Significance
Knowledge of resource and habitat use by wildlife is essential to support conservation actions. Stable isotope analysis (SIA) is a useful method for the acquisition of this type of information. Samples for SIA can be obtained through indirect and noninvasive methods, which is favorable for studies of threatened species. We used SIA to compare the resource and habitat use and trophic structure of mammals between preserved areas and human-modified landscapes in a tropical rainforest. Our study shows that mammals in human-modified landscapes present an altered trophic structure and use food items from the agricultural matrix, while they depend on forest resources in preserved areas. Our findings stress the need for favorable management of the agricultural matrix to support wildlife survival.
Keywords: stable isotope analysis, landscape matrix, agriculture, diet, noninvasive sampling
Abstract
The broad negative consequences of habitat degradation on biodiversity have been studied, but the complex effects of natural–agricultural landscape matrices remain poorly understood. Here we used stable carbon and nitrogen isotopes to detect changes in mammal resource and habitat use and trophic structure between preserved areas and human-modified landscapes (HMLs) in a biodiversity hot spot in South America. We classified mammals into trophic guilds and compared resource use (in terms of C3- and C4-derived carbon), isotopic niches, and trophic structure across the 2 systems. In HMLs, approximately one-third of individuals fed exclusively on items from the agricultural matrix (C4), while in preserved areas, ∼68% depended on forest remnant resources (C3). Herbivores, omnivores, and carnivores were the guilds that most incorporated C4 carbon in HMLs. Frugivores maintained the same resource use between systems (C3 resources), while insectivores showed no significant difference. All guilds in HMLs except insectivores presented larger isotopic niches than those in preserved areas. We observed a complex trophic structure in preserved areas, with increasing δ15N values from herbivores to insectivores and carnivores, differing from that in HMLs. This difference is partially explained by species loss and turnover and mainly by the behavioral plasticity of resilient species that use nitrogen-enriched food items. We concluded that the landscape cannot be seen as a habitat/nonhabitat dichotomy because the agricultural landscape matrix in HMLs provides mammal habitat and opportunities for food acquisition. Thus, favorable management of the agricultural matrix and slowing the conversion of forests to agriculture are important for conservation in this region.
Agriculture is one of the main drivers of habitat loss and fragmentation (1), especially in tropical forests (2). Landscape conversion can cause drastic changes in landscape composition, which have selective effects on species, particularly those that are forest dependent (3), and have reduced species richness and diversity worldwide (4). Species with high body mass, large home ranges, and sensitivity to habitat loss and fragmentation are the first to vanish from human-modified landscapes (HMLs) (5, 6). Nonetheless, some species, including various mammals, persist and use the agricultural landscape matrix (landscape matrix hereafter) (7). The landscape matrix has the potential to offer food resources to animals (8, 9), especially where agricultural fields are present. Use of such resources might be harder to detect, particularly among elusive species, such as mammals in tropical forests.
Studies on feeding ecology can contribute to our understanding of these changes. However, despite providing essential knowledge, the use of traditional methods (e.g., direct observation, fecal analysis, and stomach content analysis) can be time consuming and expensive, especially if the aim is to determine resource origin and assess temporal variation in the diet. As an alternative and complementary method, stable isotope analysis (SIA) has gained prominence in recent decades in applied animal ecology (10), becoming a reliable tool to unravel individual-level ecological processes and complex community interactions (11).
In comparison to traditional methods, SIA presents many advantages, including potential for noninvasive sample collection and assessing several temporal windows with a single sampling event while also generating information on elusive species (11–13). Stable carbon (12C and 13C) and nitrogen (14N and 15N) isotopes are commonly used in studies of feeding ecology, species movement, and trophic processes (11, 13, 14). Specifically, stable carbon isotopes allow the evaluation of changes in species resource use according to the differences in the isotopic values of plants exhibiting the C3 and C4 photosynthetic cycles, which are reflected in animal tissues along the trophic chain (15). Stable nitrogen isotopes are used to obtain information on feeding ecology that is complementary to that obtained from stable carbon isotope analysis but are mainly applied to elucidate trophic processes and interactions between species and communities (14, 16).
Isotopic ecology studies assess changes in species feeding patterns in distinct environments, and by considering the difference between C3 (e.g., trees and shrubs in forests) and C4 plants (e.g., grasses in savannas and grasslands), they can identify the origin of food resources (see ref. 12). In this study, we used SIA to evaluate this difference in the Brazilian Atlantic Forest, which is considered to be a biodiversity hotspot (17) that is extensively modified by human activities, particularly agriculture (18). Since it is a forest ecosystem, i.e., predominantly composed of C3 plants, the contrast with C4 plants is facilitated by the presence of agricultural areas (e.g., sugarcane and corn) and cattle pastures, which occupy a large extension of this biome (18).
