Abstract
Background
Essential tremor (ET) is a common movement disorder characterized by kinetic and postural tremor in the upper extremities and frequently in the midline. Persons with ET often also exhibit gait ataxia. Previous studies have observed associations between midline tremor severity and gait ataxia in persons with ET, suggesting a common pathophysiology distinct from that of upper extremity tremor. However, a causal link between midline tremor and gait impairment has not been established.
Methods
We investigated tremor and gait in 24 persons with ET before and after implantation of unilateral deep brain stimulation into the ventralis intermedius nucleus of the thalamus.
Results
Stimulation significantly improved tremor in the targeted upper extremity and midline. However, gait was unaffected at the cohort level. Furthermore, improvement in midline tremor was not significantly associated with gait improvement.
Discussion
These findings revealed that midline tremor and gait impairment may be dissociable in persons with ET.
Keywords: Essential tremor, gait, deep brain stimulation, Thalamus, Ataxia
Introduction
Essential tremor (ET) is a prevalent movement disorder that affects approximately 7 million people in the United States.1 ET is classically characterized by the presence of upper extremity kinetic and postural tremor. However, motor symptoms in ET extend beyond the upper extremities. Persons with ET also commonly exhibit axial motor symptoms, notably midline tremor2 (e.g., tremor of the head, jaw, voice, or trunk) and gait ataxia3–5 in addition to occasional nonmotor symptoms.6
Several studies have reported associations between midline tremor and gait impairment in persons with ET,5,7,8 collectively suggesting that the pathophysiology underlying these symptoms may be distinct from the pathophysiology underlying upper extremity tremor.9 These studies provided correlational evidence relating clinical tremor scores to common measures of gait ataxia (e.g., number of missteps during tandem walking, step width, step-to-step variability). However, a causal relationship between midline tremor severity and gait impairment in ET has not been established.
In this study, we assessed tremor, gait, and tandem gait in persons with medication-refractory ET before and after unilateral implantation of thalamic deep brain stimulation (DBS), a proven treatment approach for reducing upper extremity and midline tremor10–13 in ET. We aimed to understand whether improvement in midline tremor following DBS was related to gait improvement in persons with ET. Based on prior work,5,7,8,9 we hypothesized that improvement in midline – but not upper extremity – tremor would be associated with gait improvement following DBS.
Methods
General methods
Seven persons with ET and 17 persons with ET plus (due to the presence of ET and tandem gait impairment14) participated (mean±standard deviation age: 68 ± 6 years, sex: 16M/8F). ET diagnosis was confirmed by standardized criteria14 and evaluation by a fellowship-trained movement disorders neurologist. As part of our Center’s standard of care, we perform a staged DBS surgery. The second DBS surgery was performed in an identical but staged fashion with a delay of at least 6 months between sides. Participants performed gait and clinical assessments on two separate days. The first day occurred approximately 3 months prior to undergoing unilateral DBS implantation into the ventralis intermedius nucleus of the thalamus (PRE). The second day occurred approximately 6 months following surgery and consisted of two testing sessions: one with the stimulation off (OFF) and one with the stimulation on at optimized parameters (ON). Details about the surgical procedure have been reported previously.15 The stimulation was deactivated at least 1 hour prior to the OFF testing and activated at least 1 hour prior to the ON testing. Prior to each gait assessment, a movement disorders neurologist assessed tremor severity using the motor portion (items 1–14) of the Fahn–Tolosa–Marin Tremor Rating Scale (TRS).16 All participants provided written consent as approved by the University of Florida Institutional Review Board.
Gait analysis
Participants underwent gait assessments using a three-dimensional, ten-camera motion capture system (120 Hz; Vicon Nexus, Vicon, Oxford, UK). Participants wore 35 passive retroreflective markers placed in accordance with the Vicon Plug-in-Gait full body marker set. Participants performed 5–10 normal walking trials along an 8-m walkway. Twenty-two of the participants also performed five tandem walking trials along the same walkway. All trials were performed at self-selected speeds.
Walking speed, step length, step time, and step width were calculated for the normal walking trials using conventional definitions.17 We calculated the mean and a measure of variability (coefficient of variation: CV=standard deviation/mean) for each parameter. During tandem walking trials, we calculated mean walking speed and percentage of steps when a misstep occurred. A misstep was defined by two or more consecutive steps with the same foot.
Clinical assessment
We calculated motor TRS score as the sum of TRS subitems 1–14 (including all resting, postural, and kinetic scores), midline TRS score as the sum of TRS subitems 1–4 and 7 (including all resting, postural, and kinetic scores), and kinetic TRS score as the sum of TRS subitems 5, 6, 8, and 9 (including kinetic scores only).
