Abstract
Cyanobacterial diazotrophs are considered to be the most important source of fixed N2 in the open ocean. Biological N2 fixation is catalyzed by the extremely O2-sensitive nitrogenase enzyme. In cyanobacteria without specialized N2-fixing cells (heterocysts), mechanisms such as decoupling photosynthesis from N2 fixation in space or time are involved in protecting nitrogenase from the intracellular O2 evolved by photosynthesis. However, it is not known how cyanobacterial cells limit O2 diffusion across their membranes to protect nitrogenase in ambient O2-saturated surface ocean waters. Here, we explored all known genomes of the major marine cyanobacterial lineages for the presence of hopanoid synthesis genes, since hopanoids are a class of lipids that might act as an O2 diffusion barrier. We found that, whereas all non−heterocyst-forming cyanobacterial diazotrophs had hopanoid synthesis genes, none of the marine Synechococcus, Prochlorococcus (non−N2-fixing), and marine heterocyst-forming (N2-fixing) cyanobacteria did. Finally, we conclude that hopanoid-enriched membranes are a conserved trait in non−heterocyst-forming cyanobacterial diazotrophs that might lower the permeability to extracellular O2. This membrane property coupled with high respiration rates to decrease intracellular O2 concentration may therefore explain how non−heterocyst-forming cyanobacterial diazotrophs can fix N2 in the fully oxic surface ocean.
Keywords: oxygen diffusion barrier, hopanoid lipids, nitrogen fixation, marine cyanobacteria
Marine cyanobacterial diazotrophs, i.e., those capable of reducing dissolved dinitrogen gas (N2) into ammonia through N2 fixation, are key suppliers of bioavailable N, a limiting nutrient for primary production in the ocean (1). Biological N2 fixation is solely performed by the O2-sensitive nitrogenase enzyme (2), and understanding how low intracellular O2 concentrations are maintained in fully oxic open waters is a long-standing question that has attracted much interest (3–6).
Although, a priori, it would seem that N2 fixation is incompatible with the O2-evolving photosynthetic lifestyle of cyanobacteria, it is known that these microorganisms have evolved a variety of strategies to protect nitrogenase from O2 inactivation. For example, some filamentous cyanobacteria, including the symbionts of marine diatoms, form specialized cells called heterocysts (7). A microaerobic environment is created inside heterocysts by inactivating oxygenic photosynthesis, by maintaining or enhancing respiration, and by the formation of an extra glycolipid cell envelope outside the cell wall (8). In contrast, non−heterocyst-forming cyanobacteria such as the filamentous Trichodesmium or the free-living unicellular Crocosphaera must separate photosynthesis and N2 fixation either spatially or temporally to avoid exposing nitrogenase to the O2 that they produce during the light hours (9, 10). In the unicellular cyanobacterial symbiont UCYN-A, all of the genes for the synthesis of the O2-evolving photosystem II (PSII) apparatus have been lost and so UCYN-A doesn’t generate O2 (11). None of the aforementioned strategies, however, can protect nitrogenase of non−heterocyst-forming cyanobacterial diazotrophs from the O2 that diffuses across cell membranes from the environment (including host photosynthesis in the case of UCYN-A). Mechanisms such as respiration, the Mehler reaction, and/or other O2 scavenging strategies have been proposed as potential ways to overcome this problem (12), but whether these mechanisms are sufficient to lower the O2 concentration in the inner cell while N2 fixation takes place remains unknown.
We have discovered a consistent pattern of distribution of hopanoid synthesis genes among marine cyanobacteria that suggests that they may play an important role in marine N2 fixation. Hopanoids are a class of membrane lipids that have been shown to confer special properties to cell membranes (13). Hopanoids can intercalate into lipid bilayers of membranes due to their planar and hydrophobic structure and might decrease their permeability to O2 (14). Approximately 10% of bacteria, including plant-associated diazotrophs, have the gene for the synthesis of hopanoids (the squalene−hopene cyclase gene shc) (13). Interestingly, the only direct evidence showing that hopanoids facilitate N2 fixation comes from studies of the terrestrial N2-fixing heterotrophic bacteria Frankia. In Frankia sp., hopanoids might serve as an O2 diffusion barrier in their N2-fixing vesicles (15), with the thickness of the vesicle envelope directly correlated to the external O2 concentration (16). However, this linkage was later questioned based on the observation of high proportions of hopanoids in membranes regardless of the N status in Frankia sp. (17).
