Abstract
Background:
There are multiple complications reported for anterior cervical diskectomy and fusion (ACDF), one of the most common cervical spine operations performed in the US (e.g. estimated at 137,000 ACDF/year).
Methods:
Multiple studies analyzed the risks and complications rates attributed to ACDF.
Results:
In multiple studies, overall morbidity rates for ACDF varied from 13.2% to 19.3%. These included in descending order; dysphagia (1.7%-9.5%), postoperative hematoma (0.4%-5.6% (surgery required in 2.4% of 5.6%), with epidural hematoma 0.9%), exacerbation of myelopathy (0.2%-3.3%), symptomatic recurrent laryngeal nerve palsy (0.9%-3.1%), cerebrospinal fluid (CSF) leak (0.5%-1.7%), wound infection (0.1-0.9%-1.6%), increased radiculopathy (1.3%), Horner’s syndrome (0.06%-1.1%), respiratory insufficiency (1.1%), esophageal perforation (0.3%-0.9%, with a mortality rate of 0.1%), and instrument failure (0.1%-0.9%). There were just single case reports of an internal jugular veing occlusion and a phrenic nerve injury. Pseudarthrosis occurred in ACDF and was dependant on the number of levels fused; 0-4.3% (1-level), 24% (2-level), 42% (3 level) to 56% (4 levels). The reoperation rate for symptomatic pseudarthrosis was 11.1%. Readmission rates for ACDF ranged from 5.1% (30 days) to 7.7% (90 days postoperatively).
Conclusions:
Complications attributed to ACDF included; dysphagia, hematoma, worsening myelopathy, recurrent laryngeal nerve palsy, CSF leaks, wound infection, radiculopathy, Horner’s Syndrome, respiratory insufficiency, esophageal perforation, and instrument failure. There were just single case reports of an internal jugular vein thrombosis, and a phrenic nerve injury. As anticipated, pseudarthrosis rates increased with the number of ACDF levels, ranging from 0-4.3% for 1 level up to 56% for 4 level fusions.
Keywords: Anterior cervical, Diskectomy, Fusion, Risks, Complications, Adverse events

INTRODUCTION
Anterior cervical diskectomy and fusion (ACDF) is one of the most commonly performed spinal operations in the U.S. Between 2006-2013, one study cited an average of 137, 000 ACDF performed/year (total of 1,059,403 in 7 years) [Tables 1-3].[14] In a focused review of the ACDF literature, we evaluated the frequency of the various reported complications of ACDF procedures.
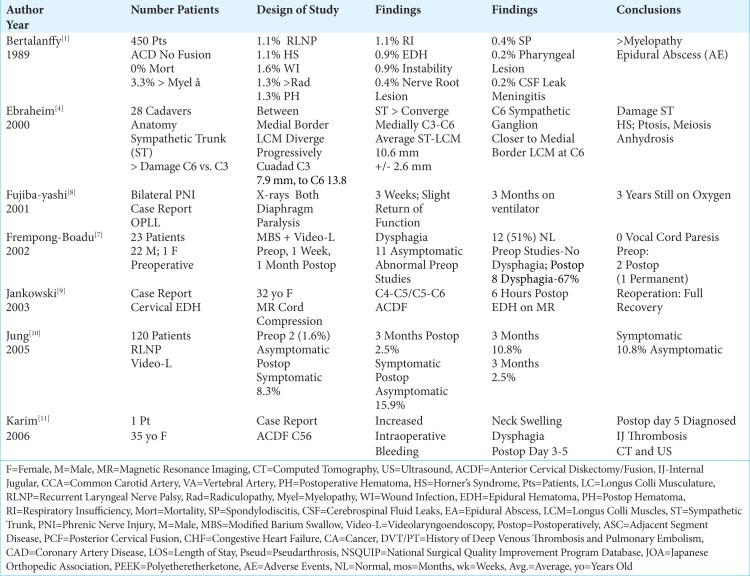
Table 1:
Literature Review ACDF Complications 1989-2006.
