Abstract
The advent of combination therapy unprecedentedly shifted the paradigm of cancer treatment by reconstructing the conventional protocols. By identifying the anti-tumoral activity for different natural products, recent interest has focused on inventing the combined- modality strategies to increase the cure rates of cancer, while reducing the toxic side effects of current intensive regimens. To evaluate whether melatonin, indolic hormone produced mainly by the pineal gland, could enhance the pro-apoptotic effect of arsenic trioxide (As2O3) in breast cancer, MCF-7 cells were treated with As2O3-plus- melatonin and then the survival, proliferative rate, caspase-3 activity, and mRNA expression level of anti- apoptosis target genes of NF-κB were investigated. Our results delineated that exposure of MCF-7 cells to As2O3 not only reduced the survival of the cells, but also induced a caspsase-3-dependent apoptotic cell death. Noteworthy, an enhanced induction of apoptosis was found using As2O3 in combination with melatonin. Moreover, RQ-PCR analysis revealed that the enhanced cytotoxic effect of As2O3 in the presence of melatonin is mediated, at least partly, through suppressing the expression of NF-κB anti-apoptotic target genes such as MCL-1, BCL-2, survivin, XIAP, and c-IAP1 in breast cancer cells. The resulting data showed that As2O3, either alone or in combination with melatonin, exerted significant cytotoxic effect against MCF-7 cells. However, further investigations are needed to provide valuable clues for expediting this combination as a therapeutic strategy for breast cancer.
Key Words: As2O3, apoptosis, combination therapy, melatonin, NF-κB
The therapeutic approaches in breast cancer, as the most common malignancy in womankind in term of morbidity and mortality (1), still remain an unsolvable dilemma; therefore, the entrance of the novel anti-tumoral agents in combination with chemotherapy or radiotherapy turns to be groundbreaking for more efficient treatment. During the last decade, identifying the potent anti-cancerous property of arsenic trioxide (As2O3) revitalizes the popularity of this agent as an effective chemotherapeutic drug not only for hematologic malignancies but also for solid tumors (2). An overwhelming number of studies imply that As2O3 exerts its variegated apoptotic effects through various mechanisms including modulation of the intracellular glutathione redox system (3), induction of mitotic arrest (4), DNA damage and inhibition of DNA repair (5). Despite its prodigious anti- tumoral activities, the clinical use of this agent became restricted due to the toxic side effects of long-term intake of high doses of As2O3. A recent renaissance of As2O3 following the novel disclosure indicating that As2O3 is an ideal agent to be used in combination therapy, has revived the interest in this ancient drug and has constructed its future direction into clinical investigations.
Melatonin, the master biologic clock neurohormone, is basically considered as the main regulator of circadian rhythm and sleep quality (6). Thus far, a plethora of biological actions have been reported for melatonin; however, recent investigations concerning its inhibitory impact on the development, promotion, and progression of various types of cancers, in particular, breast cancer have attracted tremendous attention (7). In the last decades, several experimental studies have outlined that melatonin not only possess a chemo-preventive property (8), but also exerts a pro-apoptotic activity in cancer cells mostly through generation of intercellular ROS (9, 10). For the nonce, multiple published reports have discussed about the intensifying effect of melatonin in combination with different chemotherapeutic drugs, especially DNA damaging agents such as cis-platin (11), cyclophosphamide (12), and doxorubicin (13). Based on the convergence mechanisms between melatonin and As2O3 (14, 15), it was tempting to evaluate whether melatonin could enhance the pro-apoptotic effect of As2O3 in MCF-7 breast cancer cells.
Materials and methods
Cell line and reagents
The human hormone-sensitive breast adenocarcinoma cell line MCF-7 (Pasteur Institute, Tehran, Iran) was maintained in Dulbecco’s modified Eagle’s culture medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco, Life Technologies, Carlsbad, CA). Cells in exponential growth phase were used for experiments. DMEM medium was renewed every 3 days and the cells were harvested with 0.05% trypsin on the sixth day after seeding. The common chemotherapeutic drug, As2O3, was purchased from Sina Darou (Tehran, Iran). A stock solution of As2O3 was dissolved in culture medium (DMEM) to attain the concentrations of 1-5 µM. For extensive experiments, MCF-7 cells were also treated with desired concentrations of an indolamine hormone, melatonin, either alone or in combination with As2O3. In all experiments, equal amount of solvent, ethanol, was added in medium as controls at the final concentration of 0.1%. All drug treatments were carried out in three independent experiments and all assays were performed in triplicate.
