Abstract
Background:
Chordoma located in the skull base is usually a challenging surgical condition. It is often not possible to achieve gross total resection. Residual tumors have been treated with adjuvant focal radiation therapy employing high-energy particles most commonly through proton beam. In this review, we systematically analyzed indications and outcomes of this treatment with respect to local control rates of the lesion and factors determining recurrence of skull base chordomas. In addition, we collected data on treatment-associated radiation-induced side effects.
Methods:
In line with the PRISMA guidelines, the authors performed a literature search algorithm for relevant articles using three databases: PubMed, Embase, and Cochrane. Inclusion and exclusion criteria were applied to evaluate all identified studies published between 1980 and 2018.
Results:
Our review included 11 studies for analysis (n = 511 patients). The mean age of the study population was 47.3 ± 5.8 years. The mean dose of postsurgical irradiation at the time of initial treatment was 71.1 ± 3.1 Gy. The mean follow-up duration was 45.0 ± 17.5 months. Within this follow-up duration, recurrence occurred in 26.8% of the patients. The mean time to recurrence was 34.5 ± 15.2 months. A significant number of patients experienced side effects varying from Grade 1 (mild dermatitis) to Grade 4 (temporal lobe necrosis and visual disorders).
Conclusion:
Despite advances in proton therapy, recurrence rates in skull base chordoma remain high. The toxicity of proton therapy may be more prevalent than generally thought. Unfortunately, there is substantial variation in the methods of data reporting.
Keywords: Analysis, Chordoma, Proton, Recurrence, Safety, Skull base

INTRODUCTION
Cranial chordoma is a rare skull neoplasm which is thought to arise from remnants of the embryonic notochord. It represents about 1%–4% of primary bone tumors.[10,45] Chordoma occurs typically on either end of the notochord, which during development transforms into the sacrum and the skull base.[45] The sacrum is the most frequent site (50%) followed by the skull base (30%). Only a small percentage originates within the mobile spine (20%).[13] The incidence of chordoma is age dependent and is very low among patients under 40 years of age.[26] A recent systematic review found that the survival rates for skull base chordoma are estimated at 63% and 16% for 5 years and 10 years, respectively.[20]
Skull base chordomas destroy bone and infiltrate to adjacent soft tissue. Neural and vascular structures such as cranial nerves, brainstem, carotid, and basilar artery can be compressed and/or encased by the expanding tumor. Since chordomas have no distinctly formed fibrous capsule, it is frequently difficult to identify tumor margins on magnetic resonance images.[16,2] Recently, the European Society for Medical Oncology published a position paper on chordoma management.[39] Recommendations include specific imaging protocols, suitable surgical strategies, and regimen for radiation therapy. However, different from sacral chordomas, the extent of surgical resection at the skull base is often limited by the surrounding critical neurovascular structures.
Subtotal resections are, therefore, common and residual tumors are meant to be treated by radiation therapy.[12] The relative biological effectiveness (RBE) of radiation can be influenced by different factors including the linear energy transfer (LET), total dose, number of fractions, and radiosensitivity of the targeted cells or tissue.[6] Chordoma cells are considered rather radioresistant when treated with conventional fractionated external beam protocols, and therefore, a high dose of proton therapy (PT) is applied.[39] Compared to photon therapy, the physical properties of collimated protons as particles loaded with kinetic energy allow more precise targeting while limiting the exposure of adjacent structures to irradiation.[4,9,11] In a cyclotron, when protons reach their range-end, they are strongly submitted to strong lateral scattering followed by a sharp fall-off of the dose known as “Bragg peak”.[23] In addition, protons are suitable due to their property of low LET. However, when kinetic protons rapidly lose energy at the end of their range, a limited zone of high-LET radiation (dense ionization) is created.[27] Consequently, the RBE and cell death will increase at this site. This phenomenon may, however, lead also to damage to normal tissue.[32] Despite advances in particle beam radiation techniques, recurrences in skull base chordomas occur.
Over the past few years, more information has become available on the effects of PT in patients with residual skull base chordomas. After PT, progression of residual tumor is often observed. In this review, we systematically analyzed the details of recurrence of skull base chordomas after PT therapy and collected data on side effects.
METHODS
We performed a literature search for relevant articles using three databases: PubMed, Embase, and Cochrane. The search covered English language publications between 1980 and 2018 (the last search was conducted on January 8, 2018). The strategy and keywords used for the search were [(Chordoma*)] AND [(Proton) OR (Particles)] AND [(Radiotherapy) OR (Therapy) OR (Beam)]. Inclusion criteria were peer-reviewed original articles in patients reporting the recurrence of skull base chordoma who had undergone PT and/or combined photon-PT after surgery. The exclusion criteria were single case studies, review articles, abstracts, animal or phantom studies, questionnaires, and pediatric studies. In case of possible double reporting of patients by the same research groups in different publications, we included the report with the highest sample size and/or longest follow-up period. Authors’ names, characteristics of patients, period, and place of treatment were all cross-checked. Papers not reporting recurrence were excluded. Furthermore, some studies included different groups of patients who had received different types of radiation therapy. Hence, if recurrence was reported as overall without considering the type of radiation therapy, the paper was excluded as well.