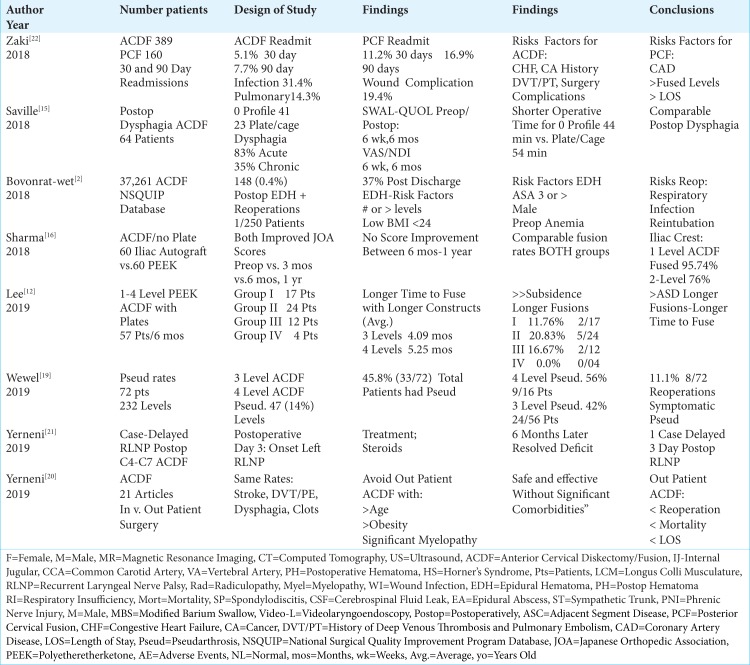
Table 3:
Literature Review ACDF Complications 2018-2019.
Rates of ACDF Performed From 2006-2013
Using the National Inpatient Sample (NIS) database, Saifi et al. (2017) reviewed the number of ACDF (1,059,403) vs. cervical disc arthroplasty (CDA; 13,099) performed in the U.S. between 2006 to 2013 [Table 2].[14] They noted a 5.7% increase in the frequency of ACDF from 2006 (average 120,617/year) to 2013 (127,500) (average 132,425/year), while CDA increased by 190% (540 in 2006, to 1,565 in 2013, averaging 1,637/year). Note the 81:1-fold difference in frequencies between the two procedures. Although ACDF patients had a longer average LOS vs. CDA (ACDF 2.3 vs. CDA 1.5 days), and were more expensive (average ACDF $16,178 vs. CDA $13,197), CDA required over double the revision rate (CDA 5.9% vs. ACDF 2.3%). The latter finding should in part explain the continued preference for ACDF, but other factors, such as reimbursement rates also likely apply.
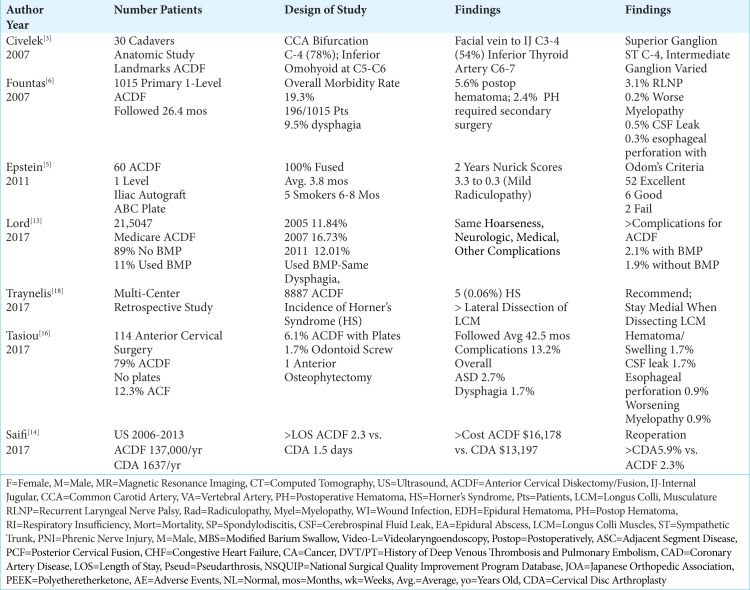
Table 2:
Literature Review ACDF Complications 2007-2017.
