-F, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, et al. oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 2018;46:W229–W234. doi: 10.1093/nar/gky352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phan MD, et al. Molecular characterization of a multidrug resistance IncF plasmid from the globally disseminated Escherichia coli ST131 clone. PLoS One. 2015;10:e0122369. doi: 10.1371/journal.pone.0122369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arndt D, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–21. doi: 10.1093/nar/gkw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple. Testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- 61.Heng ST, Chen SL, Wong JGX, Lye DC, Ng TM. No association between resistance mutations, empiric antibiotic, and mortality in ceftriaxone-resistant Escherichia coli and Klebsiella pneumoniae bacteremia. Sci. Rep. 2018;8:12785. doi: 10.1038/s41598-018-31081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ng TM, et al. Empiric Piperacillin-Tazobactam versus Carbapenems in the Treatment of Bacteraemia Due to Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae. PLoS One. 2016;11:e0153696. doi: 10.1371/journal.pone.0153696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris PNA, et al. Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp (the MERINO trial): study protocol for a randomised controlled trial. Trials. 2015;16:24. doi: 10.1186/s13063-014-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantón R, González-Alba JM, Galán JC. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peirano G, Pitout JDD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int. J. Antimicrob. Agents. 2010;35:316–321. doi: 10.1016/j.ijantimicag.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Hannan TJ, et al. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol. Microbiol. 2008;67:116–128. doi: 10.1111/j.1365-2958.2007.06025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sabaté M, Moreno E, Pérez T, Andreu A, Prats G. Pathogenicity island markers in commensal and uropathogenic Escherichia coli isolates. Clin. Microbiol. Infect. 2006;12:880–886. doi: 10.1111/j.1469-0691.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 68.Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prs-fimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol. Lett. 1995;126:189–195. doi: 10.1111/j.1574-6968.1995.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 69.Clermont O, et al. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 2009;64:274–277. doi: 10.1093/jac/dkp194. [DOI] [PubMed] [Google Scholar]
- 70.Pitout, J. D. D. & DeVinney, R. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000Res. 6 (2017). [DOI] [PMC free article] [PubMed]
- 71.Matsumura Y, et al. CTX-M-27- and CTX-M-14-producing, ciprofloxacin-resistant Escherichia coli of the H30 subclonal group within ST131 drive a Japanese regional ESBL epidemic. J. Antimicrob. Chemother. 2015;70:1639–1649. doi: 10.1093/jac/dkv017. [DOI] [PubMed] [Google Scholar]
- 72.Holt KE, et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat. Genet. 2012;44:1056–1059. doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vashishtha VM. Emergence of multidrug resistant pneumococci in India. BMJ. 2000;321:1022–1023. doi: 10.1136/bmj.321.7267.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pham Thanh D, et al. A novel ciprofloxacin-resistant subclade of H58 Salmonella Typhi is associated with fluoroquinolone treatment failure. Elife. 2016;5:e14003. doi: 10.7554/eLife.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben-Ami R, et al. 151 Arpin C, Pascual A, Livermore DM, Garau J, Carmeli Y. A multinational survey 152 of risk factors for infection with extended-spectrum beta-lactamase producing 153 enterobacteriaceae in nonhospitalized patients. Clin. Infect. Dis. 2009;49:682–690. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- 76.Doi Y, et al. Community-associated extended-spectrum $\beta$-lactamase-producing Escherichia coli infection in the United States. Clin Infect Dis. 2013;56:641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cagnacci S, Gualco L, Debbia E, Schito GC, Marchese A. European Emergence of Ciprofloxacin-Resistant Escherichia coli Clonal Groups O25:H4-ST 131 and O15:K52:H1 Causing Community-Acquired Uncomplicated Cystitis. J. Clin. Microbiol. 2008;46:2605–2612. doi: 10.1128/JCM.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hansen, D. S., Nilsson, F. & Frimodt-Møller, J. Prevalence and characteristics of the epidemic multi-resistant Escherichia coli ST131 clonal group among extended-spectrum β-lactamase (ESBL)-producing E. coli in …. Journal of clinical (2013). [DOI] [PMC free article] [PubMed]
- 79.Cho SY, et al. Clinical Features and Treatment Outcomes of Bloodstream Infections Caused by Extended-Spectrum β-Lactamase-Producing Escherichia coli Sequence Type 131. Microb. Drug Resist. 2015;21:463–469. doi: 10.1089/mdr.2014.0261. [DOI] [PubMed] [Google Scholar]
- 80.Banerjee, R., Johnston, B. & Lohse, C. The clonal distribution and diversity of extraintestinal Escherichia coli varies according to patient characteristics. Antimicrob. Agents Chemother (2013). [DOI] [PMC free article] [PubMed]
- 81.Chung H-C, et al. Bacteremia caused by extended-spectrum-β-lactamase-producing Escherichia coli sequence type ST131 and non-ST131 clones: comparison of demographic data, clinical features, and mortality. Antimicrob. Agents Chemother. 2012;56:618–622. doi: 10.1128/AAC.05753-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Köves B, et al. Rare emergence of symptoms during long-term asymptomatic Escherichia coli 83972 carriage without an altered virulence factor repertoire. J. Urol. 2014;191:519–528. doi: 10.1016/j.juro.2013.07.060. [DOI] [PubMed] [Google Scholar]
- 83.Nagamatsu K, et al. Dysregulation of Escherichia coli α-hemolysin expression alters the course of acute and persistent urinary tract infection. Proc. Natl. Acad. Sci. USA. 2015;112:E871–80. doi: 10.1073/pnas.1500374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rippere-Lampe KE, O’Brien AD, Conran R, Lockman HA. Mutation of the gene encoding cytotoxic necrotizing factor type 1 (cnf(1)) attenuates the virulence of uropathogenic Escherichia coli. Infect. Immun. 2001;69:3954–3964. doi: 10.1128/IAI.69.6.3954-3964.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Michaud JE, Kim KS, Harty W, Kasprenski M, Wang M-H. Cytotoxic Necrotizing Factor-1 (CNF1) does not promote E. coli infection in a murine model of ascending pyelonephritis. BMC Microbiol. 2017;17:127. doi: 10.1186/s12866-017-1036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bondì, R. et al. The Gene tia, Harbored by the Subtilase-Encoding Pathogenicity Island, Is Involved in the Ability of Locus of Enterocyte Effacement-Negative Shiga Toxin-Producing Escherichia coli Strains To Invade Monolayers of Epithelial Cells. Infect. Immun. 85 (2017). [DOI] [PMC free article] [PubMed]
- 87.Fleckenstein JM, Kopecko DJ, Warren RL, Elsinghorst EA. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fagan RP, Lambert MA, Smith SGJ. The hek outer membrane protein of Escherichia coli strain RS218 binds to proteoglycan and utilizes a single extracellular loop for adherence, invasion, and autoaggregation. Infect. Immun. 2008;76:1135–1142. doi: 10.1128/IAI.01327-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McNally A, et al. Combined Analysis of Variation in Core, Accessory and Regulatory Genome Regions Provides a Super-Resolution View into the Evolution of Bacterial Populations. PLoS Genet. 2016;12:e1006280. doi: 10.1371/journal.pgen.1006280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khong WX, et al. Local transmission and global dissemination of New Delhi Metallo-Beta-Lactamase (NDM): a whole genome analysis. BMC Genomics. 2016;17:452. doi: 10.1186/s12864-016-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw sequencing data described in this manuscript is available in the Genbank repository, under BioProject PJRNA488345.