The following details were extracted from the included papers: authors, year of publication, number of patients, gender, age, follow-up period, type of radiation therapy, volume of residual tumor postsurgery, radiation dose applied, dose per fraction, radiation-induced side effects, rate of recurrences, number of deaths, and control rates. The variations found in units of PT dosage were kept as reported. The dosage of PT in old studies is usually expressed as cobalt gray equivalent (CGE) units, which is a factor of 1.1 compared to photon dose (60Co). Recent reports used the units of Gy RBE (with 1.1 relative to that of 60Co) more frequently as required by the International Commission on Radiation Units and Measurements.[35] Based on symptoms, toxicity grades were categorized into five categories according to the Cancer Therapy Evaluation Program (Grade 1 – mild or asymptomatic; Grade 2 – moderate, not interfering with activities of daily living (ADLs); Grade 3 – severe interference with ADLs, possible intervention; Grade 4 – life-threatening or disabling, intervention indicated; and Grade 5 – death).[7] Any missing data were indicated as not applicable. Data were extracted by two assessors independently (the first and last author), and all disagreements were resolved through discussion until consensus was reached. The search strategy is summarized in Figure 1.
Figure 1:
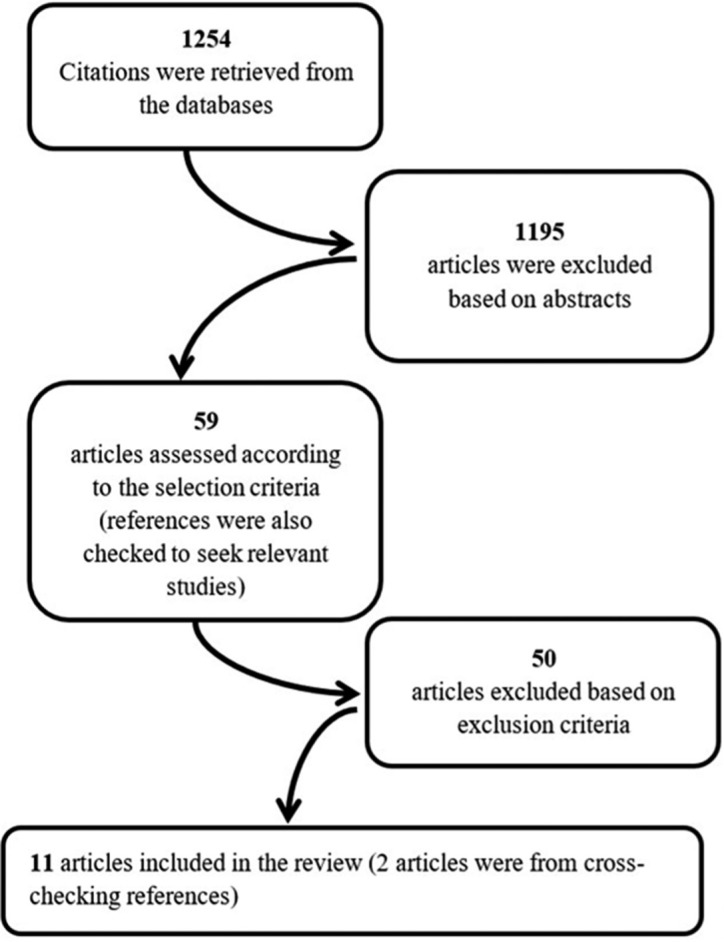
Flow chart showing the selection process of papers.
Data presentation
Eleven studies qualified for the final overall analysis. These studies will be briefly discussed in the next paragraphs. All mean values and standard deviations were analyzed using the IBM Statistical Package for the Social Science software, version 24.
RESULTS
First, we will provide a summary of included papers. These studies are described chronologically. Then, we will describe the results of the overall analysis.
1980–1989
In 1989, Sen et al. published on the treatment of eight patients with skull base chordoma.[28] Three of eight chordoma patients received combined proton-photon therapy with a dose of 70.2, 68.4, and 68.4 CGE, respectively. The other patients received conventional radiotherapy. In two patients who had received proton-photon therapy, recurrence occurred after 6 months and 3 years. Unfortunately, some patient characteristics were not available such as age of the patients, sex, control rate, and residual tumor volume. Side effects were also not mentioned.
1990–1999
In 1999, Terahara et al. described the outcome of 115 patients with skull base chordomas.[40] After initial surgery, residual tumor was irradiated with combined proton-photon radiation. The mean residual tumor volume at the time of radiotherapy was 57 cm3. In 42 cases, recurrence occurred. The reported control rates were 59% and 44% at 5 and 10 years, respectively. The mean time for tumors to relapse was 42 months. Remarkably, recurrence was more present among females than males. Larger target volumes had less durable local control. There were no adverse effects reported.