Anatomical Considerations When Performing ACDF
Knowing the anatomy before performing an ACDF is critical to avoid unnecessary complications. In a study of 30 cadaveric specimens, Civelek et al. (2007) documented several common critical anatomical landmarks for performing ACDF surgery.[3] They found; the common carotid artery bifurcation mostly occurred at the C-4 level (78%); the inferior omohyoid belly crossed the field largely at the C5-6 level; the facial vein drained into the internal jugular vein predominantly at the C3-4 (54%) level; the superior sympathetic ganglion was located in most cases at C-4, while the location of the intermediate ganglion varied; the vertebral artery entered the transverse foramen at C-6 (90%), followed by C-7 (7%), and C4 (3%) respectively; finally, the inferior thyroid artery was usually found at the C6-7 level.[3]
Esophageal Perforation Rate of 0.3%-0.9% with One Mortality
An esophageal perforation rate of 0.3%-0.9% was noted in several studies, accompanied by a single mortality.[1,7,17] In 1989, Bertalanffy and Eggert discussed a 0-mortality rate for 450 consecutive anterior cervical discectomy (ACD) without fusion.[1] Fountas et al. (2007), out of a 0.3% incidence of esophageal perforations occurring in 1015 ACDF, had 1 mortality (0.1%).[6] Although in Tasiou et al. study (2017) esophageal perforations occurred 0.9% of the 114 patients, there were no mortalities.[17]
Morbidity Rates for ACDF Varied from 13.2%-19.3%
Several studies documented morbidity/complication rates of ACD and ACDF ranging from 13.2%-15.3%-19.3% [Tables 1-3].[1,6,17] In 1989, Bertalanffy and Eggert cited a morbidity rate of 15.3% for anterior discectomy without fusion (ACD) performed in 450 consecutive patients [Table 1].[1] Fountas et al. in 2007 observed a higher morbidity rate of 19.3% (196 patients) out of a series of 1015 patients undergoing ACDF utilizing Smith-Robinson autograft/allograft, with or without plates, followed an average of 26.4 mos. [Table 2].[6] Over a 6-year period, Tasiou et al. (2017) retrospectively reported a 13.2% morbidity rate for 114 patients undergoing ACDF (79% no plates, 6.1% ACDF with plates, 12.3% anterior corpectomy with fusion(ACF), two (1.7%) odontoid screws, and one anterior osteophytectomy) [Table 2].[17]
In descending order, the following complications rates were encountered in these three studies; dysphagia (1.7%-9.5%), worsening of the pre-existing myelopathy (3.3%), wound infection (0.1-0.9%-1.6%), new radicular symptoms (1.3%), postoperative wound hematoma (1.3%-5.6% (2.4% requiring surgery), and 0.9% epidural hematoma), symptomatic recurrent laryngeal nerve palsy (0.9%-1.1%-3.1%) adjacent disc degeneration (2.7%), postoperative soft tissue swelling/hematoma (1.7%), respiratory insufficiency (1.1%), cerebrospinal fluid (CSF) leak (0.5%-1.7%), Horner’s syndrome (0.06%-0.1%-1.1%), aggravation of preexisting myelopathy (0.9%), instrument/mechanical failure/instability (0.1%-0.9%), esophageal perforation (0.3%-0.9%; one mortality 0.1%), new nerve root lesions (0.4%), aseptic spondylodiscitis (0.4%), exacerbation of myelopathy (0.2%), meningitis due to a dural perforation (0.2%), transient additional myelopathy (0.2%), and an epidural abscess(0.2%) [Tables 1-3].[1,6,17]
Rates of Immediate Postoperative Dysphagia After ACDF Ranged from 1.7% to 67%
Several series documented that for patients undergoing ACDF, the most common and immediate postoperative complaint was dysphagia, ranging in frequency from 1.7%-67% [Tables 1-3].[6,7,15,17] Frempong-Boadu et al. in 2002 evaluated ACDF-related postoperative dysphagia, vocal cord paralysis, and speech dysfunction in 23 patients averaging 59 years of age [Table 1].[7] Patients underwent modified barium swallows and videolaryngoendoscopy preoperatively, plus 1 week, and 1 month postoperatively. Preoperative swallowing studies were abnormal in 11 (48%) asymptomatic patients, but normal in 12 patients (52%). Postoperatively, 8 patients exhibited new swallowing abnormalities (67%). Preoperative vocal cord function was normal in all patients, but postoperatively 2 showed vocal cord paresis (one transient; one permanent). Interestingly, swallowing dysfunction correlated with; advanced age, a prior history of such dysfunction, longer surgical procedures, and a trend toward increased dysphagia with multilevel surgery attributed to greater soft tissue swelling/retraction injury. In 2007, Fountas et al. observed postoperative dysphagia in 9.5% of 1015 patients undergoing ACDF [Table 2].[6] The postoperative dysphagia rate in Tasiou’s et al. study (2017) was 1.7% out of 114 patients undergoing anterior cervical procedures [Table 2].[17] Other reports had cited immediate postoperative dysphagia rates of 93% and chronic rates of 35%.