Trypan blue exclusion assay
Breast cancer-derived MCF-7 cells were seeded at the density of 450 × 105 cells and were incubated with various concentrations of As2O3 and melatonin, either alone or in combined modality. After 48 h, drug treated-cells were trypsinized, centrifuged and the cell pellets were re-suspended in phosphate-buffered saline (PBS) in combination with 0.4 % trypan blue (Invitrogen, New Zealand). By using a Neubauer hemocytometer, the number of viable cells was counted and afterwards the percentage of viable cells was assessed using the following formula: viability (%)= viable cell count/total cell count×100.
MTT assay
Microculture tetrazolium assay (MTT) was applied to explore the impeding effect of As2O3, either as a single agent or in combination with melatonin, on the ability of breast cancer cells to metabolize thiazolyl blue tetrazolium bromide into formazan crystals. After incubation of cells with agents up to 48 h, cells were incubated with 100 μl of MTT (0.5 mg/ml) solution for further 3 h in a humidified incubator. The optical densitometry (OD) of a resulting formazan solubilized with dimethyl sulfoxide was measured in an enzyme-linked immunosorbent assay (ELISA) reader (BioTek® Instruments, Inc., USA) at the wavelength of 570 nm.
BrdU cell proliferation assay
The effect of As2O3 on the proliferative capacity of MCF-7 cells was assessed using bromodeoxyuridine (BrdU) kit (Roche, Germany). Initially, MCF-7 cells were seeded into 6-well plates overnight and then were treated with As2O3 for the next 48 h. In the final 12 h of the desired incubation times, 10 µl of BrdU labeling solution was added. For cell fixation and denaturation of DNA, 200 μl of FixDenat solution was added to each well. After 30 min and discarding FixDenat, 100 μl peroxidase-conjugated anti-BrdU (anti-BrdU-POD) antibody was added to each well. Finally, the cells were exposed to 100 μl of substrate tetramethyl-benzidine (TMB) for 3 min at room temperature and the reaction product was quantified by measuring the absorbance at 450 nm in an ELISA reader.
Median-effect analysis of drug combinations
To evaluate whether there was a synergistic
effect between As2O3 and melatonin, we computed the combination index (CI) using the method developed by Chou and Talalay (16). The dose which may be reduced in a combination for a given level of effect as compared to the concentration of individual drug alone was defined as dose reduction index (DRI) and calculated as follow: (DRI)1 = (Dx)1/(D)1 and (DRI)2 =(Dx)2/(D)2, where (Dx)1 and (Dx)2 indicate the individual dose of As2O3 and melatonin required to inhibit a given level of viability index, respectively. (D)1 and (D)2 are the doses of As2O3 and melatonin necessary to produce the same effect in combination, respectively.
Phosphatidylserine (PS) externalization (annexin- V assay)
Annexin-V/PI staining was applied to investigate whether As2O3 as a single agent or in combination with melatonin could induce apoptotic cell death in breast cancer cells. After 48 h incubation of MCF-7 cells with designated concentrations of As2O3 and melatonin, the trypsinized adherent cells were centrifuged and then were washed twice with cold PBS. Cells were re-suspended in 1X Binding Buffer at a concentration of 1×106 cells/ml. In the next step, annexin-V-Flous and PI stain were added to each sample, the cell suspension was mixed gently and incubated for 20 min in the dark. Finally, 400 µl binding buffer was added to each sample and the amount of externalization of phosphatidylserine was analyzed by flow cytometry.
Caspase -3 activity assay
The caspase-3 assay (Sigma, USA) is based on spectrophotometric detection of the p-nitroaniline (pNA) after the hydrolysis of the peptide substrate acetyl-Asp-Glu-ValAsp p-nitroanilide (Ac-DEVD-pNA) by caspase 3. For evaluating whether As2O3 and melatonin could reduce the survival rate of MCF-7 cells through activation of caspase-3, breast cancer cells were treated with each agent, either alone or in combined modality. Following 48 h treatment, the trypsinized adherent cells were centrifuged at 600 x g for 5 min and washed with 1 ml PBS. After gently discarding the supernatant, the cell pellets were re-suspended in lysis buffer at the concentrations of 1×107 cells/100 µl and were incubated on ice for 20 min. Next, the lysed cells were centrifuged at 20000 x g for 10 min. Next, 5 μg of the supernatant was added to 100 µl solution consisting of 85 μl assay buffer and 10 μl caspase-3 substrate acetyl-Asp-Glu-Val-Asp pnitroanilide. The concentration of the pNA released from the substrate was quantified spectrophotometrically from the absorbance values at 405 nm.
