Significance
Little is known about the neural dynamics underlying previously reported associations of organophosphate (OP) pesticides with adverse neurodevelopment. We used functional near-infrared spectroscopy (fNIRS) to examine cortical brain activation in relation to residential proximity to OP use during pregnancy among 95 adolescents enrolled in a longitudinal birth cohort. We found that prenatal OP exposure was associated with altered brain activation during tasks of executive function. We also found sex differences for OPs and brain activation during a language comprehension task. Use of fNIRS, an inexpensive and easily accessible technology, enhances our efforts to assess the impact of environmental exposures on brain function.
Keywords: organophosphates, prenatal exposure, neurodevelopment, functional neuroimaging, fNIRS
Abstract
We have reported consistent associations of prenatal organophosphate pesticide (OP) exposure with poorer cognitive function and behavior problems in our Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a birth cohort of Mexican American youth in California’s agricultural Salinas Valley. However, there is little evidence on how OPs affect neural dynamics underlying associations. We used functional near-infrared spectroscopy (fNIRS) to measure cortical activation during tasks of executive function, attention, social cognition, and language comprehension in 95 adolescent CHAMACOS participants. We estimated associations of residential proximity to OP use during pregnancy with cortical activation in frontal, temporal, and parietal regions using multiple regression models, adjusting for sociodemographic characteristics. OP exposure was associated with altered brain activation during tasks of executive function. For example, with a 10-fold increase in total OP pesticide use within 1 km of maternal residence during pregnancy, there was a bilateral decrease in brain activation in the prefrontal cortex during a cognitive flexibility task (β = −4.74; 95% CI: −8.18, −1.31 and β = −4.40; 95% CI: −7.96, −0.84 for the left and right hemispheres, respectively). We also found that prenatal OP exposure was associated with sex differences in brain activation during a language comprehension task. This first functional neuroimaging study of prenatal OP exposure suggests that pesticides may impact cortical brain activation, which could underlie previously reported OP-related associations with cognitive and behavioral function. Use of fNIRS in environmental epidemiology offers a practical alternative to neuroimaging technologies and enhances our efforts to assess the impact of chemical exposures on neurodevelopment.
Over 800 million pounds of pesticide active ingredients are applied in the United States each year, with organophosphates (OPs) the most commonly applied class of insecticides (1). Exposure to OP pesticides, which are endocrine-disrupting compounds (2), is widespread in the US population, including among pregnant women and children (3–6). The predominant route of exposure to OP pesticides is diet, including pesticide residues on fruits and vegetables (7). Individuals living in proximity to agriculture or living with individuals working in agricultural settings are also exposed to pesticides via other routes, including from residue on clothing and drift from nearby fields (8, 9).
The Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study is a longitudinal cohort study based in California’s Salinas Valley, known as “America’s Salad Bowl” for its agricultural production. Since 1999, we have followed CHAMACOS mothers and children to investigate pesticides as they relate to childhood growth and development. We previously reported associations of children’s prenatal OP pesticide exposure, measured as dialkyl phosphate (DAP) metabolites in their mothers’ pregnancy urine samples—a matrix that primarily reflects dietary OP exposure (10)—with a number of neurodevelopmental outcomes, including poorer cognitive development, attention problems, and autistic traits such as poor social cognition (11–14). We have also reported that higher amounts of OP pesticides applied to agricultural crops within 1 km of mothers’ homes during pregnancy—a matrix which reflects potential pesticide drift exposure (15)—are associated with poorer intellectual development (15, 16), although not with autistic traits (14).
The observed associations between prenatal OP exposure and neurobehavioral outcomes imply that OPs impact children’s brain structure and/or function. To date, the only neuroimaging study that explored this hypothesis is a volumetric MRI study conducted on 40 school-age children: 20 with high and 20 with low prenatal exposure to the OP insecticide chlorpyrifos. This study reported systematic volume differences for higher-exposed versus lower-exposed children in the brain structures summarized in the first column of Table 1 (17). In this table, we parallel these structural neuroimaging findings with domains associated with prenatal OP exposure in epidemiologic studies that have used neuropsychological testing, including CHAMACOS (11–13) and other cohorts (18–20) (Table 1, column 2).
Table 1.
Regions of interest for fNIRS study based on structures identified in an MRI study of chlorpyrifos-exposed children and epidemiologic studies of OPs and neurodevelopment
| Brain structures associated with chlorpyrifos from MRI study (17) | Domains associated with OPs in epidemiologic studies with neuropsychological testing | Regions of interest for fNIRS |
| Posterior temporal regions (enlarged) | Attention (12, 13, 19), language comprehension (11, 15, 18, 20) | Temporal, parietal |
| Superior temporal gyrus, medial superior frontal gyrus, cuneus and procuneus (enlarged) | Social cognition (12), language comprehension (11, 15, 18, 20) | Temporal, frontal, parietal |
| Gyrus rectus, orbitofrontal regions (enlarged) | Response inhibition (13), working memory (11, 18, 20) | Frontal |
| Dorsal and medial surfaces of the superior frontal gyrus (inward deformations) | Working memory (11, 18, 20) | Frontal |
| Prefrontal cortex (reduced cortical thickness) | Attention (12, 13, 19), working memory (11, 18, 20), response inhibition (13) | Frontal |
| Dorsal parietal (reduced cortical thickness) | Visuospatial (11, 18, 20) | Parietal |
| Orbitofrontal (reduced cortical thickness) | Working memory (11, 18, 20) | Frontal |
To investigate OP-related effects on cortical brain activation, and to localize these effects, we conducted functional neuroimaging with a subset of 95 adolescent CHAMACOS participants using functional near-infrared spectroscopy (fNIRS), a technology well-suited to a nonclinical research environment (21) that correlates well with functional MRI (fMRI) (22, 23). Our primary objective was to examine neural activity during tasks of cognitive flexibility, working memory, attention, social cognition, and language comprehension in relation to exposure to OP pesticides applied to agricultural crops in close proximity to mothers’ homes during pregnancy. Results from this preliminary research of 95 participants form the basis for conducting neuroimaging among the full CHAMACOS cohort of ∼600 participants.
Results
Table 2 shows sociodemographic data for the n = 95 participants who participated in fNIRS data collection compared to those who did not (n = 214). Similar to those without fNIRS, most fNIRS participants were born to mothers who did not graduate high school (70.5%) and to families at or below the poverty level (74.7%). fNIRS participants were more likely to be born to older mothers (46.3% were age 30+ y at delivery) compared with nonparticipants (24.7%). The vast majority of fNIRS participants were right hand-dominant (93.7%) and did not report alcohol or marijuana use in the 24 h prior to their fNIRS (91.6%).
Table 2.
Characteristics of n = 95 participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| fNIRS participants (n = 95) | Nonparticipants (n = 214) | |||
| Characteristics | n | (%) | n | (%) |
| Maternal age at delivery,* y | ||||
| 17–24 | 32 | (33.7) | 93 | (44.3) |
| 25–29 | 19 | (20.0) | 65 | (31.0) |
| 30–34 | 27 | (28.4) | 36 | (17.1) |
| 35–45 | 17 | (17.9) | 16 | (7.6) |
| Maternal education† | ||||
| ≤Sixth grade | 41 | (43.1) | 81 | (39.1) |
| Some middle/high school | 26 | (27.4) | 69 | (33.3) |
| High school graduate | 28 | (29.5) | 57 | (27.5) |
| Family poverty level at 14 y | ||||
| ≤100% federal poverty level | 71 | (74.7) | 155 | (77.1) |
| >100% federal poverty level | 24 | (25.3) | 46 | (22.9) |
| Child’s sex | ||||
| Female | 49 | (51.6) | 97 | (45.3) |
| Male | 46 | (48.4) | 117 | (54.7) |
| Child’s age at assessment, y | ||||
| 15 | 54 | (56.8) | N/A | |
| 16 | 40 | (42.1) | ||
| 17 | 1 | (1.1) | ||
| Child’s handedness | ||||
| Right | 89 | (93.7) | N/A | |
| Left | 6 | (6.3) | ||
| Child’s self-reported substance use (alcohol or marijuana use) in previous 24 h of fNIRS | ||||
| No | 87 | (91.6) | N/A | |
| Yes | 8 | (8.4) | ||
N/A, not applicable.