Saville et al. (2018) evaluated postoperative dysphagia rates after 64 ACDF utilizing 41 zero-profile devices, and 23 plate/cage constructs. [Table 3].[15] They utilized dysphagia and SWAL-QOL scores (i.e. preoperatively, postoperatively at 6 weeks 12 weeks), along with outcomes analyzed with the Visual Analog Scale (VAS), and the Neck Disabilityu Index (NDI) (i.e. preoperatively, 6 weeks and at 6 months postoperatively). For the zero-profile group, surgical time was reduced vs. plate/cages (e.g.44.88 ± 6.54 min vs 54.43 ± 14.71 min), but postoperative, dysphagia rates were the same.
Acute (0.9%-8.3%) and Chronic (2.5%) Symptomatic Postoperative Recurrent Laryngeal Nerve Palsy (RLNP) After ACDF
In multiple series, the rates of symptomatic postoperative recurrent laryngeal nerve palsies (RLNP) ranged from 0.9%-8.3% [Tables 1-2].[1, 6,10,17] Bertalanffy and Eggert (1989) found a 1.1% incidence of RLNP out of 450 ACD [Table 1].[1] Jung et al. (2005) prospectively evaluated the frequency of RLNP before and after 120 ACDF utilizing preoperative and postoperative laryngoscopy [Table 1].[10] Preoperatively, 2 (1.6%) patients had asymptomatic RLNP (e.g. hoarseness). Postoperatively, symptomatic RLNP was found in 8.3% of patients (e.g. hoarseness), but asymptomatic RLNP was also documented in 15.9% of patients (i.e. total: 24.2%). At 3 postoperative months, the incidence of symptomatic RNLP was a lesser 2.5%, while asymptomatic RNLP was still documented in 10.8% of cases. The incidence of RLNP in Fountas et al. (2007) was 3.1% out of 1015 primary single-level ACDF [Table 2].[6] Further, in Tasiou et al. (2017), RLNP occurred in 0.9% of 114 anterior cervical cases [Table 2].[17]
Delayed RLNP
In Yemeni et al. a 75-year-old female, 3 days after a C4-7 ACDF, developed a delayed recurrent laryngeal nerve palsy (RLNP) [Table 3].[21] The left vocal cord paralysis was treated with a course of steroids, and 6 months later, her deficit resolved. The most likely etiology of the delayed RLNP was “neurapraxia caused by intraoperative compression or traction on the nerve”, but other potential factors included; direct nerve injury, ischemia, vasospasm, or viral factors
Postoperative Hematomas with ACDF
Incidence of Postoperative Hematomas Following ACDF (1.3-5.6%)
Multiple ACDF series demonstrated that postoperative wound hematomas occurred in from 1.3%-5.6% of cases, with epidural clots specifically found in 0.9% of patients [Tables 1, 2].[1,6,17] Out of 450 ACD without fusion performed in 1989, Bertalanffy and Eggert found a 1.3% incidence of postoperative wound hematomas, and a 0.9% frequency of epidural hematomas [Table 1].[1] Tasiou et al. (2017) observed postoperative hematomas/significant wound swelling in 1.7% of 114 patients undergoing ACDF [Table 2].[17] Out of 1015 patients, Fountas et al. (2007) found postoperative hematomas in 5.6% of patients [Table 2].[6]
Secondary Surgery Required for Postoperative Hematomas after ACDF (0.4%-2.4%)
One case study and several large series documented that following ACDF, there was a 0.4%-2.4% incidence of postoperative cervical hematomas warranting secondary surgery [Table 1-3].[2,6,9] In 2003, Jankowski et al. evaluated a 32-year-old female who 6 hours following a C4-C5/C5-C6 ACDF became quadriplegic; following resection of a large epidural hematoma, the patient fully recovered neurological function [Table 1].[9] Fountas et al. (2007) found postoperative hematomas in 5.6% of patients undergoing 1015 ACDF; 2.4% (slightly less than half of the 5.6%) warranted secondary surgery [Table 2].[6] Using the National Surgical Quality Improvement Program database (NSQUIP) to assess 37,261 ACDF performed between 2012-2016, Bovonratwet et al. (2018) determined that 1 of 250 (0.4%) patients undergoing ACDF developed a postoperative hematoma warranting secondary surgery within 30 postoperative days [Table 3].[2] There were 148 (0.4%) patients with hematomas who required reoperations; notably, 37 occured after discharge. Risk factors for hematomas requiring secondary surgery included; 3 or more level surgery, lower body mass index (BMI ≤24), American Society of Anesthesiologists classification ≥3, anemia, and male sex. Those having reoperations prior to discharge had longer LOS, and were at greater risk for respiratory complications (e.g. pneumonia, reintubation), and infections.