Quantitative real-time PCR
To perform qRT-PCR, the cells were treated with As2O3 and As2O3-plus-melatonin, and then RNA was extracted by high pure RNA isolation kit (Roche, Germany) and measured by Nanodrop ND-1000 (Nanodrop Technologies, Wilmington, Delaware, USA).1 µg of RNA from each sample was used for reverse transcription using the revertAid First Strand cDNA synthesis kit (Takara BIO, Japan). The synthesized cDNA was used to carry out qRT-PCR using SYBR Premix Ex Taq technology (Takara BIO, Japan) on a light cycler instrument (Roche, Germany). Table 1 summarizes the nucleotide sequences of the primers used in qRT-PCR analysis. The alteration in the mRNA expression level of NF-κB anti-apoptotic target genes, such as myeloid cell leukemia-1 (MCL-1), B cell lymphoma-2 (BCL-2), survivin, X-linked inhibitor of apoptosis protein (XIAP), and cellular inhibitor of apoptosis protein 1 (c-IAP1) were analyzed in comparison with the expression of HPRT, as the housekeeping gene.
Table 1.
Nucleotide sequences of primers used for real-time RT-PCR
| Gene |
Accession
number |
Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplicon size (bp) |
|---|---|---|---|---|
| HPRT | NM_000194 | TGGACAGGACTGAACGTCTTG | CCAGCAGGTCAGCAAAGAATTTA | 111 |
| BCL-2 | NM_000633 | CGGTGGGGTCATGTGTGTG | CGGTTCAGGTACTCAGTCATCC | 90 |
| Survivin | NM_001168 | CCAGATGACGACCCCATAGAG | TTGTTGGTTTCCTTTGCAATTTT | 152 |
| MCL-1 | NM_021960 | AGAAAGCTGCATCGAACCAT | CCAGCTCCTACTCCAGCAAC | 183 |
| XIAP | NM_001167 | ATAGTGCCACGCAGTCTACAA | AGATGGCCTGTCTAAGGCAAA | 101 |
| c-IAP1 | NM_001166 | AGCACGATCTTGTCAGATTGG | GGCGGGGAAAGTTGAATATGTA | 102 |
Statistical analysis
Data are expressed as the mean ± SD of three independent experiments. The significance of differences between experimental variables was determined by the use of two-tailed Student’s t-test and by one-way variance analysis. To compare the control group and the drugs- treated cells, the Dunnett’s multiple comparison test was used. A probability level of P<0.05 was considered statistically significant.
Results
Oncocytic effect of As2O3 on MCF-7 breast cancer cells
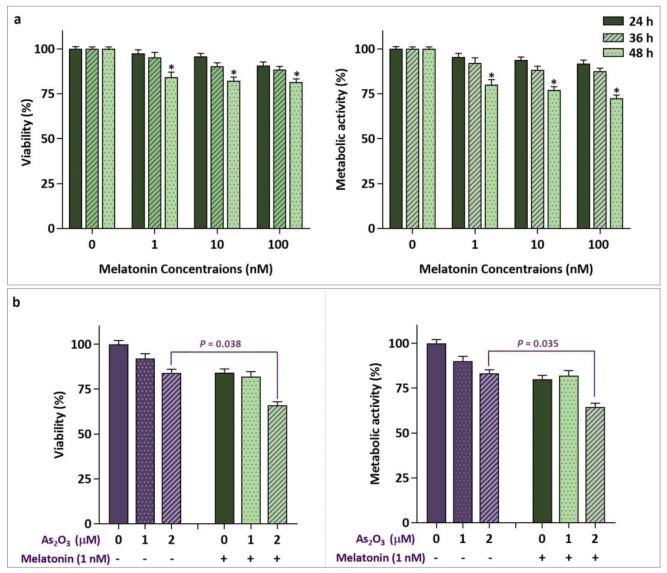
To ascertain whether MCF-7 cells treatment with increasing concentrations of As2O3 (0-5 µM) up to 48 h could reduce the survival and proliferative rate of breast cancer cells, trypan blue, MTT, and BrdU cell proliferation assays were performed. As shown in figure 1, unlike 24 and 36 h treatment with As2O3 which had no significant effect on cell survival and growth kinetics, culturing cells for 48 h decreased the viability, cell count, and metabolic activity in a dose-dependent manner. Noteworthy, calculation of IC50 value for different methods of survival assessment after 48 h revealed that As2O3 is capable to reduce 50% of MCF-7 cell viability at the concentration of 8 µM (data not shown). The results of trypan blue and MTT assay were further substantiated by investigating the amount of DNA synthesis rate using BrdU incorporation assay which revealed that As2O3-plus-meltonin remarkably hampered the proliferative capacity of breast cancer cells. As presented in figure 1d, treatment of the cells at this point in time resulted in a significant inhibitory effect on DNA synthesis (nearly by 32%) at the concentration of 5 µM.