Thus, this landscape context offers a unique opportunity to explore the use of natural and anthropogenic habitats by mammals, permitting the determination of the role of anthropogenic matrices as a location to find food and a habitat within which to live. Moreover, the use of organic fertilizers and some cultivation practices (e.g., slash and burn) may alter the values of stable nitrogen isotopes in plants occurring in the matrix (14), which are reflected in animal tissues and act as an indicator of these changes. Since the Atlantic Forest still has some large continuous remnants, we have a chance to compare areas with more complete species assemblages (i.e., similar to pristine areas) to the modified and defaunated areas in HMLs. This dichotomy allows us to assess the impact of the landscape matrix on species diet and habitat use and to detect changes in the trophic structure of the species assemblages using SIA.
As a target group, we chose medium- (between 1 and 7 kg) and large-sized mammals (>7 kg) (19, 20) because these species generally have large home ranges (6) and can access both natural and anthropogenic areas. Moreover, comprehensive knowledge about their diet and ecology exists (e.g., refs. 20 and 21). Thus, the objectives of this study were to detect changes in the resource and habitat use of mammal species by comparing assemblages in preserved areas and HMLs in the Atlantic Forest, Brazil, and to obtain insight into differences in their trophic structure.
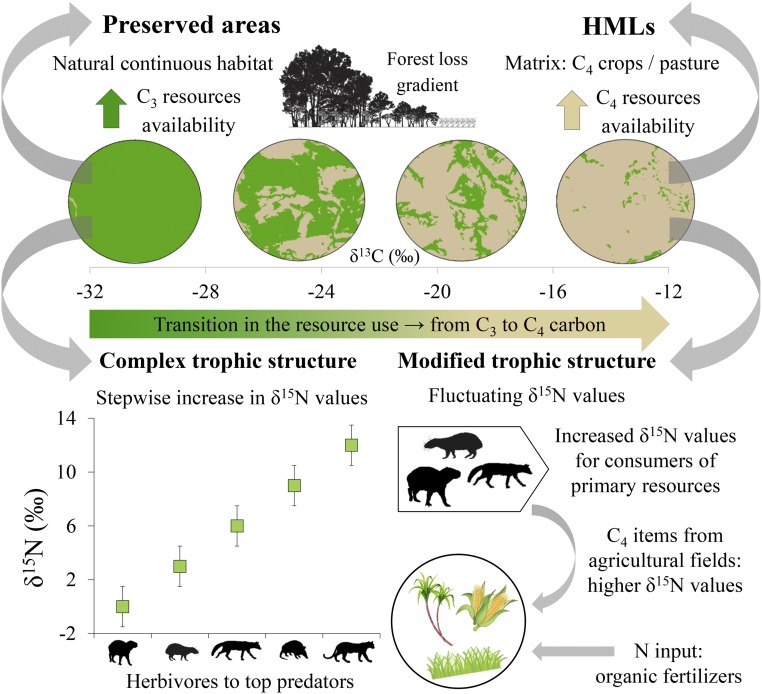
Our hypotheses were as follows: 1) Regarding resource use, as a result of forest cover reduction, mammals increase the intake of C4 carbon by consuming food items from the agricultural matrix over carbon from forest resources (C3 carbon) (Fig. 1). 2) Regarding trophic structure, in preserved areas with more complete assemblages, there is a stepwise increase in mammal δ15N values from primary consumers to top predators, while in HMLs, due to the loss and turnover of species and the influence of nitrogen-enriched food items from the agricultural matrix, the δ15N values fluctuate, and the trophic structure is modified (Fig. 1).
Fig. 1.
Hypotheses of resource use and trophic structure. Regarding resource use, the reduction in forest cover causes a transition in the use of resources by mammals between preserved areas (predominantly C3) and HMLs (predominantly C4). Regarding trophic structure, in preserved areas, with more complete assemblages than HMLs, the trophic structure is more complex, and there is a stepwise increase in δ15N values from herbivores to carnivores. In HMLs, due to species loss and turnover in combination with the presence of agricultural matrices, which offers nitrogen-enriched food items, δ15N values fluctuate among trophic guilds, resulting in a modified trophic structure.
Results
We analyzed 126 samples belonging to 23 mammal species collected from the preserved areas and 194 samples of 20 species collected from the HMLs (SI Appendix, Table S1), for a total of 29 different species. We observed a wider range of δ13Ccorrected and δ15Ncorrected values for mammals in the HMLs (δ13Ccorrected = 19.1‰; δ15Ncorrected = 10.9‰) than in the preserved areas (δ13Ccorrected = 14‰; δ15Ncorrected = 9.3‰) (SI Appendix, Table S2). We tested the robustness of our results by reanalyzing our data including only species that were common between systems (n = 16; SI Appendix, Fig. S1), showing that our results for trophic guilds were not influenced by interspecific differences in resource use.
Resource Use.
