Abstract
Structural and vibrational properties of free base, cationic and hydrochloride species derived from both S(-) and R(+) enantiomers of antihistaminic promethazine (PTZ) agent have been theoretically evaluated in gas phase and in aqueous solution by using the hybrid B3LYP/6-31G* calculations. The initial structures of S(-) and R(+) enantiomers of hydrochloride PTZ were those polymorphic forms 1 and 2 experimentally determined by X-ray diffraction. Here, all structures in aqueous solution were optimized at the same level of theory by using the polarized continuum (PCM) and the universal solvation model. As was experimentally reported, variations in the unit cell lead to slight energy, density, and melting point differences between the two forms but, this behavior is not carried through in isotropic condition, like in solution with non-chiral solvents. Hence, the N–C distances, Mulliken, atomic natural population (NPA) and Merz-Kollman (MK) charges, bond orders, stabilization and solvation energies, frontier orbitals, some descriptors and their topological properties were compared with the antihistaminic cyclizine agent. The frontier orbitals studies show that the free base species of both forms in solution are more reactive than cyclizine. Higher electrophilicity indexes are observed in the cationic and hydrochloride species of PTZ than cyclizine while the cationic species of cyclizine have higher nucleophilicity index than both species of PTZ. The presences of bands attributed to cationic species of both enantiomers are clearly supported by the infrared and Raman spectra in the solid phase. The expected 114, 117 and 120 vibration normal modes for the free base, cationic and hydrochloride species of both forms were completely assigned and the force constants reported. Reasonable concordances among the predicted infrared, Raman, UV-Vis and Electronic Circular Dichroism (ECD) with the corresponding experimental ones were found.
Keywords: Computer science, Theoretical chemistry, Electronic, Structural properties, DFT calculations, Promethazine, Descriptor properties
1. Introduction
Species containing in their structures the N–CH3 group presenting a wide range of pharmacological and medicinal properties such as tropane alkaloids whose known biologics effects can cause from pain cure up to addiction [1, 2, 3, 4, 5, 6, 7]. However, there are another groups of species that also contain that group but that present other different biological properties such as, diphenhydramine and cyclizine, where both species are broadly used in pharmacology as antihistaminic agents [8, 9]. Nevertheless, the most remarkable differences among the free base, cationic and hydrochloride species of those two antihistaminic agents are that in the species derived from diphenhydramine their two N–CH3 groups are not linked to rings while in the cyclizine species those groups are linked to piperazine rings [8, 9]. Previous theoretical studies on structures and properties of alkaloids have evidenced that when the N–CH3 group is linked to fused rings as in scopolamine, cocaine and tropane some properties are slightly different from those where the N–CH3 group is linked to only one ring as in heroin and morphine [1, 2, 3, 5, 6, 7]. Besides, the stabilities of these series of alkaloids are strongly dependent on the N–C distances [6, 7]. On the other hand, the reactivities predicted for the three species of diphenhydramine practically are the same than that reported for cationic form of cocaine [3, 7] while lowest solvation energy value was observed for the free base of cyclizine, as compared with the corresponding to tropane alkaloids [9]. Evidently, there is an important connection between the quantity of N–CH3 groups and the type of groups linked to N atom, that is, >N- tertiary or >N< quaternary. Consequently, the biological activities and effects of these types of species on human health are obviously resulted of their nature and structural, electronic and topological properties. Hence, the interest to study another antihistaminic agent, in this case promethazine (PTZ) [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33], which has two N–CH3 groups (as diphenhydramine) linked to a chiral carbon and, as a consequence two enantiomeric S and R structures are expected for their three free base, cationic and hydrochloride species. PTZ hydrochloride is a drug used to treatment of nausea, vomiting, and dizziness associated with motion sickness and, besides possesses anti-pruritic, anti-allergic, anticholinergic, antihistaminic, central nervous system depressant, and with general anaesthetics effects. Their metabolic and clinic effects were studied from long time together with their side effects [13, 29, 30, 31, 32, 33]. Some known chemical names of promethazine are proazamine, diphergan, phenargan or phensedyl while its IUPAC name is N,N-dimethyl-1-phenothiazin-10-ylpropan-2-amine. PTZ has structurally two >N–CH3 groups, three fused six members rings (two phenyl and one phenothiazine) and a chiral carbon and where experimentally Borodi et al [19] have determined by X-ray diffraction two enantiomeric disordered structures of promethazine hydrochloride but, so far, the structural properties and vibrational assignments of those three species of PTZ were not published. The vibrational analyses of the three species of PTZ are actually of great interest and significance taking into account that the infrared, Raman and SERS spectroscopies are practically the most used spectroscopic techniques to identify these species in different systems and preparations [10, 12, 15, 17, 22, 23, 24, 25, 26, 27, 28]. Hence, the aims of this work are: (i) to study the structural, electronic, topological and vibrational properties of free base, cationic and hydrochloride species of S(-) and R(+)-PTZ, (ii) to find some correlations between their properties that can explain the differences between its biological properties, as compared with alkaloids and other antihistaminic agents, (iii) to perform the complete vibrational assignments of those three species of PTZ because, so far, these are not reported. In accordance to previous studies, the infrared spectra of many hydrochloride species show clearly the presence of their cationic forms in the solid phase and in aqueous solution [1, 2, 3, 5, 7, 8]. To achieve those purposes, the theoretical structures of free base, cationic and hydrochloride species of both S(-) and R(+)-PTZ enantiomers were optimized in gas phase and in aqueous solution by using the hybrid B3LYP/6-31G* method [34, 35] while experimental infrared and Raman spectra available from the literature were used to perform the vibrational analyses [17, 24, 25, 26, 27, 28]. The studies in solution were performed with the integral equation formalism variant polarised continuum method (IEFPCM) because it scheme contemplates the solvent effects while the solvation energies were computed with the universal solvation model [36, 37, 38]. Hence, for those three S(-) and R(+)-PTZ species, atomic charges, molecular electrostatic potential, bond orders, frontier orbitals and topological properties were calculated together with the harmonic force fields by using the scaled quantum mechanical force field (SQMFF) and transferable scaling factors [39, 40]. Then, the complete assignments for the three species were performed by using the corresponding force fields, internal normal coordinates and the experimental available vibrational spectra of PTZ hydrochloride [41] together with the Molvib program [42]. Taking into account the wide range of biological activities that presents PTZ, the reactivities and behaviours of those three S(-) and R(+)-PTZ species were predicted in both media by using the frontier orbitals [43, 44] and global descriptors [45, 46, 47, 48, 49, 50, 51, 52, 53]. Finally, the predicted properties of both enantiomeric series of S(-) and R(+)-PTZ were evaluated and then compared with the available data reported for alkaloids, diphenhydramine and cyclizine [1, 2, 3, 4, 5, 6, 7, 8, 9].
2. Methodology
2.1. Ab-initio calculations
The initial structure of S(-) enantiomer of PTZ hydrochloride was that experimental polymorphic form 1 determined by X-ray diffraction by Borodi et al [19] and taken from the available CIF file. The corresponding cationic and free base species were modelled respectively by using the GaussView program [54] where the Cl atom was first removed from that initial structure of PTZ hydrochloride and, later, the H atom. A similar procedure was employed to obtain the three species of R(+) enantiomer but in this case the structures were built from that experimental polymorphic form 2 determined by X-ray diffraction by Borodi et al [19]. The Revision A.02 of Gaussian program was employed to optimize those six species in both media [55] by using the hybrid B3LYP/6-31G* method [34, 35]. In solution, the three species were optimized by using PCM and SMD calculations [36, 37, 38] while their volumes changes were evaluated with the Moldraw program [56]. In Fig. 1 can be seen the six S(-) and R(+)-PTZ structures as free base, cationic and hydrochloride together with the atoms labelling and the identifications of their three rings. The solvation energies corrected by zero point vibrational energy (ZPVE) were computed for all species of S(-) and R(+)-PTZ with the universal solvation model [36, 37, 38]. Besides, atomic natural population (NPA), Mulliken and Merz-Kollman (MK) charges [57], molecular electrostatic potentials, bond orders and topological properties were calculated by using the NBO program [58] and with the Bader's theory of atoms in molecules (AIM) by using AIM2000 program [59, 60]. On the other hand, the evaluation of reactivities and behaviours of S(-) and R(+)-PTZ species were performed calculating the gap values [43, 44] and some useful and known global descriptors with the frontier orbitals [45, 46, 47, 48, 49, 50, 51, 52, 53]. The harmonic force fields and force constants in gas phase and in aqueous solution were computed at the B3LYP/6-31G* level by using the normal internal coordinates and transferable scaling factors with the scaled quantum mechanical force field (SQMFF) and the Molvib program [39, 40, 42]. Here, the predicted Raman activities for all species were corrected to intensities by using recommended equations [61, 62] while the scale factors used were those reported for the B3LYP/6-31G* method. At this point, it is necessary to clarify that all studied properties were computed for six S(-) and R(+)-PTZ species by using only the B3LYP/6-31G* level because they are compared with properties reported at the same level of theory for other species containing N–CH3 groups, such as alkaloids, diphenhydramine and cyclizine [1, 2, 3, 6, 7, 8, 9].
Fig. 1.
Theoretical molecular structures of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine.
3. Results and discussion
3.1. Properties of species of S(-) and R(+)-PTZ in both media
The structural studies in solution of these species are of great interest because the >N–CH3 group can present fast N-methyl inversion in this medium, as suggested by Lazni et al [63]. Here, in Table 1 are summarized calculated total uncorrected and corrected by ZPVE energies, dipole moments and volumes (V) of three species of both enantiomers S(-) and R(+)-PTZ in gas and aqueous solution phases by using the B3LYP/6-31G* method. Analyzing deeply the results, it is observed that the total energy values corrected by ZPVE decrease for all species in both media while the dipole moment and volume values increase in solution, as expected because these species are possibly hydrated in solution. The exception is observed only for the cationic form of S(-)-PTZ because the E and V values decrease in solution. Here, the imaginary frequencies obtained for that species could justify clearly these differences. Note that the cationic species of both enantiomers have higher dipole moments in solution while the hydrochloride forms present the higher volumes in both media having the S(-) species slightly the higher values in the two media. In Table 2 can be observed corrected and uncorrected solvation energies by the total non-electrostatic terms and by zero point vibrational energy (ZPVE) of free base, cationic and hydrochloride species of S(-) and R(+)-PTZ by using the B3LYP/6-31G* method. The variations observed experimentally in the unit cell lead to small displacements of the molecules in the crystal structures and, consequently, to slight energy, density, and melting point differences between the forms. Note that these obtained values are closer to those observed in the study of interaction of gelatin with promethazine hydrochloride [64]. These values are compared in the same table with morphine, cocaine, scopolamine, heroin and tropane alkaloids and with cyclizine [1, 2, 3, 4, 5, 6, 7, 9]. In particular, due to the imaginary frequencies predicted for the cationic form of cyclizine in solution the value for cyclizine was obtained by using B3LYP/6-31+G* calculations while for S(-)-PTZ in solution the value of -14,48 kJ/mol was obtained directly from Table 1. The ΔGc values for the three species of tropane were calculated in this work. Fig. 2 shows clearly the variations of ΔGc for all compared species by using the solvation model [38]. In general, it is observed that all cationic species have more negative values while the free bases the less negative values. The cationic forms of morphine, scopolamine and heroin have the most negative values while the S(-) form of PTZ the most low ΔGc value. Probably, this resulted change if other basis set is used. Interesting results are observed for cyclizine (-244,36 kJ/mol) and tropane (-244,33 kJ/mol) because their cationic forms have practically the same values. In both species, the N–CH3 groups are linked to rings, in cyclizine to piperazine ring while in tropane a two fused piperidine and pyrrolidine rings. The heroin hydrochloride species present the most negative ΔGc value while the R(+)-PTZ the lower value. On the other hand, the free base of heroin presents the most negative ΔGc value while the tropane species the lowest value. Evidently, the acetyl groups in heroin increase the solvation energies of their three species, as compared with morphine. Obviously, these comparisons show easily why the hydrochloride species are highly used in pharmacology, as compared with their free base and cationic ones. Besides, the hydrochloride species in solution are in their cationic forms and show clearly high solubility in this medium. Evidently, the solubility limits visibly the drug absorption, as mentioned by Bohloko studying the formulation of an intranasal dosage form for cyclizine hydrochloride [65].
Table 1.
Calculated total energies (E), dipole moments (μ) and volumes (V) of three species of S(-) and R(+)-promethazine in gas and aqueous solution phases.
| B3LYP/6-31G* Method | ||||
|---|---|---|---|---|
| Medium | E (Hartrees) | ZPVE | μ (D) | V (Å3) |
| S(-)-Free base | ||||
| GAS | -1167.5298 | -1167.1923 | 2.18 | 312.7 |
| PCM |
-1167.5383 |
-1167.2000 |
3.75 |
314.2 |
| S(-)-Cationic | ||||
| GAS | -1167.9143 | -1167.5615 | 14.62 | 316.3 |
|
PCM# |
-1167.9121 |
-1167.5588 |
15.20 |
315.1 |
| S(-)-Hydrochloride | ||||
| GAS | -1628.3493 | -1627.9992 | 9.33 | 342.1 |
| PCM |
-1628.3849 |
-1628.0312 |
14.16 |
342.8 |
| R(+)-Free base | ||||
| GAS | -1167.5263 | -1167.1907 | 1.92 | 312.3 |
| PCM |
-1167.5277 |
-1167.1894 |
3.03 |
312.2 |
| R(+)-Cationic | ||||
| GAS | -1167.9127 | -1167.5599 | 14.77 | 315.9 |
| PCM |
-1168.0075 |
-1167.6532 |
19.73 |
319.0 |
| R(+)-Hydrochloride | ||||
| GAS | -1628.3509 | -1628.0002 | 7.50 | 338.6 |
| PCM | -1628.3836 | -1627.9920 | 11.72 | 341.4 |
Imaginary frequencies.
Table 2.
Corrected and uncorrected solvation energies by the total non-electrostatic terms and by zero point vibrational energy (ZPVE) of three species of S(-) and R(+)-promethazine by using the B3LYP/6-31G* method compared with other similar species.
| B3LYP/6-31G* methoda | |||
|---|---|---|---|
| Solvation energy (kJ/mol) | |||
| Condition | ΔGun# | ΔGne | ΔGc |
| Free base | |||
| S(-)-Promethazinea | -20.19 | 15.88 | -36.07 |
| R(+)-Promethazinea | -3.41 | 14.46 | -17.87 |
| Cyclizineb | -23.60 | 5.93 | -29.53 |
| Morphinec | -47.74 | 13.17 | -60.91 |
| Cocained | -42.75 | 28.51 | -71.26 |
| Scopolaminee | -56.66 | 18.81 | -75.47 |
| Heroinf | -59.54 | 29.13 | -88.67 |
| Tropanea,g |
-11.80 |
0.75 |
-12.55 |
| Cationic | |||
| S(-)-Promethazinea | -7.08 | 7.40 | -14.48 |
| R(+)-Promethazinea | -255.22 | 7.59 | -262.81 |
| Cyclizineb,# | -238.43 | 5.93 | -244.36 |
| Morphinec | -282.23 | 26.96 | -309.19 |
| Cocained | -216.66 | 38.58 | -255.24 |
| Scopolaminee | -279.87 | 30.47 | -310.34 |
| Heroinf | -280.13 | 43.01 | -323.14 |
| Tropanea,g |
-228.99 |
15.34 |
-244.33 |
| Hydrochloride | |||
| S(-)-Promethazinea | -101.25 | 30.81 | -70.44 |
| R(+)-Promethazinea | -21.51 | 30.51 | -52.02 |
| Cyclizineb | -81.57 | 23.49 | -105.06 |
| Morphinec | -118.82 | 25.92 | -144.74 |
| Cocained | -99.94 | 38.20 | -138.14 |
| Scopolaminee | -95.19 | 27.55 | -122.74 |
| Heroinf | -118.56 | 43.38 | -161.94 |
| Tropanea,g | -72.13 | 15.05 | -87.18 |
ΔGun# = uncorrected solvation energy: defined as the difference between the total energies in aqueous solutions and the values in gas phase. ΔGun = Solvation energy (kJ/mol) corrected by ZPVE.
ΔGne = total non electrostatic terms: due to the cavitation, dispersion and repulsion energies.
ΔGc = corrected solvation energies: defined as the difference between the uncorrected and non-electrostatic solvation energies.
This work.
From Ref [9].
From Ref [1].
From Ref [3].
From Ref [7].
From Ref [5].
From Ref [2].
Cation cyclizine: 6-31+G*.
Fig. 2.
Corrected solvation energies of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine by using the B3LYP/6-31G* method.
3.2. Geometries of species of S(-) and R(+)-PTZ in both media
Calculated geometrical parameters for three species of S(-) and R(+)-PTZ in both media are compared with the corresponding experimental polymorphic forms 1 and 2 [19] in Tables 3 and 4, respectively by using the root-mean-square deviation (RMSD) values. Despite theoretical B3LYP/6-31G* calculations show visibly overestimated values, as compared with the corresponding experimental ones, the results for all species of S(-)-PTZ forms show very good correlations for bond lengths (0.020–0.012 Å) but the three species of R(+)-PTZ evidence the better correlations for bond (1.7–1.3°) and dihedral angles (6.1–3.7°) than the S(-) ones. On the other hand, the higher differences in dihedral angles are predicted for the three species of S(-) form (176.1–137.9°), as can be seen in Table 3. Here, it is necessary to remember that those two polymorphic conformations found by Borodi et al [19] are experimentally the same where the two forms are present in the unit cell but our theoretical calculations show slight differences in the dihedral angles of both S(-) and R(+)-PTZ forms. Thus, the calculated bonds N2–C6 and N2–C7 lengths of phenothiazine rings belong to the three species of both S(-) and R(+) enantiomers are practically predicted with same values but different from the bond N2–C5 lengths of side chain. In the same way, the calculated S1–C9 bonds of phenothiazine rings are approximately the same than the S1–C10 bonds while the predicted N3–C11 bonds are practically the same than the N3–C12 bonds. The predicted values for both pairs bonds are different from the corresponding experimental ones.
Table 3.
