Abstract
Background:
The PLATelet inhibition and patient Outcomes (PLATO) trial showed that treatment with ticagrelor reduced the rate of death due to vascular causes, myocardial infarction and stroke when compared to clopidogrel in patients with ST-elevation or non-ST-elevation acute coronary syndrome (ACS). While the comparative benefit of ticagrelor over clopidogrel increased over time, event rates accrued in both groups during the study period. The purpose of our biomarker-based exploratory analysis was to determine whether long-term platelet inhibition may be associated with platelet adaptation.
Methods:
A sample of 4000 participants from the PLATO trial also consented to participate in a prospectively designed Biomarker Substudy. Blood samples were procured at baseline, immediately prior to hospital discharge and at one month and six months. Markers of platelet activity, including platelet count, serum CD40-ligand and soluble P-selectin were analyzed. Mean levels were compared at discharge, 1 month and 6 months following study drug initiation – first for all patients and subsequently stratified by treatment group. A linear mixed model was used to estimate the short-term change rate (baseline to 1 month) and long-term change rate (1 month to 6 month) for each biomarker. A Cox proportional hazards model was used to calculate hazard ratios for each change in biomarker over the two time periods examined: baseline to one month and one month to six months.
Results:
Prior to randomized treatment (baseline), sCD40 ligand and sP-selectin levels were elevated above the normal range of the assay (0.39 ug/L and 33.5 ug/L, respectively). The mean level of each biomarker was significantly different at one month compared to baseline (p<0.0001). When stratified by treatment group, at one month patients treated with ticagrelor had a larger increase in platelet count compared to those treated with clopidogrel (p<0.0001). Similarly, when comparing biomarker levels for all patients at six months with those at one month, each differed significantly (p<0.05). There was no significant difference between treatment groups during this time period. The rate of change for both platelet count and sP-selectin were significantly different between baseline and one month when compared to the one to six-month time period (p<0.0001). When comparing treatment groups, the rate of increase in platelets from baseline to one month was greater for patients treated with ticagrelor (p<0.0001). This was no longer observed in the one to six-month interval. Using a Cox proportional hazard model, the increase in platelet count from 1 month to 6 months was associated with ischemic-thrombotic events, while sCD40 ligand decrease from 1 month to 6 months was associated with hemorrhagic events. There were no differences between treatment groups for the associations with clinical endpoints.
Conclusion:
Dynamic changes in platelet count, sCD-40 ligand and sP-selectin occur over time among patients with ACS. Platelet-directed therapy with a P2Y12 receptor inhibitor in combination with aspirin modestly impacts the expression of these biomarkers. Platelet count and sCD40 ligand may offer modest overall predictive value for future ischemic-thrombotic or hemorrhagic clinical events, respectively. The existence of a platelet adaptome and its overall clinical significance among patients at risk for thrombotic events will require a more in-depth and platelet-biology specific investigation.
Keywords: Acute Coronary Syndrome, P2Y12 Inhibitor, Ticagrelor, Platelet adaptome
Introduction
Platelets play a fundamental role in hemostasis, preventing blood loss after vascular injury. Because of this, the number of platelets within the circulation far exceeds what is necessary for day-to-day hemostasis and vascular repair; dynamic systems are in place for adaption to heritable or acquired conditions. For example, platelet P2Y12 receptor deficiency is neither lethal in utero nor is it associated with a strong bleeding phenotype, suggesting that existing redundancies or responses in platelet number, activation potential and/or “platelet adaptation” offer host protection. One such redundancy includes the presence of at least two distinct ADP receptors, including P2Y12 and P2Y1, that initiate ADP-induced aggregation of platelets[1]. Further, patients receiving aspirin have heightened platelet gene expression and function of non-cyclooxygenase (COX) 1 pathways within two weeks of initiation[2].
Clopidogrel is a pro-drug that was developed as a second generation in the thienopyridine class of P2Y12 inhibitors which bind irreversibly at the platelet ADP-binding site. Ticagrelor was subsequently developed as the first oral, reversible P2Y12 receptor antagonist with binding at a site that is distinct from the position of ADP-binding site. Ticagrelor has been found to have more consistent platelet inhibition than clopidogrel[3]. The PLATelet inhibition and patient Outcomes (PLATO, www.ClinicalTrials.gov ) trial showed that ticagrelor reduced the incidence of cardiovascular death, myocardial infarction and stroke compared to clopidogrel in patients with ST-elevation and non-ST-elevation acute coronary syndrome (ACS)[4]. In both treatment groups, the number of primary outcome events increased over time, albeit more so with clopidogrel. Whether platelet adaptation to prolonged drug-induced inhibition was a contributing factor is unknown.
