Abstract
Autism is a heterogeneous neurodevelopmental disorder with a 3–4 times higher sex ratio in males than females. X chromosome genes may contribute to this higher sex ratio through unusual skewing of X chromosome inactivation. We studied X chromosome skewness in 30 females with classical autism and 35 similarly aged unaffected female siblings as controls using the polymorphic androgen receptor (AR) gene. Significantly, increased X chromosome skewness (e.g., >80:20%) was detected in our autism group (33%) compared to unaffected females (11%). X chromosome skewness was also seen in 50% of the mothers with autistic daughters. No mutation was seen in the promoter region of the XIST gene reported to be involved in X chromosome inactivation in our subjects. X chromosome skewness has been reported in female carriers of other neurological disorders such as X-linked mental retardation, adrenoleukodystrophy and Rett syndrome.
Keywords: Autism, X inactivation, females, AGRE
INTRODUCTION
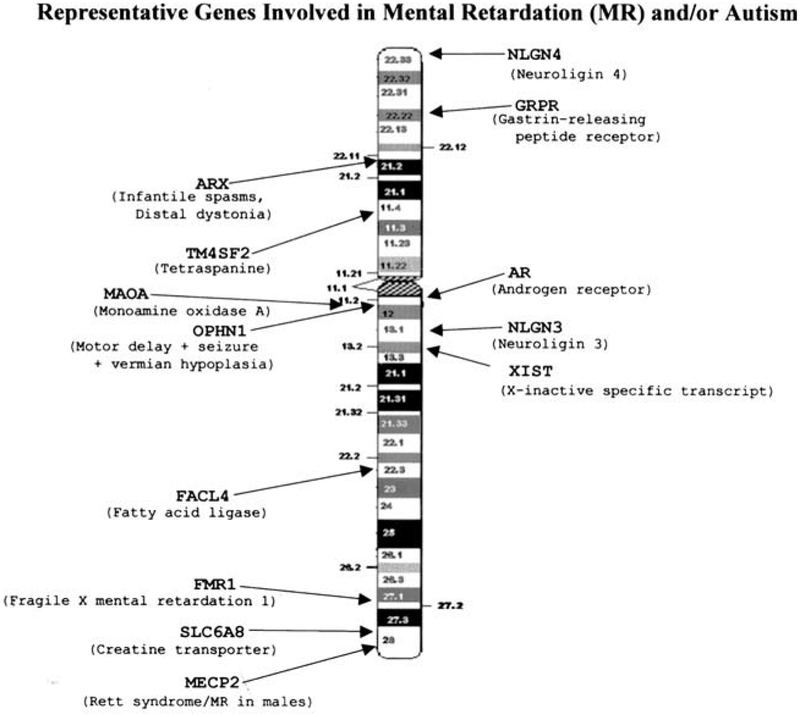
Autism (MIM 209850) is genetically heterogeneous with an early onset of neurodevelopmental problems defined by significant impairment in communication and social interaction accompanied by a pattern of repetitive or stereotypical behaviors and interests (Lord, Rutter, & Le Couteur, 1994). Mental retardation can also be a characteristic in subjects with autism. Linkage analysis has shown the involvement of genes from several chromosomes (Yonan et al., 2003) including the X (see Fig. 1).
Fig. 1.
Schematic of the human X chromosome with known location of representative genes.
An excess of males with autism in a proportion of about four to one is reported (Volkmar, Szatmari, & Sparrow, 1993) which further supports the involvement of genes on the X chromosome. In addition to genetic linkage data, several lines of evidence such as X chromosome rearrangements indicate that the X chromosome should be further studied in autism. For example, autistic patients have been reported with Xp22 duplications and/or deletions (Rao et al., 1994; Thomas et al., 1999) and an autistic female was described with a translocation involving the X chromosome (Ishikawa-Brush et al., 1997). This female showed that the breakpoint at Xp22.13 occurred in the first intron of the GRPR gene (gastrin-releasing peptide receptor). Mutations in two other X-linked genes encoding neuroligins, NLGN3 (at Xq13) and NLGN4 (at Xp22.3), have been reported in two families with autism (Jamain et al., 2003).