Comparison of calculated geometrical parameters for three species of S(-)-promethazine in both media with the corresponding experimental ones.
| B3LYP/6-31G* Methoda |
Form 1 | |||||
|---|---|---|---|---|---|---|
| Parameter | Free base |
Cation |
Hydrochloride |
Experimentalb | ||
| Gas | PCM | Gas | Gas | PCM | ||
| Bond lengths (Å) | ||||||
| S1–C9 | 1.783 | 1.786 | 1.786 | 1.783 | 1.786 | 1.772 |
| S1–C10 | 1.783 | 1.786 | 1.785 | 1.784 | 1.786 | 1.781 |
| N2–C5 | 1.464 | 1.471 | 1.445 | 1.457 | 1.465 | 1.435 |
| N2–C6 | 1.416 | 1.419 | 1.427 | 1.420 | 1.420 | 1.422 |
| N2–C7 | 1.416 | 1.418 | 1.424 | 1.420 | 1.419 | 1.418 |
| C6–C9 | 1.406 | 1.409 | 1.404 | 1.407 | 1.408 | 1.379 |
| C7–C10 | 1.406 | 1.409 | 1.406 | 1.407 | 1.409 | 1.389 |
| C4–C5 | 1.553 | 1.552 | 1.547 | 1.545 | 1.543 | 1.545 |
| N3–C4 | 1.472 | 1.482 | 1.550 | 1.511 | 1.525 | 1.513 |
| N3–C11 | 1.454 | 1.463 | 1.505 | 1.485 | 1.495 | 1.502 |
| N3–C12 | 1.456 | 1.465 | 1.505 | 1.484 | 1.495 | 1.491 |
| C6–C13 | 1.401 | 1.405 | 1.399 | 1.402 | 1.404 | 1.398 |
| C9–C15 | 1.392 | 1.395 | 1.395 | 1.395 | 1.396 | 1.394 |
| C13–C17 | 1.393 | 1.396 | 1.396 | 1.395 | 1.396 | 1.396 |
| C15–C19 | 1.393 | 1.396 | 1.395 | 1.395 | 1.396 | 1.392 |
| C17–C19 | 1.391 | 1.394 | 1.394 | 1.393 | 1.395 | 1.366 |
| C7–C14 | 1.401 | 1.404 | 1.400 | 1.402 | 1.404 | 1.402 |
| C14–C18 | 1.393 | 1.396 | 1.397 | 1.396 | 1.396 | 1.387 |
| C16–C10 | 1.392 | 1.395 | 1.395 | 1.394 | 1.395 | 1.394 |
| C16–C20 | 1.393 | 1.396 | 1.395 | 1.395 | 1.396 | 1.382 |
| C18–C20 | 1.391 | 1.395 | 1.394 | 1.393 | 1.394 | 1.379 |
|
RMSDb |
0.020 |
0.019 |
0.014 |
0.012 |
0.013 |
|
| Bond angles (°) | ||||||
| C9–S1–C10 | 97.8 | 97.8 | 98.2 | 98.0 | 98.0 | 96.4 |
| C6–C9–S1 | 118.6 | 118.5 | 118.8 | 118.7 | 118.6 | 119.0 |
| C7–C10–S1 | 118.6 | 118.5 | 118.7 | 118.6 | 118.6 | 117.9 |
| C5–N2–C6 | 119.5 | 118.7 | 120.2 | 119.5 | 119.0 | 118.9 |
| C5–N2–C7 | 119.3 | 119.2 | 119.8 | 119.6 | 119.3 | 119.1 |
| C6–N2–C7 | 117.5 | 117.0 | 118.3 | 117.9 | 117.7 | 115.6 |
| N2–C5–C4 | 112.7 | 113.2 | 108.6 | 111.3 | 110.6 | 108.9 |
| C5–C4–N3 | 113.1 | 113.0 | 111.8 | 111.6 | 111.1 | 106.9 |
| C4–N3–C11 | 114.3 | 112.4 | 113.1 | 114.3 | 113.2 | 111.7 |
| C4–N3–C12 | 116.2 | 114.1 | 114.4 | 115.8 | 114.9 | 112.1 |
| C11–N3–C12 | 111.9 | 109.8 | 111.2 | 111.2 | 110.7 | 111.4 |
| N2–C7–C14 | 122.5 | 122.5 | 122.6 | 122.5 | 122.5 | 122.4 |
| N2–C6–C13 | 122.6 | 122.6 | 122.5 | 122.6 | 122.5 | 121.9 |
| S1–C10–C16 | 120.4 | 120.3 | 120.8 | 120.6 | 120.3 | 120.0 |
| S1–C9–C15 | 120.4 | 120.3 | 120.7 | 120.5 | 120.3 | 120.0 |
|
RMSDb |
2.4 |
2.1 |
1.8 |
2.0 |
1.6 |
|
| Dihedral angles (°) | ||||||
| C11–N3–C4–C5 | 75.9 | 72.8 | 75.7 | 71.0 | 73.8 | 167.0 |
| C12–N3–C4–C5 | -56.6 | -53.1 | -53.0 | -60.3 | -54.9 | -67.0 |
| N3–C4–C5–N2 | -168.3 | -167.7 | -165.4 | -169.6 | -165.2 | 175.4 |
| C4–C5–N2–C6 | -137.3 | -142.2 | -127.8 | -135.3 | -136.5 | -68.6 |
| C4–C5–N2–C7 | 63.8 | 63.4 | 66.3 | 64.2 | 66.2 | 140.3 |
| C14–C7–N2–C6 | -135.8 | -134.5 | -134.5 | -135.2 | -136.0 | -129.1 |
| C15–C9–S1–C10- | -144.8 | -144.5 | -144.2 | -144.7 | -144.8 | -139.1 |
| C8–C4–C5–N2 | 65.1 | 66.0 | 69.7 | 64.9 | 70.4 | -65.9 |
| RMSDb | 138.9 | 139.5 | 137.9 | 176.1 | 223.0 | |
Table 4.
Comparison of calculated geometrical parameters for three species of R(+)-promethazine in both media with the corresponding experimental ones.
| B3LYP/6-31G* Methoda |
Form 2 Experimentalb |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Free base |
Cation |
Hydrochloride |
||||
| Gas | PCM | Gas | PCM | Gas | PCM | ||
| Bond lengths (Å) | |||||||
| S1–C9 | 1.783 | 1.785 | 1.786 | 1.785 | 1.784 | 1.785 | 1.772 |
| S1–C10 | 1.783 | 1.786 | 1.785 | 1.786 | 1.782 | 1.785 | 1.781 |
| N2–C5 | 1.464 | 1.470 | 1.443 | 1.462 | 1.457 | 1.464 | 1.435 |
| N2–C6 | 1.418 | 1.418 | 1.427 | 1.420 | 1.423 | 1.421 | 1.422 |
| N2–C7 | 1.417 | 1.418 | 1.425 | 1.420 | 1.418 | 1.421 | 1.418 |
| C6–C9 | 1.408 | 1.409 | 1.404 | 1.408 | 1.407 | 1.409 | 1.379 |
| C7–C10 | 1.408 | 1.409 | 1.406 | 1.408 | 1.409 | 1.409 | 1.389 |
| C4–C5 | 1.551 | 1.549 | 1.554 | 1.547 | 1.552 | 1.548 | 1.545 |
| N3–C4 | 1.479 | 1.486 | 1.551 | 1.533 | 1.520 | 1.527 | 1.513 |
| N3–C11 | 1.460 | 1.468 | 1.508 | 1.502 | 1.487 | 1.496 | 1.502 |
| N3–C12 | 1.460 | 1.468 | 1.508 | 1.503 | 1.486 | 1.496 | 1.491 |
| C6–C13 | 1.403 | 1.404 | 1.399 | 1.403 | 1.402 | 1.404 | 1.398 |
| C9–C15 | 1.394 | 1.395 | 1.395 | 1.396 | 1.395 | 1.395 | 1.394 |
| C13–C17 | 1.395 | 1.396 | 1.396 | 1.396 | 1.395 | 1.396 | 1.396 |
| C15–C19 | 1.395 | 1.396 | 1.395 | 1.396 | 1.395 | 1.395 | 1.392 |
| C17–C19 | 1.393 | 1.395 | 1.394 | 1.395 | 1.393 | 1.394 | 1.366 |
| C7–C14 | 1.403 | 1.404 | 1.400 | 1.403 | 1.403 | 1.403 | 1.402 |
| C14–C18 | 1.395 | 1.396 | 1.397 | 1.396 | 1.396 | 1.396 | 1.540 |
| C16–C10 | 1.394 | 1.395 | 1.395 | 1.395 | 1.394 | 1.395 | 1.540 |
| C16–C20 | 1.395 | 1.396 | 1.396 | 1.396 | 1.395 | 1.396 | 1.540 |
| C18–C20 | 1.393 | 1.395 | 1.394 | 1.394 | 1.393 | 1.394 | 1.325 |
|
RMSDb |
0.060 |
0.059 |
0.058 |
0.015 |
0.058 |
0.058 |
|
| Bond angles (°) | |||||||
| C9–S1–C10 | 97.8 | 97.8 | 98.2 | 98.0 | 98.0 | 98.0 | 96.4 |
| C6–C9–S1 | 118.6 | 118.5 | 118.7 | 118.6 | 118.8 | 118.7 | 119.0 |
| C7–C10–S1 | 118.6 | 118.4 | 118.7 | 118.6 | 118.7 | 118.7 | 117.9 |
| C5–N2–C6 | 119.2 | 119.3 | 120.0 | 118.9 | 119.1 | 118.7 | 118.9 |
| C5–N2–C7 | 119.4 | 119.1 | 119.7 | 119.2 | 119.4 | 119.1 | 119.1 |
| C6–N2–C7 | 117.6 | 117.3 | 118.2 | 117.8 | 118.0 | 117.6 | 115.6 |
| N2–C5–C4 | 113.1 | 112.4 | 109.5 | 111.2 | 110.9 | 111.2 | 108.9 |
| C5–C4–N3 | 107.7 | 109.1 | 109.4 | 108.2 | 108.6 | 108.6 | 106.9 |
| C4–N3–C11 | 114.8 | 111.9 | 113.6 | 113.5 | 114.5 | 113.5 | 112.1 |
| C4–N3–C12 | 111.9 | 110.5 | 112.8 | 112.8 | 113.2 | 112.7 | 111.7 |
| C11–N3–C12 | 108.9 | 107.2 | 109.2 | 108.8 | 109.6 | 108.9 | 111.4 |
| N2–C7–C14 | 122.5 | 122.5 | 122.7 | 122.5 | 122.6 | 122.5 | 122.4 |
| N2–C6–C13 | 122.5 | 122.6 | 122.6 | 122.5 | 122.5 | 122.5 | 121.9 |
| S1–C10–C16 | 120.4 | 120.3 | 120.8 | 120.3 | 120.5 | 120.2 | 121.0 |
| S1–C9–C15 | 120.4 | 120.3 | 120.8 | 120.3 | 120.3 | 120.1 | 120.0 |
|
RMSDb |
1.6 |
1.7 |
1.4 |
1.3 |
1.4 |
1.3 |
|
| Dihedral angles (°) | |||||||
| C11–N3–C4–C5 | 157.1 | 165.9 | 165.5 | 164.2 | 160.7 | 163.5 | 167.0 |
| C12–N3–C4–C5 | -77.8 | -74.4 | -69.3 | -71.2 | -72.5 | -71.9 | -67.0 |
| N3–C4–C5–N2 | 172.2 | 165.7 | 170.5 | 171.6 | 171.4 | 166.4 | 175.4 |
| C4–C5–N2–C6 | 137.0 | 136.7 | 130.3 | 137.3 | 133.5 | 137.6 | 139.9 |
| C4–C5–N2–C7 | -64.6 | -66.7 | -65.9 | -65.5 | -67.5 | -66.7 | -69.0 |
| C14–C7–N2–C6 | 135.9 | 135.5 | 134.3 | 136.0 | 136.5 | 136.0 | 131.7 |
| C15–C9–S1–C10- | 144.6 | 144.1 | 144.1 | 144.7 | 144.7 | 144.8 | 140.1 |
| C8–C4–C5–N2 | -64.5 | -71.0 | -65.8 | -65.7 | -65.9 | -70.7 | -62.7 |
| RMSDb | 6.1 | 5.8 | 4.6 | 3.7 | 4.8 | 5.4 | |
Another interesting comparisons are observed in the average bond N–C lengths of the N–CH3 groups belonging to the three species of S(-) and R(+)-PTZ with those observed for cyclizine, morphine, heroin, cocaine, scopolamine and tropane where the results in gas phase and in aqueous solution by using B3LYP/6-31G* calculations can be seen in Table 5. Here, due to the presence of two N–CH3 groups the average of N–C distances between both groups were considered. In Fig. 3 are easily observed the behaviours of N–C distances of all compared species in both media. In gas phase, the comparisons between the free base and cationic species show that cationic form of cyclizine has the lowest value (1.453 Å) while the highest value is observed in the cationic species of R(+)-PTZ (1.508 Å). In solution, it is observed that the free base species have low values and different from the hydrochloride ones. Evidently, the presence of charged cationic species and electronegative Cl atoms in all hydrochloride species produce increase in the N–C distances. The tropane hydrochloride has the shorter value while the species corresponding to R(+)-PTZ the higher value.
Table 5.
Bond lengths observed between the N and C atoms of the N–CH3 bonds belonging to the three S(-) and R(+)-promethazine species in gas phase and in aqueous solution by using B3LYP/6-31G* calculations.
| N–CH3 bonds () | ||||||
|---|---|---|---|---|---|---|
| Species | Gas phase |
Aqueous solution |
||||
| Free base | Cationic | Hydrobromide | Free base | Cationic | Hydrobromide | |
| R(+)-promethazineγ | 1.460 | 1.508 | 1.487 | 1.468 | 1.501 | 1.496 |
| S(-)-Promethazineγ | 1.455 | 1.505 | 1.485 | 1.464 | # | 1.495 |
| Cyclizine | 1.453 | 1.453 | # | 1.459 | # | 1.489 |
| Scopolamine | 1.462 | 1.492 | 1.491 | 1.466 | 1.491 | 1.493 |
| Heroin | 1.453 | 1.501 | 1.483 | 1.460 | 1.498 | 1.492 |
| Morphine | 1.453 | 1.500 | 1.483 | 1.460 | 1.497 | 1.493 |
| Cocaine | 1.459 | 1.493 | 1.487 | 1.467 | 1.492 | 1.494 |
| Tropane | 1.458 | 1.496 | 1.478 | 1.467 | 1.491 | 1.486 |
Imaginary frequencies.
average.
Fig. 3.
Calculated N–C distances corresponding to N–CH3 groups of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine in both media by using the B3LYP/6-31G* method.
3.3. Atomic charges, molecular electrostatic potentials and bond orders
Mulliken, Merz-Kollman (MK) and atomic natural population (NPA) charges, molecular electrostatic potentials (MEP) and bond orders (BO), expressed as Wiberg indexes were calculated for the three forms of S(-) and R(+)-PTZ in gas phase and in aqueous solution by using B3LYP/6-31G* calculations. The resulted only for the S1, N2, N3, C8, C11 and C12 atoms can be seen in Table 6 because these atoms present the higher variations in all species while the behaviours of MK charges on these atoms are represented in Fig. 4. Analyzing first the MK charges for the free base species of S(-) and R(+)-PTZ we observed from Fig. 4 that: (i) the MK charges on the N2, C8 and C11 atoms of all free base species undergoes important changes, presenting the highest change on N2 of free base of R(+)-PTZ in solution and (ii) the charges on the S1, N3 and C12 atoms of all species in both media have practically the same values. In the cationic species, the lower MK charges values are observed on those five atoms of S(-)-PTZ in gas phase while on N2 atoms of R(+) species in the two media are observed the higher changes. Different behaviours are observed on the MK charges of those five atoms corresponding to the hydrochloride species in both media. Hence, the charges on the N3 atoms have the higher values, as expected due to the presences in these species of electronegative Cl atoms. The Mulliken charges on those five atoms of free base species show practically the same behaviours but, in particular, on the N2 and C8 atoms are observed the most negative values while the NPA charges on C8 atoms of free base, cationic and hydrochloride species show the lower values in both enantioners. The Mulliken charges in the cationic and hydrochloride species present basically the same behaviours but on the N2 atoms are observed the lower values.
Table 6.