The purpose of our sub-study was to explore changes in platelet-related biomarkers over time that may represent an adaptation response and their association with clinical events among patients participating in the PLATO trial who received treatment with aspirin and either ticagrelor or clopidogrel.
Methods
The PLATO study (www.ClinicalTrials.gov ) has been described in detail previously[4, 5]. In brief, PLATO was a randomized, double-blind, double-dummy, parallel group, international, multicenter study with 18,624 participants. Patients with ACS were randomized to either clopidogrel or ticagrelor as soon as possible after admission and within 24 hours of the presenting event. Patients who had already received a loading dose of clopidogrel were included in randomization and subsequently received treatment per study group protocol. Ticagrelor was given as a 180mg loading dose followed by 90mg twice daily. Clopidogrel was administered as a 75mg dose once daily. In patients not previously treated with clopidogrel, a 300mg loading dose was given and an additional 300mg was allowed prior to percutaneous coronary intervention (PCI); for patients on clopidogrel at baseline, a maintenance dose of 75mg of clopidogrel was given as the first dose. All patients received aspirin daily unless they were allergic or intolerant. Treatment was continued for a minimum of 6 months and up to 12 months.
Blood samples were collected from all patients participating in PLATO at randomization. A biomarker sub-study was also conducted in a group of patients who consented to additional blood tests and included a prospective evaluation with serial sampling in an effort to further elucidate underlying mechanisms correlating to clinical data. The three platelet-related biomarkers chosen were platelet count, soluble CD40-ligand (sCD40L) and soluble P-selectin (sP-selectin). Platelet count was selected as a general measure of systemic conditions. sCD40-ligand was selected to represent the interface of platelets, thrombosis, and inflammation. Similarly, soluble P-selectin was chosen as a measure of platelet and endothelial cell activation. Platelet number, sCD40-ligand and soluble P-selectin were evaluated at baseline, hospital discharge, one month and six months from the time of study initiation. An initial evaluation of the PLATO biomarker data set did not reveal associations between baseline measures of platelet-related biomarkers and either primary or secondary outcomes at 1 year.
The current analysis is based on serial samples procured from patients enrolled in the PLATO biomarker sub-study who were on either clopidogrel or ticagrelor and had a valid baseline and two follow-up measurements for any biomarker. Blood samples were collected and analyzed at a central location. Platelet count was determined using an Electronic Cell Counter by Quintiles Laboratories. Soluble CD40L was analyzed using a Sandwich enzyme immunoassay (ELISA; Freedom EVOlyzer) using Human sCD40L kit (BenderMedSystems) at the UCR laboratory, Uppsala. Soluble P-selectin was analyzed using Sandwich enzyme immunoassay (ELISA; Freedom EVOlyzer) using Human sP-selectin/CD62P kit (R&D Systems®) at the UCR laboratory, Uppsala.
A mixed model was used to estimate the short-term change rate (baseline to 1 month) and long-term change rate (1 month to 6 month) for each biomarker. We tested whether those change rates were different. We also tested if those change rates were different for patients treated with ticagrelor compared to patients with clopidogrel. The analyses were stratified according to the presence or absence of clopidogrel loading prior to randomization and baseline sampling.
A Cox proportional hazards model was used to evaluate the predictive utility of changes in biomarkers over time. Each biomarker was assessed against clinical endpoints of myocardial infarction, all-cause death, cardiovascular death, the composite of cardiovascular death, myocardial infarction or stroke, major bleeding, and non-CABG-related major bleeding. Hazard ratios (HR) were estimated for absolute changes in biomarkers for the baseline to one-month time period as a reflection of whether immediate changes after an ischemic event were predictive of outcomes. Similarly, hazard ratios were estimated for absolute changes in biomarkers for the one month to six-month time period as an indication of whether levels after recovery from an ischemic event were predictive of future events.
Results
Of the 18,624 patients in PLATO, a subset participated in the sub-study with 5693 patients who had a baseline and two follow-up measures of platelet count, 1897 with sCD40 ligand and 1898 with sP-selectin (Table 1). Prior to randomized treatment (baseline), sCD40 ligand and sP-selectin levels were elevated above the normal range of the assay (0.39 ug/L and 33.5 ug/L, respectively). Platelet levels were within the normal range at 232 ×10E9/L.
Table 1.