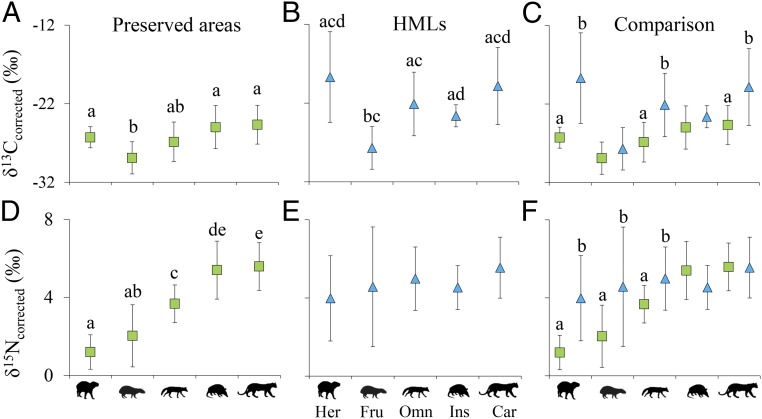
The frugivores in both systems had the lowest δ13Ccorrected values (Fig. 2 A and B), indicating that this guild is the most dependent on resources from forest remnants (SI Appendix, Table S2). The herbivores and carnivores in the HMLs showed relatively wide ranges of δ13Ccorrected values, while the herbivores in preserved areas and insectivores in the HMLs presented the smallest ranges (SI Appendix, Table S2). Overall, the δ13Ccorrected values and ranges were smaller in the preserved areas than in the HMLs (except in the case of insectivores), with significant differences among herbivores [ANOVA; F(1,32) = 16.75; P = 0.0003], omnivores [F(1,52) = 10.25; P = 0.0023], and carnivores [F(1,140) = 30.59; P < 0.0001] (Fig. 2C).
Fig. 2.
Comparison of δ13C and δ15N values among mammal trophic guilds in the Atlantic Forest, state of São Paulo, Brazil. Mean δ13Ccorrected and δ15Ncorrected values ±SD for mammal trophic guilds in (A and D) preserved areas, (B and E) HMLs, and (C and F) both systems together. Lowercase letters indicate relationships with significant differences (P < 0.01, P < 0.05). Her, herbivores; Fru, frugivores; Omn, omnivores; Ins, insectivores; Car, carnivores.
We observed a significant difference in mean δ15Ncorrected values between the C3 and mixed groups in preserved areas [ANOVA; F(1,124) = 134.83; P < 0.0001] and among the C3, mixed, and C4 groups in HMLs [F(2,191) = 12.2; P < 0.0001] (SI Appendix, Fig. S2). In both systems, individuals that incorporated C4 carbon into their body structures presented the highest δ15Ncorrected values, as observed for all trophic guilds except insectivores (SI Appendix, Table S3), suggesting their consumption of nitrogen-enriched food items from the agricultural matrix (e.g., sugarcane).
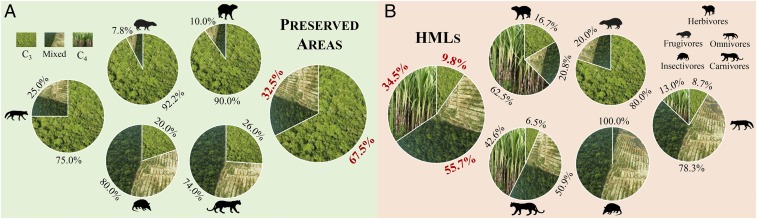
We observed a distinct difference in resource use by mammals between systems (Fig. 3), supporting our hypothesis. In the preserved areas, most species predominantly fed on C3 resources (Fig. 3A), showing dependency on the forest remnants and a low contribution of C4 resources. Conversely, in HMLs, ∼34.5% of individuals fed exclusively on C4 resources, indicating the intense use of items from the agricultural matrix and few individuals feeding only on C3 resources (Fig. 3B). Most species in HMLs used a mixture of C3/C4 resources, indicating the use of both the remnants and the matrix as feeding areas.
Fig. 3.
Resource use of mammals in the Atlantic Forest, state of São Paulo, Brazil. Percentages of individuals with C3, mixed, or C4 diets in trophic guilds (as indicated) in (A) preserved areas and (B) HMLs.
In both systems, frugivores depended on C3 resources, and insectivores presented small variation, using a mixture of C3/C4 resources. We observed drastic differences for 3 guilds between the 2 systems: herbivores switched from C3 to C4 resources, omnivores changed from predominantly C3 consumers to showing mixed use, and a relatively high proportion of carnivores consumed only C4 resources. No species in preserved areas presented δ13Ccorrected values corresponding to the C4 group.
Isotopic Niches.
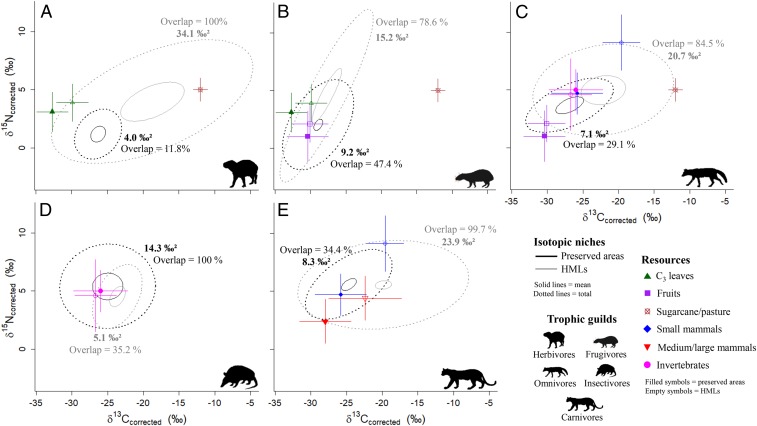
Isotopic niches in preserved areas were smaller than in HMLs in all cases except for insectivores (Fig. 4). The pattern presented by insectivores was reversed in comparison to those shown by the other trophic guilds, with a niche almost 3 times larger in preserved areas. The largest difference in niche size was observed among herbivores, with an isotopic niche more than 8 times larger in HMLs than in preserved areas. The isotopic niches of all guilds in HMLs overlapped most of the niches in preserved areas, except in the case of insectivores, which showed the reverse pattern, highlighting the high diversity of prey consumed in preserved areas (Fig. 4).