Plenge, Stevenson, Lubs, Schwartz, and Willard (2002) reported that skewed X chromosome inactivation (i.e., one X chromosome may be more or less active than the second X, for example, >80:20%) was a common finding among X-linked mental retardation female carriers using the human androgen receptor (AR) gene located at Xq11.2. The human AR gene contains a highly polymorphic in-frame CAG repeat encoding 11–31 glycine residues in exon 1 of the gene. X inactivation patterns can be assessed using the AR gene in females informative at the CAG repeat. Additionally, a rare cytosine to guanine mutation in the XIST gene promoter region has been reported to contribute to skewed X chromosome inactivation in females (Plenge et al., 1997).
X chromosome inactivation occurs early in embryonic development of somatic cells in human females to achieve gene dosage compensation with males (Lyon, 1961). Therefore, one of the two X chromosomes is inactivated in each female cell at random which then results in an equal number of active X chromosome genes in both male and female cells. The X inactivation is a complex process and requires three main steps: initiation, spreading, and maintenance (Penny, Kay, Sheardown, Rastan, & Brockdorrf, 1996; Willard, 1995). During the initiation step, one of the two X chromosomes is selected to be inactivated which requires the presence in cis of the X inactivation center (XIC) (Brown et al., 1991; Russell, 1963). In humans, the candidate region for XIC is located on the proximal long arm of the X chromosome (at Xq13). In 1991, the XIST gene (X-inactive specific transcript) was discovered in the XIC region and became a candidate gene for initiation of inactivation (Brown et al., 1991). The XIST gene is constitutively expressed from the inactive X chromosome and encodes a transcript but does not code for a protein (Brown et al., 1992). The untranslated RNA product of the XIST gene “coats” the presumptive inactive X chromosome which results in spreading of inactivation from the XIC region. However, certain X-linked genes have been found to escape inactivation and are expressed from both the active and the inactive X chromosome (Brown, Carrel, & Willard, 1997). Genes that are not subject to X inactivation are distributed non-randomly along the X chromosome with the majority clustered on the short arm of the human X (Carrel, Cottle, Goglin, & Willard, 1999).
Because an unequal sex ratio in autism could be explained by X chromosome gene involvement in a subset of affected females, we studied X chromosome inactivation patterns. We compared X inactivation patterns in 77 autistic and control female siblings from the Autism Genetics Resource Exchange (AGRE) to determine if X chromosome skewness exists in females with autism which may account for expression of genes for autism on the X chromosome.
SUBJECTS AND METHODS
Subjects
Seventy-seven females were analyzed in our study consisting of 35 females with classical autism (eight from simplex and 27 from multiplex families) and 42 female sibling controls without a history of autism or mental retardation. The average age for the autism group was 10 years and 11 years for the controls. The age range for both groups was 5–12 years. All subjects were ascertained from the Autism Genetics Resource Exchange (AGRE), a publicly available biomaterials repository located in Los Angeles, CA. The diagnosis of autism was established in the affected females with the use of the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). Chromosome analysis and fragile X testing were reportedly normal. The control females were unaffected sibs of the autistic females from selected AGRE families. DNA and clinical information (medical and pedigree data, diagnostic assessments and scores) were obtained on each subject from AGRE as well as DNA from the mothers of those subjects (autistic and control) showing significant X chromosome skewness.