Statistical analysis
We performed repeated measures ANOVAs (with Bonferroni post hoc corrections for multiple comparisons, where appropriate) to compare TRS scores (motor, midline, and kinetic), gait measures, and tandem gait measures among sessions (PRE, OFF, ON). We also performed two sets of Pearson’s correlations. First, we performed Pearson’s correlations to assess relationships between TRS scores (motor, midline, and kinetic) and gait parameters (walking speed, step length, step time, step width, step length CV, step time CV, step width CV, tandem missteps, and tandem walking speed) at baseline (PRE). Next, we performed Pearson’s correlations to assess relationships between changes in TRS scores (motor, midline, kinetic) and changes in gait parameters (walking speed, step length, step time, step width, step length CV, step time CV, step width CV, tandem missteps, and tandem walking speed) from PRE to ON (change scores calculated as ON minus PRE). We performed all tests with α = 0.05 and performed Shapiro–Wilk tests for normality and Mauchly’s tests of sphericity on all variables. Greenhouse–Geisser corrections were used when sphericity was violated. We performed all analyses using SPSS software (IBM SPSS Statistics 25, Armonk, NY).
Results
Efficacy of DBS for tremor suppression and effects of DBS on gait
As previously reported,15 we observed significant main effects of condition on all TRS scores (motor, midline, and kinetic) and also step time CV (all p < 0.01). Post hoc analyses revealed that the motor, midline, and kinetic TRS scores improved significantly when ON as compared to PRE (all p < 0.03) and OFF (all p < 0.01). TRS scores were not significantly different between PRE and OFF.
We also observed a significant main effect of condition on step time CV (p < 0.001). Post hoc analyses revealed a significant increase in step time CV from PRE to ON (p = 0.001), indicating that step times were more variable during ON than PRE. There were no significant differences in walking speed, step length, step time, step width, step length CV, step width CV, tandem walking speed, or tandem missteps among conditions (all p > 0.05). These TRS and gait data were reported previously15 and are included here only to establish that DBS caused the expected suppression of midline tremor but minimal change in gait at the cohort level.
Relationships between tremor severity and gait at baseline
At baseline (PRE), we did not observe significant relationships between any measures of normal or tandem gait and motor TRS, midline TRS, or kinetic TRS scores (Table 1; all p > 0.05).
Table 1.
Baseline (PRE) relationships between Fahn-Tolosa-Marin Tremor Rating Scale (TRS) subscores and gait parameters
| Motor TRS | Midline TRS | Kinetic TRS | |
|---|---|---|---|
| Walking speed (m/s) | r=0.01, p=0.95 | r=0.31, p=0.14 | r=-0.11, p=0.60 |
| Step length (m) | r=0.10, p=0.63 | r=0.31, p=0.14 | r=-0.09, p=0.68 |
| Step time (s) | r=0.11, p=0.62 | r=-.13, p=0.55 | r=0.05, p=0.81 |
| Step width (m) | r=0.14, p=0.52 | r=0.07, p=0.74 | r=0.26, p=0.21 |
| Step length CV | r=0.22, p=0.30 | r=-0.07, p=0.73 | r=0.16, p=0.45 |
| Step time CV | r=0.08, p=0.71 | r=-0.35, p=0.09 | r=0.09, p=0.67 |
| Step width CV | r=-0.13, p=0.55 | r=-0.22, p=0.31 | r=-0.15, p=0.49 |
| Tandem missteps (%) | r=0.04, p=0.85 | r=0.08, p=0.71 | r=0.23, p=0.31 |
| Tandem speed (m/s) | r=-0.03, p=0.90 | r=-0.07, p=0.75 | r=-0.02, p=0.95 |
r indicates Pearson’s rho, p indicates p-value.
Abbreviations: CV: coefficient of variation.