Distribution different between participants and nonparticipants (P < 0.01).
Imputed maternal education for 1 participant using data collected at another time point.
We present estimates of exposure to OP pesticides as wind-adjusted kilograms of OP pesticide use within a 1-km radius of maternal residence during pregnancy in Table 3. Diazinon use was substantially higher than any of the other individual OPs, followed by malathion. Spearman correlation coefficients between these individual pesticides ranged from moderate to high (0.19 to 0.84) (Table 3).
Table 3.
OP pesticide use (wind-adjusted kilograms) within a 1-km radius of maternal residence during pregnancy from the California Pesticide Use Reporting program for n = 95 participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| Spearman correlation coefficients | |||||||||||
| Exposure, kg | Mean | (SD) | P25 | P50 | P75 | Total OPs | Acephate | Chlorpyrifos | Diazinon | Malathion | Oxydementon-methyl |
| Total OPs | 56.4 | (147.5) | 2.2 | 13.8 | 41.2 | 1 | 0.79 | 0.68 | 0.90 | 0.55 | 0.79 |
| Acephate | 6.1 | (13.0) | 0.2 | 1.5 | 4.3 | 1 | 0.67 | 0.74 | 0.30 | 0.84 | |
| Chlorpyrifos | 6.4 | (17.0) | 0.2 | 0.8 | 4.3 | 1 | 0.76 | 0.19 | 0.76 | ||
| Diazinon | 15.7 | (47.2) | 0.7 | 3.3 | 8.4 | 1 | 0.44 | 0.76 | |||
| Malathion | 8.1 | (25.2) | 0.0 | 0.4 | 3.0 | 1 | 0.24 | ||||
| Oxydemeton-methyl | 6.8 | (23.1) | 0.2 | 1.4 | 3.4 | 1 | |||||
OPs and fNIRS Activation.
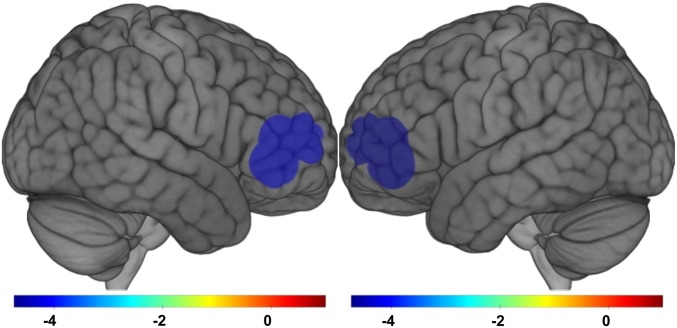
We present associations of total OP pesticide exposure and measures of brain activation, measured with fNIRS, for the 6 tasks in Tables 4 and 5. We found that with every 10-fold increase in total OP pesticide use within a 1-km radius of maternal residence during pregnancy there was a bilateral decrease in brain activation in the inferior frontal poles of the prefrontal cortex (localization clusters 1 and 9) during the Wisconsin Card Sorting Test (covariate-adjusted but not false discovery rate [FDR]-corrected β = −4.74; 95% CI: −8.18, −1.31 and β = −4.40; 95% CI: −7.96, −0.84 for the left and right hemispheres, respectively) (Table 4 and Fig. 1). There was also slightly lower bilateral activation across other regions of the frontal, temporal, and parietal lobes during the Wisconsin Card Sorting Test. We found similar, albeit much weaker, decreases in activation in regions of the frontal lobe during the visuospatial working memory N-back task, also in the inferior frontal poles of the prefrontal cortex (β = −2.27; 95% CI: −4.82, 0.28 and β = −2.78; 95% CI: −5.58, 0.02 for the left and right hemispheres, respectively) (Table 4). In contrast to the Wisconsin and N-back task findings, we found increased activation for the Sternberg working memory task, with significantly higher activation in the left superior parietal lobe (β = 4.00; 95% CI: 0.90, 7.10) and the right posterior superior/middle temporal sulcus (β = 2.21; 95% CI: 0.03, 4.38) (Table 4). We found no strong or consistent associations of OP pesticides with brain activation during the other 3 tasks (attention/impulsivity, language comprehension, and social cognition), with effect estimates all hovering at the null (Table 5). There were no statistically significant associations after adjusting for multiple comparisons with FDR correction. In addition, effect sizes estimated with the f2 were all in the small range (<0.15). Associations were more or less the same for individual OPs (SI Appendix, Tables S1–S5).
Table 4.
Adjusted* associations for a 10-fold increase in of total OP pesticide use within a 1-km radius of maternal residence during pregnancy and fNIRS brain activation by task and region of interest for cognitive flexibility and working memory tasks among participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| Region (localization cluster) | Wisconsin Card Sort (cognitive flexibility) | Sternberg (letter-retrieval working memory) | N-back (visuospatial working memory) | |||||||||
| n | β (95% CI)* | f2 | n | β (95% CI)* | f2 | n | β (95% CI)* | f2 | ||||
| Left hemisphere | ||||||||||||
| Inferior frontal pole (1) | 91 | −4.74 | (−8.18, −1.31)† | 0.09 | 87 | −0.59 | (−3.43, 2.26) | <0.01 | 93 | −2.27 | (−4.82, 0.28) | 0.04 |
| Superior frontal pole (2) | 89 | −1.79 | (−4.91, 1.32) | 0.02 | 80 | 2.28 | (−0.71, 5.27) | 0.03 | 86 | −1.62 | (−3.97, 0.74) | 0.03 |
| Broca’s/BA 44/45 (3) | 90 | −2.98 | (−6.81, 0.86) | 0.03 | 85 | 1.58 | (−0.94, 4.09) | 0.02 | 90 | −0.03 | (−3.14, 3.09) | <0.01 |
| Dorsolateral PFC (4) | 90 | −2.17 | (−5.70, 1.35) | 0.02 | 85 | 2.34 | (−0.38, 5.05) | 0.04 | 88 | −1.49 | (−4.06, 1.07) | 0.02 |
| Broca’s/BA 44 and 6 (5) | 92 | −3.48 | (−7.34, 0.39) | 0.04 | 88 | 0.97 | (−1.69, 3.64) | <0.01 | 92 | 0.34 | (−2.98, 3.66) | <0.01 |
| Superior/inferior temporal /postcentral gyrus (6) | 92 | −2.54 | (−6.22, 1.14) | 0.02 | 88 | 1.18 | (−0.83, 3.19) | 0.02 | 93 | −1.11 | (−4.42, 2.19) | <0.01 |
| Inferior parietal lobule (7) | 92 | −1.81 | (−4.65, 1.02) | 0.02 | 88 | 2.07 | (−0.95, 5.10) | 0.02 | 91 | 1.55 | (−0.71, 3.82) | 0.02 |
| Superior parietal lobule (8) | 87 | −0.51 | (−3.49, 2.46) | <0.01 | 81 | 4.00 | (0.90, 7.10)† | 0.09 | 86 | 0.57 | (−1.43, 2.57) | <0.01 |
| Right hemisphere | ||||||||||||
| Inferior frontal pole (9) | 92 | −4.40 | (−7.96, −0.84)† | 0.07 | 87 | 0.81 | (−2.33, 3.94) | <0.01 | 93 | −2.78 | (−5.58, 0.02) | 0.05 |
| Broca’s/BA 44/45 (10) | 91 | −2.93 | (−6.86, 0.99) | 0.03 | 84 | 0.44 | (−2.22, 3.10) | <0.01 | 93 | −0.23 | (−3.26, 2.80) | <0.01 |
| Superior frontal pole/dorsolateral PFC (11) | 90 | −1.27 | (−4.42, 1.88) | <0.01 | 82 | −0.20 | (−3.22, 2.81) | <0.01 | 92 | −0.67 | (−3.10, 1.75) | <0.01 |
| Premotor/somatosensory cortex (12) | 92 | −2.39 | (−5.94, 1.17) | 0.02 | 87 | 1.04 | (−1.79, 3.86) | <0.01 | 94 | −1.26 | (−3.92, 1.40) | 0.01 |
| Posterior superior/middle temporal sulcus (13) | 92 | −2.78 | (−6.16, 0.61) | 0.03 | 88 | 2.21 | (0.03, 4.38)† | 0.05 | 93 | 0.12 | (−2.24, 2.48) | <0.01 |
| Inferior parietal lobule (14) | 90 | −1.47 | (−4.07, 1.13) | 0.02 | 87 | 1.13 | (−1.76, 4.03) | <0.01 | 93 | 0.07 | (−2.37, 2.51) | <0.01 |
| Superior parietal lobule (15) | 85 | −0.01 | (−2.87, 2.85) | <0.01 | 73 | 2.38 | (−1.29, 6.04) | 0.03 | 84 | −0.99 | (−3.37, 1.40) | <0.01 |
Abbreviations: BA = Brodmann areas, PFC = prefrontal cortex.