In the same year, another group described the results of their treatment of 33 chordoma patients.[17] Residual tumors were categorized in two groups: ≤25 ml (n = 7) and >25 ml (n = 26). Brainstem involvement was noted in more than half of the patients in this cohort. Thirty patients underwent PT and three patients were treated by combined proton-photon therapy with a mean dose of 71.9 ± 2.93 CGE. The mean follow-up period was 33.2 months. In eight patients, recurrence was observed with four of those located in the field of the high-dose volume and two cases who showed recurrence in the field of the low-dose volume. One lesion recurred in the marginal area and one along the surgical route. The local control rates at 3 and 5 years were 67% and 59%, respectively. Patients with residual tumors>25 ml and involvement of the brainstem were found to have poor control. At around the time of radiotherapy, all patients developed varying degrees of side effect including, hair loss, headaches, loss of appetite, fatigue, nausea, and vomiting. Grade 3 and 4 late toxicities were recorded in seven patients.
2000–2009
In 2004, Igaki et al. evaluated 13 cases of skull base chordomas who underwent either combined proton-photon therapy or PT alone.[18] Resections were performed in seven patients and biopsy only in six patients. Tumor regrowth/progression was seen in six patients none of them was from the biopsy group. This might be due to the relatively small tumor volumes of these patients. The authors suggested that, in small chordomas, biopsy followed by radiation therapy might be a valid approach. The local control rates at 3 and 5 years were 67% and 46%, respectively. Concerning side effects, one patient suffered from brain necrosis (Grade 4) and another patient died after the development of oral ulceration (Grade 4) as well as some brain necrosis (Grade 5). Both patients underwent PT after resection.
Recurrences were noticed more frequently in females than in males. Patterns of failure were local recurrences outside the clinical target volumes in two patients, local recurrence at dose-limited regions near radiation-sensitive structures in two patients, and in-field local recurrence within the gross tumor volume in one patient as well as out-of-field regional nodal recurrence in one patient.
In 2005, Noël. et al. reported their data of 100 irradiated patients with chordomas in the base of the skull or upper cervical spine.[30] This group has published four studies with overlapping patient groups.[30,31,33,34] We included the latest study as it contained the largest number of patients and the longest follow-up period.[30] In this cohort, males represented 60% of the study population and females 40%. Irradiated tumor volumes were between 1 and 125 cm3. All patients received a combination of photon-proton treatment either immediately after surgery (n = 70) or at relapse (n = 30). The median follow-up period in this study was 31 months. The imaging follow-up after radiotherapy was every 6 months for the first 5 years and then annually. Local recurrence was observed in 28 cases (of note: 17 relapses occurred in volumes receiving >55 CGE, nine in areas receiving <55 CGE, and in two relapses, the dose could not be identified). The calculated time for the recurrence manifestation after radiotherapy ranged between 3 and 71 months. A tumor volume exceeding 23 cm3 was found to be a positive predictor for tumor regrowth after irradiation. Due to restrictions of the adjuvant critical structures surrounding the treated target volume, recurrences were also significantly more frequent when <90% of the GTV was within the 95% isodose. No correlations were found between relapse rate and gender, age, number of prior surgeries, or cumulative dose. The 2- and 4-year local control rates were 86.3% and 53.8%, respectively. Nine patients of the 28 relapsed patients died due to the progression of disease. All patients experienced typical early side effects during irradiation including fatigue, loss of appetite, mild erythema, and sometimes nausea. Forty-two patients experienced late complications consisting of visual disorders, clinically relevant neuropsychological disorders, temporal lobe necrosis, hearing problems, and pituitary dysfunctions.
2010–2017
In 2013, Deraniyagala et al. published their results of a cohort of 33 chordoma patients who had undergone adjuvant PT with a mean dose of 78.4 CGE.[8] Those patients were followed up for a median period of 21 months. Three patients experienced local tumor relapse after treatment and one progressed before finishing the course. The estimated local control rate for 2 years was 86%. Eighteen patients suffered from significant unilateral hearing loss (grade 2 toxicity) requiring a hearing aid. Toxicity higher than Grade 2 was not seen among their population. According to our review scope and goals, the following aspects were not reported in cases which recurred irradiated tumor volume, male-to-female ratio, brainstem involvement as well as the manifestation time of relapse.
Grosshans et al. published, in 2014, the results of 15 patients who were treated by PT following surgery.[15] Their cohort consisted of 10 chordomas and 5 chondrosarcomas. The mean dose received by chordoma patients was 71.8 Gy (RBE) with a median follow-up period of 27 months. Seven patients had small residual tumor volumes (15 cm3) with the other three chordomas being somewhat larger (26.2 cm3). After 21 months, local recurrence occurred in one of these patients. The 3-year local control and the overall survival rates were estimated to be 87.5% and 100%, respectively. With regard to radiation-related adverse effects, most of the patients experienced only Grade 1 and 2 early complications including fatigue and nausea. Grades 3–5 of toxicities were not reported.