Fig. 1.
As2O3 induced cytotoxic and anti-proliferative effects on breast cancer cells. a and b: breast cancer-derived MCF-7 cells were treated with increasing concentrations of As2O3 (0-5 µM) up to 48 h. The results of trypan blue exclusion assay showed that As2O3 not only reduced cell survival in a dose- and time-dependent manner, but also decreased the number of MCF-7 cells; c: cells metabolic activity was inhibited upon treatment with As2O3; d: MCF-7 cells were incubated with the inhibitor up to 48 h and the suppressive effect on DNA synthesis rate was determined using BrdU cell proliferation assay. Values are given as mean ± SD of three independent experiments. *: P ≤ 0.05 represents significant changes from untreated control
Melatonin reinforced the cytotoxic effect of As2O3
Given the IC50 value of As2O3 on breast cancer cells, which is considerably higher than its maximum dose used in clinical applications (17), we aimed to evaluate whether melatonin could enhance the effectiveness of As2O3 through reducing its effective concentration on MCF-7. Time- and dose- dependent experiments showed that melatonin alone exerted its cytotoxic and anti-proliferative effects only in a time- dependent manner. As depicted in figure 2a, 48 h exposure of MCF-7 cells to the lowest and highest concentrations of melatonin (1 nM and 100 nM) reduced cell viability by 16% and 19%, respectively. Therefore, we chose the physiological concentration of this indolamine (1 nM) for synergistic effect assessment. Interestingly, As2O3 in combination with melatonin reduced more effectively the number of MCF-7 viable cells (P=0.038) in comparison with either agents alone. This finding was further substantiated by MTT assay, showing that simultaneous treatment of cells with both agents efficiently hampered the metabolic activity (P=0.035) (Fig.2b). Determination of combination index (CI) and dose reduction index (DRI) values also clarified that melatonin boosted the cytotoxic and anti-proliferative effect of As2O3. Values of CI and DRI after 48 h treatment of MCF-7 cells are summarized in Table 2.
Fig. 2.
The synergistic effect of melatonin and As2O3. a: treatment of MCF-7 with increasing concentrations of melatonin (1-100 nM) up to 48 h resulted in the reduction of cell viability and metabolic activity; b: MCF-7 cells were treated with melatonin (1 nM) in combination with As2O3 (1 and 2 µM). The results of synergism evaluation experiments showed that melatonin could sensitize breast cancer cells to the anti-leukemic effect of As2O3. Values are given as mean ± SD of three independent experiments.*: P≤ 0.05 represents significant changes from untreated control
Table 2.