Adjusted for age of child at assessment (continuous variable), child’s sex, maternal age at delivery (continuous), maternal education at delivery (<sixth grade, 7th to 12th grade, completed high school), and quality of the home environment at the 10.5-y visit (continuous HOME z-score).
Non-FDR-corrected P < 0.05.
Table 5.
Adjusted* associations for a 10-fold increase in total OP pesticide use within a 1-km radius of maternal residence during pregnancy and fNIRS brain activation by task and region of interest for attention/impulse control, semantic language, and social cognition tasks among participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| Go/No-Go (attention/impulsivity) | Pyramids and Palm Trees (semantic language) | Dynamic Social Gestures (social cognition) | ||||||||||
| Region (localization cluster) | n | β (95% CI)* | f2 | n | β (95% CI)* | f2 | n | β (95% CI)* | f2 | |||
| Left hemisphere | ||||||||||||
| Inferior frontal pole (1) | 94 | −1.29 | (−3.54, 0.97) | 0.02 | 93 | 0.33 | (−1.97, 2.62) | <0.01 | 91 | −0.40 | (−1.69, 0.89) | <0.01 |
| Superior frontal pole (2) | 83 | −0.61 | (−2.88, 1.66) | <0.01 | 90 | −1.17 | (−3.43, 1.08) | 0.01 | 90 | −1.25 | (−2.54, 0.05) | 0.05 |
| Broca’s/BA 44/45 (3) | 92 | 0.18 | (−2.25, 2.61) | <0.01 | 91 | 0.40 | (−1.93, 2.72) | <0.01 | 92 | 0.80 | (−0.50, 2.10) | 0.02 |
| Dorsolateral PFC (4) | 92 | −0.77 | (−2.80, 1.26) | <0.01 | 90 | −0.34 | (−2.35, 1.66) | <0.01 | 92 | −0.12 | (−1.34, 1.11) | <0.01 |
| Broca’s/BA 44 and 6 (5) | 93 | −0.08 | (−2.55, 2.38) | <0.01 | 94 | 0.38 | (−1.81, 2.57) | 0.01 | 94 | 0.39 | (−0.80, 1.58) | <0.01 |
| Superior/inferior temporal /postcentral gyrus (6) | 94 | −1.11 | (−3.36, 1.15) | 0.01 | 93 | 1.26 | (−1.09, 3.61) | <0.01 | 93 | 0.66 | (−0.67, 2.00) | 0.01 |
| Inferior parietal lobule (7) | 92 | −0.49 | (−2.40, 1.42) | <0.01 | 92 | −0.36 | (−2.17, 1.46) | <0.01 | 94 | −0.83 | (−2.07, 0.42) | 0.02 |
| Superior parietal lobule (8) | 85 | 0.09 | (−1.96, 2.14) | <0.01 | 83 | −1.04 | (−2.68, 0.60) | 0.02 | 84 | −1.08 | (−2.24, 0.08) | 0.05 |
| Right hemisphere | ||||||||||||
| Inferior frontal pole (9) | 93 | −0.75 | (−2.91, 1.40) | <0.01 | 94 | 0.35 | (−1.80, 2.50) | <0.01 | 93 | 0.33 | (−0.88, 1.54) | <0.01 |
| Broca’s/BA 44/45 (10) | 92 | −0.80 | (−3.13, 1.52) | <0.01 | 91 | 0.48 | (−1.99, 2.96) | <0.01 | 91 | 0.95 | (−0.43, 2.32) | 0.02 |
| Superior frontal pole /dorsolateral PFC (11) | 93 | −1.50 | (−3.59, 0.59) | 0.02 | 92 | −1.12 | (−3.05, 0.81) | 0.02 | 84 | −1.00 | (−2.21, 0.22) | 0.04 |
| Premotor/somatosensory cortex (12) | 93 | −0.86 | (−2.92, 1.21) | <0.01 | 94 | 0.35 | (−1.86, 2.56) | <0.01 | 94 | −0.09 | (−1.36, 1.19) | <0.01 |
| Posterior superior/middle temporal sulcus (13) | 94 | −0.16 | (−2.30, 1.98) | <0.01 | 94 | −0.31 | (−2.44, 1.81) | <0.01 | 94 | −0.04 | (−1.28, 1.20) | <0.01 |
| Inferior parietal lobule (14) | 93 | −1.44 | (−3.53, 0.66) | 0.02 | 93 | −1.18 | (−3.02, 0.67) | 0.02 | 94 | −1.00 | (−2.19, 0.18) | 0.03 |
| Superior parietal lobule (15) | 85 | 0.21 | (−1.77, 2.19) | <0.01 | 85 | −1.36 | (−2.99, 0.27) | 0.04 | 87 | −1.50 | (−2.56, −0.44)† | 0.10 |
Abbreviations: BA = Brodmann Areas, PFC = prefrontal cortex.
Adjusted for age of child at assessment (continuous variable), child’s sex, maternal age at delivery (continuous), maternal education at delivery (<sixth grade, 7th to 12th grade, completed high school), and quality of the home environment at the 10.5-y visit (continuous HOME z-score).
Non-FDR-corrected P < 0.05.
Fig. 1.
Regions with significant (non-FDR corrected P < 0.05) associations of total OPs with brain activation (reduced activation) during the Wisconsin Card Sorting Test for n = 95 participants of the CHAM2 study, enrolled 2009 in Salinas Valley, California.
We found predominantly null associations for prenatal total OP use and performance (e.g., accuracy, errors, and reaction time) on the tasks administered with the fNIRS (SI Appendix, Table S6). The 1 exception was for Sternberg letter-retrieval working memory, where we observed that higher prenatal total OP use was associated with slightly longer reaction time (β = 0.25; 95% CI: 0.06, 0.45).
When we stratified OP–fNIRS associations by task performance, we observed differences only for the Wisconsin Card Sorting Test. As shown in Table 6 and SI Appendix, Fig. S1, we found that OP–fNIRS associations were much stronger among high performers (fewer total and perseverative errors) vs. low performers. For example, for the left inferior frontal pole, brain activation was reduced by 10.84 (95% CI: −16.01, −5.66) per 10-fold increase in prenatal OP use among high performers vs. only a reduction of 1.35 (95% CI: −6.26, 3.55) among low performers (Table 6). A number of these estimates remained statistically significant after adjusting for multiple comparisons (FDR-corrected P value <0.05). We did not see a strong or consistent pattern of differences in OP–fNIRS associations across performance for any of the other fNIRS tasks (SI Appendix, Tables S7–S9).
Table 6.