METHODS
Genomic DNA previously extracted from peripheral blood from each subject was amplified with polymerase chain reaction (PCR) in the presence of forward and reverse primers for the polymorphic AR gene. The polymorphic CAG repeat size was determined by capillary electrophoresis using an ABI 310 DNA sequencer (Foster City, CA). Subsequently, 200 ng of genomic DNA were digested with the methyl sensitive HpaII restriction enzyme as described elsewhere (Allen, Zoghbi, Moseley, Rosenblatt, & Belmont, 1992). The 5′ end of the reverse primer was fluorescently labeled with 6-FAM (6-carboxyfluorescein) and the resulting PCR fragments analyzed by capillary electrophoresis following established protocols (Karasawa et al., 2001; Villard et al., 2001). X chromosome inactivation was calculated as the ratio of the height of the shorter peak to the sum of the two peaks using genotyping software after digestion (see Fig. 2). Similar studies were undertaken on the mothers of the subjects showing significant X chromosome skewness. X chromosome skewness was classified into three groups: randomly skewed (50:50%–64:36%), moderately skewed (65:35%–80:20%) and highly skewed or significant skewness (>80:20%) as reported in other studies (Harris, Collins, Vetrie, Cole, & Bobrow, 1992; Maier et al., 2002).
Fig. 2.
X inactivation analysis by genotyping of the CAG repeat in the AR gene after digestion. An example of the random and highly skewed X inactivation is shown. The peak representing the active (unmethylated) X chromosome allele would be digested by the methyl sensitive enzyme and reduced in size. If skewness is present the peak height would differ between the two peaks representing each X chromosome (methylated-inactive and unmethylated-active).
Three sets of control experiments were performed to confirm complete digestion of genomic DNA and to ensure correct assessment of the ratio of an active (unmethylated) vs. an inactive (methylated) X chromosome: (1) a serial dilution of DNA from two males carrying a different AR allele as a control experiment, (2) genomic DNA from a male with a different CAG repeat size was added to the digestion reaction as an internal control, and (3) the digestion, PCR and genotyping were repeated up to three times in several samples to ensure reproducibility and equal amplification of both alleles.
The presence of a known mutation in the promoter region of the XIST gene was examined in all females with X inactivation skewness greater than 80%. A 276-bp fragment comprising the mutation was amplified and subsequently digested with HhaI enzyme following the protocol reported by Plenge et al. (1997). After digestion, a normal promoter region would produce two fragments while the mutation produces three DNA bands on an agarose gel.
RESULTS
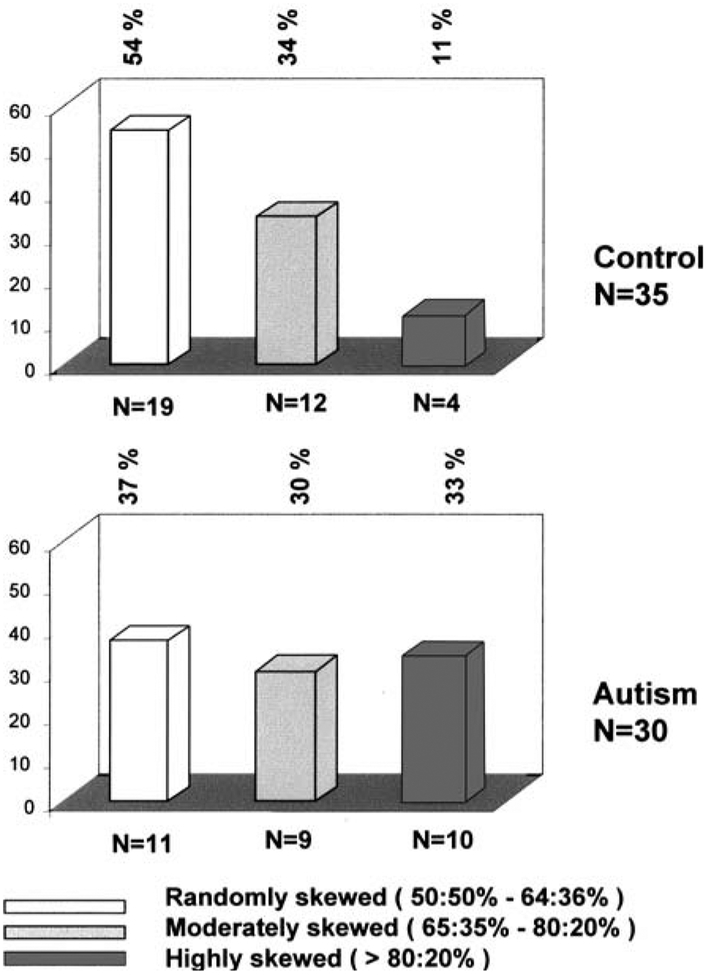