Mulliken, Merz-Kollman and NPA charges, molecular electrostatic potentials (MEP) and bond orders, expressed as Wiberg indexes for three forms of S(-) and R(+)-promethazine in gas phase and in aqueous solution by using B3LYP/6-31G* calculations.
| S(-)-Free base | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
MK |
Mulliken |
NPA |
MEP |
BO |
| S1 | -0.120 | 0.157 | 0.330 | -59.182 | 2.335 | -0.118 | 0.156 | 0.328 | -59.182 | 2.333 |
| N2 | -0.311 | -0.581 | -0.452 | -18.312 | 3.305 | -0.360 | -0.581 | -0.449 | -18.311 | 3.305 |
| N3 | -0.346 | -0.365 | -0.506 | -18.356 | 3.127 | -0.357 | -0.367 | -0.501 | -18.354 | 3.115 |
| C8 | -0.272 | -0.455 | -0.685 | -14.757 | 3.844 | -0.267 | -0.455 | -0.685 | -14.756 | 3.844 |
| C11 | -0.222 | -0.300 | -0.468 | -14.719 | 3.819 | -0.266 | -0.305 | -0.473 | -14.719 | 3.820 |
| C12 |
-0.138 |
-0.308 |
-0.475 |
-14.719 |
3.819 |
-0.124 |
-0.311 |
-0.479 |
-14.720 |
3.820 |
| S(-)-Cationic | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
|||||
| S1 | -0.097 | 0.186 | 0.348 | -59.085 | 2.343 | |||||
| N2 | -0.122 | -0.587 | -0.471 | -18.197 | 3.264 | |||||
| N3 | -0.025 | -0.492 | -0.450 | -18.052 | 3.469 | |||||
| C8 | -0.279 | -0.498 | -0.718 | -14.593 | 3.809 | |||||
| C11 | -0.335 | -0.348 | -0.475 | -14.519 | 3.713 | |||||
| C12 |
-0.368 |
-0.351 |
-0.479 |
-14.519 |
3.715 |
|||||
| S(-)-Hydrochloride | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
MK |
Mulliken |
NPA |
MEP |
BO |
| S1 | -0.106 | 0.171 | 0.340 | -59.167 | 2.339 | -0.101 | 0.174 | 0.340 | -59.164 | 2.338 |
| N2 | -0.215 | -0.583 | -0.456 | -18.291 | 3.291 | -0.257 | -0.586 | -0.453 | -18.283 | 3.294 |
| N3 | 0.370 | -0.481 | -0.497 | -18.250 | 3.341 | 0.452 | -0.480 | -0.483 | -18.223 | 3.383 |
| C8 | -0.212 | -0.488 | -0.708 | -14.733 | 3.816 | -0.180 | -0.490 | -0.711 | -14.725 | 3.811 |
| C11 | -0.400 | -0.321 | -0.477 | -14.673 | 3.756 | -0.357 | -0.328 | -0.474 | -14.660 | 3.745 |
| C12 |
-0.348 |
-0.325 |
-0.481 |
-14.673 |
3.759 |
-0.337 |
-0.334 |
-0.479 |
-14.658 |
3.748 |
| R(+)-Free base | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
MK |
Mulliken |
NPA |
MEP |
BO |
| S1 | -0.344 | 0.155 | 0.329 | -59.182 | 2.334 | -0.126 | 0.154 | 0.327 | -59.183 | 2.332 |
| N2 | -0.344 | -0.584 | -0.455 | -18.313 | 3.303 | -0.018 | -0.583 | -0.454 | -18.312 | 3.304 |
| N3 | -0.344 | -0.387 | -0.511 | -18.354 | 3.112 | -0.336 | -0.390 | -0.504 | -18.353 | 3.104 |
| C8 | -0.330 | -0.484 | -0.695 | -14.753 | 3.836 | -0.341 | -0.484 | -0.694 | -14.752 | 3.837 |
| C11 | -0.255 | -0.296 | -0.472 | -14.723 | 3.815 | -0.215 | -0.300 | -0.476 | -14.723 | 3.816 |
| C12 |
-0.123 |
-0.306 |
-0.469 |
-14.720 |
3.821 |
-0.127 |
-0.309 |
-0.473 |
-14.720 |
3.822 |
| R(+)-Cationic | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
MK |
Mulliken |
NPA |
MEP |
BO |
| S1 | -0.106 | 0.188 | 0.349 | -59.085 | 2.343 | -0.090 | 0.190 | 0.351 | -59.088 | 2.341 |
| N2 | 0.033 | -0.586 | -0.471 | -18.197 | 3.263 | -0.048 | -0.591 | -0.460 | -18.193 | 3.276 |
| N3 | 0.046 | -0.495 | -0.449 | -18.050 | 3.470 | 0.033 | -0.490 | -0.447 | -18.047 | 3.471 |
| C8 | -0.145 | -0.499 | -0.722 | -14.597 | 3.805 | -0.155 | -0.492 | -0.719 | -14.593 | 3.806 |
| C11 | -0.308 | -0.343 | -0.476 | -14.521 | 3.708 | -0.298 | -0.343 | -0.476 | -14.519 | 3.707 |
| C12 |
-0.384 |
-0.351 |
-0.473 |
-14.519 |
3.713 |
-0.358 |
-0.353 |
-0.473 |
-14.517 |
3.711 |
| R(+)-Hydrochloride | ||||||||||
| GAS |
PCM |
|||||||||
| Atoms |
MK |
Mulliken |
NPA |
MEP |
BO |
MK |
Mulliken |
NPA |
MEP |
BO |
| S1 | -0.129 | 0.158 | 0.331 | -59.173 | 2.335 | -0.122 | 0.160 | 0.332 | -59.170 | 2.335 |
| N2 | -0.132 | -0.588 | -0.457 | -18.299 | 3.295 | -0.226 | -0.588 | -0.456 | -18.292 | 3.293 |
| N3 | 0.407 | -0.481 | -0.492 | -18.244 | 3.353 | 0.454 | -0.482 | -0.479 | -18.221 | 3.389 |
| C8 | -0.208 | -0.496 | -0.710 | -14.725 | 3.818 | -0.190 | -0.497 | -0.712 | -14.715 | 3.816 |
| C11 | -0.319 | -0.315 | -0.476 | -14.671 | 3.753 | -0.314 | -0.322 | -0.475 | -14.660 | 3.743 |
| C12 | -0.448 | -0.324 | -0.472 | -14.668 | 3.760 | -0.415 | -0.332 | -0.471 | -14.657 | 3.749 |
Fig. 4.
Calculated Merz-Kollman charges of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine by using the B3LYP/6-31G* method.
The bond orders (BO) expressed as Wiberg indexes in the three species of both enantiomers in the two media have approximately the same values and behaviours, observing the higher values in the C8, C11 and C12 atoms and the lower values in the S1 atoms. In general, higher values are observed for the N2 atoms of the free base and hydrochloride species of both S(-) and R(+)-PTZ in the two media than for the N3 atoms and only in the cationic species are observed higher values in the N3 atoms.
The molecular electrostatic potentials (MEP) presented in Table 6 show practically the same values and behaviours in the three species of both enantiomers, however, when the surfaces of these species are mapped the colorations show important differences among them, as can be seen in Fig. 5. Thus, the cationic species of both enantiomers in gas phase show blue colours on the entire surface but, in particular, strong blue colours it is observed on the protonated N–H region. In the free base species the strong red colours are observed on the N3 atoms and S1 atoms while in the hydrochloride species the strong red colours are observed on the Cl atoms. Hence, the typical nucleophilic sites are clearly identified with red colours while the electrophilic sites with blue colours, as observed in other species [6, 7, 8, 9].
Fig. 5.
Calculated electrostatic potential surfaces on the molecular surfaces of the free base, cationic and hydrochloride species of both S(-) and R(+) enantiomers of promethazine. B3LYP functional and 6-31G* basis set. Isodensity value of 0.005.
3.4. NBO study
For the three species of both S(-) and R(+)-PTZ enantiomers the main delocalization energies in gas and aqueous solution were calculated by using B3LYP/6-31G* calculations with the NBO program [58]. The resulted for the three species of S(-) and R(+)-PTZ are summarized in Tables 7 and 8, respectively. Different interactions can be observed in the three species and, especially in the hydrochloride species due to the presence of Cl atoms where in particular, the π*→π* and π→π* interactions present the higher values in the S(-) and R(+)-PTZ forms, respectively. Thus, the free base (3509.36–3522.22 kJ/mol) and hydrochloride (6253.53–5840.28 kJ/mol) species present higher total energies than the cationic ones (1541.01 kJ/mol) and, for these reasons, these two species are most stable than the cationic ones. However, the hydrochloride species of R(+)-PTZ have higher values in both media than the corresponding to other enantiomer (7527.88–7332.02 kJ/mol). Nevertheless, the free base of R(+)-PTZ present lower values than the corresponding to S(-)-PTZ (3484.4–3193.04 kJ/mol) while the cationic form of R(+)-PTZ is most stable than the corresponding to S(-)-PTZ (1540.08–1612.71 kJ/mol). These studies shows clearly that the hydrochloride species are most stable than the other two species of both forms and in the two media studied but, in particular the species of R(+)-PTZ show higher total energy values evidencing a slight higher stability than the S(-) one. The three PTZ species show higher stabilities than the corresponding to cyclizine [9].
Table 7.
Main delocalization energies (in kJ/mol) for three species of S(-)-promethazine in gas and aqueous solution by using B3LYP/6-31G* calculations.
| B3LYP/6-31G*a | ||||
|---|---|---|---|---|
| Delocalization | Free base |
Hydrochloride |
||
| Gas | PCM | Gas | PCM | |
| πC6-C13→ π*C9–C15 | 74.32 | 74.28 | 73.40 | 73.19 |
| πC6-C13→ π*C17–C19 | 88.41 | 88.49 | 85.98 | 85.77 |
| πC7-C14→ π*C10–C16 | 74.49 | 74.61 | 72.73 | 72.02 |
| πC7-C14→ π*C18–C20 | 88.28 | 88.49 | 85.23 | 85.19 |
| πC9-C15→ π*C6–C13 | 83.56 | 83.39 | 85.27 | 85.27 |
| πC9-C15→ π*C17–C19 | 71.18 | 71.27 | 72.02 | 71.98 |
| πC10-C16→ π*C7–C14 | 83.81 | 83.60 | 85.90 | 86.19 |
| πC10-C16→ π*C18–C20 | 71.52 | 71.60 | 72.23 | 72.10 |
| πC17-C19→ π*C6–C13 | 79.59 | 79.59 | 81.34 | 81.97 |
| πC17-C19→ π*C9–C15 | 93.84 | 93.67 | 94.09 | 94.26 |
| πC18-C20→ π*C7–C14 | 80.05 | 80.21 | 82.05 | 82.26 |
| πC18-C20→ π*C10–C16 | 93.75 | 93.67 | 94.26 | 94.30 |
| Σπ→π* | 982.8 | 982.87 | 984.5 | 984.5 |
| LP(2)S1→ π*C9–C15 | 45.98 | 45.44 | 46.27 | 46.02 |
| LP(2)S1→ π*C10–C16 | 45.98 | 45.23 | 46.36 | 46.27 |
| LP(1)N2→ π*C6–C13 | 99.86 | 99.36 | 92.96 | 94.89 |
| LP(1)N2→ π*C7–C14 | 100.74 | 99.44 | 94.30 | 97.56 |
| ΣLP→π* | 292.56 | 289.47 | 279.89 | 284.74 |
| π*C9–C15→ π*C17–C19 | 1106.65 | 1113.09 | ||
| π*C10–C16→ π*C18–C20 | 1127.35 | 1136.79 | ||
| π*C6–C13→ π*C17–C19 | 1101.14 | 978.58 | ||
| π*C7–C14→ π*C18–C20 | 909.65 | 805.44 | ||
| π*C9–C15→ π*C17–C19 | 1057.33 | 1045.67 | ||
| π*C10–C16→ π*C18–C20 | 1040.23 | 1046.42 | ||
| Σπ*→π* | 2234 | 2249,88 | 4108,35 | 3876,11 |
| σN3-C4→ LP(1)*H41 | 44,60 | 62,57 | ||
| σN3-C11→ LP(1)*H41 | 50,08 | 62,82 | ||
| σN3-C12→ LP(1)*H41 | 47,61 | 60,19 | ||
| Σσ→LP* | 142,29 | 185,58 | ||
| LP(1)N3→ LP(1)*H41 | 1158,49 | 1456,02 | ||
| LP(1)Cl42→ LP(1)*H41 | 46,94 | 16,51 | ||
| LP(4)Cl42→ LP(1)*H41 | 797,46 | 306,06 | ||
| ΣLP→LP* | 2002,89 | 1778,59 | ||
| ΣTOTAL | 3509.36 | 3522,22 | 6253,53 | 5840,28 |
| Cationica | ||||
| Delocalization | Gas | |||
| πC13-C17→ π*C6–C9 | 47.86 | |||
| πC15-C19→ π*C6–C9 | 43.22 | |||
| πC15-C19→ π*C13–C17 | 46.98 | |||
| Σπ→π* | 138.06 | |||
| πC7-C10→ LP(1)*C16 | 93.51 | |||
| πC18-C20→ LP(1)*C16 | 107.22 | |||
| Σπ→LP* | 200.73 | |||
| LP(1)C14→ π*C7–C10 | 171.17 | |||
| LP(1)C14→ π*C18–C20 | 125.57 | |||
| LP(1)C16→ π*C7–C10 | 176.65 | |||
| LP(1)C16→ π*C18–C20 | 133.97 | |||
| ΣLP→π* | 607.36 | |||
| π*C6–C9→ π*C13–C17 | 361.99 | |||
| π*C6–C9→ π*C15–C19 | 232.87 | |||
| Σπ*→π* | 594.86 | |||
| ΣTOTAL | 1541.01 | |||
The letters bold indicated RMSD values.
This work.
Table 8.
Main delocalization energies (in kJ/mol) for three species of R(+)-promethazine in gas and aqueous solution by using B3LYP/6-31G* calculations.
| B3LYP/6-31G*a | ||||
|---|---|---|---|---|
| Delocalization | Free base |
Hydrochloride |
||
| Gas | PCM | Gas | PCM | |
| πC6-C13→ π*C9–C15 | 74.70 | 74.65 | 76.53 | 76.07 |
| πC6-C13→ π*C17–C19 | 88.41 | 88.49 | 86.23 | 85.65 |
| πC7-C14→ π*C10–C16 | 74.65 | 72.48 | 72.23 | |
| πC7-C14→ π*C18–C20 | 88.45 | 86.82 | 86.57 | |
| πC9-C15→ π*C6–C13 | 83.43 | 83.35 | 83.06 | 82.51 |
| πC9-C15→ π*C17–C19 | 71.18 | 71.14 | 70.56 | 70.30 |
| πC10-C16→ π*C7–C14 | 83.68 | 84.98 | 85.77 | |
| πC10-C16→ π*C18–C20 | 71.44 | 72.15 | 72.56 | |
| πC17-C19→ π*C6–C13 | 79.80 | 79.88 | 82.26 | 82.93 |
| πC17-C19→ π*C9–C15 | 93.97 | 93.97 | 95.89 | 96.14 |
| πC18-C20→ π*C7–C14 | 80.09 | 80.59 | ||
| πC18-C20→ π*C10–C16 | 93.84 | 93.13 | ||
| Σπ→π* | 983.64 | 491.48 | 810.96 | 984.45 |
| πC10-C16→ LP(1)*C7 | 202.39 | |||
| πC10-C16→ LP(1)*C20 | 167.07 | |||
| πC14-C18→ LP(1)*C7 | 219.66 | |||
| πC14-C18→ LP(1)*C20 | 183.38 | |||
| Σπ→LP* | 772.5 | |||
| LP(2)S1→ π*C9–C15 | 45.73 | 45.02 | 44.73 | 44.77 |
| LP(2)S1→ π*C10–C16 | 45.81 | 45.27 | 47.23 | 47.02 |
| LP(1)N2→ π*C6–C13 | 99.32 | 99.44 | 91.37 | 92.13 |
| LP(1)N2→ π*C7–C14 | 101.03 | 101.78 | 100.74 | |
| LP(1)C20→ π*C10–C16 | 337.28 | |||
| LP(1)C20→ π*C14–C18 | 305.43 | |||
| ΣLP→π* | 291.89 | 832.44 | 285.11 | 284.66 |
| LP(1)*C7→ π*C10–C16 | 267.60 | |||
| LP(1)*C7→ π*C14–C18 | 258.91 | |||
| ΣLP*→π* | ||||
| π*C9–C15→ π*C17–C19 | 1084.83 | |||
| π*C10–C16→ π*C18–C20 | 1123.92 | |||
| π*C6–C13→ π*C17–C19 | 1247.39 | 1083.04 | ||
| π*C7–C14→ π*C18–C20 | 1071.67 | 978.87 | ||
| π*C9–C15→ π*C17–C19 | 1096.62 | 843.40 | 817.61 | |
| π*C10–C16→ π*C18–C20 | 1184.32 | 1207.85 | ||
| Σπ*→π* | 2208.87 | 1096.62 | 4346.78 | 4087.37 |
| σN3-C4→ LP(1)*H41 | 49.70 | 61.65 | ||
| σN3-C11→ LP(1)*H41 | 49.16 | 59.73 | ||
| σN3-C12→ LP(1)*H41 | 46.48 | 57.85 | ||
| Σσ→LP* | 145.34 | 179.23 | ||
| LP(1)N3→ LP(1)*H41 | 1234.86 | 1491.17 | ||
| LP(1)Cl42→ LP(1)*H41 | ||||
| LP(4)Cl42→ LP(1)*H41 | 704.83 | 305.14 | ||
| ΣLP→LP* | 1939.69 | 1796.31 | ||
| ΣTOTAL | 3484.4 | 3193.04 | 7527.88 | 7332.02 |
| Cationica | ||||
| Delocalization | Gas | PCM | ||
| πC13-C17→ π*C6–C9 | 47.90 | 46.98 | ||
| πC15-C19→ π*C6–C9 | 43.30 | 43.43 | ||
| πC15-C19→ π*C13–C17 | 47.07 | 47.61 | ||
| Σπ→π* | 138.27 | 138.02 | ||
| πC7-C10→ LP(1)*C16 | 93.63 | 94.47 | ||
| πC18-C20→ LP(1)*C16 | 107.30 | 107.05 | ||
| Σπ→LP* | 200.93 | 201.52 | ||
| LP(1)N2→ π*C6–C9 | 42.72 | |||
| LP(1)N2→ π*C7–C10 | 44.68 | |||
| LP(1)C14→ π*C7–C10 | 171.67 | 168.95 | ||
| LP(1)C14→ π*C18–C20 | 125.57 | 125.69 | ||
| LP(1)C16→ π*C7–C10 | 176.65 | 177.86 | ||
| LP(1)C16→ π*C18–C20 | 133.72 | 133.84 | ||
| ΣLP→π* | 607.61 | 693.74 | ||
| π*C6–C9→ π*C13–C17 | 361.78 | 356.18 | ||
| π*C6–C9→ π*C15–C19 | 231.49 | 223.25 | ||
| Σπ*→π* | 593.27 | 579.43 | ||
| ΣTOTAL | 1540.08 | 1612.71 | ||
The letters bold indicated RMSD values.
This work.
3.5. AIM studies
According to the Bader's theory the topological properties are interesting parameters to predict different types of interactions, such as intra or inter-molecular, ionic and hydrogen bonds interactions [59]. Hence, these properties can be easily computed in the bond critical points (BCPs) and in the ring critical points (RCPs) with the AIM2000 program [60]. Here, the electron density, ρ(r), the Laplacian values, ∇2ρ(r), the eigenvalues (λ1, λ2, λ3) of the Hessian matrix and, the |λ1|/λ3 ratio calculated by using the B3LYP/6-31G* method for the three forms of both S(-) and R(+)-PTZ enantiomers can be observed from Tables 9, 10 and 11. Note that the ionic and hydrogen bonds interactions are observed when λ1/λ3< 1 and ∇2ρ(r) > 0 [9]. Here, RCPN1, RCPN2 and RCPN3 are new RCPs formed as a consequence of C⋯H and H⋯H interactions while RCP1, RCP2 and RCP3 are RCPs corresponding to the R1, R2 and R3 rings, as defined in Fig. 1. In all species, the topological properties of RCP1 and RCP3 are practically the same in the two phenyl rings but different from RCP2 because this ring is the phenothiazine ring. First, analyzing the free bases species of both enantiomers, we observed that S(-)-PTZ present two C14⋯H21 and H⋯H interactions in both media but the involved atoms change of H24--H32 in gas phase to H23--H33 in solution. In R(+)-PTZ, the free base presents in gas phase the C14⋯H21 and H22⋯H31 interactions while in solution are observed three different H⋯H interactions. In the cationic species of S(-)-PTZ are not observed interactions while in R(+)-PTZ is observed a H⋯H interaction in gas phase while in solution are observed two C⋯H and a H⋯H interactions. The hydrochloride species of S(-)-PTZ present two interactions in gas phase and three different in solution while in the R(+)-PTZ enantiomer in gas phase (Table 11) are observed five interactions and only three in solution. In the hydrochloride species the Cl⋯H are ionic interactions where in S(-)-PTZ the Cl–H distances are 1.716 Å in gas phase and 2.032 Å in solution while in R(+)-PTZ the distances change to 1.748 Å in gas phase and 2.029 Å in solution. Evidently, both hydrochloride species are the most stable due to the higher values of their topological properties. These results are in agreement with those analyzed by NBO studies. The hydrochloride species of both forms of PTZ reveals higher stabilities than the corresponding to cyclizine [9].