In 2016, Weber et al. reported the outcome of utilizing PT in 151 cases of skull base chordomas.[43] This group had published two such papers previously. We included their 2016 paper since it reported on the largest cohort of patients with the longest follow-up.[3,43,44] Following irradiation with a mean dose of 72 ± 2 Gy (RBE), 30 of 151 patients experienced local failure. Half of these failures occurred within 29.1 months after PT. Recurrence was found to be significantly more frequent among patients with tumors ≥25 cm3 and when patients had presented with brainstem or optic pathway compression. The estimated local control rates for 5 and 7 years were 75.8% and 70.9%, respectively. High grades (≥Grade 3) of late toxicity were seen in 18 of their patients. These late effects consisted of Grade 3 and 4 toxicities including unilateral optic neuropathy, temporal lobe necrosis, spinal cord necrosis, and unilateral hearing loss. Interestingly, univariate analysis performed by the authors showed no correlation between the tumor coverage radiation dose (V95%) and high-grade toxicities. However, complication-free survival was found to be influenced by age, hypertension, number of weekly fractions, and surgical complications before PT.
In the same year, McDonald et al. demonstrated that larger residual tumors and tumors receiving a lower dose were associated with poor control.[25] They reviewed 39 chordomas who underwent PT after surgery (median prescription dose= 77.4 Gy RBE). At the time of radiotherapy, 34 patients had confirmed residual tumors (median volume= 8.1 cm3, range: 0 cm3–79.7 cm3). Twenty-seven of these cases presented with volumes smaller than 20 cm3, while the remaining harbored tumors larger than 20 cm3. After a median follow-up of 51 months, nine recurrences were identified. The analysis showed that no recurrence (n = 30) occurred in patients who had tumors with smaller residual volumes at the time of RT (median = 6.4 cm3, P = 0.012). Furthermore, failures (n = 9) were noted among patients receiving low D1 cm3 (median = 66.8 Gy RBE) when compared with the median D1 cm3 received by patients whose tumors did not develop any recurrence (median= 76.3 Gy RBE). Gender was not found to be correlated with the control rate. Acute and late irradiation side effects were described as follows, 32 patients experienced acute Grade 1 and 2 acute toxicities in a form of mild dermatitis, nausea, mucositis, or sinusitis. Acute Grade 4 toxicity was seen in one patient with severe headache and visual deterioration requiring hospitalization. Late Grade 1 and 2 toxicities were observed in 13 patients including asymptomatic/symptomatic radiation necrosis, radiation necrosis, sensorineural hearing loss, chronic mastoid effusions, or pituitary dysfunction. Two patients experienced late Grade 3 late toxicity (sensorineural hearing loss requiring a hearing aid), and one patient displayed late Grade 4 late toxicity (radiation necrosis requiring hospitalization).
In 2017, Jägersberg et al. published the analysis of 13 skull base chordomas treated between 2005 and 2015.[19] Two of their patients had been treated with photon radiotherapy, whereas the remaining ten patients received PT (total dose of 74 Gy RBE). The residual tumor volume was not mentioned. Five of the ten patients who have received PT developed recurrence in a time ranging from 41 to 161 months (mean= 75 months). We calculated the 5-year tumor control and survival rates from the individuals’ data provided in the paper and found them to be 70%. Complications from radiotherapy were as follows: two patients experienced unilateral loss of functional hearing, one patient lost >50% of vision, and an oculomotor deficit was seen in one patient.
Another recent study described the treatment of 22 clival chordomas.[14] PT was applied in only five patients of this series. Since the focus of this study was on gamma-knife (GK) radiosurgery, some details on the patients treated with PT were unavailable. In three patients, recurrence occurred after a mean follow-up period of 38 months. Two patients died after serious radiation-induced complications.
Overall statistical analysis
Our review resulted in a total of 511 patients which could be included for pooled analysis. The mean age of the study population was 47.3 ± 5.8 years. The mean dose of irradiation was 71.1 ± 3.1 Gy (RBE). The mean follow-up duration was 45.0 ± 17.5 months. Within this follow-up duration, recurrence was reported in 137 patients (26.8%). The mean time to recurrence was 34.5 ± 15.2 months. Forty-seven patients died due to their chordoma, and three deaths found to be due to radiation-induced side effects. From the dataset collected, we could not reconstruct a Kaplan–Meier plot illustrating local control rate. This is largely due to the lack of a given end time which is required for any survival analysis and which was missing here for individual patients in whom no recurrences were reported. Too many missing observations made it impossible to compute the Chi-squared contingency test to test for associations between recurrence and either the gross residual tumor volume, radiotherapy type, radiation dose, or gender.