Adjusted* associations for a 10-fold increase in total OP pesticide use within a 1-km radius of maternal residence during pregnancy and fNIRS brain activation during the Wisconsin Card Sorting Test, stratified by test performance (dichotomized at the median for perseverative errors [median = 2] and total errors [median = 24]), by region of interest for participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| Total errors | Perseverative errors | |||||||||||
| High performers | Low performers | High performers | Low performers | |||||||||
| Region (localization cluster) | n | β (95% CI)* | n | β (95% CI)* | n | β (95% CI)* | n | β (95% CI)* | ||||
| Left hemisphere | ||||||||||||
| Inferior frontal pole (1) | 46 | −10.84 | (−16.01, −5.66)†, ‡ | 45 | −1.35 | (−6.26, 3.55) | 40 | −8.57 | (−14.85, −2.29)†, ‡ | 51 | −2.01 | (−6.07, 2.05) |
| Superior frontal pole (2) | 44 | −6.90 | (−11.95, −1.84)†, ‡ | 45 | 0.51 | (−3.46, 4.48) | 38 | −3.78 | (−9.26, 1.69) | 51 | 0.61 | (−2.95, 4.17) |
| Broca’s/BA 44/45 (3) | 45 | −9.06 | (−14.56, −3.57)†, ‡ | 45 | 2.49 | (−3.24, 8.23) | 39 | −8.63 | (−14.96, −2.31)†, ‡ | 51 | 1.74 | (−3.17, 6.64) |
| Dorsolateral PFC (4) | 46 | −6.84 | (−12.76, −0.91)†, ‡ | 44 | 1.16 | (−3.55, 5.88) | 40 | −4.90 | (−11.59, 1.78) | 50 | 0.79 | (−3.24, 4.82) |
| Broca’s/BA 44 and 6 (5) | 46 | −9.97 | (−15.49, −4.45)†, ‡ | 46 | 2.55 | (−3.24, 8.34) | 40 | −10.98 | (−17.41, −4.55)†, ‡ | 52 | 2.37 | (−2.28, 7.03) |
| Superior/inferior temporal /postcentral gyrus (6) | 46 | −8.76 | (−13.76, −3.75)†, ‡ | 46 | 2.58 | (−3.17, 8.33) | 40 | −8.76 | (−14.28, −3.24)†, ‡ | 52 | 2.52 | (−2.17, 7.21) |
| Inferior parietal lobule (7) | 46 | −4.52 | (−8.78, −0.26)†, ‡ | 46 | 0.07 | (−4.15, 4.30) | 40 | −2.37 | (−7.44, 2.71) | 52 | −0.78 | (−4.29, 2.72) |
| Superior parietal lobule (8) | 46 | −1.83 | (−6.60, 2.95) | 41 | −0.77 | (−5.25, 3.72) | 40 | −1.37 | (−6.62, 3.87) | 47 | 0.46 | (−3.41, 4.33) |
| Right hemisphere | ||||||||||||
| Inferior frontal pole (9) | 46 | −9.83 | (−14.77, −4.89)†, ‡ | 46 | −1.23 | (−6.86, 4.39) | 40 | −7.44 | (−13.41, −1.48)†, ‡ | 52 | −1.92 | (−6.31, 2.48) |
| Broca’s/BA 44/45 (10) | 45 | −8.26 | (−14.10, −2.43)†, ‡ | 46 | 1.39 | (−4.42, 7.20) | 40 | −7.07 | (−13.48, −0.65) | 51 | 0.21 | (−4.54, 4.97) |
| Superior frontal pole /dorsolateral PFC (11) | 45 | −6.08 | (−10.90, −1.26)†, ‡ | 45 | 0.88 | (−3.37, 5.13) | 40 | −2.67 | (−8.25, 2.92) | 50 | 0.04 | (−3.56, 3.65) |
| Premotor/somatosensory cortex (12) | 46 | −7.97 | (−13.63, −2.31)†, ‡ | 46 | 2.09 | (−2.96, 7.14) | 40 | −4.27 | (−10.20, 1.65) | 52 | 0.17 | (−4.21, 4.55) |
| Posterior superior/middle temporal sulcus (13) | 46 | −8.99 | (−13.79, −4.19)†, ‡ | 46 | 1.73 | (−3.21, 6.66) | 40 | −7.99 | (−13.34, −2.65)†, ‡ | 52 | 1.66 | (−2.11, 5.43) |
| Inferior parietal lobule (14) | 45 | −4.96 | (−8.80, −1.12)†, ‡ | 45 | 1.77 | (−2.06, 5.60) | 39 | −2.22 | (−6.40, 1.95) | 51 | 0.18 | (−3.26, 3.61) |
| Superior parietal lobule (15) | 42 | −2.74 | (−7.51, 2.04) | 43 | 0.60 | (−3.31, 4.50) | 38 | −0.57 | (−6.02, 4.89) | 47 | 0.58 | (−2.98, 4.14) |
Abbreviations: BA = Brodmann Areas, PFC = prefrontal cortex.
Adjusted for age of child at assessment (continuous variable), child’s sex, maternal age at delivery (continuous), maternal education at delivery (<sixth grade, 7th to 12th grade, completed high school), and quality of the home environment at the 10.5-y visit (continuous HOME z-score).
Non-FDR-corrected P < 0.05.
FDR-corrected P < 0.05.
Sex Differences.
When we stratified OP use and fNIRS associations by child sex, we found the strongest sex differences for the Pyramids and Palm Trees (semantic language) task (Table 7). Total OP use was associated with increased activation in males and reduced activation in females across nearly all of the 15 localization clusters (P value for sex interaction was <0.05 for 7 of the 15 clusters and <0.20 for all but 1 cluster) but was particularly marked for the frontal and temporal regions. There were a few suggestive (though many were not statistically significant) patterns across cognitive flexibility/working memory tasks (SI Appendix, Table S10) and no notable sex differences for the Go/No-Go and Dynamic Social Gestures tasks (SI Appendix, Table S11).
Table 7.
Sex-specific adjusted* associations of total OP pesticide use within a 1-km radius of maternal residence during pregnancy and fNIRS brain activation by region of interest for Pyramids and Palm Trees (semantic language) among participants with fNIRS data in the CHAM2 study, enrolled 2009 in Salinas Valley, California
| Region (localization cluster) | Pyramids and Palm Trees (semantic language) | |||||
| Males | Females | |||||
| n | β (95% CI)* | β (95% CI)* | P† | |||
| Left hemisphere | ||||||
| Inferior frontal pole (1) | 93 | 3.11 | (−0.26, 6.47) | −1.67 | (−4.55, 1.21) | 0.03 |
| Superior frontal pole (2) | 90 | 0.70 | (−2.73, 4.12) | −2.46 | (−5.33, 0.41) | 0.16 |
| Broca’s/BA 44/45 (3) | 91 | 3.30 | (−0.13, 6.73) | −1.66 | (−4.57, 1.25) | 0.03 |
| Dorsolateral PFC (4) | 90 | 1.01 | (−1.97, 4.00) | −1.37 | (−3.98, 1.24) | 0.23 |
| Broca’s/BA 44 and 6 (5) | 94 | 2.39 | (−0.86, 5.64) | −1.07 | (−3.84, 1.71) | 0.10 |
| Superior/inferior temporal /postcentral gyrus (6) | 93 | 4.50 | (1.11, 7.89)‡ | −1.11 | (−4.03, 1.82) | 0.01 |
| Inferior parietal lobule (7) | 92 | 1.82 | (−0.84, 4.48) | −1.92 | (−4.19, 0.35) | 0.03 |
| Superior parietal lobule (8) | 83 | 0.72 | (−1.72, 3.16) | −2.32 | (−4.41, −0.23)‡ | 0.06 |
| Right hemisphere | ||||||
| Inferior frontal pole (9) | 94 | 3.55 | (0.44, 6.66)‡ | −1.95 | (−4.61, 0.71) | <0.01 |
| Broca’s/BA 44/45 (10) | 91 | 4.75 | (1.22, 8.29)‡ | −2.56 | (−5.56, 0.45) | <0.01 |
| Superior frontal pole /dorsolateral PFC (11) | 92 | 0.42 | (−2.42, 3.27) | −2.31 | (−4.82, 0.20) | 0.15 |
| Premotor/somatosensory cortex (12) | 94 | 4.18 | (1.04, 7.32)‡ | −2.40 | (−5.08, 0.29) | <0.01 |
| Posterior superior/middle temporal sulcus (13) | 94 | 1.83 | (−1.32, 4.98) | −1.85 | (−4.54, 0.84) | 0.07 |
| Inferior parietal lobule (14) | 93 | 0.55 | (−2.19, 3.28) | −2.42 | (−4.75, −0.08)‡ | 0.10 |
| Superior parietal lobule (15) | 85 | −0.29 | (−2.55, 1.97) | −2.36 | (−4.56, −0.17)‡ | 0.18 |
Abbreviations: BA = Brodmann Areas, PFC = prefrontal cortex.
Adjusted for age of child at assessment (continuous variable), child’s sex, maternal age at delivery (continuous), maternal education at delivery (<sixth grade, 7th to 12th grade, completed high school), and quality of the home environment at the 10.5-y visit (continuous HOME z-score).