Combination index (CI) and dose reduction index (DRI) for drug combination by As2O3 and melatonin
| As 2 O 3 | Melatonin | CI | ||
|---|---|---|---|---|
| Concentration (µM) | DRI | Concentration (nM) | DRI | |
| 1 | 2.163 | 1 | 0.895 | 1.58 |
| 2 | 3.051 | 1 | 1.956 | 0.839 |
Melatonin potentiated As 2 O 3 - induced apoptosis in MCF-7 cells
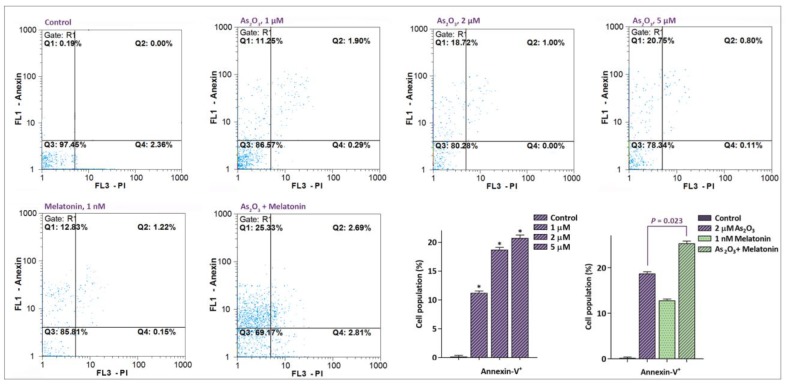
To shed light on the mechanisms through which As2O3 induced its inhibitory effect on the survival rate of mammary adenocarcinoma cells, MCF-7 were exposed to escalated concentrations of the agent (1-5 µM) and the percentage of the annexin-V and annexin-V/PI positive cells were examined using annexin-V/PI staining assay. The results of flow cytometry analysis revealed that treating the cells with As2O3 increased the percentage of annexin-V positive cells. As presented in figure 3, the highest percentage of apoptotic MCF-7 cells was detected at the concentration of 5 µM, which elevated the proportion of annexin-V positive cells to 20.75%. Our finding was even noticeable in the synergistic effect assessment, where an enhanced induction of apoptosis was found using As2O3 in combination with melatonin. As depicted in this figure, 48 h treatment of cells with melatonin and As2O3 (2 µM) remarkably increased the percentage of annexin-V positive cells from 18.72% in As2O3- treated to 25.33% in melatonin/As2O3- treated group (P= 0.023), which indicated that melatonin at its physiological concentration is able to enhance the pro-apoptotic properties of As2O3.
Fig. 3.
Treatment of MCF-7 cells with As2O3, either alone or in combination with melatonin, increased the percentage of apoptotic cells in MCF-7 cells. After incubation of breast cancer-derived MCF-7 cells with As2O3 and melatonin, the population of Annexin-V+ and Annexin-V/PI+ cells were investigated by using flow cytometry analysis. Values are given as mean ± SD of three independent experiments. *, P ≤ 0.05 represents significant changes from untreated control
Stimulatory effect of melatonin on As2O3-induced apoptosis is mediated through caspase-3 activity
In response to death stimuli, activated form of caspase-3 catalyzes the cleavage of many key cellular proteins, which eventually commit to cell apoptosis (18). To ascertain the involvement of caspase-3 activation in As2O3-induced apoptosis, we investigated the activity of the enzyme after treating MCF-7 cells with drug concentrations similar to those used for the annexin-V assay. Our results showed a concentration- dependent increase in the caspase -3 activity upon treatment with As2O3. Although treatment with 1 μM As2O3 did not induce significant enzymatic activity, exposing cells to 2 and 5 μM for 48 h increased caspase-3 activity by 9-and 27- fold, respectively (Fig. 4a). Noteworthy, co- treatment of the cells with melatonin and As2O3 also resulted in apoptotic cell death through a caspase-3-dependent cascade However, similar to annexin-V staining assay data, enzymatic activation of caspase-3 significantly increased when As2O3 was used in combination with melatonin (P =0.044) (Fig. 4b), substantiating the potentiating effect of melatonin for As2O3 treatment.
Fig. 4.
Activation of caspase-3-dependent apoptotic pathway in response to As2O3 and its combination with melatonin. a: As2O3 increased dose-dependently the enzymatic activity of caspase-3; b: a notable increase in caspase-3 activity was observed when MCF-7 cells were treated simultaneously with As2O3 and melatonin. Values are given as mean ± SD of three independent experiments. *: P ≤ 0.05 represents significant changes from untreated control
Downregulation of NF-kB anti-apoptotic target genes following cell treatment with As2O3 and melatonin
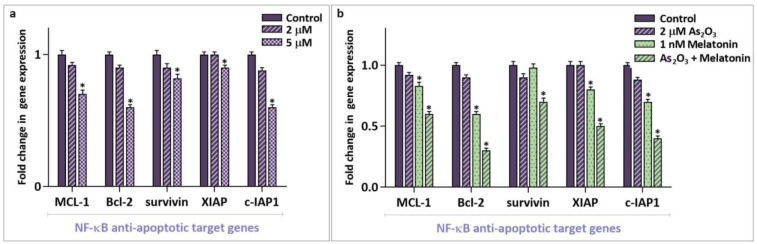
Conclusive evidence has stated that perturbed activation of NF-κB, a potent inducer of anti-apoptotic genes, contributes to the process of chemo-resistance in tumor cells (19). Our results revealed that while 2 µM As2O3 had minimal effect on NF-κB death repressor genes transcription (Fig.5a), physiological dose of melatonin robustly reinforced the suppressive effect of As2O3 on NF-κB downstream anti- apoptotic target genes. As illustrated in figure 5b, when MCF-7 cells were treated with both As2O3 and melatonin, the mRNA expression levels of MCL-1, BCL-2, survivin, XIAP, and c-IAP1 reduced significantly (Fig. 5b).