Fig. 4.
Mammal isotopic niches in the Atlantic Forest, state of São Paulo, Brazil. Corrected standard ellipse area (SEAc in ‰2; dotted lines), mean resource use (solid lines), and the percentage overlap of the different mammal trophic guilds in preserved areas (black) and HMLs (gray). (A) Herbivores, (B) frugivores, (C) omnivores, (D) insectivores, and (E) carnivores. Mean δ13C and δ15N values ±SD of the main resources consumed by each guild are included in the panels (filled forms indicate preserved areas; empty forms indicate HMLs).
Trophic Structure.
We observed a distinct difference in the trophic structure of mammals between the 2 systems (Fig. 2), supporting our hypothesis. In preserved areas, there were significant differences in mean δ15Ncorrected values among trophic guilds [ANOVA; F(4,121) = 25.99; P < 0.0001] (Fig. 2D), while the differences were not significant for HMLs [F(4,189) = 1.16; P = 0.16] (Fig. 2E). Comparing systems, we observed significant differences for herbivores [ANOVA; F(1,32) = 15.23; P = 0.0005], frugivores [F(1,67) = 9.93; P = 0.0024], and omnivores [F(1,52) = 4.92; P = 0.0309] (Fig. 2F) in addition to a wider range of values for all guilds in HMLs except for insectivores (SI Appendix, Table S2). These results indicate that mammals in HMLs that consume primary resources (e.g., fruits and leaves) present elevated δ15Ncorrected values.
Discussion
Resource Use.
Differences in landscape composition (i.e., the availability of C3/C4 resources) explain the changes in resource use by mammals between systems. In HMLs, more than one-third of the mammals exclusively used C4 resources, while the preserved areas were the closest representations of pristine Atlantic Forest remnants. Overall, there was a transition in the resource use of mammals between systems, from C3 to C4, demonstrating the impact of land use changes on species diets and habitat use. The size of the isotopic niches strengthens our findings, reflecting the plasticity of mammals in terms of their use of C4 resources from the matrix in HMLs (large niches) and the greater constraint in preserved areas (smaller niches).
These changes were most evident for herbivores, omnivores, and carnivores, which incorporated high proportions of C4 carbon in the HMLs. Grazing species, such as Hydrochoerus hydrochaeris, benefit from changes in landscape composition, presenting C4-dominated diets, and the high availability of C4 resources (e.g., sugarcane and corn) may stimulate the population growth of this species (22). Even arboreal browsers, such as Coendou spinosus, had individuals with C4-dominated diets, which contrasts with observations in the preserved areas. Moreover, the high δ15N values for herbivores in HMLs corroborate the consumption of items from the agricultural matrix, which have elevated δ15N values due to the use of organic fertilizers and cultivation methods (12, 14, 16, 23).
Omnivores presented C3-dominated diets in preserved areas, which is explained by the high availability of fruits (24, 25), an abundant C3 resource in these areas. Fruits are less available in HMLs; thus, omnivores may increase their consumption of small vertebrates (e.g., rodents, birds, and reptiles) and invertebrates, prey that feed on items from the agricultural matrix (7) and C4 crops (e.g., sugarcane and corn). These animals thus change to having a mixed diet, such as observed in species in the genus Didelphis. The elevated δ15N values of omnivores in HMLs are a consequence of this high intake of animal matter and nitrogen-enriched items. HMLs favor the presence of omnivores such as Didelphis albiventris and Chrysocyon brachyurus, which are tolerant to open habitats and absent from preserved areas, and support the augmentation of the populations of others, such as Cerdocyon thous (26), and the presence of these species contributes to the increased δ15N values of this guild.
Carnivores were the second guild that most consumed C4 resources in HMLs. This incorporation of C4 carbon is a reflection of the prey base, which includes several species with C4-dominant diets. Similar to the results obtained for pumas (Puma concolor) between landscapes with different levels of forest cover (7), other carnivore species in HMLs also increased the intake of C4 carbon in comparison to those in preserved areas. For example, small felids (genus Leopardus and Puma yagouaroundi) feed mainly on small mammals, which present higher δ13C values in HMLs (7) than in preserved areas (27). Even species considered to be sensitive to habitat loss, such as Leopardus pardalis and Leopardus wiedii (28), contained individuals with C4-dominant diets, indicating their plasticity and resilience to survive in HMLs. Nonetheless, most felids are threatened in Brazil, and trends indicate declines in their populations in response to anthropogenic pressure (28).