Thirty of the 35 females with classical autism were informative for the AR gene polymorphism (i.e., demonstrating two different allele sizes) while 35 of the 42 unaffected female controls were informative. A greater percentage of X inactivation skewness (highly skewed >80:20%) was seen in the autism group (10 of 30 or 33%) compared with the control group (4 of 35 or 11%) (p=.04; Fisher’s exact test, two-sided) (see Table I). The subjects were classified into three subgroups representing highly, moderately, and randomly skewed X inactivation patterns (see Fig. 3). When X inactivation patterns were compared using this subgroup classification, as reported in other studies, differences were observed between the autism and control groups (p=.03; Exact test of Wilcoxon–Mann–Whitney, one-sided).
Table I.
X Inactivation Result for the Two Studied Groups
| Androgen receptor (AR) genotyping | Autism (n = 35) |
Control (n = 42) |
|---|---|---|
| Non-informative (one allele size) | 5 (14%) | 7 (17%) |
| Informative (two different allele sizes) | 30 (86%) | 35 (83%) |
| Randomly skewed (50:50%−64:36%) | 11 (37%) | 19 (54%) |
| Moderately skewed (65:35%−80:20%) | 9 (30%) | 12 (34%) |
| Highly skewed (>80:20%) | 10 (33%) | 4 (11%) |
Fig. 3.
Distribution of patterns of X inactivation in 30 autistic females and 35 unaffected female siblings. X inactivation pattern was divided into random (50:50%–64:36%), moderately skewed (65:35%–80:20%), and highly skewed (>80:20%). A significantly greater percentage of highly skewed X inactivation (>80:20%) was detected in the autism group compared with the female sibling group (p=.04; Fisher exact test, two-sided). When comparing X inactivation patterns classified into three subgroups (random, moderately skewed, highly skewed), we found significant differences in the two subject groups (p=.03; Exact test of Wilcoxon–Mann–Whitney, one-sided).
Furthermore, we studied X inactivation patterns in the mothers of the highly skewed females (both autism and controls) to determine whether the skewness was familial in nature. Five of the 10 mothers of the autistic daughters with highly skewed X chromosome inactivation also showed skewness but none of the mothers of the four control females with highly skewed inactivation showed skewness (see Table II). The promoter region of the XIST gene was amplified in those females with skewness and examined for the presence of a mutation reportedly associated with X chromosome skewness (Plenge et al., 1997). The reported mutation was not detected in the 14 subjects (10 with autism and four control females) with highly skewed X inactivation patterns in our study (data not shown).
Table II.
X Inactivation Ratio Using the Methylation Status of the Androgen Receptor (AR) Gene in Highly Skewed Subjects (Autism and Control) and Their Mother
| Daughter | Mother | |
|---|---|---|
| Autism subject | ||
| 1 | 95:5%a | 95:5%a |
| 2 | 92:8%a | 73:27% |
| 3 | 90:10%a | 83:17%a |
| 4 | 88:12%a | 95:5%a |
| 5 | 88:12%a | 50:50% |
| 6 | 87:13%a | 94:6%a |
| 7 | 83:17%a | 59:41% |
| 8 | 82:18%a | 66:34% |
| 9 | 82:18%a | 94:6%a |
| 10 | 82:18%a | 56:44% |
| Control subject | ||
| 1 | 89:11%a | 74:26% |
| 2 | 87:13%a | 56:44% |
| 3 | 87:13%a | 51:49% |
| 4 | 82:18%a | 56:44% |
Highly skewed X inactivation pattern (>80:20%).