30 (5.1%) and 90 Day (7.7%) Readmissions Rates Following ACDF
Zaki et al. (2018 Spine) retrospectively evaluated unplanned readmissions 30 and 90 days after 389 ACDF vs. 160 posterior cervical fusions (PCF) (2013-2014) [Table 3].[22] The 30- and 90-day unplanned readmissions respectively occurred at lower rates for ACDF (5.1% and 7.7%) vs. PCF (11.2% and 16.9%). Typically, readmissions addressed; infection/sepsis for 31.4% having ACDF, and 25.8% for PCF, pulmonary adverse events for ACDF (14.3%), and wound complications with PCF (19.4% readmitted). Risks factors for ACDF readmissions included; “heart failure, history of malignancy, history of deep vein thrombosis/pulmonary embolism, and intraoperative untoward events”. Risks for complications after PCF included; a history of cardiac disease, more fusion levels, and more prolonged LOS.
Anatomy of Sympathetic Trunk and Risk of Horner’s Syndrome (0.06%-0.1%-1.1%) with ACDF
Anatomy of the Sympathetic Trunk
Damage to the sympathetic trunk (i.e. Horner’s syndrome characterized by ptosis, miosis, and anhidrosis) is more likely to occur during more caudad anterior cervical surgery. In their anatomic study of 28 adult cadavers, Ebraheim et al. (2000) found the longus colli muscles (LCM) progressively diverged laterally from C3 (distance between LCM 7.9 mm) to C6 (distance between LCM 13.8 mm), while the sympathetic trunk locations (average 10.6 mm) progressively converged medially [Table 1].[4] Therefore, at lower cervical levels, the sympathetic trunk was more susceptible to injury during ACDF.
Frequency of Horner’s Syndrome with ACDF
In three clinical studies, the frequency of Horner’s Syndromes ranged from 0.06-0.1%-1.1% [Tables 1-2].[1,6,18] In 1989, Bertalanffy and Eggert noted a 1.1% risk of a Horner’s Syndrome out of 450 ACD [Table 1].[1] When Fountas et al. (2007) evaluated 1015 patients undergoing initial ACDF, over an average of 26.4 postoperative months, 0.1% of patients developed Horner’s syndromes [Table 2].[6] A multicenter retrospective study of 8887 ACDF performed by Traynelis et al. (2017), revealed a 0.06% (5 patients) rate of Horner’s Syndromes occuring most frequently with C5-C6 level surgery; the majority of these deficits partially recovered [Table 2].[18] To reduce these injures in the future, Traynelis et al. recommended more restricted medial dissection of the LCM with very limited lateral retraction, particularly at the more distal cervical levels.
Worsening Myelopathy (0.2%-3.3%) and Radiculopathy (1.3%) Attributed to ACDF
Worsening of preoperative myelopathy and/or radiculopathy were variously reported in different ACDF studies [Table 1-2].[1,6,17] One study (1989) documented worsening of the pre-existing myelopathy in 3.3% of cases, 1.3% exhibited worsening of preoperative radiculopathy, while new nerve root lesions (iatrogenic) occurred in 0.4% of cases [Table 1].[1] Two other ACDF studies reported worsening of preexisting myelopathy; one study cited a 0.2% incidence (2 out of 1015; 2017), while the other reported this in 0.9% of cases (out of 114; 2017) [Table 2].[7,17]
Other Complications of ACDF (Up to 2.7%)
Multiple other complications were reported following ACDF [Tables 1, 2].[1,17] A 1989 study of 450 ACD reported; respiratory insufficiency (1.1%), instability (0.9%), aseptic spondylodiscitis (0.4%), and a pharyngeal lesion (0.2%) [Table 1].[1] Nearly three decades later, Tasiou et al. (2017) observed several additional complications following 114 anterior cervical procedures; a 2.7% incidence of adjacent segment degeneration (ASD), and a 0.9% frequency of mechanical failures of instrumentation [Table 2].[17]
Higher Complications Rates (2.1%) for ACDF Performed with Bone Morphogenetic Protein (BMP/Infuse)
Lord et al. (2017) retrospectively evaluated the trends, costs, and complication occurring within 90 days of 215,047 ACDF performed in Medicare patients using BMP/Infuse “off-label” (2005-2011) [Table 2].[13] In 2005, BMP was used in 11.84% of cases; this increased to 16.73% in 2007, and decreased to 12.01% by 2011. Notably, the FDA had required that Medtronic issue a warning against using BMP/Infuse for anterior cervical surgery in 2008. The overall complication rate was a higher 2.1% with BMP vs. 1.9% without; there was also a higher wound complication rate with BMP. In the 90-day perioperative period, BMP charges were 17.6% greater than those for non-BMP users. Notably, similar rates of “dysphagia/hoarseness, neurologic, medical, or other complications” were observed for both groups.