Wald P value for interaction by sex.
Non-FDR-corrected P < 0.05.
Sensitivity Analyses.
We did not see any material differences in total OP use and fNIRS associations when we restricted our sample to right-handed individuals, those that reported no substance use in the previous 24 h, or individuals whose mothers did not move during the pregnancy. Estimates were also unchanged when we adjusted our models for family poverty level.
Discussion
Most of the previous epidemiologic evidence for associations of prenatal OP pesticide exposure with neurodevelopment comes from studies that employ neuropsychological testing or behavioral rating scales administered to the parent, teacher, or the individual. While these assessments are critical for identifying the impact of pesticides on cognitive and behavioral function, they provide limited information on the brain structures or neural functions targeted by these exposures. In the current study, we took a first step toward examining the impact of prenatal OP pesticide exposure on brain activation as measured with fNIRS, a convenient and cost-effective neuroimaging technique.
We found several noteworthy associations in this preliminary study of 95 participants. Our most salient finding was that prenatal OP exposure was associated with altered brain activation patterns during tasks of executive function, including cognitive flexibility and working memory. We also found strong sex differences in prenatal OP and brain activation associations during a language comprehension task. However, we did not observe any consistent associations of prenatal OPs with brain activation during tasks of attention/response inhibition or social cognition.
Only 1 published study of 40 children aged 6 to 12 y examined the influence of prenatal pesticide exposure on brain structure using volumetric MRI (17). This study reported associations of exposure to the OP chlorpyrifos with reduced cortical thickness of frontal, temporal, and parietal regions using MRI (Table 1). In addition, 2 studies have examined functional neuroimaging in children in relation to other early life environmental exposures. Higher childhood blood lead levels among 42 young adults in the Cincinnati Lead Study were linked with reduced activation on fMRI in left hemisphere regions associated with semantic language during a language task (left frontal gyrus and left middle temporal gyrus) and increased activation in the right hemisphere regions (Wernicke’s area) (24). A study in a subset of 12 adolescent boys in the Faroe Islands birth cohort showed that adolescents with higher prenatal exposure to methylmercury and polychlorinated biphenyls had greater brain activation during tasks requiring visual processing (photic stimulation) and manual motor movement (finger tapping) than those with lower exposure (25). This growing body of neuroimaging literature complements studies of environmental exposures and neurodevelopment by identifying the neural underpinnings of previously observed associations with cognitive and behavioral function. These neuroimaging studies may also detect subtle exposure-induced impacts on neural structure and function that could be missed in traditional studies of neuropsychological assessment due to compensatory mechanisms and brain plasticity.
We found both an increase and decrease in brain activation in association with prenatal OPs during tests of executive function. We observed a negative association (i.e., lower relative activation) during tests of cognitive flexibility (Wisconsin Card Sorting Test) and visuospatial working memory (N-back) in relation to OP exposure that were most pronounced in the inferior frontal poles. We also observed a positive association (i.e., greater relative activation) during a test of letter-retrieval working memory (Sternberg) with higher OP exposure, though in different brain regions–primarily the left parietal lobule and right temporal/parietal regions. The inconsistent directions of these associations, aside from their emergence in different regions of the brain, could be explained by the variable cognitive demands of these tasks. For example, higher activation with increased exposure could indicate an increase in recruitment of neural resources to effectively meet the demands of a straightforward working memory task (Sternberg), as has been observed in older persons with beta-amyloid deposition (26–28). Reduced activation, on the other hand, could indicate that exposure has altered the overall neural response, including the ability of a region or network to marshal a typical response to a task, particularly one with higher complexity (e.g., Wisconsin Card Sorting Test). Similar findings have also been observed in cognitively impaired neurogenetic groups previously assessed with fMRI (29, 30).
We previously reported associations of working memory with nonspecific OPs measured via urinary DAP metabolites during pregnancy, where a 10-fold increase in DAPs was associated with reduced age 7 y Wechsler Intelligence Scale for Children, fourth edition (WISC-IV) working memory subtest scores of 4.3 points (95% CI: −7.7 to −0.9) (11). This is consistent with associations with poorer working memory found in associations with cord blood chlorpyrifos, a single OP pesticide, in the Columbia Center for Children's Environmental Health (20). More relevant to our current findings, we also found associations of OP use within a 1-km radius of maternal residence during pregnancy (estimated using Pesticide Use Reporting [PUR] data) and poorer age 10 y WISC-IV working memory in CHAMACOS (working memory scores decreased by 2.8 points [95% CI: −5.6, −0.1] for the fourth vs. first quartile of pesticide use) (16). Notably, the latter associations were independent of our prenatal DAP metabolite associations.
In contrast with our previous OP–working memory findings (11, 16), we did not detect associations of prenatal OP use with performance (e.g., errors, accuracy, and reaction time) on any of the working memory or executive function tasks administered with the fNIRS. This could be explained by our smaller sample size, or the differences between these experimental tasks, optimized for fNIRS testing, and standardized neuropsychological tests. Notably, while there was insufficient sensitivity to detect associations of OPs with task performance, we did observe associations with brain activation. This underscores the potential for neuroimaging to detect very subtle impacts of pesticide exposure on the brain.
We found strong sex differences for prenatal OPs and brain activation during a semantic language task (Pyramids and Palm Trees); increased OP exposure was associated with higher activation among males and lower activation among females across nearly all brain regions assessed. We previously reported associations of prenatal urinary OP metabolites with lower age 7 y WISC-IV verbal comprehension scores in CHAMACOS (β = −5.3; 95% CI:−8.6,−2.0 per 10-fold increase in DAPs) (11); associations were similar for PUR OP use within a 1-km radius of maternal residence during pregnancy (β = −2.9; 95% CI: −4.4, −1.3 for an SD increase in OP pesticide use) (16). However, we did not report sex differences for any of these associations, nor did the Columbia study, which also reported main effects with verbal comprehension (20). Previous studies show sexual dimorphism in brain activation when engaged in semantic language processing tasks (31–33). In addition, there is rationale for differences in the impact of endocrine-disrupting chemicals like OP pesticides among males and females (34). However, there was no a priori reason to expect that associations of OPs with brain activation during a semantic language task would be different across child sex. These findings should therefore be interpreted with caution and replicated in larger study samples.
We did not detect any strong or consistent associations of prenatal OP exposure with alterations in brain activation during tasks of attention/response inhibition (Go/No-Go task) and social cognition (Dynamic Social Gestures task), despite our previous findings that higher prenatal DAPs were associated with poorer attention and social cognition (including traits related to autism spectrum disorders) in CHAMACOS (12–14). Inconsistent with DAPs, but perhaps more consistent with the current findings, we reported null associations of PUR-estimated OPs with social cognition (14). This could be due to different exposure routes (DAPs are predominantly from diet, vs. PUR estimates which represent ambient exposure). In addition, since PUR-estimated OP exposure does not consider exposures that may have occurred through diet, agricultural work, or home pesticide use, this measure may underestimate exposure or lead to exposure measurement error that may have attenuated findings, reducing our ability to detect associations of OPs with brain activation. Nonetheless, PUR-estimated exposure is well correlated with environmental pesticide concentrations (8) and has been linked with intelligence quotient at age 7 y in CHAMACOS (15).
There were other notable limitations of our study. First, fNIRS measures activity at the cortical surface and therefore cannot detect hemodynamic changes in subcortical, deep-brain regions where OP exposure may exert an effect. Second, our sample size, while large for a neuroimaging study, which typically measures brain activation in a case group with a specific disorder vs. a control group, was modest for a study examining sometimes very subtle effects of environmental toxicant exposures. This may have limited our ability to detect OP-related associations with precision. Third, we took a rather crude approach to controlling for test performance in our OPs–brain activation findings. Adjusting for performance in our multivariable models was inappropriate as performance could be affected by OP exposure and by brain activation, and conditioning on a common effect of both exposure and outcome could induce bias in effect estimates (35). The same problem could be present in our stratified analysis (high vs. low performers), in addition to the problem of small numbers in our strata which reduced the precision of our estimates. We therefore interpret these results with caution and continue to explore methods for appropriately accounting for test performance when examining associations of exposures with brain activation. Finally, due to the large number of tasks (6), localization clusters (15), and OP exposure measures (6, including total OPs) as well as many other comparisons, multiple testing is certainly a concern when interpreting these data. When we applied an FDR correction, we found no statistically significant associations, likely due to our small sample size, but also because FDR correction is an extremely conservative approach to adjusting for multiple comparisons. We therefore interpret our non-FDR-corrected estimates with caution, not highlighting any isolated findings but rather focusing on patterns of associations.