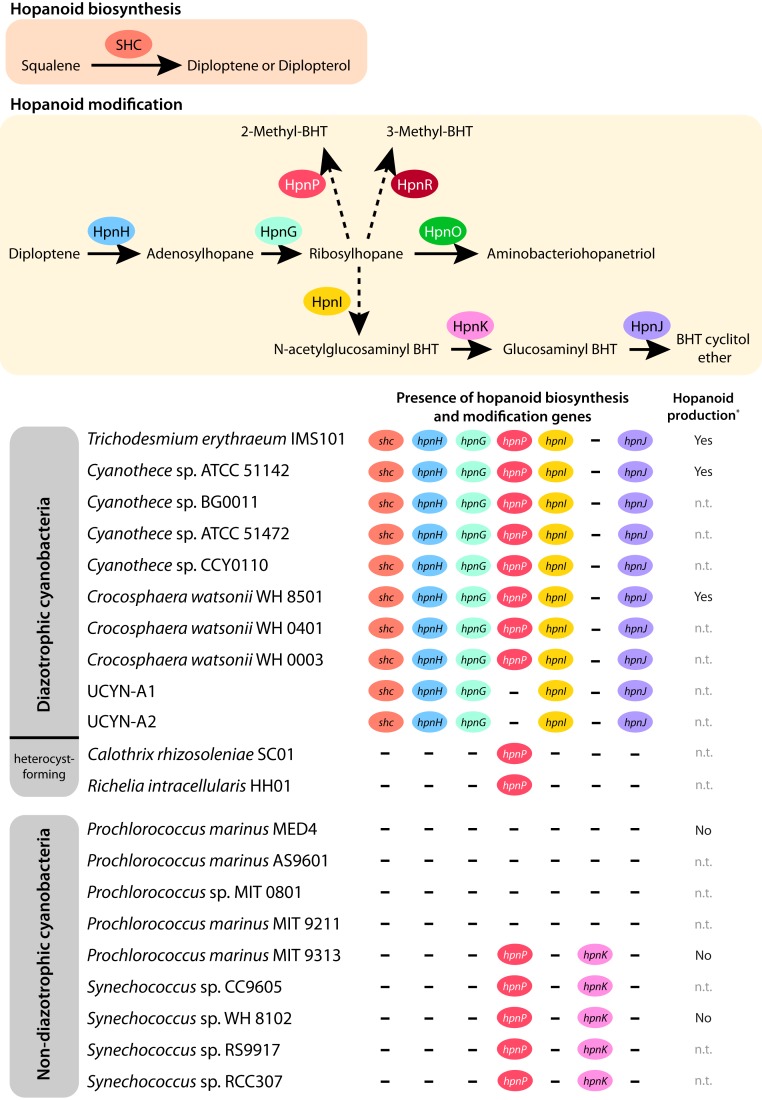
We compiled data on hopanoid production and mined the publicly available genomes of marine cyanobacteria to provide an exploration of the presence of hopanoid biosynthetic and modification genes across all of the major marine cyanobacterial lineages, including both diazotrophs and non-diazotrophs (Fig. 1). We found that the shc gene for synthesizing hopanoids was consistently present in all of the non−heterocyst-forming cyanobacterial diazotrophs, including unicellular cyanobacterial symbionts with extremely reduced genomes such as UCYN-A. In contrast, none of the non-diazotrophic marine Synechococcus and Prochlorococcus, which are the dominant cyanobacteria in the ocean (18, 19), nor the heterocyst-forming marine cyanobacteria Calothrix rhizosoleniae SC01 and Richelia intracellularis HH01 had the shc gene in their genomes. The same pattern was observed for almost all of the hopanoid modification genes except for the hpnK and hpnP genes (Fig. 1). These observations suggest that, whereas the capacity of allocating hopanoids into cell membranes may be universal across all marine non−heterocyst-forming diazotrophic cyanobacteria, it is absent from all marine Synechococcus and Prochlorococcus (which do not fix N2) and from heterocyst-forming marine cyanobacteria (which already protect nitrogenase from O2 by heterocysts). Furthermore, in Crocosphaera and Cyanothece, the transcription of the shc gene peaks right before the nitrogenase-encoding gene (nifH) starts increasing its expression level (data collected from ref. 20), and simultaneous expression of both markers has also been detected in UCYN-A (21). These patterns are further supported by previous observations of hopanoid production in the cyanobacterium Crocosphaera watsonii WH8501 in the context of N2 fixation (22, 23). However, the role of hopanoids in N2 fixation was discarded because C. watsonii WH8501 showed constant levels of hopanoids regardless of light−dark periods or the availability of fixed N (23).