Table 9.
Analysis of the Bond Critical Points (BCPs) and Ring critical point (RCPs) for three species of S(-)-promethazine in gas and aqueous solution by using the B3LYP/6-31G* method.
| B3LYP/6-31G* Method | ||||||||
|---|---|---|---|---|---|---|---|---|
| Free base | ||||||||
| Gas phase | ||||||||
| Parameter# |
C14--H21 |
RCPN1 |
H24--H32 |
RCPN2 |
RCP1 |
RCP2 |
RCP3 |
|
| ρ(r) | 0.0088 | 0.0088 | 0.0095 | 0.0095 | 0.0198 | 0.0170 | 0.0198 | |
| ∇2ρ(r) | 0.0333 | 0.0357 | 0.0400 | 0.0421 | 0.1580 | 0.1104 | 0.1580 | |
| λ1 | -0.0043 | -0.0036 | -0.0084 | -0.0080 | -0.0146 | -0.0055 | -0.0145 | |
| λ2 | -0.0011 | 0.0013 | -0.0014 | 0.0015 | 0.0815 | 0.0552 | 0.0813 | |
| λ3 | 0.0388 | 0.0379 | 0.0500 | 0.0485 | 0.0910 | 0.0608 | 0.0911 | |
| |λ1|/λ3 | 0.1108 | 0.0950 | 0.1680 | 0.1649 | 0.1604 | 0.0905 | 0.1592 | |
| Distances (Å) |
2.693 |
2.190 |
||||||
| Aqueous solution |
||||||||
| Parameter# |
C14--H21 |
RCPN1 |
H23--H33 |
RCPN2 |
RCP1 |
RCP2 |
RCP3 |
|
| ρ(r) | 0.0093 | 0.0090 | 0.0133 | 0.0133 | 0.0198 | 0.0169 | 0.0198 | |
| ∇2ρ(r) | 0.0344 | 0.0398 | 0.0626 | 0.0656 | 0.1573 | 0.1103 | 0.1572 | |
| λ1 | -0.0049 | -0.0026 | -0.0084 | -0.0077 | -0.0145 | -0.0055 | -0.0144 | |
| λ2 | 0.0035 | 0.0050 | -0.0019 | 0.0020 | 0.0809 | 0.0569 | 0.0808 | |
| λ3 | 0.0429 | 0.0375 | 0.0729 | 0.0712 | 0.0909 | 0.0590 | 0.0908 | |
| |λ1|/λ3 | 0.1142 | 0.0693 | 0.1152 | 0.1081 | 0.1595 | 0.0932 | 0.1586 | |
| Distances (Å) |
2.646 |
2.086 |
||||||
| Cationic | ||||||||
| Gas phase | ||||||||
| Parameter# |
RCP1 |
RCP2 |
RCP3 |
|||||
| ρ(r) | 0.0199 | 0.0173 | 0.0199 | |||||
| ∇2ρ(r) | 0.1584 | 0.1084 | 0.1586 | |||||
| λ1 | -0.0146 | -0.0050 | -0.0146 | |||||
| λ2 | 0.0832 | 0.0469 | 0.0835 | |||||
| λ3 | 0.0896 | 0.0665 | 0.0896 | |||||
| |λ1|/λ3 |
0.1629 |
0.0752 |
0.1629 |
|||||
| Hydrochloride | ||||||||
| Gas phase | ||||||||
| Parameter# |
Cl42--H25 |
Cl42--H41 |
RCPN1 |
RCP1 |
RCP2 |
RCP3 |
||
| ρ(r) | 0.0080 | 0.0804 | 0.0080 | 0.0198 | 0.0171 | 0.0198 | ||
| ∇2ρ(r) | 0.0263 | 0.0866 | 0.0284 | 0.1582 | 0.1100 | 0.1582 | ||
| λ1 | -0.0062 | -0.1359 | -0.0062 | -0.0146 | -0.0053 | -0.0145 | ||
| λ2 | -0.0017 | -0.1357 | 0.0018 | 0.0822 | 0.0530 | 0.0820 | ||
| λ3 | 0.0342 | 0.3583 | 0.0327 | 0.0905 | 0.0624 | 0.0906 | ||
| |λ1|/λ3 | 0.1813 | 0.3793 | 0.1896 | 0.1613 | 0.0849 | 0.1600 | ||
| Distances (Å) |
2.908 |
1.716 |
||||||
| Aqueous solution | ||||||||
| Parameter# |
C13--H23 |
RCPN1 |
H22---28 |
RCPN2 |
Cl42--H41 |
RCP1 |
RCP2 |
RCP3 |
| ρ(r) | 0.0134 | 0.0133 | 0.0090 | 0.0090 | 0.0416 | 0.0198 | 0.0169 | 0.0198 |
| ∇2ρ(r) | 0.0617 | 0.0666 | 0.0384 | 0.0398 | 0.0764 | 0.1574 | 0.1094 | 0.1574 |
| λ1 | -0.0093 | -0.0081 | -0.0079 | -0.0074 | -0.0534 | -0.0145 | -0.0056 | -0.0145 |
| λ2 | -0.0031 | 0.0036 | -0.0014 | 0.0014 | -0.0532 | 0.0813 | 0.0552 | 0.0811 |
| λ3 | 0.0742 | 0.0711 | 0.0476 | 0.0458 | 0.1828 | 0.0906 | 0.0597 | 0.0907 |
| |λ1|/λ3 | 0.1253 | 0.1139 | 0.1660 | 0.1616 | 0.2921 | 0.1600 | 0.0938 | 0.1599 |
| Distances (Å) | 2.508 | 2.189 | 2.032 | |||||
# This symbol implies values in a.u. units.
Table 10.
Analysis of the Bond Critical Points (BCPs) and Ring critical point (RCPs) for free base and cationic species of R(+)-promethazine in gas and aqueous solution by using the B3LYP/6-31G* method.
| B3LYP/6-31G* Method | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Free base | |||||||||
| Gas phase | |||||||||
| Parameter# |
C14--H21 |
RCPN1 |
H22--H31 |
RCPN2 |
RCP1 |
RCP2 |
RCP3 |
||
| ρ(r) | 0.0084 | 0.0084 | 0.0120 | 0.0110 | 0.0198 | 0.0170 | 0.0198 | ||
| ∇2ρ(r) | 0.0315 | 0.0332 | 0.0483 | 0.0571 | 0.1580 | 0.1105 | 0.1580 | ||
| λ1 | -0.0037 | -0.0033 | -0.0127 | 0.0078 | -0.0146 | -0.0055 | -0.0145 | ||
| λ2 | -0.0008 | 0.0009 | -0.0083 | 0.0107 | 0.0815 | 0.0551 | 0.0813 | ||
| λ3 | 0.0362 | 0.0355 | 0.0694 | 0.0542 | 0.0911 | 0.0609 | 0.0911 | ||
| |λ1|/λ3 | 0.1022 | 0.0930 | 0.1830 | -0.1439 | 0.1603 | 0.0903 | 0.1592 | ||
| Distances (Å) |
2.727 |
2.024 |
|||||||
| Aqueous solution | |||||||||
| Parameter# |
H31--H34 |
H22--H31 |
RCPN1 |
H23--H33 |
RCPN2 |
RCP1 |
RCP2 |
RCP3 |
|
| ρ(r) | 0.0057 | 0.0128 | 0.0057 | 0.0132 | 0.0132 | 0.0198 | 0.0170 | 0.0198 | |
| ∇2ρ(r) | 0.0212 | 0.0511 | 0.0208 | 0.0605 | 0.0656 | 0.1573 | 0.1103 | 0.1572 | |
| λ1 | -0.0041 | -0.0137 | -0.0038 | -0.0093 | -0.0080 | -0.0145 | -0.0055 | -0.0144 | |
| λ2 | -0.0022 | -0.0092 | 0.0029 | -0.0033 | 0.0039 | 0.0809 | 0.0559 | 0.0807 | |
| λ3 | 0.0275 | 0.0742 | 0.0216 | 0.0732 | 0.0697 | 0.0909 | 0.0598 | 0.0909 | |
| |λ1|/λ3 | 0.1491 | 0.1846 | 0.1759 | 0.1270 | 0.1148 | 0.1595 | 0.0920 | 0.1584 | |
| Distances (Å) |
2.353 |
1.995 |
2.072 |
||||||
| Cationic | |||||||||
| Gas phase | |||||||||
| Parameter# |
H22--H31 |
RCPN1 |
RCP1 |
RCP2 |
RCP3 |
||||
| ρ(r) | 0.0120 | 0.0108 | 0.0199 | 0.0173 | 0.0199 | ||||
| ∇2ρ(r) | 0.0478 | 0.0533 | 0.1584 | 0.1084 | 0.1588 | ||||
| λ1 | -0.0131 | -0.0087 | -0.0146 | -0.0049 | -0.0146 | ||||
| λ2 | -0.0086 | 0.0107 | 0.0832 | 0.0472 | 0.0836 | ||||
| λ3 | 0.0696 | 0.0513 | 0.0897 | 0.0664 | 0.0896 | ||||
| |λ1|/λ3 | 0.1882 | 0.1696 | 0.1628 | 0.0738 | 0.1629 | ||||
| Distances (Å) |
2.008 |
||||||||
| Aqueous solution | |||||||||
| Parameter# |
C14--H21 |
RCPN1 |
C13--H23 |
RCPN2 |
H22--H31 |
RCPN3 |
RCP1 |
RCP2 |
RCP3 |
| ρ(r) | 0.0085 | 0.0085 | 0.0131 | 0.0131 | 0.0124 | 0.0112 | 0.0198 | 0.0169 | 0.0198 |
| ∇2ρ(r) | 0.0326 | 0.03369 | 0.0617 | 0.0639 | 0.0495 | 0.0556 | 0.1576 | 0.1095 | 0.1575 |
| λ1 | -0.0033 | -0.0030 | -0.0085 | -0.0080 | -0.0135 | -0.0090 | -0.0145 | -0.0056 | -0.0145 |
| λ2 | -0.0006 | 0.0006 | -0.0014 | 0.0015 | -0.0087 | 0.0108 | 0.0815 | 0.0553 | 0.0811 |
| λ3 | 0.0366 | 0.0360 | 0.0716 | 0.0704 | 0.0717 | 0.0538 | 0.0906 | 0.0598 | 0.0908 |
| |λ1|/λ3 | 0.0902 | 0.0833 | 0.1187 | 0.1136 | 0.1883 | 0.1673 | 0.1600 | 0.0936 | 0.1597 |
| 2.718 | 2.520 | 1.996 | |||||||
# This symbol implies values in a.u. units.
Table 11.
Analysis of the Bond Critical Points (BCPs) and Ring critical point (RCPs) for three species of R(+)-promethazine in gas and aqueous solution by using the B3LYP/6-31G* method.
| B3LYP/6-31G* Method | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrochloride | ||||||||||||
| Gas phase | ||||||||||||
| Parameter# |
C5⋯H34 |
RCPN1 |
C13⋯H23 |
RCPN2 |
H22⋯H31 |
RCPN3 |
Cl42⋯H23 |
Cl42⋯H41 |
RCPN3 |
RCP1 |
RCP2 |
RCP3 |
| ρ(r) | 0.0112 | 0.0112 | 0.0130 | 0.0130 | 0.0120 | 0.0109 | 0.0093 | 0.0746 | 0.0082 | 0.0198 | 0.0170 | 0.0199 |
| ∇2ρ(r) | 0.0508 | 0.0508 | 0.0624 | 0.0624 | 0.0484 | 0.0548 | 0.0311 | 0.0935 | 0.0343 | 0.1580 | 0.1096 | 0.1584 |
| λ1 | -0.0092 | -0.0092 | -0.0090 | -0.0090 | -0.0130 | -0.0087 | -0.0072 | -0.1220 | -0.0057 | -0.0146 | -0.0055 | -0.0146 |
| λ2 | -0.0005 | -0.0005 | -0.0006 | -0.0006 | -0.0085 | 0.0107 | -0.0051 | -0.1219 | 0.0068 | 0.0815 | 0.0535 | 0.0820 |
| λ3 | 0.0606 | 0.0606 | 0.0720 | 0.0720 | 0.0699 | 0.0528 | 0.0435 | 0.3374 | 0.0331 | 0.0910 | 0.0615 | 0.0909 |
| |λ1|/λ3 | 0.1518 | 0.1518 | 0.1250 | 0.1250 | 0.1860 | 0.1648 | 0.1655 | 0.3616 | 0.1722 | 0.1604 | 0.0894 | 0.1606 |
| Distances (Å) |
2.637 |
2.520 |
2.008 |
2.814 |
1.748 |
|||||||
| Aqueous solution | ||||||||||||
| Parameter# |
C13⋯H23 |
RCPN1 |
H22⋯H31 |
RCPN2 |
Cl42⋯H41 |
RCP1 |
RCP2 |
RCP3 |
||||
| ρ(r) | 0.0134 | 0.0134 | 0.0122 | 0.0111 | 0.0418 | 0.0198 | 0.0169 | 0.0198 | ||||
| ∇2ρ(r) | 0.0633 | 0.0668 | 0.0494 | 0.0558 | 0.0771 | 0.1575 | 0.1093 | 0.1576 | ||||
| λ1 | -0.0094 | -0.0085 | -0.0131 | -0.0088 | -0.0536 | -0.0145 | -0.0057 | -0.0145 | ||||
| λ2 | -0.0023 | 0.0025 | -0.0085 | 0.0106 | -0.0535 | 0.0813 | 0.0557 | 0.0808 | ||||
| λ3 | 0.0750 | 0.0726 | 0.0711 | 0.0537 | 0.1843 | 0.0907 | 0.0592 | 0.0911 | ||||
| |λ1|/λ3 | 0.1253 | 0.1171 | 0.1842 | 0.1639 | 0.2908 | 0.1599 | 0.0963 | 0.1592 | ||||
| Distances (Å) | 2.507 | 1.999 | 2.029 | |||||||||
# This symbol implies values in a.u. units.
3.6. Frontier orbitals and global descriptors studies
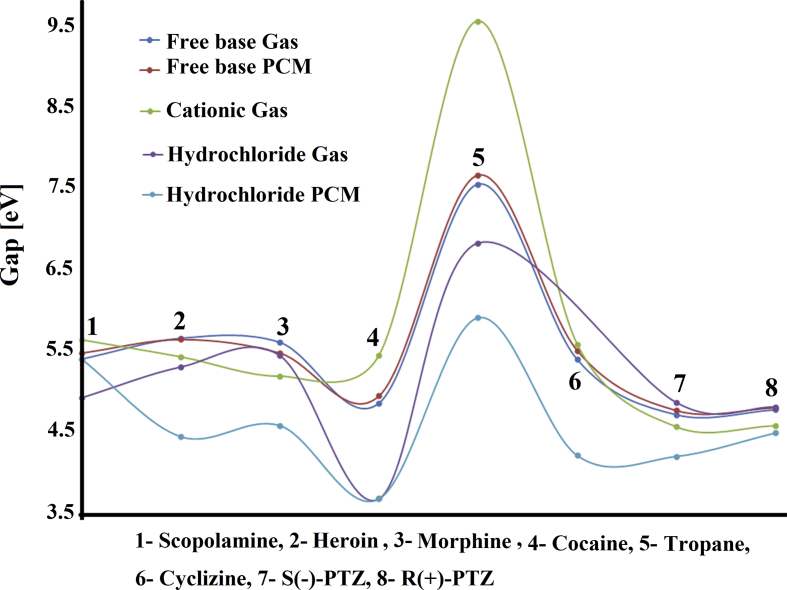
To predict reactivities and behaviours of both S(-) and R(+)-PTZ forms are of interest to understand why the presence of two N–CH3 groups in their structures present the same biological activities than cyclizine despite those two groups in PTZ are not linked to rings. Hence, from the frontier orbitals and their differences is possible to compute the gap values [43, 44] and later, by using known equations the chemical potential (μ), electronegativity (χ), global hardness (η), global softness (S), global electrophilicity index (ω) and global nucleophilicity index (Ε) descriptors can be calculated by using the hybrid B3LYP/6-31G* level of theory [45, 46, 47, 48, 49, 50, 51, 52, 53]. The gap and descriptors values for both PTZ enantiomers in the two media are presented in Table 12. The evaluation of gap values for the three species show easily that the hydrochloride species of both S(-) and R(+)-PTZ forms in solution have low gap values and, for these reasons, the two species are more reactive but the S(-) form is most reactive than the R(+)-PTZ one, as expected because this latter form presents higher stability by NBO analysis (>Total energy). Moreover, the free base and cationic species of S(-) form are most reactive than the corresponding to the R(+) form. Comparisons of these results with the observed for similar species containing N–CH3 groups, as scopolamine, heroin morphine, cocaine, tropane and cyclizine are presented in Table 13 while their behaviours can be seen in Fig. 6. This figure shows that the hydrochloride species of cocaine in both media present the lower gap values and, obviously, are the most reactive species while in all media the tropane species are the less reactive being, the cationic one in gas phase the less reactive. Note that the free base and cationic species of two forms of PTZ are most reactive than the corresponding to cyclizine, however, the hydrochloride species of cyclizine is most reactive than both forms of PTZ. If now the descriptors are analyzed it is observed from Table 12 that the three species of S(-)-PTZ have higher electrophilicity indexes than the corresponding to R(+) form while, on the contrary, the species of R(+) form have higher nucleophilicity indexes than the species of S(-)-PTZ. The only exception is the hydrochloride species in gas phase of S(-) form because it present a higher value (-7.6061 eV) than the corresponding to R(+) form (7.1020 eV). If both electrophilicity and nucleophilicity indexes of the two S(-) and R(+)-PTZ are compared with other species from Table 14 the behaviours can easily be seen in Fig. 7. Higher electrophilicity indexes are observed in the cationic and hydrochloride species of PTZ than cyclizine while the cationic species of cyclizine have higher nucleophilicity index than both species of PTZ. The higher electrophilicity indexes are observed for all cationic forms in gas phase and, in particular, for cocaine while tropane in both media presents the lowest values. In relation to nucleophilicity indexes, the cationic species of tropane in gas phase presents the highest negative value indicating probably that for these two reasons, this species is the less reactive than the other ones (see Table 13).