Baseline Characteristics by Biomarker-Defined Populations
| Populations | ||||
|---|---|---|---|---|
| Characteristic | Plato Population (N=18624) | Platelet Subgroup (N=5963) | CD 40 Subgroup (N=1897) | sP-Selectin Subgroup (N=1898) |
| Female Gender | 5288 / 18624 (28.4%) | 1660 / 5963 (27.8%) | 533 / 1897 (28.1%) | 534 / 1898 (28.1%) |
| Age, years (Median, 25th-75th) | 62 (54–71) | 62 (54–70) | 61 (53–70) | 61 (53–70) |
| Body Mass Index, (Median, 25th-75th) | 27.4 (24.7–30.4) | 27.5 (24.9–30.5) | 27.7 (25.0–30.9) | 27.7 (25.1–30.9) |
| Systolic Blood Pressure, mmHg (Median, 25th-75th) | 133 (120–150) | 133 (120–150) | 135 (120–150) | 135 (120–150) |
| Diastolic Blood Pressure, mmHg (Median, 25th-75th) | 80 (70–90) | 80 (70–90) | 80 (70–90) | 80 (70–90) |
| Heart Rate, bpm (Median, 25th-75th) | 73 (64–84) | 72 (64–82) | 72 (63–82) | 72 (63–82) |
| Medical History | ||||
| Myocardial Infarction | 3824 / 18613 (20.5%) | 1163 / 5963 (19.5%) | 379 / 1897 (20.0%) | 379 / 1898 (20.0%) |
| Coronary Artery Disease | 5126 / 18613 (27.5%) | 1580 / 5963 (26.5%) | 542 / 1897 (28.6%) | 543 / 1898 (28.6%) |
| Percutaneous Coronary Intervention | 2492 / 18612 (13.4%) | 788 / 5963 (13.2%) | 234 / 1897 (12.3%) | 234 / 1898 (12.3%) |
| Coronary Artery Bypass Graft | 1106 / 18613 (5.9%) | 330 / 5963 (5.5%) | 84 / 1897 (4.4%) | 84 / 1898 (4.4%) |
| Transient Ischemic Attack | 499 / 18613 (2.7%) | 156 / 5963 (2.6%) | 39 / 1897 (2.1%) | 39 / 1898 (2.1%) |
| Non-Hemorrhagic Stroke | 722 / 18612 (3.9%) | 203 / 5963 (3.4%) | 69 / 1897 (3.6%) | 69 / 1898 (3.6%) |
| Hypertension | 12183 / 18613 (65.5%) | 3822 / 5963 (64.1%) | 1255 / 1897 (66.2%) | 1256 / 1898 (66.2%) |
| Diabetes | 4662 / 18613 (25.0%) | 1393 / 5963 (23.4%) | 423 / 1897 (22.3%) | 424 / 1898 (22.3%) |
| Dyslipidemia | 8689 / 18612 (46.7%) | 2765 / 5962 (46.4%) | 811 / 1897 (42.8%) | 812 / 1898 (42.8%) |
| Gastrointestinal Bleeding | 265 / 18613 (1.4%) | 86 / 5963 (1.4%) | 24 / 1897 (1.3%) | 24 / 1898 (1.3%) |
| Chronic Renal Disease | 785 / 18613 (4.2%) | 190 / 5963 (3.2%) | 57 / 1897 (3.0%) | 57 / 1898 (3.0%) |
| Treatment Approach | ||||
| Invasive | 13408 / 18624 (72.0%) | 4200 / 5963 (70.4%) | 1353 / 1897 (71.3%) | 1353 / 1898 (71.3%) |
| Medically Managed | 5216 / 18624 (28.0%) | 1763 / 5963 (29.6%) | 544 / 1897 (28.7%) | 545 / 1898 (28.7%) |
| STEMI at presentation | 7544 / 18618 (40.5%) | 2392 / 5963 (40.1%) | 832 / 1897 (43.9%) | 832 / 1898 (43.8%) |
| Aspirin at time of randomization | 17428 / 18601 (93.7%) | 5575 / 5963 (93.5%) | 1817 / 1897 (95.8%) | 1818 / 1898 (95.8%) |
When biomarker levels for all patients at one month – 0.35 ug/L and 29.1 ug/L for sCD40 ligand and sP-selectin, respectively – were compared with baseline values, each had changed significantly (Table 2, p <0.0001). While platelet counts increased for all patients, levels of sCD40 ligand and sP-selectin both decreased (Figure 1). Additionally, patients treated with ticagrelor had a larger increase in platelet count compared to those treated with clopidogrel (Table 3a, p<0.0001). The rate of change for platelet count from baseline to one month also differed between treatment groups.