The resource use of frugivores and insectivores was less affected by land use changes; in the HMLs, frugivores were the only guild to preferentially use C3 resources. This feeding preference shows that even small, low-quality forest remnants are essential for providing food and habitat. Although they feed mainly on C3 resources in preserved areas, even threatened frugivores, such as Tapirus terrestris and Tayassu pecari (28), had individuals incorporating C4 carbon into their body structures. T. terrestris is known to feed in orchards and cultivated areas (29), while T. pecari herds raid crops and plantations (30), corroborating our results. Individuals of these species also present high δ15N values, similar to individuals of the genus Mazama and Cuniculus paca in HMLs, indicating the consumption of nitrogen-enriched food items (e.g., fruits in orchards).
Comparing systems, mammal insectivores showed small variation in resource use, reflecting the feeding specialization of armadillos (Dasypus novemcinctus and Cabassous tatouay) and anteaters (Myrmecophaga tridactyla and Tamandua tetradactyla), which feed mainly on termites and ants (20, 21). Termite δ13C values vary with the foraging substrate, which affects whether they have C3- or C4-dominant diets or mixed diets (31, 32), while ants have lower δ13C values (33, 34) depending on the C3 resources available. In both systems, insectivores presented mixed diets, but a few individuals in preserved areas fed exclusively on C3 prey. This variation is explained by the higher diversity of prey in preserved areas (35, 36); such diversity is lower in HMLs, but there is a higher abundance of generalist prey, increasing diet specificity. Insectivores in preserved areas presented a wider range of δ13C and δ15N values than those in HMLs, resulting in an isotopic niche that was 3 times larger, which is a reversed pattern compared to that found in the other guilds.
Trophic Structure.
In preserved areas, mammals showed an ordered trophic structure with increasing δ15N values, indicating 15N bioaccumulation from herbivores to carnivores. This scaling of 15N enrichment is expected for longer trophic chains (37), which commonly occur as an ecosystem increases in size (38). Our results for preserved areas point toward a structure similar to that in the Eltonian pyramid, which has also been observed among other terrestrial organisms, such as birds (39) and ants (40), and more commonly among aquatic organisms, such as fish (41), supporting our hypothesis.
Conversely, the mean δ15N values for different guilds in the HMLs were similar, especially considering the high δ15N values for primary consumers. Animals in HMLs are expected to have higher δ13C and δ15N values than those in preserved areas (23). In particular, mammals with mixed or C4-dominant diets presented higher δ15N values, as also noted in other studies (7, 16). Changes in trophic structure, similar to those reported here, were also recorded for other taxa in response to environmental disturbance (40–42). The results for carnivores were very similar between systems despite some individuals in HMLs having higher δ15N values than those in preserved areas. Nonetheless, HMLs also presented carnivorous individuals with the lowest δ15N values for this guild, resulting in similar mean values between systems. These findings show that human activities cause impacts beyond the loss of species richness and diversity, also changing species diets, habitat use, and trophic structure.
We considered 2 explanations for the distinct difference in trophic structure between the 2 systems. The first is based on species plasticity in terms of thriving in modified habitats, as we observed for most species in the HMLs, which consumed nitrogen-enriched items from the agricultural matrix. A second explanation for the difference between the 2 systems is related to species turnover, as most sensitive species disappear from HMLs, being replaced by generalist species and those tolerant to open habitats, an effect already detected in the Atlantic Forest (43).
Conclusions.
The scenario we assessed is similar to those in other biomes and ecosystems worldwide, particularly tropical areas dominated by agriculture. The agricultural landscape matrix, despite negatively affecting the richness, diversity and abundance of mammals (44–46), acts as a complementary or predominant feeding area and habitat for several species, showing that the habitat/nonhabitat dichotomy (44) is not the most adequate classification. Matrix management aimed at wildlife conservation has been recently discussed (9, 44–46) but is still an incipient matter. The replacement of natural ecosystems with agriculture has created conflict between production and conservation, and an understanding between these areas is necessary for biodiversity maintenance (47, 48). Based on the diets of mammal species, our study provides a first step in determining which species have the highest likelihood of surviving in HMLs. Future studies should address knowledge gaps regarding how items originating from the agricultural matrix may affect species diet quality and the long-term effects on population growth and species survival. Even if such effects are not hugely negative, continuous and large habitat remnants represent the last refuges for wildlife, are the most similar habitats to pristine areas, and are irreplaceable for the persistence of sensitive species (49). These habitats support assemblages with a more complex trophic structure than those in HMLs, acting as source areas for the maintenance of biodiversity in HMLs.
Materials and Methods
Study Areas.