Relationships between changes in midline tremor severity and changes in gait following DBS
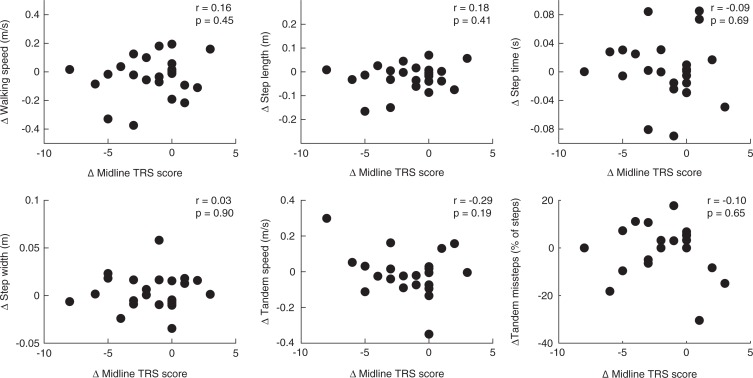
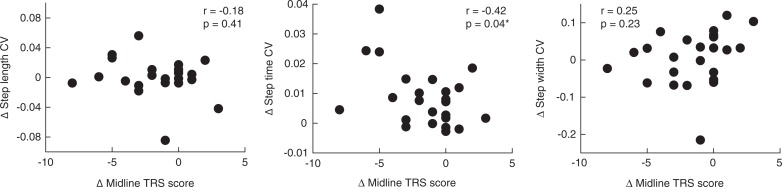
We did not observe significant relationships between changes in mean gait parameters (walking speed, step length, step time, step width, and tandem gait measures) and changes in midline TRS scores from PRE to ON (Figure 1). When assessing relationships between changes in gait variability (step length CV, step time CV, and step width CV) and changes in midline TRS scores, we observed only a significant negative relationship between changes in step time CV and midline TRS score (r = −0.42, p = 0.04; Figure 2).
Figure 1.
Correlations between Changes in Midline TRS Score and Changes in Mean Gait and Tandem Gait Parameters. Change = ON minus PRE.
Figure 2.
Correlations between Changes in Midline TRS Score and Changes in Gait Variability Parameters. Change = ON minus PRE.
Discussion
ET is a common movement disorder characterized by kinetic and postural tremor of the upper extremities. Persons with ET also frequently exhibit midline tremor and gait ataxia. Previous findings indicated that midline tremor severity and gait dysfunction may be linked in persons with ET. We tested this directly in persons with medication-refractory ET who underwent unilateral thalamic DBS to improve tremor. Midline tremor showed significant improvement following DBS; however, gait was unaffected at the cohort level. Contrary to our hypothesis, midline tremor improvement showed no significant association with gait improvement. Our results demonstrated that improvement in midline tremor occurred independently of changes in walking in persons with ET, suggesting that these symptoms are dissociable.
Previous studies have suggested that a link between midline tremor severity and gait ataxia could represent a shared pathophysiology between these symptoms that is distinct from the pathophysiology underlying upper extremity tremor.9 More specifically, midline tremor and gait ataxia have been hypothesized to be caused by dysfunction in cerebellar regulation of axial motor control.9This hypothesis is bolstered by many studies that observed Purkinje cell dysfunction18–25 and cerebellar-like gait disturbances7,26–29 in persons with ET. Our results do not question the potential cerebellar origin of motor control deficits in ET; however, they do suggest that these deficits can be differentially modulated with unilateral stimulation of the ventralis intermedius nucleus of the thalamus.
Our results are supported by prior studies of motor outcomes following unilateral thalamic DBS for ET. Prior work has shown that unilateral thalamic DBS improves midline tremor in persons with ET.11–13,30 However, the gait response to DBS has been much less consistent.31 Some evidence supports gait improvement following thalamic DBS for ET,32,33 but gait worsening is more commonly reported.13,15,34,35 Furthermore, one of the studies that observed gait improvement also revealed that the gait benefits occurred independently of changes in tremor.30 In sum, current evidence suggests that the gait response to thalamic DBS for ET varies regardless of the reliable improvement in upper extremity and midline tremor.
It is also notable that our study did not observe relationships between midline tremor severity and gait impairment at baseline as have been previously reported.4,7–9 Our study differs from previous studies in several ways. The gait data presented here were collected in a gait laboratory using three-dimensional motion capture instead of clinical gait assessments. We assessed midline tremor severity using the Fahn-Tolosa-Marin TRS rather than a binary tremor score. Our sample size is smaller than previous studies; however, even with a smaller sample size, we observed robust, significant improvement in tremor following DBS that was not accompanied by gait improvement.
Our study had several limitations. We recruited participants based on a convenience sample of patients wishing to undergo DBS implantation at a single center. We did not select participants based on severity of midline tremor or gait ataxia. DBS parameters were set to optimize tremor reduction and were not set with the goal of gait improvement. Further studies are needed to clarify the difference of stimulation parameters between tremor reduction and gait improvement. Our study included only participants with unilateral DBS. Prior work suggests that bilateral DBS may have stronger effects on gait and balance.34 We only tested participants at two time points. It is possible that tremor and gait impairment in ET have a shared pathophysiology, but gait features become gradually unresponsive to DBS. Studies that include a longer follow-up period may dissociate effects of disease progression and long-term efficacy of DBS. However, the lack of differences between PRE and OFF suggests that disease progression (over approximately 9 months) was unlikely to be a major factor in our results. Finally, we measured only spatiotemporal features of walking and did not include kinematic or kinetic measurements.