Intraoperative Cerebrospinal Fluid Fistulas During ACDF (0.2%- 1.7%)
Three studies documented dural fistulas/cerebrospinal fluid leaks as occurring in from 0.2%-1.7% of ACDF [Tables 1, 2].[1,6,17] In Bertalanffy and Eggert (1989), one patient with a dural fistula developed meningitis (0.2% out of 450 non-plated ACD) [Table 1].[1] A 0.5% incidence of CSF fistulas occurred out of 1015 primary ACDF in Fountas et al. (2007) study [Table 2].[6]. Dural tears occurred in 1.7% of 114 anterior cervical procedure performed by Tasiou et al. (2017) [Table 2].[17]
Wound Infections after ACDF (0.1%- 1.6%)
Three studies documented different infection rates following ACDF ranging from 0.1%-0.2% up to 1.6% [Tables 1-2].[1,7,17] In one study involving 450 ACD, the wound infection rate was 1.6%, there was one instance of meningitis (0.2%) attributed to a dural perforation, and another patient developed an epidural abscess (0.2%) [Table 1].[1] Two other series (2007), documented superficial postoperative wound infections in 0.1% (out of 1015 ACDF; 2007), and 0.9% of patients resepctively (out of 114 anterior cervical procedures) [Table 2].[7,17]
Fusion and Pseudarthrosis Rates Following ACDF
Fusion Rates and Pseudarthrosis Rates for 1-2 Level ACDF
Higher fusion rates were typically noted following 1-level vs. 2-level ACDF.[5,16] When Epstein (2011) evaluated fusion rates for 60 ACDF utilizing dynamic plates and iliac crest autograft, both CT and dynamic X-rays confirmed a 100% fusion rate over an average 3.8-months duration (range 2.5-8 months); the 5 smokers exhibited delayed fusions (i.e. over 6-8 months) [Table 2].[5] In another study, Sharma et al. (2018) documented comparable fusion rates for 1-2 level ACDF using stand-alone tricortical iliac crest autograft (60 patients) vs. stand-alone polyetheretherketone (PEEK) cages (60 patients); however, statistically significant higher fusion rates occurred for 1-level ACDF (95.74%) vs. 2-level ACDF (76.00%) [Table 3].[16].
Higher Pseudarthrosis Rates for 3-4 Level ACDF
For 3 and 4-level ACDF, higher pseudarthrosis rates were observed [Table 3].[12,19] Lee et al. (2019) looked at fusion rates for 1-level vs. multilevel ACDF using polyetheretherketone (PEEK) cage-plate fusions in 57 patients followed at least 6 months [Table 3].[12] It took significantly longer for 3-4 multilevel constructs to fuse vs. single level ACDF (e.g. 3-level (4.09 mos.) and 4-level (5.25 mos.)), and there was greater caudal adjacent segmental disc disease following multilevel procedures. Wewel et al. (2019) also retrospectively evaluated fusion and pseudarthrosis rates for 72 patients undergoing 3 and 4-level ACDF (232 levels fused) [Table 3].[19] Pseudarthrosis was observed at 47 (14%) total levels, with 45.8% of patients (33/72) exhibiting at least one level that failed to fuse (e.g. typically, the most caudal level). Furthermore, 4-level ACDF incurred a higher pseudarthrosis rate of 56% (9/16 patients) vs. a rate of 42% for 3-level ACDF (24/56 patients). Additionally, 11.1% (8/72 patients) of patients with symptomatic postoperative pseudarthrosis required secondary surgery.
Internal Jugular Venous Thrombosis After ACDF: Single Case Study
Karim et al. (2006) presented what they considered to be the first case of internal jugular venous thrombosis attributed to an ACDF.[11] This involved a 35-year-old female, without significant comorbidities, who underwent a C5-C6 ACDF. During the surgery, there was “significant venous bleeding” that was routinely controlled. The patient was discharged 1 day postoperatively without complaints. On postoperative day 3 and progressing by postoperative day 5, she developed mild dysphagia and increased neck swelling on the right side, the site of surgical access, without symptoms/signs of respiratory distress. Although they anticipated finding a postoperative hematoma, the cervical CT “suggested” and the ultrasound “confirmed” right internal jugular venous thrombosis. She was seen by vascular surgery, and sent home after a 3-day hospital stay, during which time she was placed on Warfarin.