Table 12.
Frontier molecular HOMO and LUMO orbitals , gap values and descriptors for the three species of S(−) and R(+)-promethazine (in eV) in gas and aqueous solution by using the B3LYP/6-31G* level of theory.
| Orbitals | Free base |
Cationic |
Hydrochloride |
|||
|---|---|---|---|---|---|---|
| Gas | PCM | Gas | PCM | Gas | PCM | |
| S(-)-promethazine | ||||||
| HOMO | -5.0096 | -5.0559 | -7.943 | -5.5593 | -5.0151 | |
| LUMO | -0.2939 | -0.2857 | -3.3769 | -0.6939 | -0.8109 | |
| ∣GAP∣ |
4.7157 |
4.7702 |
4.5661 |
4.8654 |
4.2042 |
|
| Descriptors | ||||||
| χ | -2.3579 | -2.3851 | -2.2831 | -2.4327 | -2.1021 | |
| μ | -2.6518 | -2.6708 | -5.6600 | -3.1266 | -2.9130 | |
| η | 2.3579 | 2.3851 | 2.2831 | 2.4327 | 2.1021 | |
| S | 0.2121 | 0.2096 | 0.2190 | 0.2055 | 0.2379 | |
| ω | 1.4911 | 1.4954 | 7.0158 | 2.0092 | 2.0184 | |
| Ε |
-6.2524 |
-6.3701 |
-12.9219 |
-7.6061 |
-6.1234 |
|
| R(+)-promethazine | ||||||
| HOMO | -5.0504 | -5.0776 | -7.9403 | -5.5593 | -5.3579 | -5.1538 |
| LUMO | -0.2748 | -0.2748 | -3.3633 | -0.6939 | -0.5469 | -0.6612 |
| ∣GAP∣ |
4.7756 |
4.8028 |
4.5770 |
4.8654 |
4.8110 |
4.4926 |
| Descriptors | ||||||
| χ | -2.3878 | -2.4014 | -2.2885 | -2.4327 | -2.4055 | -2.2463 |
| μ | -2.6626 | -2.6762 | -5.6518 | -3.1266 | -2.9524 | -2.9075 |
| η | 2.3878 | 2.4014 | 2.2885 | 2.4327 | 2.4055 | 2.2463 |
| S | 0.2094 | 0.2082 | 0.2185 | 0.2055 | 0.2079 | 0.2226 |
| ω | 1.4845 | 1.4912 | 6.9790 | 2.0092 | 1.8118 | 1.8817 |
| Ε | -6.3578 | -6.4266 | -12.9341 | -7.6061 | -7.1020 | -6.5311 |
χ = - [E(LUMO)- E(HOMO)]/2; μ = [E(LUMO) + E(HOMO)]/2; η = [E(LUMO) - E(HOMO)]/2.
S = ½η; ω = μ2/2η; Ε = μ*η.
Table 13.
Frontier molecular HOMO and LUMO orbitals and gap values for the three species of S(-) and R(+)-promethazine compared with other species in gas and aqueous solution phases by using the B3LYP/6-31G* level of theory.
| Orbital | Scopolamine#,b | Heroinc | Morphined | Cocainee | Tropanef | Cyclizineg | Promethazinea |
|
|---|---|---|---|---|---|---|---|---|
| S(-) | R(+) | |||||||
| Free base/Gas phase | ||||||||
| ∣GAP∣ |
5.4004 |
5.6563 |
5.6044 |
4.8580 |
7.5506 |
5.3946 |
4.7157 |
4.7756 |
| Free base/Aqueous solution | ||||||||
| ∣GAP∣ |
5.4758 |
5.6414 |
5.4750 |
4.9487 |
7.6611 |
5.5067 |
4.7702 |
4.8028 |
| Cationic/Gas phase | ||||||||
| ∣GAP∣ |
5.6356 |
5.4268 |
5.1889 |
5.4468 |
9.5595 |
5.5823 |
4.5661 |
4.5770 |
| Hydrochloride/Gas phase | ||||||||
| ∣GAP∣ |
4.9239 |
5.3024 |
5.4417 |
3.6813 |
6.8246 |
4.8654 |
4.8110 |
|
| Hydrochloride/Aqueous solution | ||||||||
| ∣GAP∣ | 5.4026 | 4.4469 | 4.5840 | 3.6813 | 5.9119 | 4.2159 | 4.2042 | 4.4926 |
Fig. 6.
Calculated gap values of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine in both media by using the B3LYP/6-31G* method compared with reported values for alkaloids and antihistaminic agents.
Table 14.
Global electrophilicity(ω) and nucleophilicity (E) indexes for the three species of S(-) and R(+)-promethazine compared with other species in gas and aqueous solution phases by using the B3LYP/6-31G* level of theory.
| Descriptor | Scopolamine#,b | Heroinc | Morphined | Cocainee | Tropanef | Cyclizineg | Promethazinea |
|
|---|---|---|---|---|---|---|---|---|
| S(-) | R(+) | |||||||
| Free base/Gas phasea | ||||||||
| ω | 1.7393 | 1.5083 | 1.3639 | 2.5183 | 0.3914 | 1.6777 | 1.4911 | 1.4845 |
| Ε |
-8.2756 |
-8.2606 |
-7.7475 |
-8.4959 |
-6.4905 |
-8.1146 |
-6.2524 |
-6.3578 |
| Free base/Aqueous solutiona | ||||||||
| ω | 1.7504 | 1.5180 | 1.2339 | 2.5297 | 0.4429 | 1.7288 | 1.4954 | 1.4912 |
| Ε |
-8.4763 |
-8.2545 |
-7.1153 |
-8.7546 |
-7.0557 |
-8.4953 |
-6.3701 |
-6.4266 |
| Cationic/gas phasea | ||||||||
| ω | 6.4529 | 6.7459 | 6.8155 | 7.9799 | 6.9598 | 6.5083 | 7.0158 | 6.9790 |
| Ε |
-16.9925 |
-16.4174 |
-15.4288 |
-17.9548 |
-38.9872 |
-16.8238 |
-12.9219 |
-12.9341 |
| Hydrochloride/Aqueous solutiona | ||||||||
| ω | 0.9799 | 1.9667 | 1.8414 | 2.6828 | 0.6421 | 1.9053 | 2.0184 | 1.8817 |
| Ε | -6.2154 | -6.5755 | -6.6589 | -5.7845 | -5.7592 | -5.9742 | -6.1234 | -6.5311 |
Fig. 7.
Calculated electrophilicity indexes of free base, cationic and hydrocloride species of both S(-) and R(+) enantiomers of promethazine in both media by using the B3LYP/6-31G* method.
3.7. Vibrational study
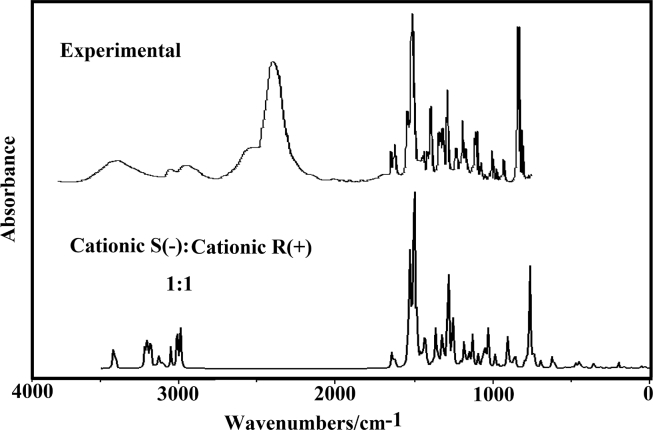
B3LYP calculations have optimized the three species of S(-) and R(+)-PTZ forms with C1 symmetries. The normal vibration modes expected for the free base, cationic and hydrochloride species are respectively 114, 117 and 120 and, where all modes are active, in both spectra. The experimental available infrared and Raman spectra for promethazine hydrochloride were taken from Refs [10] and [66]. The experimental IR from Ref [66] was compared with the corresponding predicted for the three species of both enantiomers in Fig. 8 while the comparisons of the corresponding predicted Raman spectra with the experimental one are given in Fig. 9. Evidently, the hydrochloride forms of both enantiomers are not present in the experimental IR spectrum because the predicted intense IR bands of both S(-) and R(+) forms at 1625 and 1713 cm−1 respectively are not observed in the experimental one with the same intensities. Besides, the predicted IR spectra in the 2000-500 cm−1 region show strong differences between the intensities of IR bands at 1459 and 759 cm−1 in the three species of both S(-) and R(+)-PTZ enantiomers but when only the average of cationic forms by using frequencies and intensities Lorentzian band shapes for a 1:1 population ratio of each species the ratio between those two bands decreases notably, as shown in Fig. 10. Note that in the higher wavenumbers region the predicted IR spectra for both cationic species are similar to the corresponding experimental ones. Hence, it is evident the presence of both cationic species of S(-) and R(+)-PTZ in the solid phase, as revealed by Borodi et al [19]. The normal internal coordinates, the SQMFF methodology [39] and the Molvib program [42] were used to calculate the harmonic force fields in order to perform the complete vibrational assignments of all species of DHC. The scale factors used were those reported in the literature [40]. In Table 15 are presented the experimental and calculated wavenumbers together with the assignments of three species of S(-) and R(+)-PTZ forms, respectively. Below, discussions of assignments for some groups are presented.
Fig. 8.
Experimental infrared spectrum of hydrocloride promethazine compared with the corresponding predicted for the free base, cationic and hydrochloride species of both S(-) and R(+) enantiomers by using B3LYP/6-31G* level of theory.
Fig. 9.
Experimental Raman spectrum of hydrocloride promethazine compared with the corresponding predicted for the free base, cationic and hydrochloride species of both S(-) and R(+) enantiomers by using B3LYP/6-31G* level of theory.
Fig. 10.
Experimental infrared spectrum of hydrocloride promethazine compared with the corresponding average predicted for the cationic species of both S(-) and R(+) enantiomers by using frequencies and intensities Lorentzian band shapes for a 1:1 population ratio of each species at B3LYP/6-31G* level of theory.
Table 15.
Observed and calculated wavenumbers (cm−1) and assignments for the three species of S(-) and R(+)-promethazine in gas phase by using B3LYP/6-31G* level of theory.
| Experimental |
B3LYP/6-31G* Methoda |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S(-)-PTZ |
R(+)-PTZ |
|||||||||||||
| Free base |
Cationic |
Hydrochloride |
Free base |
Cationic |
Hydrochloride |
|||||||||
| IRc | IRd | Ramane | SQMb | Assignmentsa | SQMb | Assignmentsa | SQMb | Assignmentsa | SQMb | Assignmentsa | SQMb | Assignmentsa | SQMb | Assignmentsa |
| 3391w,br | 3448w | 3411vw | 3295 | νN3-H41 | 3273 | νN3-H41 | ||||||||
| 3104w | 3092 | νC14-H34 | 3092 | νC19-H39 | ||||||||||
| 3091 | νC20-H40 | |||||||||||||
| 3104w | 3090 | νaCH3(C11) | 3090 | νC13-H33 | ||||||||||
| 3087 | νC13-H33 | 3088 | νaCH3(C12) | 3087 | νC14-H34 | |||||||||
| 3081 | νC15-H35 | |||||||||||||
| 3079 | νC20-H40 | 3080 | νC17-H37 | 3080 | νC20-H40 | |||||||||
| 3078 | νC19-H39 | 3078 | νC16-H36 | 3078 | νaCH3(C11) | |||||||||
| 3073 | νC15-H35 | 3071 | νaCH3(C12) | |||||||||||
| 3071 | νC14-H34 | 3072 | νC18-H38 | 3070 | νC17-H37 | |||||||||
| 3066 | νC13-H33 | 3069 | νC20-H40 | 3067 | νC14-H34 | 3066 | νC15-H35 | 3067 | νaCH3(C12) | 3067 | νC16-H36 | |||
| 3069 | νC19-H39 | 3065 | νC19-H39 | 3066 | νC16-H36 | 3063 | νaCH3(C11) | 3060 | νC19-H39 | |||||
| 3058sh | 3059 | νC20-H40 | 3057 | νC17-H37 | 3059 | νC13-H33 | 3058 | νC18-H38 | ||||||
| 3058sh | 3058 | νC19-H39 | 3056 | νC16-H36 νC18-H38 |
3060 | νC20-H40 | 3057 | νC18-H38 | ||||||
| 3051 | νaCH3(C12) | 3057 | νC13-H33 | 3056 | νC17-H37 | 3055 | νC14-H34 | |||||||
| 3050 | νC16-H36 νC15-H35 |
3049 | νC16-H36 | |||||||||||
| 3046 | νC16-H36 | 3050 | νC15-H35 | 3047 | νC15-H35 | |||||||||
| 3045 | νC15-H35 | 3044 | νaCH3(C11) | 3040 | νC18-H38 | |||||||||
| 3046w,br | 3044m | 3039 | νC13-H33 | 3039 | νC17-H37 | 3039 | νaCH3(C12) | |||||||
| 3037 | νC17-H37 | 3037 | νC14-H34 | 3036 | νaCH3(C11) | |||||||||
| 3035 | νaCH3(C12) | |||||||||||||
| 3035sh | 3037 | νC18-H38 | 3031 | νaCH3(C11) | 3032 | νaCH3(C12) | 3030 | νaCH3(C8) | 3031 | νaCH2 | ||||
| 3019 | νaCH3(C11) | 3021 | νaCH3(C11) | 3025 | νaCH3(C8) | |||||||||
| 3006 | νaCH3(C11) | 3013 | νaCH3(C8) | 3014 | νaCH3(C8) | |||||||||
| 3018w | 3004 | νaCH3(C12) | 3012 | νaCH2 | 3012 | νaCH3(C8) | ||||||||
| 2986 | νaCH3(C8) | 2995 | νaCH3(C8) | 2999 | νaCH3(C8) | 3000 | νaCH3(C12) | 2989 | νC4-H21 | |||||
| 2980w | 2984 | νaCH2 | 2984 | νaCH2 | 2986 | νaCH2 | 2999 | νaCH3(C8) | 2982 | νsCH3(C12) | ||||
| 2966sh | 2978 | νaCH3(C12) | 2974 | νaCH3(C8) | 2979 | νaCH3(C8) | 2978 | νsCH3(C11) | ||||||
| 2948w | 2968 | νaCH3(C8) | 2955 | νC4-H21 | 2962 | νaCH3(C11) | 2958 | νsCH3(C12) | ||||||
| 2962 | νaCH3(C11) | 2952 | νsCH3(C12) | 2953 | νC4-H21 | 2957 | νaCH3(C12) | 2956 | νsCH3(C11) | |||||
| 2938w,br | 2930sh | 2928 | νC4-H21 | 2947 | νsCH3(C11) | 2931 | νsCH3(C12) | 2974 | νaCH2 | 2945 | νsCH3(C8) | |||
| 2925 | νsCH3(C12) | 2926 | νsCH3(C11) | 2929 | νsCH3(C8) | 2933 | νsCH3(C8) | 2941 | νC4-H21 | |||||
| 2918 | νsCH3(C11) | 2913 | νsCH3(C8) | 2915 | νsCH2 | 2926 | νsCH2 | |||||||
| 2907sh | 2907 | νsCH3(C8) | 2908 | νsCH3(C8) | ||||||||||
| 2872sh | 2888sh | 2887sh | 2875 | νsCH2 | 2863 | νsCH2 | 2872 | νsCH2 | 2837 | νC4-H21 | 2894 | νsCH2 | ||
| 2824m | 2793 | νaCH3(C12) | 2820 | νsCH3(C12) | ||||||||||
| 2747m | 2782 | νaCH3(C11) | 2812 | νsCH3(C11) | ||||||||||
| 2673w | ||||||||||||||
| 2370s | 2508w | |||||||||||||
| 1688w | 1625 | νN3-H41 | 1713 | νN3-H41 | ||||||||||
| 1638vw | 1633w | 1630vw | 1600 | νC13-C17 | 1596 | νC13-C17 νC14-C18 |
1599 | νC14-C18 | ||||||
| 1596w | 1586w | 1581sh | 1581 | νC14-C18 | 1580 | νC14-C18 | 1581 | νC18-C20 νC17-C19 |
1584 | νC17-C19 νC18-C20 |
1581 | νC17-C19 νC15-C19 |
||
| 1558s | 1577 | νC13-C17 νC14-C18 |
1577 | νC14-C18 | 1578 | νC7-C14 | 1578 | νC13-C17 | ||||||
| 1573w | 1582sh | 1558s | 1561 | νC7-C14 | 1566 | νC17-C19 | 1564 | νC7-C14 | 1570 | νC17-C19 νC18-C20 |
1573 | νC18-C20 νC17-C19 |
1571 | νC7-C14 |
| 1558s | 1556 | νC13-C17 | 1558 | νC7-C14 | 1557 | νC13-C17 | ||||||||
| 1550w | 1552sh | 1550 | νC17-C19 νC18-C20 |
1554 | νC17-C19 | 1552 | νC17-C19 νC18-C20 |
1498 | ρN3-H41 | |||||
| 1489m | 1500w | 1489 | βC13-H33 βC16-H36 |
1486 | δaCH3(C8) | 1488 | βC14-H34 | |||||||
| 1480sh | 1483 | δaCH3(C8) | 1484 | δaCH3(C8) | 1480 | δaCH3(C8) | ||||||||
| 1466sh | 1470w | 1473 | ρN3-H41 | 1474 | δCH2 | 1478 | δaCH3(C12) | 1476 | δCH2 δaCH3(C8) |
|||||
| 1466sh | 1470w | 1470 | βC16-H36 βC14-H34 |
1471 | δaCH3(C11) δaCH3(C12) | 1473 | δaCH3(C8) | |||||||
| 1466sh | 1470w | 1469 | βC16-H36 βC14-H34 βC13-H33 |
1467 | βC15-H35 βC13-H33 βC14-H34 |
1468 | δaCH3(C8) | 1468 | δaCH3(C8) | |||||
| 1459vs | 1454sh | 1463 | δaCH3(C12) | 1463 | δaCH3(C11) | 1464 | δaCH3(C11) δaCH3(C12) | |||||||
| 1459vs | 1454sh | 1456 | δaCH3(C12) δaCH3(C11) |
1456 | δaCH3(C11) | 1458 | δaCH3(C11) | 1460 | βC14-H34 βC13-H33 |
1461 | δaCH3(C12) | |||
| 1459vs | 1454sh | 1452 | δaCH3(C11) | 1454 | δCH2 | 1455 | δCH2 | 1453 | δCH2 | 1454 | δCH2 | |||
| 1451sh | 1451 | δCH2 | 1451 | δaCH3(C12) | 1451 | δaCH3(C8) | 1450 | δaCH3(C12) | 1451 | δaCH3(C11) | 1449 | δaCH3(C11) | ||
| 1447sh | 1447sh | 1444 | δaCH3(C8) | 1446 | δaCH3(C8) δaCH3(C12) |
1449 | δaCH3(C11) | 1447 | βC20-H40 βC18-H38 |
1447 | βC20-H40 βC18-H38 |
|||
| 1447sh | 1447sh | 1440 | δaCH3(C8) | 1444 | δaCH3(C12) | 1442 | βC13-H33 | 1446 | βC20-H40 βC18-H38 |
1446 | βC19-H39 | 1446 | βC19-H39 | |