Despite these limitations, we found upper extremity and midline tremor severity improved significantly following DBS, while gait was not significantly affected at the cohort level. Improvement in midline tremor was not significantly associated with the improvement in gait following DBS. Our study offers evidence that gait impairment can be dissociated from tremor in persons with ET. Future prospective studies should examine the underlying causes of gait impairment in ET and its lack of responsiveness to DBS.
Acknowledgments
The authors thank Jared Skinner and Hyokeun Lee for assistance with data collection.
Footnotes
Citation: Higuchi M, Roemmich RT, Martinez-Ramirez D, Roper JA, Zukowski LA, Foote KD, et al. Changes in midline tremor and gait following deep brain stimulation for essential tremor. Tremor Other Hyperkinet Mov. 2019; 9. doi: 10.7916/tohm.v0.684
Editor: Ruth H. Walker, Mount Sinai School of Medicine, USA
Funding: None.
Financial Disclosures: Dr. Roemmich has received research grants from NIH and the National Center for Simulation in Rehabilitation Research at Stanford University. He has received honoraria from the Michael J. Fox Foundation and the Research Grant Council of Hong Kong.
Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books).
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
Authors’ contribution
MH, RTR, DMR, CJH, and MSO were responsible for the conception of this research article. MH, RTR, CJH, and MSO were responsible for the organization of the article. All authors executed the study. For the statistical analysis, RTR and LAZ were responsible for the design and RTR for the execution of the study. MH and RTR wrote the first draft of the article and DMR, JAR, LAZ, CJH, and MSO critically reviewed the article.
References
- 1.Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (NY) 2014;4:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koller WC, Glatt S, Biary N, Rubino FA. Essential tremor variants: effect of treatment. Clin Neuropharmacol 1987;10(4):342–350. doi: 10.1097/00002826-198708000-00004 [DOI] [PubMed] [Google Scholar]
- 3.Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain 2001;124(11):2278–2286. doi: 10.1093/brain/124.11.2278 [DOI] [PubMed] [Google Scholar]
- 4.Roemmich RT, Zeilman PR, Vaillancourt DE, Okun MS, Hass CJ. Gait variability magnitude but not structure is altered in essential tremor. J Biomech 2013;46(15):2682–2687. doi: 10.1016/j.jbiomech.2013.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao AK, Gilman A, Louis ED. Balance confidence and falls in nondemented essential tremor patients: the role of cognition. Arch Phys Med Rehabil 2014;95(10):1832–1837. doi: 10.1016/j.apmr.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fois AF, Briceño HM, Fung VSC. Nonmotor symptoms in essential tremor and other tremor disorders. Int Rev Neurobiol 2017;134:1373–1396. doi: 10.1016/bs.irn.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 7.Hoskovcová M, Ulmanová O, Sprdlík O, et al. Disorders of balance and gait in essential tremor are associated with midline tremor and age. Cerebellum 2013;12(1):27–34. doi: 10.1007/s12311-012-0384-4 [DOI] [PubMed] [Google Scholar]
- 8.Louis ED, Rao AK, Gerbin M. Functional correlates of gait and balance difficulty in essential tremor: balance confidence, near misses and falls. Gait Posture 2012;35(1):43–47. doi: 10.1016/j.gaitpost.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louis ED, Rios E, Rao AK. Tandem gait performance in essential tremor: clinical correlates and association with midline tremors. Mov Disord 2010;25(11):1633–1638. doi: 10.1002/mds.23144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337(8738):403–406. doi: 10.1016/0140-6736(91)91175-T [DOI] [PubMed] [Google Scholar]
- 11.Obwegeser AA, Uitti RJ, Witte RJ, Lucas JA, Turk MF, Wharen RE. Quantitative and qualitative outcome measures after thalamic deep brain stimulation to treat disabling tremors. Neurosurgery 2001;48(2):274–281. doi: 10.1227/00006123-200102000-00004 [DOI] [PubMed] [Google Scholar]