Fig 5.
Effects of As2O3 alone or in combination with melatonin on the transcription of anti-apoptotic target genes of NF-κB in MCF-7 cells. a: the results of RQ-PCR analysis revealed that As2O3 at its highest concentration (5 µM) was able to reduce the mRNA level of different anti-apoptotic target genes of NF-κB in MCF-7 cells; b: combined treatment with melatonin and As2O3 potently reduced the mRNA expression level of NF-κB anti- apoptotic target genes. Values are given as mean ± SD of three independent experiments. *: P ≤ 0.05 represents significant changes from untreated control
Discussion
During the ages, natural products have catered the basic needs of humans in the provision of medicines for the treatment of a broad spectrum of diseases (20). A traditional Chinese medicine As2O3 has long been of biomedical interest, and is largely investigated for treatment of a variety of cancers, ranging from hematological malignancies to solid tumors (21-23) However, its prodigious anti-cancerous efficacy has been rigorously overshadowed by the unfavorable side effects. In this regard, several strategies have been established thus far, to reinforce the cytotoxicity of this agent, especially for use in combination therapy. The recent discovery of the roles deviating from the canonical activities of melatonin has added a new perspective to reevaluate the application of this natural product of pineal gland in cancer therapeutic approaches (24). The results obtained in the present study showed that As2O3 reduced the survival and proliferative rate of MCF-7 cells and induced a caspase-3- dependent apoptotic pathway in this cell line. This finding was even more evident in the combined treatment, where we found that in the presence of melatonin the ability of As2O3 to decrease cell survival increased significantly in comparison with either drug alone. Consistently, previous studies conducted on breast cancer declared that melatonin may ameliorate the adriamycin-induced cardiac dysfunction through free radical scavenging or abrogating lipid peroxidation (25, 26). Additionally, it has been indicated that melatonin enhanced the apoptotic effect of the conventional chemotherapeutic drugs, such as tamoxifen (27), cis-platin (28), and 5‐fluorouracil (29). Although multiple lines of evidence have introduced melatonin as a promising candidate for adjuvant therapy (29, 31), further studies are now under way to characterize more precisely both the efficacy and the molecular mechanisms of action of this hormone in combination with anti-cancerous agents.
Conclusive evidence has stated that the activation of NF-κB, a potent inducer of anti-apoptotic genes, could be responsible for unexpected side effects of many commonly used chemotherapeutic drugs (19). Moreover, perturbation of NF-κB pathway in the pathogenesis of most human malignancies has attracted a great interest to the suppression of this nuclear transcription factor (32). Our study delineated that As2O3 at the concentration of 2 µM had minimal effect on the expression level of NF-κB target genes such as MCL-1, BCL-2, and the anti-apoptotic proteins of IAP family. Noteworthy, when As2O3 was used in combination with melatonin, its suppressive effect on the expression level of aforementioned genes turned to be more strengthened, indicative of the negative contributory role of melatonin on the transcription of anti-apoptotic target genes of NF-κB. This finding was in accordance with a study demonstrating that over-expression of MCL-1, a well-known member of anti-apoptotic genes of BCL-2 family, prevented the induction of apoptosis in multiple myeloma cells (33). The results of recent studies also revealed that suppression of either telomerase (34) or neurokinin-1 receptor (35) potentiated As2O3-induced cytotoxicity in APL-derived NB4 cells through down-regulating NF-κB anti-apoptotic target genes. In another study, Qiu et al. introduced asymmetric curcuminoid analogs as potent anticancer agents which not only reduced the survival of gastric cancer cells, but also enhanced the sensitivity of the cells to the common chemotherapeutic drug via downregulation of NF-κB activation (36). Moreover, it has been indicated that over-expression of anti-apoptotic proteins of BCL-2 family endows cancer cells a survival advantage (37-39). Herein, based on the suppressive effect of melatonin on the expression level of anti-apoptotic target genes of NF-κB, it is tempting to hazard a conjecture for the first time that probably the adjuvant effect of melatonin on the anti-tumoral activity of As2O3 is mediated through repressing the NF-κB activation. Consistently, the study by Lu et al. revealed that melatonin is capable of inhibiting nuclear translocation of NF-κB in lung cancer cells (40).