We selected 2 systems in the Atlantic Forest biome for sampling: preserved areas and HMLs. We considered preserved areas to be those in a better conservation state with high forest cover. We selected 2 landscapes with similar structural and floristic compositions within the largest area of continuous forest in the Atlantic Forest (>1 Mha), state of São Paulo, Brazil (SI Appendix, Fig. S3 and Table S4). For the second system, we selected 2 HMLs with similar structural, floristic, and compositional characteristics, with low forest cover, and dominated by anthropogenic matrices in the state of São Paulo, Brazil (SI Appendix, Fig. S4 and Table S5).
Samples for SIA.
We used mammal hair for the SIA. For the preserved areas, we used hair originating from carnivore fecal samples, hair traps, and opportunistic collection from the field. For the HMLs, we used hair samples collected by the authors and collaborators who conducted other studies (e.g., refs. 7 and 50), including carnivore fecal samples and opportunistically collected samples; the methods used for sample collection and identification for the HMLs are described in detail in the aforementioned studies. Descriptions of the sampling procedures used in the preserved areas, fecal sample screening, hair identification, and preparation of samples for SIA are provided in SI Appendix.
SIA.
The prepared material was submitted to combustion in a CHN-1110 elemental analyzer (Carlo Erba), and the resultant gases were separated in a chromatographic column. Later, the gases were inserted into a coupled continuous flow isotope ratio mass spectrometer (Delta Plus; Thermo Scientific) to evaluate the isotopic composition of the samples. The isotopic values of carbon and nitrogen were expressed in delta notation (δ13C, δ15N) in per mil (‰) relative to the V-PDB (Vienna Pee Dee Belemnite) and atmospheric N2 standards, respectively. The delta values were calculated based on the standards using the following equation: δX = [(Rsample/Rstandard) − 1] multiplied by 1,000, where X represents the stable carbon or nitrogen isotopes (13C or 15N), and R represents the isotope ratio (13C/12C or 15N/14N).
We performed the replication of the same individual material for only 10% of the samples, but the precision of the analytical method for 22 replicates of an internal standard in all batches was estimated to be 0.09‰ for carbon and nitrogen. The samples were compared to international standards by the use of international reference materials: NBS-19 and NBS-22 for carbon and IAEA-N1 and IAEA-N2 for nitrogen.
Data Analysis.
Considering the difference in mammal assemblage composition between systems, we grouped species into trophic guilds following Paglia et al. (21) and Magioli et al. (50): herbivores, frugivores, omnivores, insectivores, and carnivores (SI Appendix, Table S6).
Resource Use.
We adapted the analytical approach used by Magioli et al. (7). The analysis consists of using a simple mixing model that interpolates the stable carbon isotopic values of the samples, accounting for specific fractionation factors, with the mean values of the different vegetation types (C3 and C4 plant photosynthetic cycles), also considering the minimum and maximum values obtained for all animal samples analyzed. To estimate fractionation factors (Δ13C and Δ15N) for most species without existing values, we used the SIDER package (51) available in R 3.4.3 (52), which estimates species-specific fractionation factors from phylogenetic regression models according to a database of fractionation values available for several species. We generated fractionation factors using the script available in Healy et al. (51) (SI Appendix, Table S6).
To determine the origin of food items consumed by mammals (i.e., forest remnants, C3, or agricultural matrix, C4), we calculated the C3 and C4 carbon content in each sample (δ13C values corrected by Δ13C values). After calculating the proportions of C3-/C4-derived carbon, we classified the samples into 3 groups: 1) C3 group, species that consumed only items from forest remnants (>70% C3 carbon; δ13C = −32 to −26‰); 2) mixed group, species that fed on items from both remnants and agricultural matrix (30 to 70% C3 carbon; δ13C = −25.9 to −18.1‰); and 3) C4 group, species that only consumed items from the agricultural matrix (<30% C3 carbon; δ13C = −18 to −12‰). Details of the calculation of the C3 and C4 carbon content are provided in SI Appendix.
We also compared the δ15N values within and between the groups in both systems. To do this, we corrected the δ15N values using the fractionation factors (Δ15N) generated by the SIDER package (SI Appendix, Table S6). We compared the percentages of individuals belonging to each group among trophic guilds and considering all samples analyzed in each system. We determined the significance of the differences in mean δ13Ccorrected and δ15Ncorrected values between guilds and systems using 1-way ANOVA and Tukey post hoc tests.
Isotopic Niche Analysis.
To assess the difference in resource use between trophic guilds, we analyzed the size of the isotopic niches using the SIBER package (53) in R 3.4.3. To account for the sample size, we used the SEA corrected (SEAc). To compare the isotopic niches between guilds, we calculated the Bayesian estimate of the SEA (SEAb). The ellipses were calculated and compared between guilds in the different systems, and their overlap was evaluated (details are provided in SI Appendix).
Trophic Structure.
To assess the differences in the trophic structure of the 2 systems, we used the mean δ15Ncorrected values. We identified significant differences in the trophic guilds within and between systems using 1-way ANOVA and Tukey post hoc tests.