Conclusions
In summary, we found that prenatal OP exposure was associated with altered brain activation patterns during tasks of executive function and sex-specific activation patterns for language comprehension in this population-based CHAMACOS study. This study of prenatal OP exposure and brain activation in humans suggests that OPs may be impacting cognitive function at the neural level. We anticipate that this work will pave the way for more widespread use of neuroimaging, and more specifically fNIRS, a relatively inexpensive and easily accessible technology, in assessing the impact of a range of environmental exposures on brain function.
Methods
Study Sample and Recruitment for Functional Neuroimaging Study.
We recruited youth who participated in the present study into the CHAMACOS cohort in years 2009 to 2011, when they were 9 y old. This constituted the second wave of enrollment to the CHAMACOS study (“CHAMACOS 2” or “CHAM2” enrollment), which was intended to augment the existing (“CHAM1”) portion of the cohort enrolled prenatally, with additional, demographically similar children. Children were eligible for CHAM2 enrollment if they were 1) born in 2000 to 2002 to mothers who were Spanish- or English-speaking, 2) had lived in the Salinas Valley during pregnancy and still resided there at child age 9 y, 3) were MediCal-eligible during pregnancy, 4) had received prenatal care, and 5) were at least 18 y old at time of delivery. We recruited eligible families through elementary schools, libraries, churches, food banks, and outreach at community events. In total, we enrolled 305 children in the CHAM2 portion of the cohort, of whom 288 completed study visits 5 y later (2014 to 2016) at age 14 y.
In 2017, we recruited a subset of participants for this preliminary fNIRS study from among these 288 active CHAM2 participants. Our target number of participants for this preliminary study was between 80 and 100. To optimize the precision of our pesticide exposure measure, which is based on the primary residential address during pregnancy as reported by the mother at child age 9 y, we recruited youth whose mothers 1) had provided a mappable (i.e., valid) primary pregnancy address at the age 9 y visit and 2) had reported living at the same address for the majority of their pregnancy (of the 95 participants in this study, 86 reported that they did not move during their pregnancy; the 9 mothers who did move spent a median of 87% of their pregnancy at the primary address).
We recruited eligible participants primarily by phone, although some who were due for a routine 16-y study visit during the data collection period were invited to participate when they attended that visit. In all cases, our research field coordinator first described the fNIRS study visit to the parent of the youth and then requested permission to describe the visit to the youth. The most common reason for nonparticipation was that the participant had a disconnected phone line or lived out of town; other reasons included refusal (n = 7 youth or their mothers refused to participate) and house arrest (n = 2). When both parent and youth were in agreement that the youth could participate, we scheduled an fNIRS study visit. Parents provided written permission and youth (ages 15 to 16 y) provided written assent to participate. All research activities were approved by the Office for the Protection of Human Subjects at University of California, Berkeley. We ultimately completed a full fNIRS visit with 95 youth.
Functional Neuroimaging Data Collection.
We measured cortical neural activation using fNIRS, a method of optical neuroimaging which characterizes hemodynamic changes at the brain’s surface (i.e., just beneath the scalp) in a priori-identified regions of the brain. Specifically, we employed the NIRScout (NIRx Medical Technologies), a versatile neuroimaging platform that allows customization of optode placement to target activity in specific regions of the brain. We positioned the optodes over standard 10 to 20 system locations using individually sized caps (Brain Products) selected based on head circumference. The 10 to 20 locations were spatially adjusted across all cap sizes to maintain consistent coverage of our regions of interest despite changes in head size across participants (36, 37). Consistent 3-cm channel distance was achieved using plastic supports between each source/detector pair that constituted a recording channel. We synchronized our fNIRS data collection with a computerized neurobehavioral test battery, monitoring hemodynamic changes in response to specific stimuli and tasks.
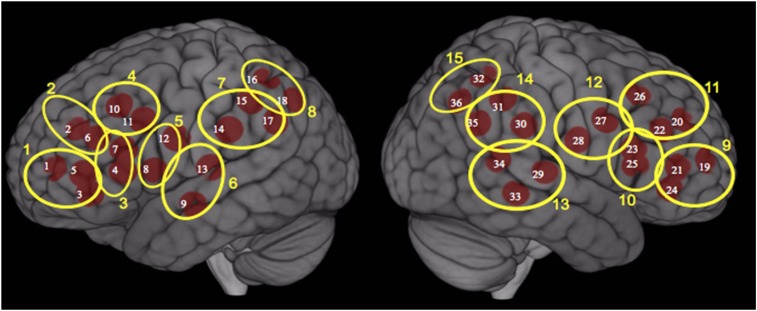
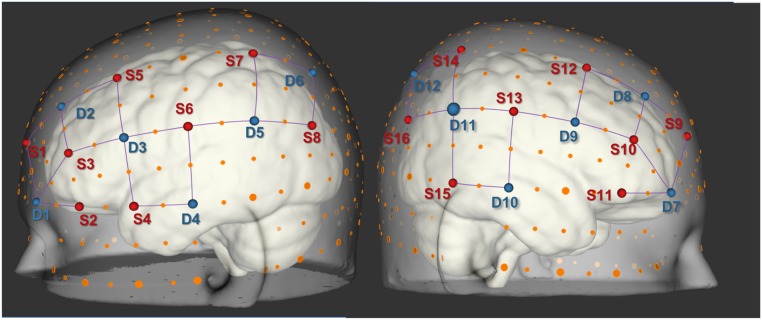
At the start of the visit, trained study staff measured participant head size, selected an appropriately sized cap, placed it on a mannequin, and fit 28 optodes (16 sources and 12 detectors) on the cap in a set configuration (Figs. 2 and 3), designed to optimize coverage of brain structures in the frontal, temporal, and parietal lobes. Fig. 3 shows source and detector locations on the scalp and Fig. 2 shows the channel locations (visualized as the midpoint between each source and detector pair) as well as the functional localization clusters (described later). We selected these regions (Table 1, column 3) based on the previous neuroimaging study of prenatal OP exposure (20) (Table 1, column 1) as well as epidemiologic studies of prenatal OP exposure and neurobehavioral outcomes (11–13, 18, 19) (Table 1, column 2). Prior to placement of the cap on the participant’s head, we asked participants to complete a brief survey (Covariate Data Collection). Staff also presented a PowerPoint-based visual practice session of each neurobehavioral task along with instructions on how to complete it. Staff then placed the optode-fitted cap on the participant’s head, performed calibration tests, and adjusted optodes as needed.
Fig. 2.
fNIRS channel (n = 36) locations and functional localization clusters (n = 15) in the CHAM2 study, enrolled 2009 in Salinas Valley, California. Red circles represent channel locations visualized as the midpoint between each source and detector pair. Yellow circles are clusters based on proximity of channels and anatomy, and include 1 = left inferior frontal pole; 2 = left superior frontal pole; 3 = left Broca’s/Brodmann areas 44 and 45; 4 = left dorsolateral prefrontal cortex; 5 = left Broca’s/Brodmann areas 44 and 6; 6 = left superior/inferior temporal gyrus/postcentral gyrus; 7 = left inferior parietal lobule; 8 = left superior parietal lobule; 9 = right inferior frontal pole; 10 = right Broca’s/Brodmann areas 44 and 45; 11 = right superior frontal pole/dorsolateral prefrontal cortex; 12 = right premotor/somatosensory cortex; 13 = right posterior superior/middle temporal sulcus; 14 = right inferior parietal lobule; 15 = right superior parietal lobule.
Fig. 3.
Source and detector locations on the scalp. Sources (16) are shown in red and detectors (12) in blue. Orange dots indicate 10 to 20 positions and purple lines indicate measurement channels. Semitransparent scalp surface rendering is shown on top of an opaque brain surface. All source and detector positions were based on a major 10 to 20 landmark. Plastic supports were used to maintain a consistent 3-cm channel distance between each source/detector pair that constituted a recording channel.