Table 2:
Summary of Biomarker Changes: For All Patients
| Characteristic | 1 Month - Baseline | p-value 1 1 Mon Vs. Baseline |
6 Month - 1 Month | p-value 2 6 Mon Vs. 1 Mon |
|---|---|---|---|---|
| Platelet (x10E9/L) N: Median (25th-75th) | 5963: 6.0 (−21.0–35.0) | <.0001 | 5963: −1.0 (−27.0–22.0) | <.0001 |
| CD40 (ug/L) N: Median (25th-75th) | 1897: −0.04 (−0.27–0.13) | <.0001 | 1897: −0.01 (−0.22–0.17) | 0.0188 |
| P-Selectin (ug/L) N: Median (25th-75th) | 1898: −4.4 (−13.0–3.7) | <.0001 | 1898: 1.3 (−5.8–8.4) | <.0001 |
Figure 1.
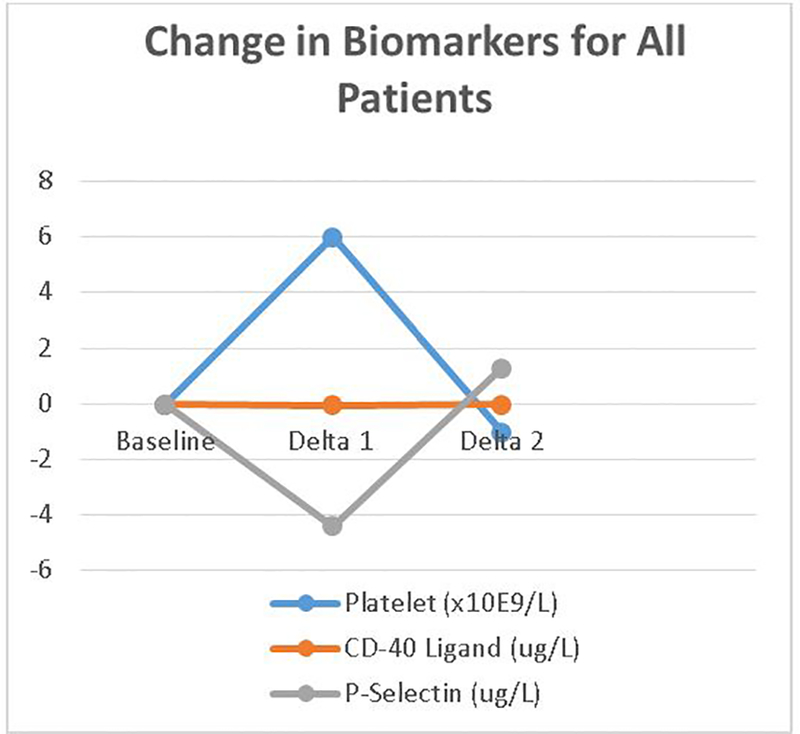
Change of each biomarker over time periods studied. Delta 1 represents value at 1 month minus baseline and delta 2 value at 6 months minus 1 month.
Table 3:
Change of Biomarkers over each Time Period by Treatment Group
| Table 3a. Baseline to 1 Month by Treatments | |||||
|---|---|---|---|---|---|
| Characteristic | Overall Levels at 1 Month | Change in Biomarker Level | |||
| Overall | Clopidogrel | Ticagrelor | p-value | ||
| Platelet (x10E9/L) N: Median (25th-75th) | 5963: 239.0 (200.0 – 288.0) | 5963: 6.0 (−21.0–35.0) | 2985: 1.0 (−23.0–31.0) | 2978: 10.0 (−16.0–39.0) | <.0001 |
| CD40 (ug/L) N: Median (25th-75th) | 1897: 0.35 (0.14 – 0.72) | 1897: −0.04 (−0.27–0.13) | 973: −0.03 (−0.26–0.14) | 924: −0.04 (−0.30–0.12) | 0.5852 |
| P-Selectin (ug/L) N: Median (25th-75th) | 1898: 29.1 (22.5 – 37.3) | 1898: −4.4 (−13.0–3.7) | 974: −4.3 (−13.6–3.1) | 924: −4.4 (−12.6–4.2) | 0.2719 |
| Table 3b. 1 Month to 6 Month by Treatments | |||||
| Characteristic | Overall Levels at 6 Months | Change in Biomarker Level | |||
| Overall | Clopidogrel | Ticagrelor | p-value | ||
| Platelet (x10E9/L) N: Median (25th-75th) | 5963: 237.0 (201.0 – 278.0) | 5963: −1.0 (−27.0–22.0) | 2985: 0.0 (−26.0–21.0) | 2978: −1.0 (−28.0–22.0) | 0.9115 |
| CD40 (ug/L) N: Median (25th-75th) | 1897: 0.31 (0.12 – 0.74) | 1897: −0.01 (−0.22–0.17) | 973: −0.02 (−0.24–0.19) | 924: −0.01 (−0.20–0.17) | 0.7871 |
| P-Selectin (ug/L) N: Median (25th-75th) | 1898: 30.1 (23.1 – 39.3) | 1898: 1.3 (−5.8–8.4) | 974: 1.5 (−5.5–8.4) | 924: 1.2 (−6.1–8.4) | 0.4223 |
Similarly, when comparing biomarker levels for all patients at six months with those at one month, each differed significantly (Table 2; p<0.05). Platelet counts and sCD40 ligand decreased, while sP-selectin increased. However, there was no significant difference in change between treatment groups during this time period (Table 3b).