| 1433sh | 1438sh | 1437 | δaCH3(C12) δaCH3(C8) |
1440 | δCH2 | 1438 | δaCH3(C8) | 1444 | βC19-H39 | 1442 | δaCH3(C12) | 1443 | δaCH3(C11) δaCH3(C12) | |
| 1433sh | 1438sh | 1435sh | 1435 | δaCH3(C8) | 1432 | βC19-H39 | 1438 | δaCH3(C12) | ||||||
| 1430 | δaCH3(C12) | 1431 | βC20-H40 βC17-H37 |
1430 | βC20-H40 | 1435 | δsCH3(C12) δsCH3(C11) | |||||||
| 1429 | βC17-H37 βC19-H39 |
1429 | δaCH3(C11) δaCH3(C12) | 1429 | βC19-H39 | 1427 | δsCH3(C12) | 1425 | wagCH2 ρ′N3–H41 |
|||||
| 1421sh | 1427 | βC19-H39 | 1423 | δaCH3(C8) | 1426 | δaCH3(C12) | 1406 | wagCH2 | 1407 | wagCH2 | 1420 | wagCH2 | ||
| 1419sh | 1417 | δaCH3(C11) | 1411 | δsCH3(C12) | 1420 | δaCH3(C11) | 1400 | ρN3-H41 | 1408 | δsCH3(C11) | ||||
| 1408vw | 1403sh | 1406 | δsCH3(C11) | 1400 | δaCH3(C8) | 1408 | ρ′N3–H41 | 1402 | wagCH2 δsCH3(C11) |
1397 | δsCH3(C11) | 1401 | δsCH3(C12) | |
| 1390vw | 1395sh | 1392 | ρ′N3–H41 wagCH2 | 1394 | wagCH2 | 1394 | ρ′N3–H41 | |||||||
| 1378w | 1387w | 1388 | wagCH2 | 1381 | ρN3-H41 | 1376 | δsCH3(C11) δsCH3(C12) | 1375 | δsCH3(C8) ρ′C4–H21 |
1380 | δsCH3(C8) | 1379 | δsCH3(C8) | |
| 1364sh | 1374vw | 1376 | δsCH3(C12) | 1379 | δsCH3(C11) | 1360 | δsCH3(C8) | |||||||
| 1354w | 1362 | ρC4-H21 | 1361 | δsCH3(C8) | 1355 | νN3-H41 δsCH3(C12) |
1357 | δsCH3(C8) | 1350 | ρ′C4–H21 ρCH2 |
1356 | ρ′C4–H21 | ||
| 1342sh | 1347sh | 1340w | 1342 | δsCH3(C8) | 1349 | ρC4-H21 | 1351 | ρC4-H21 | ||||||
| 1334m | 1327sh | 1326sh | 1323 | ρ′C4–H21 | 1335 | ρ′C4–H21 | 1330 | ρC4-H21 νN2-C6 |
1327 | ρCH2 νN2-C6 |
||||
| 1320w | 1319 | ρCH2 νN2-C6 |
1320 | ρ′C4–H21 | 1318 | ρC4-H21 | 1320 | ρ′C4–H21 | 1313 | ρC4-H21 | ||||
| 1292sh | 1312sh | 1315sh | 1309 | νN2-C6 ρCH2 |
1307 | ρ′C4–H21 | 1315 | νN2-C6 | 1301 | νC6-C13 | 1300 | νC6-C13 | 1301 | νC6-C13 |
| 1285m | 1294s | 1296sh | 1286 | βC15-H35 νC16-C10 |
1282 | νC9-C15 νC6-C9 νC7-C10 |
1288 | βC15-H35 βC13-H33 |
||||||
| 1285m | 1294s | 1296sh | 1283 | νC6-C13 | 1282 | νC6-C13 | 1283 | νC6-C13 | 1285 | νC9-C15 νC6-C9 |
1286 | βC15-H35 | 1285 | νC16-C10 νC7-C10 νC6-C9 |
| 1270m | 1279sh | 1289m | 1273 | βC15-H35 νC16-C10 |
1274 | νC16-C10 νC9-C15 |
1275 | νC16-C10 | 1270 | νN3-C4 ρCH3(C12) |
||||
| 1274sh | 1267 | νC9-C15 νC6-C9 νC7-C10 |
||||||||||||
| 1256m | 1253sh | 1265 | νN3-C4 | 1264 | νC7-C10 νC6-C9 |
1266 | νC9-C15 νC7-C10 νC6-C9 |
1267 | ρCH2 | 1266 | ρCH2 | 1269 | ρCH2 βC16-H36 |
|
| 1249sh | 1247m | 1253 | ρCH2 | 1255 | ρCH2 βC16-H36 |
1260 | ρCH2 | |||||||
| 1228m | 1233sh | 1236sh | 1233 | νN2-C5 νN2-C7 |
1234 | νN2-C5 νN2-C7 |
1243 | νN2-C6 βC14-H34 νC7-C14 |
1248 | νN2-C5 νN2-C7 |
1242 | νN2-C6 | ||
| 1228m | 1223sh | 1228 | νN2-C6 | 1228 | ρ′CH3(C12) ρ′CH3(C11) |
1224 | νN3-C12 | 1226 | ρCH3(C12) | 1238 | ρCH3(C12) | |||
| 1218sh | 1208s | 1218w | 1217 | νN2-C7 | 1216 | ρ′CH3(C12) | 1221 | νN2-C7 | 1216 | ρ′CH3(C11) | 1223 | νN2-C7 | ||
| 1218sh | 1208s | 1209sh | 1210 | νN2-C6 | 1211 | νN2-C7 βC15-H35 |
||||||||
| 1170w | 1189vs | 1209sh | 1187 | νN3-C11 | 1187 | ρ′CH3(C11) | 1181 | ρCH3(C11) δC8C4N3 |
1178 | ρ′CH3(C12) | 1200 | ρ′CH3(C11) | ||
| 1170w | 1189vs | 1171sh | 1166 | βC17-H37 | 1167 | βC17-H37 | 1172 | ρCH3(C11) | ||||||
| 1162sh | 1167sh | 1164m | 1153 | βC18-H38 βC20-H40 |
1156 | βC17-H37 | 1155 | βC17-H37 | 1164 | ρCH3(C11) ρ′CH3(C12) |
1166 | βC18-H38 βC20-H40 |
1167 | βC17-H37 |
| 1156sh | 1157sh | 1151 | βC17-H37 | 1155 | βC18-H38 βC20-H40 |
1153 | βC18-H38 | 1163 | βC18-H38 | 1157 | ρ′CH3(C11) ρCH3(C11) |
1164 | βC18-H38 βC20-H40 |
|
| 1142w | 1143 | ρCH3(C11) ρCH3(C12) |
1141 | ρ′CH3(C12) | 1138 | ρCH3(C12) | 1137 | βC20-H40 | 1138 | βC19-H39 | 1138 | νC15-C19 | ||
| 1128m | 1129sh | 1126 | βC19-H39 βC20-H40 |
1128 | νC15-C19 | 1128 | βC19-H39 βC20-H40 |
1120 | βC19-H39 βC20-H40 |
1121 | νC15-C19 | 1121 | νC16-C20 | |
| 1106w | 1117sh | 1118m | 1109 | νC15-C19 | 1111 | νC16-C20 νC15-C19 |
1111 | νC16-C20 | 1111 | ρCH3(C8) | 1100 | ρCH3(C8) νN2-C5 |
||
| 1091sh | 1103m | 1105m | 1097 | ρ′CH3(C8) | 1094 | ρ′CH3(C8) | 1103 | ρCH3(C11) ρ′CH3(C12) |
1095 | ρCH3(C8) | 1107 | ρCH3(C8) | ||
| 1091sh | 1103m | 1105m | 1084 | ρCH3(C12) ρCH3(C11) |
1089 | νN2-C5 | 1089 | νN2-C5 νC7-C10 |
1091 | νN2-C5 | ||||
| 1082sh | 1075sh | 1088sh | 1080 | νN2-C5 νC9-S1 |
1084 | νN2-C5 | 1082 | νN2-C5 | 1079 | ρ′CH3(C8) | 1079 | νC4-C8 | ||
| 1066vw | 1066sh | 1073 | νC4-C8 | 1069 | νC4-C8 | 1067 | νC4-C8 | 1072 | νC4-C8 | |||||
| 1059vw | 1058sh | 1060 | ρ′CH3(C11) | 1057 | ρ′CH3(C8) | 1052 | βR1(A3) | 1057 | βR1(A3) | 1057 | ρ′CH3(C12) | |||
| 1048sh | 1047 | βR1(A3) | 1054 | βR1(A1) | 1050 | ρCH3(C11) | 1052 | βR1(A3) | 1053 | βR1(A3) | ||||
| 1043m | 1040sh | 1044sh | 1043 | βR1(A1) | 1048 | βR1(A3) | 1048 | βR1(A1) | 1048 | βR1(A1) | 1051 | βR1(A1) | 1051 | βR1(A1) |
| 1034m | 1027vs | 1030 | ρ′CH3(C12) νN3-C11 |
1023 | νC17-C19 ρCH3(C12) |
1040 | βR1(A3) | 1036 | ρ′CH3(C11) νN3-C11 |
1031 | νC17-C19 | 1034 | νC4-C8 βR1(A3) |
|
| 1034m | 1027vs | 1023 | νC17-C19 | 1020 | ρCH3(C11) ρCH3(C12) |
1033 | νC17-C19 νC15-C19 |
1029 | νC18-C20 νC16-C20 |
1033 | νC17-C19 νC18-C20 |
|||
| 1034m | 1027vs | 1021 | νC18-C20 νC16-C20 |
1019 | νC18-C20 νC16-C20 |
1024 | νC17-C19 | 1031 | νC18-C20 νC16-C20 |
1025 | ρCH3(C11) | 1032 | νC18-C20 νC17-C19 νC15-C19 |
|
| 1009sh | 1012s | 1008sh | 1015 | νC4-C8 νC4-C5 |
1021 | νC18-C20 νC15-C19 |
||||||||
| 1005w | 1012s | 1008sh | 1006 | ρ′CH3(C8) | 1002 | νN3-C11 | ||||||||
| 987s | 996sh | 987 | γC19-H39 γC17-H37 |
988 | νN3-C11 νN3-C12 |
981 | γC19-H39 γC17-H37 |
|||||||
| 988vw | 973 | γC18-H38 γC20-H40 |
980 | γC20-H40 | ||||||||||
| 976w | 971vw | 971 | γC17-H37 | 986 | γC20-H40 | 976 | γC20-H40 γC18-H38 |
968 | νN3-C12 νN3-C11 |
971 | γC17-H37 | |||
| 975 | γC19-H39 γC17-H37 |
964 | γC17-H37 | |||||||||||
| 957sh | 955s | 957 | νN3-C12 | 963 | γC18-H38 | 958 | νN3-C11 νN3-C12 |
965 | γC20-H40 γC18-H38 |
|||||
| 950w | 949vw | 949 | νN3-C11 νN3-C12 |
|||||||||||
| 935sh | 941sh | 935 | γC16-H36 | 943 | γC15-H35 | 937 | γC15-H35 | 937 | γC15-H35 | 939 | ρCH3(C8) | |||
| 930w | 930sh | 934 | γC15-H35 | 941 | γC16-H36 γC18-H38 |
936 | γC16-H36 | 928 | γC15-H35 γC13-H33 |
930 | γC16-H36 γC18-H38 |
932 | γC15-H35 | |
| 924sh | 929w | 924 | ρCH3(C8) | 928 | νN3-C4 ρCH3(C8) |
923 | γC16-H36 | 928 | ρCH3(C8) γC15-H35 |
922 | γC16-H36 | |||
| 902w | 893m | 917sh | 915 | ρCH3(C8) | 918 | ρCH3(C8) | 920 | γC15-H35 | 915 | νN3-C12 | ||||
| 884vw | 893m | 875 | νN3-C4 | 866 | νC4-C5 | 873 | νC4-C5 | 867 | γC13-H33 νC4-C5 |
|||||
| 874vw | 873sh | 854 | γC13-H33 | 856 | γC13-H33 | 862 | νC4-C5 | 861 | γC13-H33 | |||||
| 859w | 856s | 856w | 851 | γC14-H34 | 854 | γC14-H34 | 853 | γC14-H34 | 856 | γC13-H33 | 854 | γC14-H34 | 857 | γC13-H33 γC15-H35 |
| 852w | 832sh | 842sh | 847 | νC4-C5 | 852 | γC14-H34 γC16-H36 |
850 | γC13-H33 | 852 | γC14-H34 | 844 | νN3-C11 νN3-C12 |
850 | γC14-H34 |
| 807vw | 817sh | 808m | 803 | τwCH2 | 808 | τwCH2 | 813 | τwCH2 | 816 | τwCH2 | 811 | τwCH2 | 813 | τwCH2 |
| 778sh | 775sh | 775sh | 778 | βR2(A1) | 774 | βR2(A1) | 777 | βR2(A1) | 794 | δC5C4N3 | 787 | νN3-C4 | 803 | νN3-C4 δC5C4N3 |
| 759vs | 758s | 761w | 752 | γC19-H39 | 756 | γC19-H39 γC17-H37 |
754 | νN3-C4 | 760 | νN3-C4 | 760 | γC19-H39 γC17-H37 |
762 | βR2(A1) |
| 759vs | 758s | 761w | 756 | γC19-H39 | 755 | γC20-H40 γC14-H34 |
757 | γC19-H39 | ||||||
| 759vs | 758s | 754sh | 751 | γC20-H40 | 751 | γC20-H40 γC18-H38 |
752 | γC20-H40 | 751 | γC20-H40 | 752 | γC20-H40 γC18-H38 |
||
| 752sh | 742sh | 745 | γC20-H40 γC19-H39 |
746 | γC20-H40 γC18-H38 |
747 | νN3-C4 | |||||||
| 734m | 737sh | 722 | τR1(A1) | 723 | τR1(A3) τR1(A1) |
722 | τR1(A1) | 723 | τR1(A3) | 722 | τR1(A3) | 722 | τR1(A3) | |
| 729m | 720 | τR1(A1) | ||||||||||||
| 712vw | 718m | 714 | τR1(A3) | 713 | τR1(A3) | 714 | τR1(A3) | 716 | τR1(A1) | 716 | τR1(A1) | 715 | τR1(A1) | |
| 695w | 687m | 688sh | 686 | βR2(A3) | 683 | τR1(A3) τR1(A1) |
686 | βR2(A3) | 688 | βR2(A3) | 685 | τR1(A3) τR1(A1) |
688 | τR1(A3) τR1(A1) |
| 675w | 655m | 672s | 676 | βR3(A1) | 675 | βR3(A1) βR2(A3) |
677 | βR3(A1) | 677 | βR3(A1) | 676 | βR3(A1) | 677 | βR3(A1) βR2(A3) |
| 646s | 616vw | 623 | βR3(A3) | 619 | βR3(A3) | 631 | βR3(A3) | 623 | βR3(A3) | 619 | βR3(A3) | 622 | βR3(A3) | |
| 613w | 594vw | 609 | βR2(A1) | 611 | βR2(A1) | 613 | βR2(A1) | 605 | βR2(A1) βR1(A2) |
601 | βR1(A2) βR2(A1) |
604 | βR2(A1) βR1(A2) |
|
| 567s | 540w | 539 | τR1(A2) τR3(A1) |
534 | τR3(A1) | 546 | τR2(A3) τR1(A2) |
537 | τR1(A2) τR3(A1) |
532 | τR1(A2) τR3(A1) |
538 | τR1(A2) γN2-C5 |
|
| 524 | βR1(A2) | 526 | βR1(A2) | 530 | βR1(A2) | 524 | τR3(A1) | 519 | τR3(A1) | 525 | τR3(A1) | |||
| 510vw | 518sh | 520 | τR3(A3) | 517 | τR3(A3) | 520 | τR3(A3) τR3(A1) |
522 | τR3(A3) | 514 | τR3(A3) | 520 | τR3(A3) | |
| 486sh | 508w | 502 | δC8C4N3 | 497 | τR3(A1) | 490 | δC8C4N3 | 494 | δC5C4C8 δC11N3C12 |
503 | δC5C4C8 νH41-Cl42 |
|||
| 486sh | 508w | 486 | δC4N3C12 τR2(A1) |
489 | δC8C4N3 | 479 | δC5C4C8 | 475 | δC11N3C12 δC4N3C11 |
|||||
| 482s | 479sh | 473 | δC8C4N3 | 466 | δC11N3C12 δC4N3C11 |
471 | δC4N3C12 | |||||||
| 470w | 451 | δC4N3C11 | 444 | τR2(A3) | 447 | τR2(A3) τR2(A1) |
445 | τR2(A3) τR2(A1) |
441 | τR2(A3) | ||||
| 440s | 440sh | 439 | δC11N3C12 | 439 | τR2(A3) ButtC6-C9 |
438 | τR2(A3) | |||||||
| 435 | τR2(A3) | 432 | τR2(A1) | 434 | τR2(A1) | 434 | τR2(A1) | 434 | τR2(A1) | 434 | τR2(A1) | |||
| 423sh | 423m | 426 | τR2(A1) νC10-S1 |
427 | νC9-S1 νC10-S1 |
429 | νC9-S1 | 427 | νC9-S1 νC10-S1 |
427 | νC10-S1 νC9-S1 βR2(A3) |
429 | νC10-S1 νC9-S1 |
|
| 423sh | 423m | 421 | δC5C4N3 | 417 | δC11N3C12 | 424 | νC10-S1 | 418 | δC8C4N3 δC4N3C12 |
|||||
| 392w | 394sh | 402 | βR2(A2) βR3(A2) |
401 | βR2(A2) | 404 | βR2(A2) | 407 | βR2(A2) βN2-C5 |
402 | βR2(A2) | 407 | βR2(A2) | |
| 392w | 394sh | 382 | δC5C4C8 | 395 | δC5C4C8 | |||||||||
| 370w | 370sh | 360 | γN2-C5 | 375 | δC11N3C12 | 379 | δC4N3C11 | 377 | δC8C4N3 | |||||
| 357sh | 357sh | 352 | δC5C4C8 | 358 | βR3(A2) | 356 | βR3(A2) δC5C4C8 |
356 | γN2-C5 βR3(A2) |
357 | βR3(A2) | 356 | βR3(A2) | |
| 357sh | 357sh | 349 | δC5C4C8 | 346 | δC4N3C12 | 347 | δC4N3C12 δC4N3C11 |
|||||||
| 340sh | 333 | βR2(A2) | 331 | τR2(A3) | 332 | τR2(A3) | ||||||||
| 337s | 325 | βN2-C5 | 337 | τR2(A3) | 336 | τR2(A3) | 340 | τR2(A3) γN2-C5 |
||||||
| 320sh | 325sh | 319 | τR2(A3) δC11N3C12 |
315 | βN2-C5 | 318 | βN2-C5 | |||||||
| 303m | 294vw | 302 | δC11N3C12 | 305 | βN2-C5 δC5C4N3 |
|||||||||
| 278sh | 281 | βN2-C5 δC4N3C12 |
288 | δC4N3C12 δC4N3C11 |
280 | τR2(A2) | ||||||||
| 278sh | 273 | δC4N3C12 | 272 | τR2(A2) | 272 | τwCH3(C8) | 273 | τR2(A2) | 284 | τR2(A2) | ||||
| 245sh | 266 | τR2(A2) | 263 | δC4N3C11 | 257 | τwCH3(C8) | 267 | τwCH3(C8) | ||||||
| 235m | 232 | νH41-Cl42 | 233 | ButtC7-C10 | 232 | ButtC7-C10 ButtC6-C9 |
||||||||
| 235m | 227 | ButtC6-C9 ButtC7-C10 |
228 | ButtC6-C9 ButtC7-C10 |
228 | ButtC6-C9 ButtC7-C10 |
||||||||
| 220 | τwCH3(C11) | 225 | τwCH3(C11) | 226 | ButtC7-C10 ButtC6-C9 |
222 | νH41-Cl42 | |||||||
| 215s | 211 | τwCH3(C11) τwCH3(C12) |
214 | τwCH3(C8) | 213 | τwCH3(C12) | 218 | τwCH3(C11) | ||||||
| 209 | τwCH3(C12) | 209 | τwCH3(C8) | |||||||||||
| 204sh | 203 | τwCH3(C11) | 201 | δN2C5C4 | 201 | δN2C5C4 | 206 | δN2C5C4 | ||||||
| 198 | δN2C5C4 | 197 | δN2C5C4 | |||||||||||
| 195sh | 194 | τwCH3(C8) | 196 | τwCH3(C12) | 194 | τwCH3(C11) | ||||||||
| 188sh | 188 | τwCH3(C11) | ||||||||||||
| 178 | τwCH3(C12) | 177 | τR2(A2) τR1(A2) |
178 | τR2(A2) τwCH3(C12) |
180 | τR2(A2) τR1(A2) |
|||||||
| 155 | τR1(A2) | 159 | δC5C4N3 | 157 | τR1(A2) τR2(A2) |
|||||||||
| 140 | τR1(A2) | 164 | τwCH3(C12) | 154 | τwCH3(C12) | |||||||||
| 144 | τR1(A2) | 143 | τR1(A2) | 143 | τR1(A2) | |||||||||
| 136 | τR3(A1) | 139 | τR1(A2) | |||||||||||
| 118 | τR3(A2) | 115 | τR3(A2) | 119 | τR3(A2) | 122 | τR3(A2) τR3(A3) |
119 | τR3(A2) | 119 | τR3(A2) | |||
| 105 | τN3-H41 | |||||||||||||
| 80 | τN3-C4 | 80 | δN3H41Cl42 | 83 | δN3H41Cl42 ρ′N3–H41 |
|||||||||
| 62 | τR2(A2) γN2-C5 |
72 | τN3-C4 | 66 | τN3-C4 | 72 | τR3(A2) | 70 | τN3-C4 τN3-H41 |
|||||
| 54 | τR2(A2) δN2C5C4 |
58 | τR2(A2) | 57 | τR2(A2) | 60 | τR2(A2) | 61 | γN2-C5 τwN2-C5 |
62 | τN3-H41 | |||
| 42 | γN2-C5 τwN2-C5 |
52 | γN2-C5 τN3-C4 |
47 | τN3-C4 | 58 | τR2(A2) | 54 | τR2(A2) τR2(A2) |
|||||
| 36 | τC4-C5 | 31 | γN2-C5 | 37 | τC4-C5 | 35 | τC4-C5 | 37 | γN2-C5 τwN2-C5 |
|||||
| 31 | τwN2-C5 τC4-C5 |
27 | τN3-C4 τC4-C5 |
27 | τwN2-C5 | 32 | τN3-C4 γN2-C5 |
31 | τC4-C5 | |||||
| 24 | γN2-C5 τwN2-C5 |
21 | τC4-C5 τwN2-C5 |
18 | τN3-C4 | 18 | τN3-C4 | |||||||
Abbreviations: ν, stretching; β, deformation in the plane; γ, deformation out of plane; wag, wagging; τ, torsion; βR, deformation ring; τR, torsion ring; ρ, rocking; τw, twisting; δ, deformation; a, antisymmetric; s, symmetric; (A1), Ring 1.