Fig. 1.
Hopanids in marine cyanobacteria. (Upper) A schematic representation of the hopanoid biosynthesis and modification pathways, including enzymes and products. (Lower) Summary of the presence/absence of the genes involved in the synthesis and modification of hopanoids across a selection of the major marine cyanobacterial lineages. All of the available marine cyanobacterial genomes in NCBI (May 2019) were screened for this analysis, yet only 21 are shown, for simplification. Asterisk (*), experimentally tested in refs. 20 and 21 (n.t., not tested). Enzymes participating in hopanoid pathways: squalene−hopene cyclase (SHC), hopanoid biosynthesis-associated radical SAM protein (HpnH), hopanoid-associated phosphorylase (HpnG), hopanoid biosynthesis-associated glycosyltransferase protein (HpnI), hopanoid biosynthesis-associated protein (HpnK), hopanoid biosynthesis-associated radical SAM protein (HpnJ), aminotransferase (HpnO), hopanoid 2-methyltransferase (HpnP), and hopanoid C3 methylase (HpnR); 3-methylhopanoid production has never been found in marine cyanobacteria (28); hpnO was absent in all of the screened strains. Dashed arrows indicate that enzymes driving intermediate steps are unknown. See ref. 13 for further details on hopanoid biosynthesis.
We thus propose that the presence of hopanoids in the whole-cell membrane is a conserved trait in marine non−heterocyst-forming cyanobacterial diazotrophs that might confer protection to nitrogenase by reducing the rate of diffusion of extracellular O2 into the cell. In parallel, as shown for Cyanothece (24), increases in respiration rates can presumably lower the intracellular O2 concentration to levels suitable for nitrogenase activity while fulfilling the adenosine 5′-triphosphate (ATP) demand required for N2 fixation. Although the constant levels of hopanoids to total lipids has previously been argued to discount a role of hopanoids in marine N2 fixation (23), we believe that hopanoids reduce O2 membrane permeability that limits the diffusion rate and facilitates respiratory protection of nitrogenase. It is also possible that hopanoids can form rafts, i.e., membrane microdomains with high hopanoid content that promote dynamic changes in membrane permeability based on redistributions of hopanoid molecules in the membrane (13). Hopanoid rafts have been detected in C. watsonii (25), which suggests that Crocosphaera might have such dynamic changes in membrane permeability.
Since members of non−heterocyst-forming freshwater cyanobacteria (e.g., Aphanothece, Pleurocapsa, endosymbionts of the diatoms Rhopalodia gibberula and Epithemia turgida) and noncyanobacterial diazotrophs (e.g., Azotobacter) also have the shc gene, we believe that our hypothesis, which provides a mechanism that restricts O2 diffusion analogous to the heterocyst, may provide an important research direction for future studies devoted to understanding N2 fixation in different environments (marine, freshwater, terrestrial) as well as other O2-sensitive processes (e.g., methanogenesis) when happening in well-oxygenated environments (26, 27).