- 12.Putzke JD, Uitti RJ, Obwegeser AA, Wszolek ZK, Wharen RE. Bilateral thalamic deep brain stimulation: midline tremor control. J Neurol Neurosurg Psychiatry 2005;76(5):684–690. doi: 10.1136/jnnp.2004.041434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell KT, Larson P, Starr PA, et al. Benefits and risks of unilateral and bilateral ventral intermediate nucleus deep brain stimulation for axial essential tremor symptoms. Parkinsonism Relat Disord 2019;60:126–132. doi: 10.1016/j.parkreldis.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 14.Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 2018;33(1):75–87. doi: 10.1002/mds.27121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemmich R, Roper JA, Eisinger RS, et al. Gait worsening and the microlesion effect following deep brain stimulation for essential tremor. J Neurol Neurosurg Psychiatry 2019;90(8):913–919. doi: 10.1136/jnnp-2018-319723 [DOI] [PubMed] [Google Scholar]
- 16.Stacy MA, Elble RJ, Ondo WG, et al. Assessment of interrater and intrarater reliability of the Fahn-Tolosa-Marin Tremor Rating Scale in essential tremor. Mov Disord 2007;22(6):833–838. doi: 10.1002/mds.21412 [DOI] [PubMed] [Google Scholar]
- 17.Whittle MW. Gait analysis: an introduction. Halley Court, Jordan Hill, Oxford: Butterworth-Heinemann Ltd; 1991. [Google Scholar]
- 18.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol 2006;63(8):1189–1193. doi: 10.1001/archneur.63.8.1189 [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor. Parkinsonism Relat Disord 2011;17(6):406–409. doi: 10.1016/j.parkreldis.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord 2012;18(8):1003–1004. doi: 10.1016/j.parkreldis.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 21.Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 2014;137(12):3149–3159. doi: 10.1093/brain/awu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 2014;137(12):3142–3148. doi: 10.1093/brain/awu314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis ED, Faust PL. Purkinje cell loss in essential tremor. Mov Disord. 2014;29(10):1329–1330. doi: 10.1002/mds.25965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis ED, Rabinowitz D, Choe M, et al. Mapping Purkinje cell placement along the Purkinje cell layer: an analysis of postmortem tissue from essential tremor patients vs. controls. Cerebellum 2016;15(6):726–731. doi: 10.1007/s12311-015-0742-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe M, Cortés E, Vonsattel JP, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 2016;31(3):393–401. doi: 10.1002/mds.26490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer C, Sanchez-Ramos J, Weiner WJ. Gait abnormality in essential tremor. Mov Disord 1994;9(2):193–196. doi: 10.1002/mds.870090212 [DOI] [PubMed] [Google Scholar]
- 27.Klebe S, Stolze H, Grensing K, Volkmann J, Wenzelburger R, Deuschl G. Influence of alcohol on gait in patients with essential tremor. Neurology 2005;65(1):96–101. doi: 10.1212/01.wnl.0000167550.97413.1f [DOI] [PubMed] [Google Scholar]
- 28.Rao AK, Gillman A, Louis ED. Quantitative gait analysis in essential tremor reveals impairments that are maintained into advanced age. Gait Posture 2011;34(1):65–70. doi: 10.1016/j.gaitpost.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez KM, Roemmich RT, Stegemöller EL, et al. Gait initiation impairments in both essential tremor and Parkinson’s disease. Gait Posture 2013;38(4):956–961. doi: 10.1016/j.gaitpost.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moscovich M, Morishita T, Foote KD, Favilla CG, Chen ZP, Okun MS. Effect of lead trajectory on the response of essential head tremor to deep brain stimulation. Parkinsonism RelatDisord 2013;19(9):789–794. doi: 10.1016/j.parkreldis.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Earhart GM, Clark BR, Tabbal SD, Perlmutter JS. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord 2009;24(3):386–391. doi: 10.1002/mds.22356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fasano A, Herzog J, Raethjen J, et al. Gait ataxia in essential tremor is differentially modulated by thalamic stimulation. Brain 2010;133(12):3635–3648. doi: 10.1093/brain/awq267 [DOI] [PubMed] [Google Scholar]
- 33.Fasano A, Herzog J, Raethjen J, et al. Lower limb joints kinematics in essential tremor and the effect of thalamic stimulation. Gait Posture 2012;36(2):187–193. doi: 10.1016/j.gaitpost.2012.02.013 [DOI] [PubMed] [Google Scholar]
- 34.Hwynn N, Hass CJ, Zeilman P, et al. Steady or not following thalamic deep brain stimulation for essential tremor. J Neurol 2011;258(9):1643–1648. doi: 10.1007/s00415-011-5986-0 [DOI] [PubMed] [Google Scholar]
- 35.Reich MM, Brumberg J, Pozzi NG, et al. Progressive gait ataxia following deep brain stimulation for essential tremor: adverse effect or lack of efficacy? Brain 2016;139(11):2948–2956. doi: 10.1093/brain/aww223 [DOI] [PubMed] [Google Scholar]