Supplementary Material
Acknowledgments
We are grateful to the Forest Sciences Department (Luiz de Queiroz College of Agriculture, University of São Paulo); the Interdisciplinary Graduate Program in Applied Ecology; and the Wildlife Ecology, Management and Conservation Lab. We thank the São Paulo Research Foundation for the grants to M.M. (2014/10192-7) and K.M.P.M.d.B.F. (2014/09300-0). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil, Finance Code 001 (grant to M.M.). We thank National Council of Technological and Scientific Development for the productivity fellowship granted to K.M.P.M.d.B.F. (308503/2014-7 and 308632/2018-4). We thank the Fundação Grupo Boticário de Proteção à Natureza for the financial support (Project 201410014).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904384116/-/DCSupplemental.
References
- 1.Foley J. A., et al. , Global consequences of land use. Science 309, 570–574 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Gibbs H. K., et al. , Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Proc. Natl. Acad. Sci. U.S.A. 107, 16732–16737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gascon C., et al. , Matrix habitat and species richness in tropical forest remnants. Biol. Conserv. 91, 223–229 (1999). [Google Scholar]
- 4.Newbold T., et al. , Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Dirzo R., et al. , Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Tucker M. A., et al. , Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 359, 466–469 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Magioli M., et al. , Stable isotope evidence of Puma concolor (Felidae) feeding patterns in agricultural landscapes in southeastern Brazil. Biotropica 46, 451–460 (2014). [Google Scholar]
- 8.Franklin J. F., Lindenmayer D. B., Importance of matrix habitats in maintaining biological diversity. Proc. Natl. Acad. Sci. U.S.A. 106, 349–350 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driscoll D. A., Banks S. C., Barton P. S., Lindenmayer D. B., Smith A. L., Conceptual domain of the matrix in fragmented landscapes. Trends Ecol. Evol. 28, 605–613 (2013). [DOI] [PubMed] [Google Scholar]
- 10.del Rio C. M., Wolf N., Carleton S. A., Gannes L. Z., Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. Camb. Philos. Soc. 84, 91–111 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Crawford K., McDonald R. A., Bearhop S., Application of stable isotope techniques to the ecology of mammals. Mammal Rev. 38, 87–107 (2008). [Google Scholar]
- 12.Hobson K. A., Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 120, 314–326 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Boecklen W. J., Yarnes C. T., Cook B. A., James A. C., On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42, 411–440 (2011). [Google Scholar]
- 14.Rubenstein D. R., Hobson K. A., From birds to butterflies: Animal movement patterns and stable isotopes. Trends Ecol. Evol. 19, 256–263 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Ben-David M., Flaherty E. A., Stable isotopes in mammalian research: A beginner’s guide. J. Mammal. 93, 312–328 (2012). [Google Scholar]
- 16.Kelly J. F., Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 78, 1–27 (2000). [Google Scholar]
- 17.Mittermeier R. A., Turner W. R., Larsen F. W., Brooks T. M., Gascon C., “Global biodiversity conservation: The critical role of hotspots” in Biodiversity Hotspots, Zachos F. E., Habel J. C., Eds. (Springer Publishers, London, 2011), pp. 3–22. [Google Scholar]
- 18.Ribeiro M. C., Metzger J. P., Martensen A. C., Ponzoni F. J., Hirota M. M., The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 142, 1141–1153 (2009). [Google Scholar]
- 19.Chiarello A. G., Density and population size of mammals in remnants of Brazilian Atlantic Forest. Conserv. Biol. 14, 1649–1657 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Emmons L. H., Feer F., Neotropical Rainforest Mammals: A Field Guide (University of Chicago Press, Chicago, 1997). [Google Scholar]
- 21.Paglia A. P., et al. , “Annotated checklist of Brazilian mammals” in Occasional Papers in Conservation Biology (Conservation International, Arlington, VA, ed. 2, 2012), no. 6. [Google Scholar]
- 22.Ferraz K. M. B., Ferraz S. F. B., Moreira J. R., Couto H. T., Verdade L. M., Capybara (Hydrochoerus hydrochaeris) distribution in agroecosystems: A cross‐scale habitat analysis. J. Biogeogr. 34, 223–230 (2007). [Google Scholar]
- 23.Nadelhoffer K. J., Fry B., “N-isotope studies in forests” in Stable Isotopes in Ecology and Environmental Sciences, Lajtha K., Michener R. H., Eds. (Blackwell, Oxford, 1994), pp. 22–62. [Google Scholar]
- 24.Pessoa M. S., et al. , Deforestation drives functional diversity and fruit quality changes in a tropical tree assemblage. Perspect Plant Ecol Syst 28, 78–86 (2017). [Google Scholar]
- 25.Pessoa M. S., et al. , Fruit biomass availability along a forest cover gradient. Biotropica 49, 45–55 (2017). [Google Scholar]
- 26.Ferraz K. M. P. M. B., Siqueira M. F., Martin P. S., Esteves C. F., Couto H. T. Z., Assessment of Cerdocyon thous distribution in an agricultural mosaic, southeastern Brazil. Mammalia 74, 275–280 (2010). [Google Scholar]