Included studies and radiotherapy approach
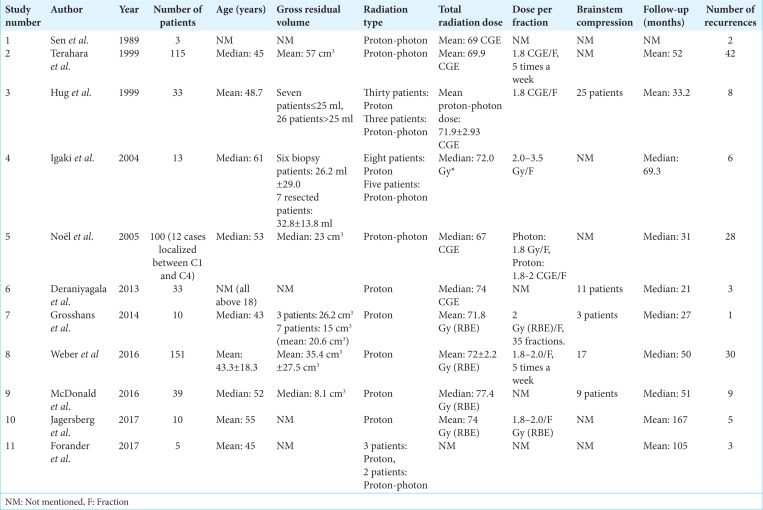
Table 1 shows the characteristics of the 11 articles which met the inclusion criteria.[8,14,15,17-19,25,28,30,40,43] As shown in Table 1, three studies have included only patients who underwent proton-photon therapy,[28,30,40] three studies divided their population size into two groups: patients who went through a proton-photon treatment regimen and patients who experienced PT alone.[14,17,18] The remaining five studies reported on PT only.[8,15,19,25,43]
Table 1:
Characteristics of the 11 eligible studies.
Chordoma recurrence
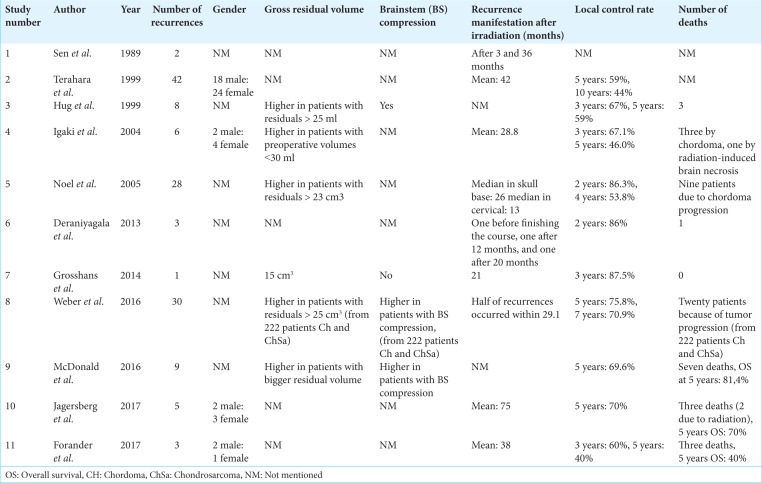
The incidences of chordoma recurrence are presented in Table 2, without highlighting the radiotherapy type. The table also presents factors that may contribute to or negatively impact disease control rates.
Table 2:
Characteristics of the recurrent chordomas.
Studies reporting on radiation-induced side effects
A summary of the reported acute and late radiation-induced effects, number of occurrences, and their toxicity grading levels are shown in Tables 3 and 4. Among studies reported on the combined radiotherapy approach, radiation adverse effects were only described by one paper which lacked grading these effects.[30] On the other hand, incidences of radiation effects were collected for a total of 294 patients included in 8 studies which reported the experience of PT alone or which divided their populations into subgroups allowing us to assess the data.[8,14,15,17-19,25,43] It may thus be possible for a single patient to develop two or more of those side effects.
Table 3:
Reported early toxicities, their grades, and number of occurrence after PT, gathered from 8 studies.
DISCUSSION
This study confirms that radiation therapy of skull base chordomas suffers from high recurrence rates despite high-intensity treatments.[38,41] Since complete surgical resection is rarely achievable in skull base chordomas and due to the radioresistant behavior of this neoplasm, a delivery of a dose of at least 74 Gy (RBE) to the tumor bed and other sites of gross disease is recommended.[39] The physics of kinetic protons that we mentioned earlier makes PT the preferred adjuvant radiotherapy modality whenever possible.[29,37] Prospective trials are currently being conducted to compare the biological effectiveness and safety of carbon-ion therapy with PT for skull base chordomas.[29] Our results suggest that, even after irradiating the residual chordomas with a high dose of PT (71.1 ± 3 Gy RBE), in 25% of the cases, regrowth is expected to occur within 3 years.