Participants engaged in 6 tasks that assess neurobehavioral functions previously linked with prenatal OP exposure in epidemiologic studies, including attention, working memory, cognitive flexibility, response inhibition, social cognition, and language comprehension. We generated all tasks using code written in-house for Psychtoolbox-3 Psychophysics Toolbox Version 3 in MATLAB 2014b, with trial order and timing optimized to maximize detectable changes in hemodynamic response using OptSeq2 (38). Tasks were presented on a MacBook Pro connected to a 20-inch light-emitting diode monitor. We collected hemodynamic activity data, including oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (HbR), with a sampling frequency of 3.9063 Hz from 36 channels, grouped into 15 functional localization clusters on both the right and left hemispheres, including 9 clusters in the prefrontal cortex, 2 in the temporal, and 4 in the parietal regions (Fig. 3). To avoid bias associated with test fatigue, we presented the 6 tasks in a randomized order across 2 testing sessions (3 tasks in each session with a break in between). The 1 exception was the Go/No-Go, a test of attention and vigilance, which was always presented at the end of 1 of the 2 testing sessions. The tasks included 1) the Wisconsin Card Sorting Test, a test of cognitive flexibility and executive function where the participant matches cards based on an unstated rule (shape, number, or color); 2) the Sternberg working memory task, a test of letter-retrieval working memory where the participant is asked to recall if a presented letter was in a previously viewed string of 7 to 8 letters; 3) the Visuospatial N-back, a test of visuospatial working memory where the participant is instructed to respond if a stimulus is presented in the same location as the previous trial (1-back) or 2 trials previous (2-back); 4) the Go/No-Go, a test of attention and response inhibition where the participant is instructed to press a button when any letter other than “X” appears (i.e., Go trials) and withhold a button press when “X” is shown (i.e., No-Go trials); 5) Pyramids and Palm Trees, a test of verbal comprehension where the participant decides which of 2 words is semantically related to a stimulus word; and 6) the Dynamic Social Gestures task, an implicit test of social cognition, where the participant views video clips portraying social gestures (e.g., friendly wave) and nonsocial gestures (e.g., looking at a book). We provide more comprehensive task descriptions in SI Appendix.
We assessed patterns of brain activation for each task of interest using a generalized linear model (GLM) approach, which has been well established for analysis of event-related as well as blocked fNIRS designs (39). The onset and duration of each condition of interest were submitted to the GLM procedure as predictor variables used to estimate standardized β coefficients for each condition and within each channel. The sign and magnitude of each β coefficient provides an indicator of the direction (positive/negative) and intensity of blood oxygen level-dependent change (i.e., brain activity) that occurs during each condition. We estimated β coefficients for all task and control conditions (we describe control conditions in SI Appendix). In order to capture the brain activation unique to the task demands, and thus not expected to be present in signals corresponding to the control conditions, we made contrasts between each β coefficient and its corresponding control. We included data from all trials in the GLM, with the exception of the Wisconsin Card Sorting task, in which we only included data collected for correct responses (6 times in a row, the number required to reach criteria).
We utilized a functional localization approach (40–42) to account for variation in cortical activation in response to our tasks. This procedure allows for minor individual variation in the location of task-responsive brain regions across participants and reduces the risk of committing type II (i.e., false negative) errors due to averaging across nonresponsive channels. We grouped channels based on proximity and anatomical location (Fig. 2) to create 15 clusters. Within each of the 15 functional localization clusters (42), we selected the channel with the greatest contrast value. Technical problems with data collection as well as data cleaning (see SI Appendix for details) led to some exclusions that reduced our sample sizes; these exclusions varied across tasks and localization clusters.
OP Pesticide Exposure Assessment.
We quantified ambient, prenatal exposure to agricultural pesticides by linking the latitude and longitude coordinates of mothers’ self-reported (at child age 9 y) primary pregnancy address with California’s unique PUR database (43). The PUR database, compiled by California’s Department of Pesticide Regulation, contains geocoded location (in square-mile sections) and date-stamped records with the amount of active ingredients used for every commercial agricultural pesticide application in the state dating back to 1990 (43). We estimated the amount (kilograms) of agricultural OP pesticide use within a 1-km buffer distance of the pregnancy address and weighted pesticide use based on the amount of time the residence was downwind of the applications using wind direction data. We selected a 1-km buffer distance for this analysis because it best captures the spatial scale most strongly correlated with measured agricultural pesticide concentrations in house dust samples (8, 44). We replaced PUR data outliers with unusually high application rates (>2 SD above the mean application rate), that were likely data entry errors, with the median application rate for that pesticide and crop combination (44). We examined exposure to the 5 most common OP pesticides (acephate, chlorpyrifos, diazinon, malathion, and oxydemeton-methyl) and the sum of all OP pesticides used in the region (15 total) (15). Note that because the participants in CHAM2 did not join the CHAMACOS study until age 9 y we do not have prenatal biomarker of their pesticide exposure, such as urinary DAPs.
Covariate Data Collection.
We obtained sociodemographic data from in-person maternal interviews at all visits between enrollment and child age 14 y. We administered the Home Observation for the Measurement of the Environment-Short Form (HOME-SF) (45) to assess the home learning environment when the child was 10.5 y of age.
In addition, just before fNIRS testing, youth completed a brief, self-administered, tablet-based survey regarding their handedness and other factors that could affect their performance. This included recent intake of nicotine, alcohol, marijuana, medications, and caffeine; how well they had slept the night before testing; whether or not they had eaten that morning; and their self-assessed levels of sleepiness and mental fatigue.
Statistical Analysis.
We used 1-sample t tests to determine whether there was significant brain activation (increased HbO and decreased HbR) in each localization cluster for each task contrast of interest. Although we used HbO for all subsequent analysis, localization based on both HbO and HbR ensured we were ascertaining valid activations and taking advantage of the full scope of the fNIRS data. Contrast conditions included maintenance/encoding vs. recall for the Sternberg working memory task; semantic meaning vs. control condition for the Pyramids and Palm Trees task; card sort vs. control for the Wisconsin Card Sorting Test; No-Go vs. Go for the Go/No-Go task; 2-back vs. 1-back vs. control, parametrically analyzed, for the N-back; and social vs. nonsocial gestures for the Dynamic Social Gesture task. We fit linear regression models to estimate associations (β and 95% CI) of log10-transformed OP pesticide exposure with brain activation; β coefficients represented the change in brain activity during a challenge vs. control task per 10-fold increase in exposure. In this preliminary study of n = 95, we primarily examined associations without correcting for multiple comparisons. In secondary analysis, to account for multiple comparisons, we controlled for type I error using the Benjamini–Hochberg FDR at <0.05 (46). We tested for linearity of the exposure–outcome associations using generalized additive models with 3 degrees of freedom cubic splines. Since linearity was justified across most associations, we present the results of our linear regression models.
We selected covariates to adjust for in multivariable regression models a priori using a causal model framework (47) (refer to SI Appendix, Fig. S2 for a directed acyclic graph). We adjusted for age of child at assessment (continuous variable), child’s sex, maternal age at delivery (continuous), maternal education at delivery (<sixth grade, 7th to 12th grade, completed high school), and quality of the home environment at the 10.5-y visit (continuous HOME score, standardized within our sample using z-scores). When available, we used data from earlier time points to replace missing covariate data.
To facilitate comparison of effect size measures from these multilevel models across other studies, we computed the proportion of variance explained by the given effect (R22) relative to the proportion of outcome variance unexplained (R21) f2 = (R22 − R21)/(1 − R22) (48), where a value of 0.02 is considered a small effect, 0.15 a medium effect, and 0.35 a large effect (49).
In addition to estimating associations of OPs with brain activation on the fNIRS, we also examined associations of total OPs with task performance, including, where applicable, errors, accuracy, and reaction time. We also examined total OPs and brain activation associations across test performance by dichotomizing performance at the median score for each task and estimating OP-fNIRS associations for those who performed above and below the median. We did not examine task performance for Dynamic Social Gestures. The performance aspect of this task (pressing a button when a red dot appeared on the screen) was to ensure that the participant was attending to the task so that we could record their neural response to a social vs. nonsocial gesture; performance accuracy therefore did not reflect social cognition.