Bilateral Phrenic Nerve Injuries with ACDF: Case Report
In 2001, Fujibayashi et al. observed bilateral phrenic nerve palsies resulting from an ACDF performed in one patient for ossification of the posterior longitudinal ligament (OPLL).[8] In this case, bilateral phrenic nerve paralysis was documented on a chest X-ray showing bilateral “laxity of the diaphragm”. Diaphragmatic motion began to reappear 3 weeks later, and the patient was off the ventilator by the third postoperative month; yet she still required oxygen 3 years later.” Various etiologies for unilateral/bilateral phrenic nerve injuries occurring during ACDF included; stretch injuries to the C4 roots, iatrogenic injury to the ventral horn, ischemia/spinal edema, or cord compression. Interestingly, the author (Epstein) could not find any other ACDF studies in the literature that cited such a deficit. However, most such injuries are reported following cardiovascular procedures.
Risks of Out-Patient Cervical Surgery
Yerneni et al. (2019) performed a met-analysis involving 21 articles (up to April 2018), most of which were retrospective studies, looking at the safety of performing outpatient ACDF [Table 3].[20] They noted no “statistically significant difference between inpatient and outpatient ACDF in overall complications; incidence of stroke, thrombolytic events, dysphagia, and hematoma development.” Nevertheless, those having outpatient ACDF were “well-selected”, a factor which they readily admitted significantly contributed to their lower reoperation rates, reduced mortality, and lesser length of stay. Furthermore, they acknowledged that; “ advanced age and comorbidities such as obesity and significant myelopathy are likely not suitable for outpatient ACDF.” Their recommendation was for future larger prospective randomized control trials to determine the safety of outpatient ACDF.
CONCLUSION
Although the mortality rates for ACDF remain low, these operations carry significant morbidity rates varying from 13.2%-19.3% [Tables 1-3].[1,7,17] Therefore, patients should be carefully selected to undergo these procedures, and operative intervention should be limited only to those who warrant it based on the correlation of neurological deficits with significant MR/CT/X-ray abnormalities.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien) 1989;99(1-2):41–50. doi: 10.1007/BF01407775. [DOI] [PubMed] [Google Scholar]
- 2.Bovonratwet P, Fu MC, Tyagi V, Bohl DD, Ondeck NT, Albert TJ, et al. Incidence, Risk Factors, and Clinical Implications of Postoperative Hematoma Requiring Reoperation Following Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2018. Sep 21. doi: 10.1097/BRS.0000000000002885 [Epub ahead of print] [DOI] [PubMed]
- 3.Civelek E, Kiris T, Hepgul K, Canbolat A, Ersoy G, Cansever T. Anterolateral approach to the cervical spine: major anatomical structures and landmarks. Technical note. J Neurosurg Spine. 2007 Dec;7(6):669–78. doi: 10.3171/SPI-07/12/669. [DOI] [PubMed] [Google Scholar]
- 4.Ebraheim NA, Lu J, Yang H, Heck BE, Yeasting RA. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine (Phila Pa 1976) 2000 Jul 1;25(13):1603–6. doi: 10.1097/00007632-200007010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Epstein NE. Efficacy and outcomes of dynamic-plated single-level anterior diskectomy/fusion with additional analysis of comparative costs. Surg Neurol Int. 2011;2:9. doi: 10.4103/2152-7806.76146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fountas KN, Kapsalaki EZ, Nikolakakos LG, Smisson HF, Johnston KW, Grigorian AA, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32(21):2310–7. doi: 10.1097/BRS.0b013e318154c57e. [DOI] [PubMed] [Google Scholar]
- 7.Frempong-Boadu A, Houten JK, Osborn B, Opulencia J, Kells L, Guida DD, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech. 2002;15(5):362–8. doi: 10.1097/00024720-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fujibayashi S, Shikata J, Yoshitomi H, Tanaka C, Nakamura K, Nakamura T. Bilateral phrenic nerve palsy as a complication of anterior decompression and fusion for cervical ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2001;26(12):E281–6. doi: 10.1097/00007632-200106150-00029. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski R, Zukiel R, Nowak S. Acute cervical epidural hematoma as a complication of anterior cervical C5-C6 diskectomy. A case report. Neurol Neurochir Pol. 2003;37(4):955–62. [Article in Polish] [PubMed] [Google Scholar]