In conclusion, our results clearly demonstrated that besides the pro-apoptotic effect of As2O3 as a single agent in breast cancer, melatonin could significantly boost the anti-tumoral activity of this well-known chemotherapeutic drug in MCF-7 cells. Furtheremore, our results showed that melatonin-plus-As2O3 decreased the survival rate of breast cancer-derived MCF-7 cells by triggering a caspase-3-dependent apoptosis probably through suppressing the expression of anti-apoptotic target genes of NF-κB. Given the safety of melatonin, as the natural secretory product of pineal gland, the resulting data suggests that using melatonin in combination with As2O3 might be advantageous for expediting this hormone as a therapeutic agent in breast cancer.
Acknowledgments
Authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Bleiker EM, van der Ploeg HM. Psychosocial factors in the etiology of breast cancer: review of a popular link. Patient Educ Couns. 1999;37:201–14. doi: 10.1016/s0738-3991(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 2.Antman KH. Introduction: the history of arsenic trioxide in cancer therapy. Oncologist. 2001;6 Suppl 2:1–2. doi: 10.1634/theoncologist.6-suppl_2-1. [DOI] [PubMed] [Google Scholar]
- 3.Jing Y, Dai J, Chalmers-Redman RM, et al. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–11. [PubMed] [Google Scholar]
- 4.Li YM, Broome JD. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 1999;59:776–80. [PubMed] [Google Scholar]
- 5.Yoo DR, Chong SA, Nam MJ. Proteome profiling of arsenic trioxide-treated human hepatic cancer cells. Cancer Genomics Proteomics. 2009;6:269–74. [PubMed] [Google Scholar]
- 6.Nooshinfar E, Safaroghli-Azar A, Bashash D, et al. Melatonin, an inhibitory agent in breast cancer. Breast Cancer. 2017;24:42–51. doi: 10.1007/s12282-016-0690-7. [DOI] [PubMed] [Google Scholar]
- 7.Hill SM, Blask DE, Xiang S, et al. Melatonin and associated signaling pathways that control normal breast epithelium and breast cancer. J Mammary Gland Biol Neoplasia. 2011;16:235–45. doi: 10.1007/s10911-011-9222-4. [DOI] [PubMed] [Google Scholar]
- 8.Ilbey YO, Ozbek E, Simsek A, et al. Potential chemoprotective effect of melatonin in cyclophosphamide- and cisplatin-induced testicular damage in rats. Fertil Steril. 2009;92:1124–32. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 9.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–32. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 10.Buyukavci M, Ozdemir O, Buck S, et al. Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol. 2006;20:73–9. doi: 10.1111/j.1472-8206.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 11.Hao J, Li Z, Zhang C, et al. Targeting NF-kappaB/AP-2beta signaling to enhance antitumor activity of cisplatin by melatonin in hepatocellular carcinoma cells. Am J Cancer Res. 2017;7:13–27. [PMC free article] [PubMed] [Google Scholar]
- 12.Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G, et al. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res. 2010;48:72–80. doi: 10.1111/j.1600-079X.2009.00727.x. [DOI] [PubMed] [Google Scholar]
- 13.Kosar PA, Naziroglu M, Ovey IS, et al. Synergic Effects of Doxorubicin and Melatonin on Apoptosis and Mitochondrial Oxidative Stress in MCF-7 Breast Cancer Cells: Involvement of TRPV1 Channels. J Membr Biol. 2016;249:129–40. doi: 10.1007/s00232-015-9855-0. [DOI] [PubMed] [Google Scholar]
- 14.Sainz RM, Mayo JC, Rodriguez C, et al. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 2003;60:1407–26. doi: 10.1007/s00018-003-2319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woo SH, Park IC, Park MJ, et al. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int J Oncol. 2002;21:57–63. [PubMed] [Google Scholar]
- 16.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 17.Fox E, Razzouk BI, Widemann BC, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–73. doi: 10.1182/blood-2007-08-107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 19.Tapia MA, Gonzalez-Navarrete I, Dalmases A, et al. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle. 2007;6:2284–92. doi: 10.4161/cc.6.18.4721. [DOI] [PubMed] [Google Scholar]