The reported local control rate at 5 years after surgery and either combined therapy or PT alone ranges between 40% and 75.8%.[14,17-19,25,40,43] This confirms that the management of skull base chordomas remains a challenge. Large case series demonstrated that unfavorable tumor control was often linked to high residual volumes (>23 cm3) and brainstem involvement.[17,30,43]
As an alternative to PT, modern technology in conformal photon irradiation can offer encouraging control rates which can be compared with those reported by PT. The intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT) are developed to escalate, shape, and deliver the respective dose more effectively and more conformally toward the targeted disease.[1] An overall 5-year local control rate of 65.3% was reported recently in a study which included 24 skull base chordoma patients treated with IMRT and IGRT, treated to a median dose of 76 Gy.[37] Another study confirmed the feasibility of GK radiosurgery either as a primary treatment modality or in the adjuvant setting after surgery for skull base chordomas, especially with small-sized tumors and younger patients.[21] Data of 71 chordoma patients were gathered from 6 GK centers in North America with a maximum prescription dose ranging from 18 Gy to 50 Gy. The 5-year control rate was estimated to be 66% for the entire group.[21] These control rates are comparable with those of PT. In spite of the improvements in such photon dose delivery systems, the possibility of high exposure to the normal tissue beyond the tumor margins is expected and remains of concern.[3]
The phenomenon of Bragg peak in PT has generated the hypothesis of minimizing treatment-related side effects. This assumption will be tested more thoroughly now with the increase in a number of available PT facilities. The question arises whether secondary malignant neoplasms can occur. The current follow-up durations are insufficient to answer this question, and longer follow-up data are required (at least 10–15 years after treatment).[22] Furthermore, some researchers stated that no significant differences in radiation-induced side effects were observed after either PT or photon therapy.[32,37]
We summarized the most frequently observed acute toxicities (Grade 1 or 2) within the duration of 6 months after PT. These included nausea and vomiting, fatigue, temporary hair loss, mucositis, headache, and loss of appetite [Table 3]. High grades of acute toxicity were rarely seen, and if they do occur, it might be in the form of a severe headache or visual deterioration. Late-occurring toxicities included unilateral hearing loss, temporal lobe necrosis, optic neuropathy, and occasionally pituitary dysfunction [Table 4]. The dose volume histogram was found to be the most valuable prognostic factor of late complications. The risk for focal brain necrosis, for example, will increase proportionally with the increase of the received dose of 10 Gy (RBE) and above [Figure 2].[18,24,36,42] However, variations in normal tissue radiosensitivity should be taken into account as these differ based on an individuals’ genetic profile.[5] These late complications highlight the importance of adhering to the recommended dose constraints for the temporal lobe, optic pathway, brainstem, spinal cord, and skin.[39]
Table 4:
Reported late toxicities, their grades, and number of occurrence after PT, gathered from eight studies.
Figure 2:
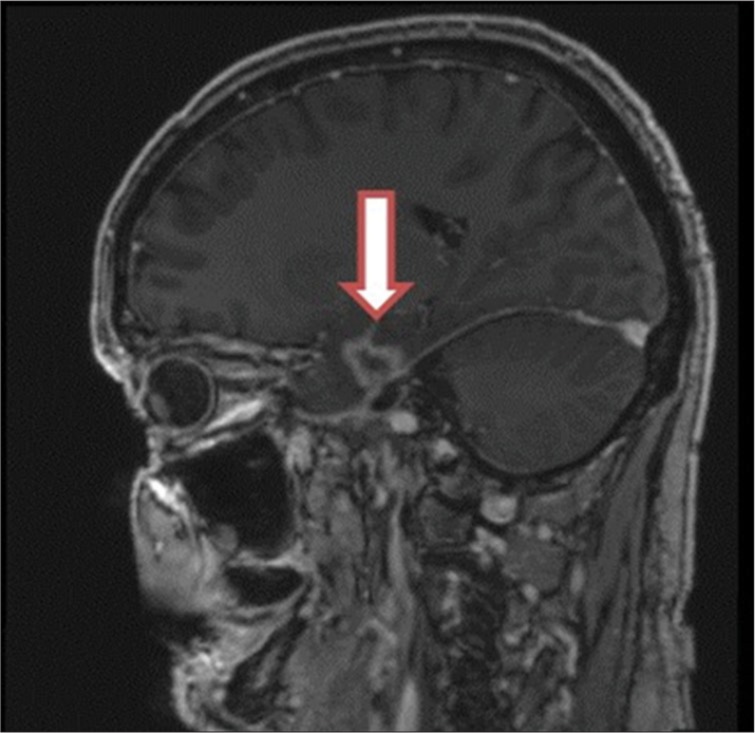
A sagittal view of brain magnetic resonance image with gadolinium enhancement showing temporal lobe necrosis after proton therapy (Courtesy Y. Temel).
Of note, during the review process, we have encountered difficulties which may limit the interpretation of our data. First, in some of the early studies, protons were combined with photon-based radiation. Second, missing data and not reporting data systematically limit performing a more extensive analysis.
CONCLUSION
Despite advances in PT, recurrence rates in skull base chordoma remain high. The toxicity of PT may be more prevalent than generally thought.
Acknowledgements
The authors are grateful to Mr. Gil Stevenson for his support in the analysis of the data.
Contributor Information
Mohammed Alahmari, Email: m.alahmari@maastrichtuniversity.nl.