This work.
From scaled quantum mechanics force field.
From Ref [66].
From Ref [10].
From Ref [10].
3.7.1. Band assignments
3.7.1.1. N–H modes
For both PTZ forms, the NH stretching modes are expected only for the cationic and hydrochloride species. For instance, in monomer and dimer of clonidine hydrochloride [67] these modes are assigned at 3427/3341 and 2584cm−1, respectively while in those two forms of diphenhydramine [8] these modes are predicted respectively at 3150 and 1748 cm−1. Here, in the cationic and hydrochloride species of S(-) form of DHC these modes are predicted to 3295 and 1638 cm−1 and in the R(+) form they are predicted to 3273 and 1713 cm−1. Then, they can be assigned in the same region. Here, the group of bands observed in IR spectrum of DHC between 2800 and 2200 cm−1 with a strong band centered at 2370 cm−1 could be assigned to the N–H stretching modes due to H bonds, as was also reported for clonidine hydrochloride [67]. The N–H rocking modes for both cationic and hydrochloride forms are predicted in different regions, as observed in Table 15. Later, these modes are assigned in accordance. The torsion τN3-H41 modes expected only in both hydrochloride forms are predicted by calculations to 105 and 70 cm−1 and they cannot be assigned because there are not observed bands in this region.
3.7.1.2. CH modes
In the three species of both S(-) and R(+)-PTZ enantomers, eight aromatics C–H stretching modes are expected and only one stretching mode (C4–H21) with aliphatic characteristic. Hence, they are predicted by the SQM/B3LYP/6-31G* calculations in different regions. Evidently, the aromatics modes are assigned at higher wavenumbers than the other ones, as shown in Tables 15 and 16. Besides, the in-plane deformation or rocking and out-of-plane deformation modes expected only for these C–H aromatics are predicted respectively between 1489/1120 and 987/745 cm−1. Hence, they can be assigned in these regions. These modes in carquejol [50] are assigned between 1483/1121 and 972/746 cm−1.
Table 16.
Scaled internal force constants for the free base, cationic and hydrochloride species of S(-) and R(+)- prometazine in gas phase by using the B3LYP/6-31G* method compared with the corresponding to cyclizine.
| Force constant | Promethazinea |
Cyclizineb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S(-) |
R(+) |
||||||||
| Free base | Cationic | HCl | Free base | Cationic | HCl | Free base | Cationic | HCl/PCM | |
| f(νN-H) | 6.02 | 2.47 | 5.94 | 2.60 | 5.91 | 4.61 | |||
| f(νN-CH3) | 4.67 | 3.92 | 4.25 | 4.70 | 3.94 | 4.94 | 4.85 | 4.06 | 4.33 |
| f(νC-N) | 4.97 | 4.65 | 4.82 | 5.05 | 4.74 | 4.96 | 4.54 | 4.13 | 4.19 |
| f(νCH2) | 4.74 | 4.72 | 4.74 | 4.85 | 4.76 | 4.89 | 4.62 | 4.82 | 4.87 |
| f(νCH3) | 4.82 | 4.83 | 4.85 | 4.90 | 4.94 | 4.95 | 4.69 | 5.06 | 5.07 |
| f(νC-H)R | 5.11 | 5.11 | 5.11 | 5.18 | 5.19 | 5.19 | 5.15 | 5.17 | 5.18 |
| f(νC-H) | 4.73 | 4.82 | 4.81 | 4.45 | 4.90 | 4.78 | 4.31 | 4.44 | 4.74 |
| f(νC=C) | 6.50 | 6.50 | 6.46 | ||||||
| f(νC-C) | 3.40 | 3.57 | 3.65 | 3.70 | 3.56 | 3.69 | |||
| f(δCH2) | 0.78 | 0.79 | 0.79 | 0.81 | 0.82 | 0.81 | 0.74 | 0.73 | 0.73 |
| f(δCH3) | 0.53 | 0.53 | 0.53 | 0.56 | 0.56 | 0.57 | 0.58 | 0.56 | 0.55 |
Units are mdyn Å−1 for stretching and mdyn Å rad−2 for angle deformations.
This work.
From Ref. [9].
3.7.1.3. CH3 modes
The three species of both S(-) and R(+)-PTZ enantiomers present three CH3 groups, where two of them are linked to N3 atoms and the other one to C4 atoms. Then, these modes are predicted in different regions and, thus, they can be easily assigned in accordance to the calculations. In carquejol [50] these stretching modes are assigned between 3031 and 2919 cm−1 while in this case these modes are assigned to the IR and Raman bands between 3411 and 2747 cm−1. Note that the symmetrical stretching modes corresponding to CH3 groups linked to N3 atoms of two free base species of both S(-) and R(+)-PTZ are predicted at lower wavenumbers and, hence, they are assigned to the IR bands at 2824 and 2747 cm−1. The CH3 deformation, rocking and twisting modes in carquejol [50] are respectively assigned between 1587/1436, 1084/1026 and 220/171 cm−1. Here, those three vibration modes are assigned to the IR and Raman bands to 1500/1340, 1289/902 and 267/154 cm−1. These latter modes between 178 and 154 couldn't be assigned due to that there are not observed bands in these regions.
3.7.1.4. CH2 modes
All PTZ species have only one CH2 group, for which, the expected antisymmetrical and symmetrical stretching, deformation, wagging, rocking and twisting modes are clearly assigned as predicted by the calculations. For the free base and hydrochloride species of R(+)-PTZ the antisymmetrical modes are predicted at higher wavenumbers than the other species of S(-) form, hence, those modes are assigned to the groups of IR and Raman bands at 3037/2872, 1470/1433, 1421/1387, 1354/1247 and 817/808 cm−1. Those vibration modes of the two CH2 groups of Carquejol are assigned in approximately the same regions [50].
3.7.1.5. Skeletal modes
In the three species of both S(-) and R(+)-PTZ enantiomers are very important the N3–C11 and N3–C12 stretching modes because their corresponding bonds are predicted by B3LYP/6-31G* calculations longer than the corresponding to N3–C4 bonds, as was experimentally observed by X-ray diffraction [19]. Therefore, the strong IR bands at 1012, 987, 955 and 893 cm−1 could be associated to the N3–C11 and N3–C12 stretching modes. Note that the IR band of medium intensity at 1256 cm−1 could be also attributed to the N3–C4 stretching mode of free base of S(-)-PTZ while the strong IR band at 1189 cm−1 could be assigned to the N3–C11 stretching mode of free base of that form. Moreover, the very strong IR band at 759 cm−1 and the band at 893 cm−1 could be associated to N3–C4 stretching modes of both forms. The IR bands at 1128, 1208 and 1105 cm−1 could be assigned to other N–C stretching modes (N2–C5, N2–C6 and N2–C7) expected for all species of PTZ because the calculations predicted these modes in those regions. The C=C stretching modes are usually assigned between 1680 and 1659 cm−1 [[1], [2], [3], [5], [6], [7], [8], [9], [45], [47], [48], [49], [50], [52], [53], [67]]; thus, the strong IR bands at 1558 cm−1 is without difficulty associated to these vibration modes of three species of both enantiomeric forms. Here, a very important result is the very strong Raman band observed at 1027 cm−1 which is attributed to C–C stretching modes of both phenyl rings of both forms, as was reported for identification of PTZ by Assi [22]. In the IR spectrum that band is observed with medium intensity at 1034 cm−1. The two C9–S1 and C10–S1 stretching modes expected in all species of both enantiomers can be associated to the IR band of medium intensity at 423 cm−1 because all species, with exception of free base of S(-) form, are predicted in this region. In the free base of S(-) form the C9–S1 stretching mode is predicted at 1080 cm−1 coupled with the N2–C5 stretching mode. The remaining skeletal modes including the deformation and torsion modes of both phenyl rings are assigned in the regions predicted by SQM calculations and according the assignments for similar compounds [1, 2, 3, 5, 6, 7, 8, 9, 45, 47, 48, 49, 50, 52, 53, 67], as detailed in Table 15.
3.8. Force fields
Both S(-) and R(+)-PTZ enantiomers have evidenced differences in the positions of IR bands because differences in their geometrical parameters are observed. Hence, it is necessary to investigate if the harmonic force constants present some changes since these parameters are also strongly dependent of their structures. Hence, the force fields for all species of both forms are calculated in gas phase by using B3LYP/6-31G* level of theory. These parameters are compared in Table 16 with the reported for the three species of cyclizine [9]. In general, the force constants for the R(+)-PTZ enantiomer have higher values than the corresponding to the S(-) form. Comparing the f(νN-H) force constants of all species, we observed that the cationic species of both forms of PTZ and cyclizine are higher than the hydrochloride ones because the presence of electronegative Cl atoms linked to H atoms generate a enlargement of N–H bonds with the consequent reduction of their f(νN-H) force constants. Note that in hydrochloride cyclizine the presence of N–CH3 group linked to two rings produces a higher value in its force constant (4.61 mdyn Å−1), as compared with both forms of PTZ. Probably, for this same reason, the f(νN-CH3) force constants of free base and cationic species of cyclizine have higher values than the corresponding to PTZ. On the other hand, the hydrochloride species of R(+) has higher value than the other ones because the distances observed for both N–CH3 groups are lower in the R(+) form than the S(-) one, as observed in Tables 3 and 4. Note that the f(νC-H) force constants corresponding to the aromatic rings in general are higher in all species than the aliphatic ones and, moreover, these values are similar to those published for the species of diphenhydramine [8]. The remaining constants have similar values in the two compared species, as is observed in Table 16.
3.9. Ultraviolet-visible spectrum
The electronic spectra of free base, cationic and hydrochloride species of both S(-) and R(+)-PTZ enantiomers were predicted in aqueous solution with the TD-DFT method and the Gaussian program [55] by using the B3LYP/6-31G* level of theory. The experimental UV-Vis spectrum of a racemic mixture of hydrochloride species of both enantiomers in ethanol solution was taken from Ref. [68] where in each enantiomer it is observed one intense band at c. a. 250 nm and where one of them is slightly most intense than the other one. In all theoretical spectra are observed one intense band, whose positions are respectively in free base, cationic and hydrochloride species of S(-) form at 247.0 (shoulder at 283.2 nm), 290.8 and 290.2 nm while and in the R(+) form the positions of those bands change at 245.7 (shoulder at 280.0 nm), 292.7 and 284.4 nm, respectively. The shifting of the bands observed in the experimental UV spectra from 250 to 290 nm, in relation to the theoretical ones, can be attributed to the different solvents. All UV spectra are compared in Fig. 11 with the corresponding experimental one. Here, it is evident that the free base species of both forms are protonated, as suggested by the shoulders at higher wavelengths and closer to the values for the cationic species. Also, the proximities between the maxima of both hydrochloride forms show that these species are as cationic species. Hence, these spectra evidence clearly the presence of both cationic S(-) and R(+) forms in solution. Obviously, the π→π* transitions due to the C=C double bonds justify the intense bands observed in the experimental spectra, as supported by NBO calculations.
Fig. 11.
Experimental electronic spectrum of hydrocloride promethazine in ethanol solution compared with the corresponding predicted for the free base, cationic and hydrochloride species of both S(-) and R(+) enantiomers in aqueous solution by using B3LYP/6-31G* level of theory.
3.10. Electronic circular dichroism (ECD)
The experimental ECD spectrum of hydrobromide prometazine was taken from Ref [66] which shows two bands with opposite polarity, one of them with cotton effect and the other one positive. This ECD spectrum is similar to that recorded in the 190–240 nm region by Rub et al. in the study of interaction of gelatin with promethazine hydrochloride [64]. On the other hand, the predicted ECD spectra for the free base of R(+) shows one positive band while in the S(-) form one negative in the same position. In the same region, in the cationic species of R(+) form can be observed two bands one positive and other negative while in the S(-) form two bands negative. The hydrochloride species of S(-) and R(+) forms show one band positive and two negative in different positions, hence, these forms evidently are not present in the experimental spectrum in solution. Here, only the predicted ECD spectra in solution for the cationic species of both enantiomers present similarity with the experimental one, for which, both species are present in a racemic sample of hydrochloride promethazine in aqueous solution. Then, the two negative and positive bands observed in the experimental spectrum at 250 nm could be assigned to π→π* transitions.