- 20.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–43. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 21.Baj G, Arnulfo A, Deaglio S, et al. Arsenic trioxide and breast cancer: analysis of the apoptotic, differentiative and immunomodulatory effects. Breast Cancer Res Treat. 2002;73:61–73. doi: 10.1023/a:1015272401822. [DOI] [PubMed] [Google Scholar]
- 22.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–60. [PubMed] [Google Scholar]
- 23.Lin CC, Hsu C, Hsu CH, et al. Arsenic trioxide in patients with hepatocellular carcinoma: a phase II trial. Invest New Drugs. 2007;25:77–84. doi: 10.1007/s10637-006-9004-9. [DOI] [PubMed] [Google Scholar]
- 24.Jung B, Ahmad N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789–93. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- 25.Arif IS, Hooper CL, Greco F, et al. Increasing doxorubicin activity against breast cancer cells using PPARgamma-ligands and by exploiting circadian rhythms. Br J Pharmacol. 2013;169:1178–88. doi: 10.1111/bph.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma C, Li LX, Zhang Y, et al. Protective and sensitive effects of melatonin combined with adriamycin on ER+ (estrogen receptor) breast cancer. Eur J Gynaecol Oncol. 2015;36:197–202. [PubMed] [Google Scholar]
- 27.Sánchez-Barceló E, Mediavilla M, Cos S. Melatonin: an endogenous antiestrogen with oncostatic properties. In: Pandi-Perumal SR, editor. Melatonin From Molecules to Therapy. 2007. pp. 261–72. [Google Scholar]
- 28.Pariente R, Pariente JA, Rodriguez AB, et al. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60:55–64. doi: 10.1111/jpi.12288. [DOI] [PubMed] [Google Scholar]
- 29.Gao Y, Xiao X, Zhang C, et al. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res. 2017:62. doi: 10.1111/jpi.12380. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Barcelo EJ, Cos S, Mediavilla D, et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38:217–22. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Lialiaris T, Lyratzopoulos E, Papachristou F, et al. Supplementation of melatonin protects human lymphocytes in vitro from the genotoxic activity of melphalan. Mutagenesis. 2008;23:347–54. doi: 10.1093/mutage/gen020. [DOI] [PubMed] [Google Scholar]
- 32.Dolcet X, Llobet D, Pallares J, et al. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 33.Derenne S, Monia B, Dean NM, et al. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–9. doi: 10.1182/blood.v100.1.194. [DOI] [PubMed] [Google Scholar]
- 34.Asghari-Kia L, Bashash D, Safaroghli-Azar A, et al. Targeting human telomerase RNA component using antisense oligonucleotide induces rapid cell death and increases ATO-induced apoptosis in APL cells. Eur J Pharmacol. 2017;809:215–23. doi: 10.1016/j.ejphar.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 35.Bashash D, Safaroghli-Azar A, Bayati S, et al. Neurokinin-1 receptor (NK1R) inhibition sensitizes APL cells to anti-tumor effect of arsenic trioxide via restriction of NF-kappaB axis: Shedding new light on resistance to Aprepitant. Int J Biochem Cell Biol. 2018;103:105–14. doi: 10.1016/j.biocel.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Qiu P, Zhang S, Zhou Y, et al. Synthesis and evaluation of asymmetric curcuminoid analogs as potential anticancer agents that downregulate NF-kappaB activation and enhance the sensitivity of gastric cancer cell lines to irinotecan chemotherapy. Eur J Med Chem. 2017;139:917–25. doi: 10.1016/j.ejmech.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Coustan-Smith E, Kitanaka A, Pui CH, et al. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996;87:1140–6. [PubMed] [Google Scholar]
- 38.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 39.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–88. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 40.Lu JJ, Fu L, Tang Z, et al. Melatonin inhibits AP-2beta/hTERT, NF-kappaB/COX-2 and Akt/ERK and activates caspase/Cyto C signaling to enhance the antitumor activity of berberine in lung cancer cells. Oncotarget. 2016;7:2985–3001. doi: 10.18632/oncotarget.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]