Yasin Temel, Email: y.temel@maastrichtuniversity.nl.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ahmed SK, Brown PD, Foote RL. Protons vs photons for brain and skull base tumors. Semin Radiat Oncol. 2018;28:97–107. doi: 10.1016/j.semradonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 2.al-Mefty O, Borba LA. Skull base chordomas: A management challenge. J Neurosurg. 1997;86:182–9. doi: 10.3171/jns.1997.86.2.0182. [DOI] [PubMed] [Google Scholar]
- 3.Amichetti M, Amelio D, Minniti G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: A review. Radiat Oncol. 2012;7:210. doi: 10.1186/1748-717X-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: First long-term report. Int J Radiat Oncol Biol Phys. 2009;75:1111–8. doi: 10.1016/j.ijrobp.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 5.Barnett GC, West CM, Dunning AM, Elliott RM, Coles CE, Pharoah PD, et al. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev Cancer. 2009;9:134–42. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014;1:24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer therapy evaluation program . In: Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. National Cancer Institute; 2009. Available from https://www.ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc 40. [Last cited on 2018 Jan 09] [Google Scholar]
- 8.Deraniyagala RL, Yeung D, Mendenhall WM, Li Z, Morris CG, Mendenhall NP, et al. Proton therapy for skull base chordomas: An outcome study from the university of florida proton therapy institute. J Neurol Surg B Skull Base. 2014;75:53–7. doi: 10.1055/s-0033-1354579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Maio S, Rostomily R, Sekhar LN. Current surgical outcomes for cranial base chordomas: Cohort study of 95 patients. Neurosurgery. 2012;70:1355–60. doi: 10.1227/NEU.0b013e3182446783. [DOI] [PubMed] [Google Scholar]
- 10.Di Maio S, Yip S, Al Zhrani GA, Alotaibi FE, Al Turki A, Kong E, et al. Novel targeted therapies in chordoma: An update. Ther Clin Risk Manag. 2015;11:873–83. doi: 10.2147/TCRM.S50526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagundes MA, Hug EB, Liebsch NJ, Daly W, Efird J, Munzenrider JE, et al. Radiation therapy for chordomas of the base of skull and cervical spine: Patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579–84. doi: 10.1016/0360-3016(95)02014-3. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Mendenhall WM, Suárez C, et al. Clival chordomas: A pathological, surgical, and radiotherapeutic review. Head Neck. 2014;36:892–906. doi: 10.1002/hed.23415. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press: Lyon. 2013:328–329. [Google Scholar]
- 14.Förander P, Bartek J, Jr, Fagerlund M, Benmaklouf H, Dodoo E, Shamikh A, et al. Multidisciplinary management of clival chordomas; long-term clinical outcome in a single-institution consecutive series. Acta Neurochir (Wien) 2017;159:1857–68. doi: 10.1007/s00701-017-3266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosshans DR, Zhu XR, Melancon A, Allen PK, Poenisch F, Palmer M, et al. Spot scanning proton therapy for malignancies of the base of skull: Treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys. 2014;90:540–6. doi: 10.1016/j.ijrobp.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Gui S, Zong X, Wang X, Li C, Zhao P, Cao L, et al. Classification and surgical approaches for transnasal endoscopic skull base chordoma resection: A 6-year experience with 161 cases. Neurosurg Rev. 2016;39:321–32. doi: 10.1007/s10143-015-0696-1. [DOI] [PubMed] [Google Scholar]
- 17.Hug EB, Loredo LN, Slater JD, DeVries A, Grove RI, Schaefer RA, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432–9. doi: 10.3171/jns.1999.91.3.0432. [DOI] [PubMed] [Google Scholar]
- 18.Igaki H, Tokuuye K, Okumura T, Sugahara S, Kagei K, Hata M, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60:1120–6. doi: 10.1016/j.ijrobp.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 19.Jägersberg M, El Rahal A, Dammann P, Merkler D, Weber DC, Schaller K, et al. Clival chordoma: A single-centre outcome analysis. Acta Neurochir (Wien) 2017;159:1815–23. doi: 10.1007/s00701-017-3163-7. [DOI] [PubMed] [Google Scholar]
- 20.Jian BJ, Bloch OG, Yang I, Han SJ, Aranda D, Parsa AT, et al. A comprehensive analysis of intracranial chordoma and survival: A systematic review. Br J Neurosurg. 2011;25:446–53. doi: 10.3109/02688697.2010.546896. [DOI] [PubMed] [Google Scholar]
- 21.Kano H, Iqbal FO, Sheehan J, Mathieu D, Seymour ZA, Niranjan A, et al. Stereotactic radiosurgery for chordoma: A report from the north american gamma knife consortium. Neurosurgery. 2011;68:379–89. doi: 10.1227/NEU.0b013e3181ffa12c. [DOI] [PubMed] [Google Scholar]
- 22.Lühr A, von Neubeck C, Krause M, Troost EGC. Relative biological effectiveness in proton beam therapy current knowledge and future challenges. Clin Transl Radiat Oncol. 2018;9:35–41. doi: 10.1016/j.ctro.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luxton G, Petrovich Z, Jozsef G, Nedzi LA, Apuzzo ML. Stereotactic radiosurgery: Principles and comparison of treatment methods. Neurosurgery. 1993;32:241–59. doi: 10.1227/00006123-199302000-00014. [DOI] [PubMed] [Google Scholar]