4. Conclusions
In this work, the molecular structures of free base, cationic and hydrochloride species of both S(-) and R(+)- enantiomers of promethazine antihistaminic agent were theoretically studied in gas phase and in aqueous solution by using the hybrid B3LYP/6-31G* method. The initial structures of S(-) and R(+) enantiomers of PTZ hydrochloride were those polymorphic forms 1 and 2 experimentally determined by X-ray diffraction. In solution, all species were optimized with the SCRF methodology by using the PCM and SD models. The corrected solvation energies (ΔGc) by the total non-electrostatic terms and by zero point vibrational energy (ZPVE) were computed for all species showing the higher value the cationic species of R(+) form. The comparisons of geometrical parameters with the corresponding experimental ones have showed slight differences in the dihedral angles of both S(-) and R(+)-PTZ forms that later they are evidenced in the different vibrational assignments of their infrared and Raman spectra and in the calculated force constants. Here, the studied MK, Mulliken and NPA charges have evidenced variations in the three species of both enantiomers observing the higher MK charges on N2 atoms of the cationic species of R(+) species in the two media. The cationic and hydrochloride species present basically the same behaviours in the Mulliken charges where the lower values are observed on N2 atoms. The mapped surfaces MEP have clearly evidenced nucleophilic sites in the free base on the N3 and S1 atoms and in the hydrochloride species on the Cl atoms. The NBO and AIM studies reveal clearly that the hydrochloride species are most stable than the other two species of both forms and in both media and, in particular, the species of R(+)-PTZ evidence a slight higher stability than the S(-) one. The frontier orbitals studies show that the free base species of both forms in solution are more reactive than cyclizine. Higher electrophilicity indexes are observed in the cationic and hydrochloride species of PTZ than cyclizine while the cationic species of cyclizine have higher nucleophilicity index than both species of PTZ. The predicted infrared, Raman, UV-Visible and ECD have showed a reasonable concordance with the corresponding experimental available spectra. The presences of cationic species of both enantiomers are clearly supported by the infrared, Raman, UV-Vis and ECD spectra. The increase in the volume of cationic and hydrochloride species in solution could suggest the H bonds formation, as supported by AIM study. The force fields were computed by using the SQMFF approach and Molvib program which were used to perform the complete vibrational analysis. Here, the 114, 117 and 120 vibration normal modes expected for the free base, cationic and hydrochloride species were assigned and the force constants reported and compared with other reported from the literature.
Declarations
Author contribution statement
María Eugenia Manzur: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Silvia A. Brandán: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by grants from CIUNT Project Nº 26/D608 (Consejo de Investigaciones, Universidad Nacional de Tucumán).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Brandán S.A. Why morphine is a molecule chemically powerful. Their comparison with cocaine. Indian J. Appl. Res. 2017;7(7):511–528. [Google Scholar]
- 2.Rudyk R.A., Brandán S.A. Force field, internal coordinates and vibrational study of alkaloid tropane hydrochloride by using their infrared spectrum and DFT calculations. Paripex - Indian J. Res. 2017;6(8):616–623. [Google Scholar]
- 3.Romani D., Brandán S.A. Vibrational analyses of alkaloid cocaine as free base, cationic and hydrochloride species based on their internal coordinates and force fields. Paripex - Indian J. Res. 2017;6(9):587–602. [Google Scholar]
- 4.MA Iramain, AE Ledesma, Brandán S.A. Analyzing the effects of halogen on properties of a halogenated series of R and S enantiomers analogues alkaloid cocaine-X, X=F, Cl, Br, I. Paripex - Indian J. Res. 2017;6(12):454–463. [Google Scholar]
- 5.Brandán S.A. Understanding the potency of heroin against to morphine and cocaine, IJSRM. Int. J. Soc. Res. Methodol. 2018;12(2):97–140. [Google Scholar]
- 6.Rudyk R.A., Checa M.A., Guzzetti K.A., Iramain M.A., Brandán S.A. Behaviour of N-CH3 group in tropane alkaloids and correlations in their properties, IJSRM. Int. J. Soc. Res. Methodol. 2018;10(4):70–97. [Google Scholar]
- 7.Rudyk R.A., Checa M.A., Catalán C.A.N., Brandán S.A. Structural, FT-IR, FT-Raman and ECD spectroscopic studies of free base, cationic and hydrobromide species of scopolamine alkaloid, submitted to. J. Mol. Struct. 2018;1180:603–617. [Google Scholar]
- 8.MA Iramain, Brandán S.A. Structural and vibrational properties of three species of anti-histaminic diphenhydramine by using DFT calculations and the SQM approach. Chem. J. 2018;1(1):105–130. [Google Scholar]
- 9.Márquez María J., Iramain Maximiliano A., Brandán Silvia Antonia. Ab-initio and vibrational studies on free base, cationic and hydrochloride species derived from antihistaminic cyclizine agent, IJSRM. Int. J. Soc. Res. Methodol. 2018;12(2):97–140. [Google Scholar]
- 10.Davies M.C., Binns J.S., Melia C.D., Hendra P.J., Bourgeois D., Church S.P., Stephenson P.J. FT Raman spectroscopy of drugs in polymers. Int. J. Pharm. 1990;66:223–232. [Google Scholar]
- 11.Lara Francisco J., García-Campaña Ana M., Al'es-Barrero F., Bosque-Sendra Juan M. Determination of thiazinamium, promazine and promethazine in pharmaceutical formulations using a CZE method. Anal. Chim. Acta. 2005;535:101–108. [Google Scholar]
- 12.Mazurek Sylwester, Szostak Roman. Quantitative determination of diclofenac sodium in solid dosage forms by FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2008;48:814–821. doi: 10.1016/j.jpba.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Page C.B., Duffull S.B., Whyte I.M., Isbister G.K. Promethazine overdose: clinical effects, predicting delirium and the effect of charcoal. Q. J. Med. 2009;102:123–131. doi: 10.1093/qjmed/hcn153. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari Ruchi, Srivastava Birendra, Tiwari Gaurav, Rai Awanik. Extended release promethazine HCl using acrylic polymers by freeze-drying and spray-drying techniques: formulation considerations. Braz. J. Pharma. Sci. 2009;45(4):829–840. [Google Scholar]
- 15.Abalde-Cela Sara, Aldeanueva-Potel Paula, Mateo-Mateo Cintia, Rodríguez-Lorenzo Laura, Alvarez-Puebla R.A., Luis M., Liz-Marzán Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface. 2010;7:S435–S450. doi: 10.1098/rsif.2010.0125.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charles J. Choi, Hsin-Yu Wu, Jonathan Weyhenmeyer, Sherine George, and Brian T. Cunningham, Nanodome Sensor Tubing for Monitoring of Intravenous Drug Infusion and Metabolites, 2011 11th IEEE International Conference on Nanotechnology Portland Marriott August 15-18, 2011, Portland, Oregon, USA.
- 17.Hargreaves Michael D., Macleod Neil A., Smith Mark R., Andrews Darren, Hammond Stephen V., Matousek Pavel. Characterisation of transmission Raman spectroscopy for rapid quantitative analysis of intact multi-component pharmaceutical capsules. J. Pharm. Biomed. Anal. 2011;54:463–468. doi: 10.1016/j.jpba.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Charles J. Choi, Wu Hsin-Yu, Weyhenmeyer Jonathan, George Sherine, Cunningham Brian T. Biochemical sensor tubing for point-of-care monitoring of intravenous drugs and metabolites. Lab Chip. 2012;3 doi: 10.1039/c2lc20586f. [DOI] [PubMed] [Google Scholar]
- 19.Borodi Gheorghe, Pop Mihaela M., Onija Oana, Filip Xenia. Distinct disordered forms of promethazine hydrochloride: a case of intergrowth of polymorphic domains? Cryst. Growth Des. 2012;12:5846–5851. [Google Scholar]
- 20.Maurya Neha, ud din Parray Mehraj, Maurya Jitendra Kumar, Kumar Amit, Patel Rajan. Interaction of promethazine and adiphenine to human hemoglobin: a comparative spectroscopic and computational analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018;199:32–42. doi: 10.1016/j.saa.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Cantisani C., Ricci S., Grieco T., Paolino G., Faina V., Silvestri E., Calvieri S. Vol. 2013. Hindawi Publishing Corporation, BioMed Research International; 2013. Topical Promethazine Side Effects: Our Experience and Review of the Literature; p. 9. Article ID 151509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Assi Sulaf. Raw material identification using dual laser handheld Raman spectroscopy. Eur. Pharma. Rev. 2013;18(5):25–31. [Google Scholar]
- 23.Xie Wei, Schlücker Sebastian. Medical applications of surface-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2013;15:5329–5344. doi: 10.1039/c3cp43858a. [DOI] [PubMed] [Google Scholar]
- 24.Li Ying-Sing, Church Jeffrey S. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J. Food Drug Anal. 2014;22:29–48. doi: 10.1016/j.jfda.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong Kenny, Kendall Catherine, Stone Nicholas, Notingher Ioan. Raman spectroscopy for medical diagnostics-From in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 2015;89:121–134. doi: 10.1016/j.addr.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Paudel Amrit, Raijada Dhara, Rantanen Jukka. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015;89:3–20. doi: 10.1016/j.addr.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Bumbrah Gurvinder Singh, Sharma Rakesh Mohan. Raman spectroscopy – basic principle, instrumentation and selected applications for the characterization of drugs of abuse. Egypt. J. Food Sci. 2016;6:209–215. [Google Scholar]
- 28.Saleh Tawfik A. Pharmaceutical characterization and detection using surface-enhanced Raman scattering. Saleh. Int. Arch. Clin. Pharmacol. 2017;3:7. 010. [Google Scholar]
- 29.Tarkkila P., Torn K., Tuominen M., Lindgren L. Premedication with promethazine and transdermal scopolamine reduces the incidence of nausea and vomiting after intrathecal morphine. Acta Anaesthesiol. Scand. 1995;39(7):983–986. doi: 10.1111/j.1399-6576.1995.tb04210.x. [DOI] [PubMed] [Google Scholar]
- 30.Vidal Pan C., Gonz'alez Quintela A., Galdos Anuncibay P., MateoVic J. Topical promethazine intoxication. Curr. Med. Res. Opin. 2012;28(4):623–642. [Google Scholar]
- 31.Promethazine should not be used for infants. Die Pharmazie. 2010;65(5):339–342. [Google Scholar]
- 32.Cohen M.R. Frequency and severity of promethazine adverse event reports obligated the ISMP alert. Jt. Comm. J. Qual. Patient Saf. 2010;36(3):143–144. doi: 10.1016/s1553-7250(10)36023-5. [DOI] [PubMed] [Google Scholar]
- 33.Kai Y., Okamoto O., Fujiwara S. Fixed drug eruption caused bythreeunrelated drugs: promethazine, pethidineand omeprazole. Clin. Exp. Dermatol. 2011;36(7):755–758. doi: 10.1111/j.1365-2230.2011.04093.x. [DOI] [PubMed] [Google Scholar]
- 34.Becke A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. 1988;A38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 35.Lee C., Yang W., Parr R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988;B37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 36.Miertus S., Scrocco E., Tomasi J. Electrostatic interaction of a solute with a continuum. Chem. Phys. 1981;55:117–129. [Google Scholar]
- 37.Tomasi J., Persico J. Molecular interactions in solution: an overview of methods based on continous distributions of the solvent. Chem. Rev. 1994;94:2027–2094. [Google Scholar]
- 38.Marenich A.V., Cramer C.J., Truhlar D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. 2009;B113:6378–6396. doi: 10.1021/jp810292n. [DOI] [PubMed] [Google Scholar]
- 39.Pulay P., Fogarasi G., Pongor G., Boggs J.E., Vargha A. Combination of theoretical ab initio and experimental information to obtain reliable harmonic force constants. Scaled quantum mechanical (QM) force fields for glyoxal, acrolein, butadiene, formaldehyde, and ethylene. J. Am. Chem. Soc. 1983;105:7073. [Google Scholar]
- 40.a) Rauhut G., Pulay P. Transferable scaling factors for density functional derived vibrational force fields. J. Phys. Chem. 1995;99:3093–3100. [Google Scholar]; b) Rauhut G., Pulay P. J. Phys. Chem. 1995;99:14572. Correction. [Google Scholar]
- 41.Available from. DL- 3,4- Methylenedioxymethamphetamine, Spectra BASE, https://spectrabase.com/spectrum/4IWjF3I2JuZ.
- 42.Sundius T. Scaling of ab-initio force fields by MOLVIB. Vib. Spectrosc. 2002;29:89–95. [Google Scholar]
- 43.Parr R.G., Pearson R.G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983;105:7512–7516. [Google Scholar]
- 44.Brédas J.-L. Mind the gap! Mater. Horizons. 2014;1:17–19. [Google Scholar]
- 45.Romani D., Brandán S.A., Márquez M.J., Márquez M.B. Structural, topological and vibrational properties of an isothiazole derivatives series with antiviral activities. J. Mol. Struct. 2015;1100:279–289. [Google Scholar]
- 46.Romani D., Tsuchiya S., Yotsu-Yamashita M., Brandán S.A. Spectroscopic and structural investigation on intermediates species structurally associated to the tricyclic bisguanidine compound and to the toxic agent, saxitoxin. J. Mol. Struct. 2016;1119:25–38. [Google Scholar]
- 47.Romano E., Castillo M.V., Pergomet J.L., Zinczuk J., Brandán S.A. Synthesis, structural and vibrational analysis of (5,7-Dichloro-quinolin-8-yloxy) acetic acid. J. Mol. Struct. 2012;1018:149–155. [Google Scholar]
- 48.Chain F.E., Ladetto M.F., Grau A., Catalán C.A.N., Brandán S.A. Structural, electronic, topological and vibrational properties of a series of N-benzylamides derived from Maca (Lepidium meyenii) combining spectroscopic studies with ONION calculations. J. Mol. Struct. 2016;1105:403–414. [Google Scholar]
- 49.Chain F., Iramain M.A., Grau A., Catalán C.A.N., Brandán S.A. Evaluation of the structural, electronic, topological and vibrational properties of N-(3,4-dimethoxybenzyl)-hexadecanamide isolated from Maca (Lepidium meyenii) using different spectroscopic techniques. J. Mol. Struct. 2016;1119:25–38. [Google Scholar]
- 50.Minteguiaga M., Dellacassa E., Iramain M.A., Catalán C.A.N., Brandán S.A. A structural and spectroscopic study on carquejol, a relevant constituent of the medicinal plant Baccharis trimera (Less.) DC. (Asteraceae) J. Mol. Struct. 2017;1150:8–20. [Google Scholar]
- 51.Iramain M.A., Davies L., Brandán S.A. Structural and spectroscopic differences among the Potassium 5-hydroxypentanoyltrifluoroborate salt and the furoyl and isonicotinoyl salts. J. Mol. Struct. 2019;1176:718–728. [Google Scholar]
- 52.Issaoui N., Ghalla H., Brandán S.A., Bardak F., Flakus H.T., Atac A., Oujia B. Experimental FTIR and FT-Raman and theoretical studies on the molecular structures of monomer and dimer of 3-thiopheneacrylic acid. J. Mol. Struct. 2017;1135:209–221. [Google Scholar]
- 53.Minteguiaga M., Dellacassa E., Iramain M.A., Catalán C.A.N., Brandán S.A. FT-IR, FT-Raman, UV-Vis, NMR and structural studies of Carquejyl Acetate, a component of the essential oil from Baccharis trimera (Less.) DC. (Asteraceae), submitted to. J. Mol. Struct. 2018 [Google Scholar]
- 54.Nielsen A.B., Holder A.J. GAUSSIAN Inc.; Pittsburgh, PA: 2000–2003. Gauss View 3.0, User’s Reference. [Google Scholar]
- 55.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., Nakatsuji H., Caricato M., Li X., Hratchian H.P., Izmaylov A.F., Bloino J., Zheng G., Sonnenberg J.L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery J.A., Jr., Peralta J.E., Ogliaro F., Bearpark M., Heyd J.J., Brothers E., Kudin K.N., Staroverov V.N., Kobayashi R., Normand J., Raghavachari K., Rendell A., Burant J.C., Iyengar S.S., Tomasi J., Cossi M., Rega N., Millam J.M., Klene M., Knox J.E., Cross J.B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R.E., Yazyev O., Austin A.J., Cammi R., Pomelli C., Ochterski J.W., Martin R.L., Morokuma K., Zakrzewski V.G., Voth G.A., Salvador P., Dannenberg J.J., Dapprich S., Daniels A.D., Farkas O., Foresman J.B., Ortiz J.V., Cioslowski J., Fox D.J. Gaussian, Inc.; Wallingford CT: 2009. [Google Scholar]
- 56.Ugliengo P. University of Torino, Dipartimento Chimica IFM; Torino, Italy: 1998. Moldraw Program. [Google Scholar]
- 57.Besler B.H., Merz K.M., Jr., Kollman P.A. Atomic charges derived from demiempirical methods. J. Comput. Chem. 1990;11:431–439. [Google Scholar]
- 58.Glendening E.D., Badenhoop J.K., Reed A.D., Carpenter J.E., Weinhold F. Theoretical Chemistry Institute, University of Wisconsin; Madison, WI: 1996. NBO 3.1. [Google Scholar]
- 59.Bader R.F.W. Oxford University Press; Oxford: 1990. Atoms in Molecules, A Quantum Theory. [Google Scholar]
- 60.Biegler-Köning F., Schönbohm J., Bayles D. AIM2000; a program to analyze and visualize atoms in molecules. J. Comput. Chem. 2001;22:545. doi: 10.1002/jcc.10085. [DOI] [PubMed] [Google Scholar]
- 61.Keresztury G., Holly S., Besenyei G., Varga J., Wang A.Y., Durig J.R. Vibrational spectra of monothiocarbamates-II. IR and Raman spectra, vibrational assignment, conformational analysis and ab initio calculations of S-methyl-N,N-dimethylthiocarbamate Spectrochim. Acta. 1993;49A:2007–2026. [Google Scholar]
- 62.Michalska D., Wysokinski R. The prediction of Raman spectra of platinum(II) anticancer drugs by density functional theory. Chem. Phys. Lett. 2005;403:211–217. [Google Scholar]
- 63.Lazny R., Ratkiewicz A., Nodzewska A., Wynimko A., Siergiejczyk L. Determination of the N-methyl stereochemistry in tropane and granatane derivatives in solution: a computational and NMR spectroscopic study. Tetrahedron Lett. 2012 [Google Scholar]
- 64.Rub M.A., Asiri A.M., Khan J.M., Khan R.H. Kabir-ud Din, Interaction of gelatin with promethazine hydrochloride: conductimetry, tensiometry and circular dichroism studies. J. Mol. Struct. 2013;1050:35–42. [Google Scholar]
- 65.Bohloko N.S. Faculty of Health Sciences at the Department of Pharmaceutics, Potchefstroom University for Christian Higher Education; Potchefstroom: 2003. Development and Formulation of an Intranasal Dosage Form for Cyclizine Hydrochloride, Doctoral Thesis. [Google Scholar]
- 66.Available from: http://webbook.nist.gov/chemistry.
- 67.Romano E., Davies L., Brandán S.A. Structural properties and FTIR-Raman spectra of the anti-hypertensive clonidine hydrochloride agent and their dimeric species. J. Mol. Struct. 2017;1133:226–235. [Google Scholar]
- 68.Sánchez F.G., Navas Díaz A., Sánchez Torreño E., Aguilar A., Medina Lama I., Algarra M. Determination of enantiomeric excess by chiral liquid chromatography without enantiomerically pure starting standards. Biomed. Chromatogr. 2012 doi: 10.1002/bmc.2685. [DOI] [PubMed] [Google Scholar]