DISCUSSION
Our study suggests that X inactivation patterns are not the same in females with autism compared to control females. Statistically, a greater X chromosome skewness was observed in females with autism (33%) compared with control females (11%). Extreme skewing of >80:20% has been reported in approximately 10% of female control subjects (Plenge et al., 2002), which compares favorably with our female controls. No known mutations were identified in the promoter region of the XIST gene in our female subjects (autistic or control) with significant skewing of X chromosome inactivation and the X chromosome skewness could not be attributed to the reported mutation of the XIST gene.
In our autism group, affected females with highly skewed X inactivation had mothers with a similar skewness pattern in 50% or five of 10 cases. No familial consistency of X inactivation pattern was reported in mother/daughter pairs from the normal population (Harris et al., 1992); however, a heritable skewing of X inactivation has been reported (Naumova et al., 1996) in unique families (e.g., with XIST mutations).
Our study is the first to report on X inactivation in females with autism compared with control females. We recognize the importance of confirming our X chromosome skewness in females with autism in additional studies. In healthy females, X chromosome inactivation is considered to follow a Gaussian or bell-shaped distribution with highly skewed patterns being uncommon events (Migeon, 1998). Although no data were previously available for females with autism, X inactivation studies have been performed in other neurological conditions. For example, a significantly higher percentage of X inactivation skewness was observed in X-linked mental retardation carriers (Plenge et al., 2002) and a high concordance of skewing of X inactivation was reported between mothers and daughters in families with dystrophinopathies (Azofeifa, Voit, & Hubner, Cremer, 1995; Yoshioka, Yorifuji, & Mituyoshi, 1998). In addition, Villard et al. (2001) described a totally skewed pattern of X inactivation in four familial cases of Rett syndrome without the MECP2 gene mutation. Female carriers of X-linked adrenoleukodystrophy (X-ALD) are also more susceptible to X chromosome inactivation skewness (Maier et al., 2002) with an equal proportion of moderate and highly skewed findings observed in X-ALD female carriers as seen in our study of females with autism. Furthermore, extreme skewing of X inactivation was observed in fetuses and newborns associated with confined placental mosaicism of an autosomal trisomy and in Prader–Willi syndrome females with uniparental disomy of chromosome 15 (Lau et al., 1997). Skewed X inactivation was also seen in female carriers of dyskeratosis congenita (Devriendt et al., 1997) and females with recurrent spontaneous abortions (Sangha, Stephenson, Brown, & Robinson, 1999). Non-random X inactivation was also suggested to explain reduced penetrance in carrier females with the fragile X gene mutation (Sun & Baumer, 1999). X chromosome inactivation is a complex, multi-process phenomenon which also involves several epigenetic factors such as: DNA methylation, X chromosome reactivation, genes escaping inactivation, parental origin effect (imprinting) and possible elements influencing X inactivation skewness.
Possible explanations for the observed X chromosome skewness in our study includes selective cell death after initial random X inactivation (e.g., carriers of X-autosome translocations, lymphocytes of carriers of X-linked immunodeficiency disease) but probably unlikely in the peripheral blood of our females with autism. A second possibility for the X chromosome skewness may be selective ascertainment of individuals from the tail of a random distribution of inactivation because of an unusual or unexpected phenotype. Examples of this phenomenon would include female carriers of Duchenne muscular dystrophy (an X-linked recessive disorder) manifesting the disease state. If there is an X-linked recessive susceptibility gene(s) for autism, this could explain the observation of X chromosome skewness in our females with autism.