For each biomarker except sCD40 ligand, there was a significant difference in the rate of change between baseline to 1 month versus the 1 month to 6 month time points (p<0.0001). When the rate of change over both time periods was compared between treatment groups, there was a significantly greater change in platelet count during the acute phase from baseline to 1 month in the ticagrelor group. Both groups showed an increase in platelet count, but the rate of change was greater in patients who received ticagrelor versus clopidogrel with a slope of 17.4 vs. 8.9 (p<0.0001) respectively (Table 4). This difference was no longer observed from 1 month to 6 months.
Table 4:
Rate of Change of Biomarkers over each Time Period
| Biomarkers | Slope (Rate of change per month) | Estimate | 95% Confidence Limit | P value for different slopes | P value for interaction with treatment |
|---|---|---|---|---|---|
| Platelet Count | Slope 1: Baseline to 1M | <.0001 | <.0001 | ||
| Ticagrelor | 17.4040 | (15.316, 19.492) | |||
| Clopidogrel | 8.9605 | (6.8754, 11.046) | |||
| Slope 2: 1Mth to 6M | 0.8663 | ||||
| Ticagrelor | −1.5535 | (−1.971, −1.136) | |||
| Clopidogrel | −1.5028 | (−1.920, −1.086) | |||
| CD40 Ligand | Slope 1: Baseline to 1M | −0.04948 | (−.1118, 0.0128) | 0.1828 | 0.7730 |
| Slope 2: 1Mth to 6M | −0.00233 | (−.0148, 0.0101) | 0.5342 | ||
| P-Selectin | Slope 1: Baseline to 1M | −4.9842 | (−6.039, −3.929) | <.0001 | 0.4251 |
| Slope 2: 1Mth to 6M | 0.3676 | (0.1566, 0.5785) | 0.2076 |
Clinical ischemic-thrombotic and hemorrhagic end point event rates were low overall in the PLATO population between the follow-up time points of 6 months and 1-year (Figure 2). Each biomarker was then assessed against ischemic-thrombotic and hemorrhagic clinical endpoints (Table 5). Platelet change from 1 month to 6 months was associated with ischemic-thrombotic events, including cardiovascular death, all-cause death and the composite of cardiovascular death, myocardial infarction or stroke. sCD40 ligand change from 1 month to 6 months was associated with hemorrhagic events. There were no differences between treatment groups for the associations with endpoints.
Figure 2.
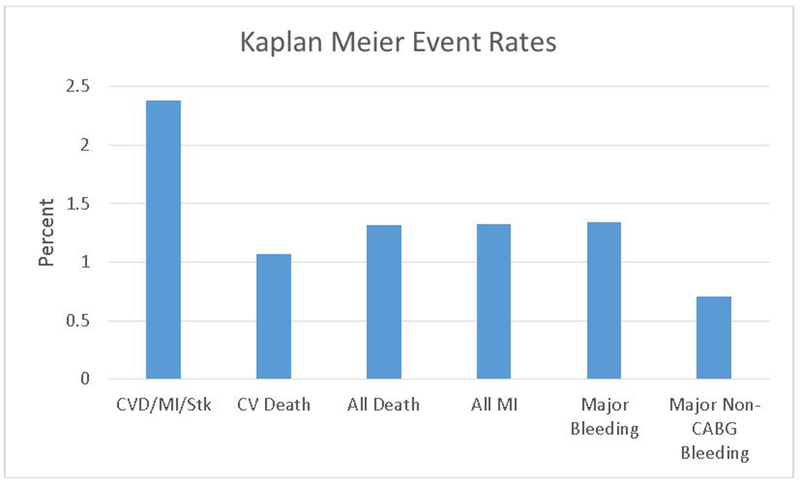
Kaplan-Meier Event rates in the PLATO population between 6 months and 1-year
Table 5:
Changes in Platelet-Related Biomarkers and Their Association with Clinical Outcomes from Cox proportional hazards models
| Outcome | Biomarker changes | Hazard Ratio | 95% Lower Confidence Limit for Hazard Ratio | 95% Upper Confidence Limit for Hazard Ratio | P Value |
|---|---|---|---|---|---|
| CVD/MI/Stk | Platelet:change per 100 from BL to 1M | 1.195 | 0.846 | 1.687 | 0.3119 |
| Platelet:change per 100 from 1m to 6 M | 1.512 | 1.094 | 2.090 | 0.0122 | |
| CD40:change from BL to 1 M | 0.914 | 0.591 | 1.414 | 0.6865 | |
| CD40:change from 1m to 6 M | 1.128 | 0.967 | 1.317 | 0.1252 | |