We examined effect modification by sex by including an interaction term between OP exposure and sex in the multivariable linear regression models and computing sex-specific effect estimates. We conducted the following sensitivity analyses to examine how robust findings were to the following adjustments: 1) restricted to right-handed individuals (n = 6 were left-handed); 2) restricted to those who reported no substance use (alcohol or marijuana) in the previous 24 h (n = 8 reported substance use); 3) restricted to individuals whose mothers did not move during the pregnancy (n = 9 moved during their pregnancy); and 4) adjusting for family poverty level.
Supplementary Material
Acknowledgments
We thank the CHAMACOS and Center for Interdisciplinary Brain Sciences Research staff and students, whose work allowed us to develop and implement this study, with special thanks to volunteer Vivian Wan. We also thank the CHAMACOS youth who volunteered their time to participate. This work was supported by NIH grants UG3 OD023356, P01 ES009605, R01 ES015572, T32 MH019908, K99 HD092883, and K99 HD092883; grants RD 83171001 and RD 83451301 from the US Environmental Protection Agency (EPA); the Stanford Maternal and Child Health Institute; and a gift from the Albert Yu and Mary Bechmann Foundation. The contents of this publication are solely the authors’ responsibility and do not necessarily represent the official views of the National Institute of Environmental Health Sciences, NIH, or EPA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1903940116/-/DCSupplemental.
References
- 1.Atwood D., Paisley-Jones C., “Pesticides industry sales and usage 2008–2012 market estimates” (US Environmental Protection Agency, Washington, DC, 2017).
- 2.Campos É., Freire C., Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health 219, 481–497 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Perla M. E., Rue T., Cheadle A., Krieger J., Karr C. J., Population-based comparison of biomarker concentrations for chemicals of concern among Latino-American and non-Hispanic white children. J. Immigr. Minor. Health 17, 802–819 (2015). Erratum in: J. Immigr. Minor. Health17, 1287 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Adgate J. L., et al. , Measurement of children’s exposure to pesticides: Analysis of urinary metabolite levels in a probability-based sample. Environ. Health Perspect. 109, 583–590 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kutz F. W., Cook B. T., Carter-Pokras O. D., Brody D., Murphy R. S., Selected pesticide residues and metabolites in urine from a survey of the U.S. general population. J. Toxicol. Environ. Health 37, 277–291 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Whyatt R. M., et al. , Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect. 110, 507–514 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradman A., et al. , Effect of organic diet intervention on pesticide exposures in young children living in low-income urban and agricultural communities. Environ. Health Perspect. 123, 1086–1093 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harnly M. E., et al. , Pesticides in dust from homes in an agricultural area. Environ. Sci. Technol. 43, 8767–8774 (2009). [DOI] [PubMed] [Google Scholar]
- 9.McKone T. E., et al. , Merging models and biomonitoring data to characterize sources and pathways of human exposure to organophosphorus pesticides in the Salinas Valley of California. Environ. Sci. Technol. 41, 3233–3240 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Bradman A., et al. , Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int. J. Environ. Res. Public Health 8, 1061–1083 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouchard M. F., et al. , Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect. 119, 1189–1195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eskenazi B., et al. , Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 115, 792–798 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks A. R., et al. , Organophosphate pesticide exposure and attention in young Mexican-American children: The CHAMACOS study. Environ. Health Perspect. 118, 1768–1774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagiv S. K., et al. , Prenatal organophosphate pesticide exposure and traits related to autism spectrum disorders in a population living in proximity to agriculture. Environ. Health Perspect. 126, 047012 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunier R. B., Bradman A., Harley K. G., Kogut K., Eskenazi B., Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ. Health Perspect. 125, 057002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe C., et al. , Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ. Res. 150, 128–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauh V. A., et al. , Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci. U.S.A. 109, 7871–7876 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel S. M., et al. , Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ. Health Perspect. 119, 1182–1188 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Handal A. J., Harlow S. D., Breilh J., Lozoff B., Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology 19, 851–859 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Rauh V., et al. , Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 119, 1196–1201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker J. M., et al. , Portable functional neuroimaging as an environmental epidemiology tool: A how-to guide for the use of fNIRS in field studies. Environ. Health Perspect. 125, 094502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui X., Bray S., Reiss A. L., Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 49, 3039–3046 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N., Cui X., Bryant D. M., Glover G. H., Reiss A. L., Inferring deep-brain activity from cortical activity using functional near-infrared spectroscopy. Biomed. Opt. Express 6, 1074–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan W., et al. , The impact of early childhood lead exposure on brain organization: A functional magnetic resonance imaging study of language function. Pediatrics 118, 971–977 (2006). [DOI] [PubMed] [Google Scholar]
- 25.White R. F., et al. , Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology 32, 975–980 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elman J. A., et al. , Neural compensation in older people with brain amyloid-β deposition. Nat. Neurosci. 17, 1316–1318 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witiuk K., et al. , Cognitive deterioration and functional compensation in ALS measured with fMRI using an inhibitory task. J. Neurosci. 34, 14260–14271 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suskauer S. J., et al. , fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. J. Am. Acad. Child Adolesc. Psychiatry 47, 1141–1150 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon H., et al. , Functional neuroanatomy of visuospatial working memory in fragile X syndrome: Relation to behavioral and molecular measures. Am. J. Psychiatry 158, 1040–1051 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Haberecht M. F., et al. , Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Hum. Brain Mapp. 14, 96–107 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baxter L. C., et al. , Sex differences in semantic language processing: A functional MRI study. Brain Lang. 84, 264–272 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Shaywitz B. A., et al. , Sex differences in the functional organization of the brain for language. Nature 373, 607–609 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Gauthier C. T., Duyme M., Zanca M., Capron C., Sex and performance level effects on brain activation during a verbal fluency task: A functional magnetic resonance imaging study. Cortex 45, 164–176 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Weiss B., Same sex, no sex, and unaware sex in neurotoxicology. Neurotoxicology 32, 509–517 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernán M. A., Hernández-Díaz S., Robins J. M., A structural approach to selection bias. Epidemiology 15, 615–625 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Okamoto M., et al. , Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10-20 system oriented for transcranial functional brain mapping. Neuroimage. 21, 99–111 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Tsuzuki D D., et al. , Stable and convenient spatial registration of stand-alone NIRS data through anchor-based probabilistic registration. Neurosci Res. 72, 163–171 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J., Computational anatomy with the SPM software. Magn. Reson. Imaging 27, 1163–1174 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Plichta M. M., Heinzel S., Ehlis A. C., Pauli P., Fallgatter A. J., Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: A parametric validation study. Neuroimage 35, 625–634 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Baker J. M., Bruno J. L., Gundran A., Hosseini S. M. H., Reiss A. L., fNIRS measurement of cortical activation and functional connectivity during a visuospatial working memory task. PLoS One 13, e0201486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruno J. L., et al. , Mind over motor mapping: Driver response to changing vehicle dynamics. Hum. Brain Mapp. 39, 3915–3927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseini S. M. H., et al. , Neural, physiological, and behavioral correlates of visuomotor cognitive load. Sci. Rep. 7, 8866 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.California Department of Pesticide Regulation , Pesticide use reporting: An overview of California’s unique full reporting system. https://www.cdpr.ca.gov/docs/pur/purovrvw/tabofcon.htm. Accessed 1 February 2019.
- 44.Gunier R. B., Harnly M. E., Reynolds P., Hertz A., Von Behren J., Agricultural pesticide use in California: Pesticide prioritization, use densities, and population distributions for a childhood cancer study. Environ. Health Perspect. 109, 1071–1078 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldwell B., Bradley R., Home Observation for Measurement of the Environment (University of Arkansas, Little Rock, 1984), rev. ed.
- 46.Hochberg Y., Benjamini Y., More powerful procedures for multiple significance testing. Stat. Med. 9, 811–818 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Greenland S., Pearl J., Robins J. M., Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999). [PubMed] [Google Scholar]
- 48.Aiken L. S., West S. G., Multiple Regression: Testing and Interpreting Interactions (Sage, Newbury Park, 1991). [Google Scholar]
- 49.Cohen J., A power primer. Psychol. Bull. 112, 155–159 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.