In summary, the results from our study suggest that, in peripheral blood cells, skewed X inactivation is a more common feature of females with autism than unaffected female subjects. Furthermore, we detected a higher percentage of heritability of X chromosome inactivation (i.e., both affected daughter and their unaffected mother with a highly skewed X inactivation pattern) than expected in the general population. However, no reported XIST mutations were seen in our subjects. Ascertainment of more families with heritable X inactivation skewing and analysis of possible candidate genes on the X chromosome and factors influencing X inactivation may lead to a better understanding of the genetics of autism in both males and females.
ACKNOWLEDGMENTS
We acknowledge the AGRE families without whom none of this research would be possible. The AGRE Program, founded by Cure Autism Now, is supported through private donations from Cure Autism Now and by a grant from the National Institute of Mental Health (NIMH) to UCLA and collaborating investigators. Continual scientific oversight is provided by the AGRE Scientific Steering Committee, chaired by Dan Geschwind, M.D., Ph.D., Director of the Neurogenetics Program, Department of Neurology, UCLA. Partial funding support for this study was from CMH Special Gift Funds (GL 01.2650).
REFERENCES
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, & Belmont JW (1992). Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. American Journal of Human Genetics, 51, 1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Azofeifa J, Voit T, Hubner C, & Cremer M (1995). X-chromosome methylation in manifesting and healthy carries of dystrophinopathies: Concordance of activation ratios among first degree female relatives and skewed inactivation as cause of the affected phenotypes. Human Genetics, 96, 167–176. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Carrel L, & Willard HF (1997). Expression of genes from the human active and inactive X chromosomes. American Journal of Human Genetics, 60, 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Hendrich BD, Rupertt JL, Lafreniere RG, Xing Y, Lawrence J, & Willard HF (1992). The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell, 71, 527–542. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Lafreniere RG, Powers VE, Sebastio G, & Ballabio A (1991). Localization of the X inactivation center on the human X chromosome in Xq13. Nature, 349, 82–84. [DOI] [PubMed] [Google Scholar]
- Carrel L, Cottle AA, Goglin KC, & Willard HF (1999). A first-generation X-inactivation profile of the human X chromosome. Proceedings of the National Academy of Sciences USA, 7, 14440–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devriendt K, Matthijs G, Legius E, Schollen E, Blockmans D, vanvan Geet C, Degreef H, Cassiman JJ, & Fryns JP (1997). Skewed X-chromosome inactivation in female carriers of dyskeratosis congenita. American Journal of Human Genetics, 60, 581–587. [PMC free article] [PubMed] [Google Scholar]
- Harris A, Collins J, Vetrie D, Cole C, & Bobrow M (1992). X inactivation as a mechanism of selection against lethal alleles: Further investigation of incontinentia pigmenti and X linked lymphoproliferative disease. Journal of Medical Genetics, 29, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Brush Y, Powell JF, Bolton P, Miller AP, Francis F, Willard HF, Lehrach H, & Monaco AP (1997). Autism and multiple exostoses associated with an X;8 translocation occurring within the GRPR gene and 3′ to the SDC2 gene. Human Molecular Genetics, 6, 1241–1250. [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Gillberg C, & Bourgeron T (2003). Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Paris Autism Research International Sibpair Study. Nature Genetics, 34, 27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa M, Tsukamoto N, Yamane A, Okamoto K, Maehara T, Yokohama A, Nojima Y, & Omine M (2001). Analysis of the distribution of CAG repeats and X-chromosome inactivation status of HUMARA gene in healthy female subjects using improved fluorescence-based assay. International Journal of Hematology, 74, 281–286. [DOI] [PubMed] [Google Scholar]