| Selectin:change per 100 from BL to 1M | 0.106 | 0.009 | 1.297 | 0.0789 | |
| Selectin:change per 100 from 1m to 6 M | 0.494 | 0.077 | 3.181 | 0.4576 | |
| CV Death | Platelet:change per 100 from BL to 1M | 1.265 | 0.804 | 1.989 | 0.3090 |
| Platelet:change per 100 from 1M to 6M | 1.972 | 1.323 | 2.939 | 0.0008 | |
| CD40:change from BL to 1M | 0.683 | 0.343 | 1.359 | 0.2768 | |
| CD40:change from 1M to 6M | 1.180 | 0.946 | 1.472 | 0.1425 | |
| Selectin:change per 100 from BL to 1M | 0.020 | 0.000 | 1.523 | 0.0768 | |
| Selectin:change per 100 from 1M to 6M | 0.226 | 0.009 | 5.873 | 0.3709 | |
| All Cause Death | Platelet:change per 100 from BL to 1M | 1.373 | 0.912 | 2.068 | 0.1287 |
| Platelet:change per 100 from 1M to 6M | 2.232 | 1.547 | 3.219 | <.0001 | |
| CD40:change from BL to 1M | 0.683 | 0.343 | 1.359 | 0.2768 | |
| CD40:change from 1M to 6M | 1.180 | 0.946 | 1.472 | 0.1425 | |
| Selectin:change per 100 from BL to 1M | 0.020 | 0.000 | 1.523 | 0.0768 | |
| Selectin:change per 100 from 1 M to 6M | 0.226 | 0.009 | 5.873 | 0.3709 | |
| MI | Platelet:change per 100 from BL to 1M | 1.247 | 0.778 | 1.999 | 0.3581 |
| Platelet:change per 100 from 1 M to 6M | 1.537 | 0.980 | 2.412 | 0.0612 | |
| CD40:change from BL to 1M | 1.155 | 0.852 | 1.566 | 0.3541 | |
| CD40:change from 1M to 6M | 1.126 | 0.889 | 1.427 | 0.3256 | |
| Selectin:change per 100 from BL to 1M | 1.519 | 0.111 | 20.798 | 0.7541 | |
| Selectin:change per 100 from 1M to 6M | 1.337 | 0.205 | 8.700 | 0.7614 | |
| Major Bleeding | Platelet:change per 100 from BL to 1M | 1.390 | 0.882 | 2.188 | 0.1556 |
| Platelet:change per 100 from 1M to 6M | 1.661 | 1.047 | 2.634 | 0.0312 | |
| CD40:change from BL to 1M | 0.929 | 0.481 | 1.794 | 0.8269 | |
| CD40:change from 1M to 6M | 1.396 | 1.174 | 1.660 | 0.0002 | |
| Selectin:change per 100 from BL to 1M | 0.583 | 0.031 | 10.907 | 0.7183 | |
| Selectin:change per 100 from 1M to 6M | 1.236 | 0.193 | 7.927 | 0.8230 | |
| Non-CABG Major Bleeding | Platelet:change per 100 from BL to 1M | 2.224 | 1.357 | 3.645 | 0.0015 |
| Platelet:change per 100 from 1M to 6M | 1.669 | 0.996 | 2.797 | 0.0518 | |
| CD40:change from BL to 1M | 0.322 | 0.047 | 2.188 | 0.2462 | |
| CD40:change from 1M to 6M | 1.299 | 1.058 | 1.594 | 0.0124 | |
| Selectin:change per 100 from BL to 1M | 0.257 | 0.003 | 25.705 | 0.5635 | |
| Selectin:change per 100 from 1M to 6M | 0.385 | 0.006 | 26.732 | 0.6591 |
Adjustments made for baseline biomarkers, baseline treatment, on/off drug at 6 month and all the covariates in baseline model.
Discussion
Our study was an exploratory analysis of platelet-related biomarkers over time among patients with ACS. While there were dynamic changes, only platelet count was associated with ischemic-thrombotic events. The decrease in platelet count between the 1 and 6 month time points was associated with ischemic-thrombotic events but not with higher rates of bleeding. sCD40 ligand correlated directly with hemorrhagic events.