- 24.McDonald MW, Linton OR, Calley CS. Dose-volume relationships associated with temporal lobe radiation necrosis after skull base proton beam therapy. Int J Radiat Oncol Biol Phys. 2015;91:261–7. doi: 10.1016/j.ijrobp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 25.McDonald MW, Linton OR, Moore MG, Ting JY, Cohen-Gadol AA, Shah MV, et al. Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int J Radiat Oncol Biol Phys. 2016;95:304–11. doi: 10.1016/j.ijrobp.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 26.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: Incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control. 2001;12:1–1. doi: 10.1023/a:1008947301735. [DOI] [PubMed] [Google Scholar]
- 27.Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol. 1999;175(Suppl 2):57–63. doi: 10.1007/BF03038890. [DOI] [PubMed] [Google Scholar]
- 28.Nath Sen C, Sekhar LN, Schramm VL, Janecka IP. Chordoma and chrondrosarcoma of the cranial base: An 8-year experience. Neurosurgery. 1989;25:931–41. doi: 10.1097/00006123-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Nikoghosyan AV, Karapanagiotou-Schenkel I, Münter MW, Jensen AD, Combs SE, Debus J, et al. Randomised trial of proton vs. Carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-study. BMC Cancer. 2010;10:607. doi: 10.1186/1471-2407-10-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noël G, Feuvret L, Calugaru V, Dhermain F, Mammar H, Haie-Méder C, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44:700–8. doi: 10.1080/02841860500326257. [DOI] [PubMed] [Google Scholar]
- 31.Noël G, Feuvret L, Ferrand R, Boisserie G, Mazeron JJ, Habrand JL, et al. Radiotherapeutic factors in the management of cervical-basal chordomas and chondrosarcomas. Neurosurgery. 2004;55:1252–60. doi: 10.1227/01.neu.0000143330.30405.aa. [DOI] [PubMed] [Google Scholar]
- 32.Noël G, Gondi V. Proton therapy for tumors of the base of the skull. Chin Clin Oncol. 2016;5:51. doi: 10.21037/cco.2016.07.05. [DOI] [PubMed] [Google Scholar]
- 33.Noël G, Habrand JL, Jauffret E, de Crevoisier R, Dederke S, Mammar H, et al. Radiation therapy for chordoma and chondrosarcoma of the skull base and the cervical spine. Prognostic factors and patterns of failure. Strahlenther Onkol. 2003;179:241–8. doi: 10.1007/s00066-003-1065-5. [DOI] [PubMed] [Google Scholar]
- 34.Noël G, Habrand JL, Mammar H, Pontvert D, Haie-Méder C, Hasboun D, et al. Combination of photon and proton radiation therapy for chordomas and chondrosarcomas of the skull base: The centre de protonthérapie D’orsay experience. Int J Radiat Oncol Biol Phys. 2001;51:392–8. doi: 10.1016/s0360-3016(01)01634-0. [DOI] [PubMed] [Google Scholar]
- 35.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–72. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 36.Pehlivan B, Ares C, Lomax AJ, Stadelmann O, Goitein G, Timmermann B, et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys. 2012;83:1432–40. doi: 10.1016/j.ijrobp.2011.10.042. [DOI] [PubMed] [Google Scholar]
- 37.Sahgal A, Chan MW, Atenafu EG, Masson-Cote L, Bahl G, Yu E, et al. Image-guided, intensity-modulated radiation therapy (IG-IMRT) for skull base chordoma and chondrosarcoma: Preliminary outcomes. Neuro Oncol. 2015;17:889–94. doi: 10.1093/neuonc/nou347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC. Incidence and relative survival of chordomas: The standardized mortality ratio and the impact of chordomas on a population. Cancer. 2013;119:2029–37. doi: 10.1002/cncr.28032. [DOI] [PubMed] [Google Scholar]
- 39.Stacchiotti S, Sommer J, Chordoma Global Consensus Group Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015;16:e71–83. doi: 10.1016/S1470-2045(14)71190-8. [DOI] [PubMed] [Google Scholar]
- 40.Terahara A, Niemierko A, Goitein M, Finkelstein D, Hug E, Liebsch N, et al. Analysis of the relationship between tumor dose inhomogeneity and local control in patients with skull base chordoma. Int J Radiat Oncol Biol Phys. 1999;45:351–8. doi: 10.1016/s0360-3016(99)00146-7. [DOI] [PubMed] [Google Scholar]
- 41.Tzortzidis F, Elahi F, Wright D, Natarajan SK, Sekhar LN. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery. 2006;59:230–7. doi: 10.1227/01.NEU.0000223441.51012.9D. [DOI] [PubMed] [Google Scholar]
- 42.Weber DC, Badiyan S, Malyapa R, Albertini F, Bolsi A, Lomax AJ, et al. Long-term outcomes and prognostic factors of skull-base chondrosarcoma patients treated with pencil-beam scanning proton therapy at the paul scherrer institute. Neuro Oncol. 2016;18:236–43. doi: 10.1093/neuonc/nov154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol. 2016;120:169–74. doi: 10.1016/j.radonc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Weber DC, Rutz HP, Pedroni ES, Bolsi A, Timmermann B, Verwey J, et al. Results of spot-scanning proton radiation therapy for chordoma and chondrosarcoma of the skull base: The paul scherrer institut experience. Int J Radiat Oncol Biol Phys. 2005;63:401–9. doi: 10.1016/j.ijrobp.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Yakkioui Y, van Overbeeke JJ, Santegoeds R, van Engeland M, Temel Y. Chordoma: The entity. Biochim Biophys Acta. 2014;1846:655–69. doi: 10.1016/j.bbcan.2014.07.012. [DOI] [PubMed] [Google Scholar]