- Lau AW, Brown CJ, Penaherrera M, Langlois S, Kalousek DK, & Robinson WP (1997). Skewed X-chromosome inactivation is common in fetuses or newborns associated with confined placental mosaicism. American Journal of Human Genetics, 61, 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism diagnostic interview—revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lyon MF (1961). Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature, 190, 372–373. [DOI] [PubMed] [Google Scholar]
- Maier EM, Kammerer S, Muntau AC, Wichers M, Braun A, & Roscher AA (2002). Symptoms in carriers of adrenoleukodystrophy relate to skewed X inactivation. Annals of Neurology, 52, 683–688. [DOI] [PubMed] [Google Scholar]
- Migeon BR (1998). Non-random X chromosome inactivation in mammalian cells. Cytogenetics and Cell Genetics, 80, 142–148. [DOI] [PubMed] [Google Scholar]
- Naumova AK, Plenge RM, Bird LM, Leppert M, Morgan K, Willard HF, & Sapienza C (1996). Heritability of X chromosome—inactivation phenotype in a large family. American Journal of Human Genetics, 58, 1111–1119. [PMC free article] [PubMed] [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, & Brockdorff N (1996). Requirement for XIST in X chromosome inactivation. Nature, 379, 131–137. [DOI] [PubMed] [Google Scholar]
- Plenge RM, Hendrich BD, Schwartz C, Arena F, Naumova A, Sapienza C, Winter RM, & Willard HF (1997). A promoter mutation in the XIST gene in two unrelated families with skewed X-chromosome inactivation. Nature Genetics, 17, 353–356. [DOI] [PubMed] [Google Scholar]
- Plenge RM, Stevenson RA, Lubs HA, Schwartz CE, & Willard HF (2002). Skewed X-chromosome inactivation is a common feature of X-linked mental retardation disorders. American Journal of Human Genetics, 71, 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PN, Klinepeter K, Stewart W, Hayworth R, Grubs R, & Pettenati MJ (1994). Molecular cytogenetic analysis of duplication Xp in a male: Further delineation of a possible sex influencing region on the X chromosome. Human Genetics, 94, 149–153. [DOI] [PubMed] [Google Scholar]
- Russell LB (1963). Mammalian X-chromosome action: Inactivation limited in spread and region of origin. Science, 140, 976–978. [DOI] [PubMed] [Google Scholar]
- Sangha KK, Stephenson MD, Brown CJ, & Robinson WP (1999). Extremely skewed X-chromosome inactivation is increased in women with recurrent spontaneous abortion. American Journal of Human Genetics, 65, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, & Baumer A (1999). Nonrandom X inactivation and selection of fragile X full mutation in fetal fibroblasts. American Journal of Medical Genetics, 86, 162–164. [DOI] [PubMed] [Google Scholar]
- Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, & Dennis NR (1999). Xp deletions associated with autism in three females. Human Genetics, 104, 43–48. [DOI] [PubMed] [Google Scholar]
- Villard L, Levy N, Xiang F, Kpebe A, Labelle V, Chevillard C, Zhang Z, Schwartz CE, Tardieu M, Chelly J, Anvret M, & Fontes M (2001). Segregation of a totally skewed pattern of X chromosome inactivation in four familial cases of Rett syndrome without MECP2 mutation: Implications for the disease. Journal of Medical Genetics, 38, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Szatmari P, & Sparrow SS (1993). Sex differences in pervasive developmental disorder. Journal of Autism and Developmental Disorders, 23, 579–591. [DOI] [PubMed] [Google Scholar]
- Willard HF (1995). The sex chromosome and X-chromosome inactivation In: Scriver CR, Beaudet AL, Sly WS, & Valle D (Eds.), The metabolic and molecular bases of inherited disease. (pp. 717–737). New York: McGraw-Hill. [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, Grunn A, Juo SH, Terwilliger JD, Liu J, Cantor RM, Geschwind DH, & Gilliam TC (2003). A genomewide screen of 345 families for autism-susceptibility loci. American Journal of Human Genetics, 73, 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M, Yorifuji T, & Mituyoshi I (1998). Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clinical Genetics, 53, 102–107. [DOI] [PubMed] [Google Scholar]