Because ticagrelor is a more potent platelet inhibitor than clopidogrel[3], one might hypothesize that it would elicit a more robust platelet adaptive response. While the rate of increase (slope) of platelet count was greater in the first month of treatment, whether this represents a marker of platelet adaptation is unknown. A prior analysis of data from the PLATO trial revealed that, in addition to lower platelet counts, there were lower levels of leukocytes and neutrophil sub-fractions in the clopidogrel group compared to the ticagrelor group[6]. This may reflect an off-target effect of the medication on bone marrow-derived progenitor cells in the clopidogrel group. As previously reported, the observations are not consistent with any off-target inhibitory effect of ticagrelor on the P2Y13 receptors, which are involved in pro-platelet formation, since this would be expected to lead to a lower platelet count compared to clopidogrel treatment[7].
The general concept of platelet adaptation in response to pharmacologic inhibition is biologically and teleologically plausible-perhaps even necessary to maintain hemostatic capacity. One might anticipate a platelet adaptome originating in megakaryocytes given their diversity and ability to respond at the molecular level to systemic conditions. Indeed, megakaryocyte adaptation has been observed after treatment with aspirin. Following administration of aspirin, there is an enhancement of the MRP4 expression, leading to decreased platelet inhibition by aspirin[8]. This does not exclude a platelet-specific adaptive transcriptome or proteome. In patients treated with aspirin daily, changes in platelet gene expression are seen as early as 2 weeks after initiation[2]. When inhibition of platelet reactivity is compared in patients after two months of treatment and two years of treatment, platelet aggregation is significantly higher at two years[9]. Similarly, impaired platelet inhibition to aspirin has been associated with platelet-specific genes and pathways that would predictably blunt the response to aspirin[10]. The aspirin response signature (ARS) is a group of 60 co-expressed genes, primarily of platelet origin, which has been shown to correlate with platelet function. Although the genes are similarly co-expressed both before and after aspirin administration, the ARS was found to only correspond with the platelet function score following treatment with aspirin. This suggests an adaptive mechanism wherein the ARS genes are only apparent in the presence of COX-1 inhibition or aspirin causes a change in the genomics and protein signature of platelets.
Preservation of platelet function has also been studied in murine P2Y12 knock-out and heterozygous knock-down models. In vivo studies revealed an inability to form an occlusive thrombus, decreased thrombus stability and increased bleeding times in knock-out mice while heterozygous mice had preserved bleeding times[11, 12]. In humans with deficiency of platelet P2Y12 receptors or loss-of-function mutations of the P2Y12 receptor gene, there is typically a mild bleeding phenotype[11, 12]. Given these observations, several different in vivo experiments have been performed to better understand the underlying mechanism(s) of adaptation. For example, while knock-out mice have decreased response to collagen and murine-TRAP, at high concentrations the response is similar to wild-type mice indicating a shift, rather than a lack, in the concentration-response. In addition, heterozygous mice have been found to have similar repression of cAMP levels by ADP to wild-type mice; thus even 50% of the level of the P2Y12 receptor remains sufficient to mediate inhibition of adenylyl cyclase, indicating presence of an innate redundancy[12].
The PLATO sub-study cohort and serial sampling provided an opportunity to test, in principle, our exploratory hypothesis. However, we acknowledge several limitations. While sCD40 ligand and sP-selectin are markers of platelet activation, they are not platelet-specific. sCD40 ligand is present within activated platelets and T cells; changes in levels can be seen in a number of clinical conditions characterized by inflammation and thrombosis; and the clinical utility of these measures is unknown. The optimal platelet-related biomarker to determine adaptive responses to either short-or long-term pharmacologic inhibition is yet to be determined, but is likely of megakaryocyte origin and is reflected in the platelet proteome or secretome.
In conclusion, dynamic changes in platelet count, CD-40 ligand and soluble P-selectin occur over time among patients with ACS. Platelet count and sCD40 ligand may offer modest overall predictive value for future ischemic-thrombotic or hemorrhagic clinical events, respectively. Platelet-directed therapy with a P2Y12 receptor inhibitor in combination with aspirin modestly impacts the expression of these biomarkers. The existence of a platelet adaptome and its overall clinical significance among patients at risk for thrombotic events will require a more in-depth and platelet-biology specific investigation.
Funding
The PLATO study was funded by AstraZeneca. Support for the analysis and interpretation of the results and preparation for the manuscript was provided through funds to the Uppsala Clinical Research Center and Duke Clinical Research Institute as part of the Clinical Study Agreement.