- 10.Jung A, Schramm J, Lehnerdt K, Herberhold C. Recurrent laryngeal nerve palsy during anterior cervical spine surgery: a prospective study. J Neurosurg Spine. 2005;2(2):123–7. doi: 10.3171/spi.2005.2.2.0123. [DOI] [PubMed] [Google Scholar]
- 11.Karim A, Knapp J, Nanda A. Internal jugular venous thrombosis as a complication after an elective anterior cervical discectomy: case report. Neurosurgery. 2006;59(3):E705. doi: 10.1227/01.NEU.0000229056.02698.6E. discussion E705. [DOI] [PubMed] [Google Scholar]
- 12.Lee HC, Chen CH, Wu CY, Guo JH, Chen YS. Comparison of radiological outcomes and complications between single-level and multilevel anterior cervical discectomy and fusion (ACDF) by using a polyetheretherketone (PEEK) cage-plate fusion system. Medicine (Baltimore) 2019;98(5):e14277. doi: 10.1097/MD.0000000000014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lord EL, Cohen JR, Buser Z, Meisel HJ, Brodke DS, Yoon ST, et al. Trends, Costs, and Complications of Anterior Cervical Discectomy and Fusion With and Without Bone Morphogenetic Protein in the United States Medicare Population. Global Spine J. 2017;7(7):603–608. doi: 10.1177/2192568217699207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saifi C, Fein AW, Cazzulino A, Lehman RA, Phillips FM, An HS, Riew KD. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. Spine J. 2018;18(6):1022–1029. doi: 10.1016/j.spinee.2017.10.072. [DOI] [PubMed] [Google Scholar]
- 15.Saville P, Vaishnav AS, McAnany S, Gang CH, Qureshi SA. Spine (Phila Pa 1976) 2018. Predictive Factors of Post-operative Dysphagia in Single-level Anterior Cervical Discectomy and Fusion (ACDF) Sep 17. doi:10.1097/BRS.0000000000002865. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma A, Kishore H, Singh V, Shawky Abdelgawaad A, Sinha S, Kamble PC, et al. Comparative Study of Functional Outcome of Anterior Cervical Decompression and Interbody Fusion With Tricortical Stand-Alone Iliac Crest Autograft Versus Stand-Alone Polyetheretherketone Cage in Cervical Spondylotic Myelopathy. Global Spine J. 2018;8(8):860–865. doi: 10.1177/2192568218780345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasiou A, Giannis T, Brotis AG, Siasios I, Georgiadis I, Gatos H, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg. 2017;3(3):444–459. doi: 10.21037/jss.2017.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traynelis VC, Malone HR, Smith ZA, Hsu WK, Kanter AS, Qureshi SA, et al. Rare Complications of Cervical Spine Surgery: Horner’s Syndrome. Global Spine J. 2017;7(1 Suppl):103S–108S. doi: 10.1177/2192568216688184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wewel JT, Kasliwal MK, Adogwa O, Deutsch H, O’Toole JE, Traynelis VC. Fusion rate following three-and four-level ACDF using allograft and segmental instrumentation: A radiographic study. J Clin Neurosci. 2019 doi: 10.1016/j.jocn.2018.11.040. Jan 25. pii: S0967-5868(18)31669-2. doi: 10.1016/j.jocn.2018.11.040. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Yerneni K, Burke JF, Chunduru P, Molinaro AM, Riew KD, Traynelis VC, et al. Neurosurgery. 2019. Safety of Outpatient Anterior Cervical Discectomy and Fusion: A Systematic Review and Meta-Analysis. Jan 23. doi: 10.1093/neuros/nyy636. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Yerneni K, Burke JF, Nichols N, Tan LA. Delayed Recurrent Laryngeal Nerve Palsy Following Anterior Cervical Discectomy and Fusion. World Neurosurg. 2019;122:380–383. doi: 10.1016/j.wneu.2018.11.066. [DOI] [PubMed] [Google Scholar]
- 22.Zaki O, Jain N, Yu E, Khan SN. Spine (Phila Pa 1976) 2018. 30- and 90-day Unplanned Readmission Rates, Causes, and Risk Factors after Cervical Fusion: A Single Institution Analysis. Nov 20. doi: 10.1097/BRS.0000000000002937. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]