- 27.Galetti M., Rodarte R. R., Neves C. L., Moreira M., Costa-Pereira R., Trophic niche differentiation in rodents and marsupials revealed by stable isotopes. PLoS One 11, e0152494 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Instituto Chico Mendes de Conservação da Biodiversidade, Ministério do Meio Ambiente , Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume II–Mamíferos (Instituto Chico Mendes de Conservação da Biodiversidade, Ministério do Meio Ambiente, Brasília, 2018). [Google Scholar]
- 29.Galetti M., Keuroghlian A., Hanada L., Inez Morato M., Frugivory and seed dispersal by the lowland tapir (Tapirus terrestris) in southeast Brazil. Biotropica 33, 723–726 (2001). [Google Scholar]
- 30.Mayer J. J., Wetzel R. M., Tayassu pecari. Mamm. Species 293, 1–7 (1987). [Google Scholar]
- 31.Spain A. V., Reddell P., δ13C values of selected termites (Isoptera) and termite-modified materials. Soil Biol. Biochem. 28, 1585–1593 (1996). [Google Scholar]
- 32.Sponheimer M., et al. , Hominins, sedges, and termites: New carbon isotope data from the Sterkfontein valley and Kruger National Park. J. Hum. Evol. 48, 301–312 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Parmentier T., Bouillon S., Dekoninck W., Wenseleers T., Trophic interactions in an ant nest microcosm: A combined experimental and stable isotope (δ13C/δ15N) approach. Oikos 125, 1182–1192 (2016). [Google Scholar]
- 34.Penick C. A., Savage A. M., Dunn R. R., Stable isotopes reveal links between human food inputs and urban ant diets. Proc. Biol. Sci. 282, 20142608 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bihn J. H., Verhaagh M., Brändle M., Brandl R., Do secondary forests act as refuges for old growth forest animals? Recovery of ant diversity in the Atlantic forest of Brazil. Biol. Conserv. 141, 733–743 (2008). [Google Scholar]
- 36.Martello F., et al. , Homogenization and impoverishment of taxonomic and functional diversity of ants in Eucalyptus plantations. Sci. Rep. 8, 3266 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perkins M. J., et al. , Application of nitrogen and carbon stable isotopes (δ15N and δ13C) to quantify food chain length and trophic structure. PLoS One 9, e93281 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Post D. M., Pace M. L., Hairston N. G. Jr, Ecosystem size determines food-chain length in lakes. Nature 405, 1047–1049 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Herrera L. G., Hobson K. A., Rodríguez M., Hernandez P., Trophic partitioning in tropical rain forest birds: Insights from stable isotope analysis. Oecologia 136, 439–444 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Blüthgen N., Gebauer G., Fiedler K., Disentangling a rainforest food web using stable isotopes: Dietary diversity in a species-rich ant community. Oecologia 137, 426–435 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Layman C. A., Quattrochi J. P., Peyer C. M., Allgeier J. E., Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa M., Hyodo F., Nakashizuka T., Effect of forest use on trophic levels of small mammals: An analysis using stable isotopes. Can. J. Zool. 85, 472–478 (2007). [Google Scholar]
- 43.Bogoni J. A., et al. , What would be the diversity patterns of medium-to large-bodied mammals if the fragmented Atlantic Forest was a large metacommunity? Biol. Conserv. 211, 85–94 (2017). [Google Scholar]
- 44.Prugh L. R., Hodges K. E., Sinclair A. R., Brashares J. S., Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. U.S.A. 105, 20770–20775 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brady M. J., McAlpine C. A., Possingham H. P., Miller C. J., Baxter G. S., Matrix is important for mammals in landscapes with small amounts of native forest habitat. Landsc. Ecol. 26, 617–628 (2011). [Google Scholar]
- 46.Watling J. I., Nowakowski A. J., Donnelly M. A., Orrock J. L., Meta‐analysis reveals the importance of matrix composition for animals in fragmented habitat. Glob. Ecol. Biogeogr. 20, 209–217 (2011). [Google Scholar]
- 47.Martinelli L. A., Naylor R., Vitousek P. M., Moutinho P., Agriculture in Brazil: Impacts, costs, and opportunities for a sustainable future. Curr. Opin. Environ. Sustain. 2, 431–438 (2010). [Google Scholar]
- 48.Ferreira J., et al. , Towards environmentally sustainable agriculture in Brazil: Challenges and opportunities for applied ecological research. J. Appl. Ecol. 49, 535–541 (2012). [Google Scholar]
- 49.Gibson L., et al. , Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Magioli M., et al. , Connectivity maintain mammal assemblages functional diversity within agricultural and fragmented landscapes. Eur. J. Wildl. Res. 62, 431–446 (2016). [Google Scholar]
- 51.Healy K., et al. , SIDER: An R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography 41, 1393–1400 (2018). [Google Scholar]
- 52.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.4.3, R Foundation for Statistical Computing, Vienna, Austria, 2018) Electronic Database. http://www.R-project.org/. Accessed 10 January 2018.
- 53.Jackson A. L., Inger R., Parnell A. C., Bearhop S., Comparing isotopic niche widths among and within communities: SIBER—Stable isotope Bayesian ellipses in R. J. Anim. Ecol. 80, 595–602 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.