Footnotes
Conflict of Interest
RFS: institutional research grants, consultancy fees, honoraria and travel support from AstraZeneca; consultancy fees from Aspen, PlaqueTec, The Medicines Company, ThermoFisher Scientific, Correvio, Bayer; travel support from Medtronic.
DJA: has received payment as an individual for: a) Consulting fee or honorarium from Amgen, Bayer, Sanofi, Eli Lilly, Daiichi-Sankyo, The Medicines Company, AstraZeneca, Merck, Pfizer, Abbott Vascular and PLx Pharma; b) Participation in review activities from CeloNova, Johnson & Johnson, St. Jude Medical. Institutional payments for grants from GlaxoSmithKline, Eli Lilly, Daiichi-Sankyo, The Medicines Company, AstraZeneca, Janssen Pharmaceuticals, Inc., Osprey Medical, Inc., Novartis, CSL Behring, Gilead.
CPC: grants and personal fees from Amgen, Arisaph, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck, and Takeda; personal fees from AstraZeneca, GlaxoSmithKline, Kowa, Lipimedix, Pfizer, Regeneron, Sanofi, Janssen; grants from Daiichi-Sankyo, Janssen.
AH: reports being an employee of AstraZeneca.
KH: lecture fees and research grant from AstraZeneca; lecture fees from Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Bayer, Daiichi Sankyo, Sanofi Aventis, The Medicines Company.
SKJ: institutional research grant, honoraria and consultant/advisory board fee from AstraZeneca; institutional research grant and consultant/advisory board fee from Medtronic; institutional research grants and honoraria from The Medicines Company; consultant/advisory board fees from Janssen, Bayer.
HAK: personal fees from AstraZeneca, Bayer Vital, Roche Diagnostics.
JM: research grant from Servier, consultant and speaker fees from AstraZeneca, Bayer Healthcare, Merck Sharp & Dohme, Boehringer Ingelheim, Jaba Recordati, Pfizer/Bristol-Myers Squibb, Daiichi Sankyo; speaker fees from Amgen, AstraZeneca.
AS: institutional research grants from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer and GlaxoSmithKline.
PGS: research grant and speaking, or consulting fees from Merck, Sanofi, Servier; speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL-Behring, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Regeneron, The Medicines Company.
LW: institutional research grants, consultancy fees, lecture fees, and travel support from Bristol-Myers Squibb/Pfizer, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim; institutional research grants from Merck & Co, Roche; consultancy fees from Abbott; holds two patents involving GDF-15.
RCB: scientific advisory board member for Janssen, Ionis Pharmaceuticals, and AstraZeneca; safety reviewing committee member for Portola.
AL, MN, J-LA: nothing to disclose.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References:
- 1.Gachet C, P2 receptors, platelet function and pharmacological implications. Thromb Haemost, 2008. 99(3): p. 466–72. [DOI] [PubMed] [Google Scholar]
- 2.Voora D, et al. , Time-dependent changes in non-COX-1-dependent platelet function with daily aspirin therapy. J Thromb Thrombolysis, 2012. 33(3): p. 246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Storey RF, et al. , Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol, 2010. 56(18): p. 1456–62. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, et al. , Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med, 2009. 361(11): p. 1045–57. [DOI] [PubMed] [Google Scholar]
- 5.James S, et al. , Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: Rationale, design, and baseline characteristics of the PLATelet inhibition and patient Outcomes (PLATO) trial. Am Heart J, 2009. 157(4): p. 599–605. [DOI] [PubMed] [Google Scholar]
- 6.Storey RF, et al. , Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets, 2014. 25(7): p. 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorquist A, et al. , Studies of the interaction of ticagrelor with the P2Y13 receptor and with P2Y13-dependent pro-platelet formation by human megakaryocytes. Thromb Haemost, 2016. 116(6): p. 1079–1088. [DOI] [PubMed] [Google Scholar]
- 8.Massimi I, et al. , Aspirin influences megakaryocytic gene expression leading to up-regulation of multidrug resistance protein-4 in human platelets. Br J Clin Pharmacol, 2014. 78(6): p. 1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulcinelli FM, et al. , Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol, 2004. 43(6): p. 979–84. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo M, et al. , Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci U S A, 2003. 100(4): p. 1978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiraga M, et al. , Impaired platelet function in a patient with P2Y12 deficiency caused by a mutation in the translation initiation codon. J Thromb Haemost, 2005. 3(10): p. 2315–23. [DOI] [PubMed] [Google Scholar]
- 12.Nergiz-Unal R, et al. , Stabilizing role of platelet P2Y(12) receptors in shear-dependent thrombus formation on ruptured plaques. PLoS One, 2010. 5(4): p. e10130. [DOI] [PMC free article] [PubMed] [Google Scholar]