Acknowledgments
We especially thank Mick Follows for inspiring discussions and for reading early versions of the manuscript. We also thank Linda Jahnke, Clara Ruiz-Gonzalez, Marine Landa, Kendra Turk-Kubo, and Ana M. Cabello for their useful comments. J.P.Z. was supported by Simons Collaboration on Ocean Processes and Ecology (SCOPE, Grant 329108), Simons Foundation (Grant 545171), and Gordon and Betty Moore Foundation (Grant 493.01). F.M.C.-C. was supported by a Marie Curie Individual Global Fellowship - Horizon 2020 European Framework Programme (UCYN2PLAST, Grant 749380).
Footnotes
The authors declare no conflict of interest.
References
- 1.Karl D., et al. , Dinitrogen fixation in the world’s oceans. Biogeochemistry 57/58, 47–98 (2002). [Google Scholar]
- 2.Gallon J. R., Reconciling the incompatible: N2 fixation and O2. New Phytol. 122, 571–609 (1992). [Google Scholar]
- 3.Bergman B., Sandh G., Lin S., Larsson J., Carpenter E. J., Trichodesmium—A widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiol. Rev. 37, 286–302 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zehr J. P., Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Stal L. J., Is the distribution of nitrogen-fixing cyanobacteria in the oceans related to temperature? Environ. Microbiol. 11, 1632–1645 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Staal M., Meysman F. J. R., Stal L. J., Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans. Nature 425, 504–507 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Villareal T. A., “Marine nitrogen-fixing diatom-cyanobacteria symbioses” in Marine Pelagic Cyanobacteria Trichodesmium Other Diazotrophs, Carpenter E. J., Capone D. G., Rueter J. G., Eds. (Springer, Dordrecht, The Netherlands, 1992), pp. 163–175. [Google Scholar]
- 8.Wolk C. P., Ernst A., Elhai J., “Heterocyst metabolism and development” in The Molecular Biology of Cyanobacteria, Bryant D. A., Ed. (Springer, Dordrecht, The Netherlands, 1994), pp. 769–823. [Google Scholar]
- 9.Fay P., Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56, 340–373 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berman-Frank I., Lundgren P., Falkowski P., Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 154, 157–164 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Zehr J. P., et al. , Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 322, 1110–1112 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Staal M., Rabouille S., Stal L. J., On the role of oxygen for nitrogen fixation in the marine cyanobacterium Trichodesmium sp. Environ. Microbiol. 9, 727–736 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Belin B. J., et al. , Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Microbiol. 16, 304–315 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poger D., Mark A. E., The relative effect of sterols and hopanoids on lipid bilayers: When comparable is not identical. J. Phys. Chem. B 117, 16129–16140 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Berry A. M., et al. , Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. U.S.A. 90, 6091–6094 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons R., Silvester W. B., Harris S., Gruijters W. T., Bullivant S., Frankia vesicles provide inducible and absolute oxygen protection for nitrogenase. Plant Physiol. 83, 728–731 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalin R., et al. , High hopanoid/total lipids ratio in Frankia mycelia is not related to the nitrogen status. Microbiology 146, 3013–3019 (2000). [DOI] [PubMed] [Google Scholar]
- 18.Flombaum P., et al. , Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. U.S.A. 110, 9824–9829 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partensky F., Hess W. R., Vaulot D., Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 63, 106–127 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muñoz-Marín M. D. C., et al. , The transcriptional cycle is suited to daytime N2 fixation in the unicellular cyanobacterium “Candidatus Atelocyanobacterium thalassa” (UCYN-A). MBio 10, e02495-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornejo-Castillo F. M., et al. , Cyanobacterial symbionts diverged in the late Cretaceous towards lineage-specific nitrogen fixation factories in single-celled phytoplankton. Nat. Commun. 7, 11071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbot H. M., et al. , Cyanobacterial bacteriohopanepolyol signatures from cultures and natural environmental settings. Org. Geochem. 39, 232–263 (2008). [Google Scholar]
- 23.Sáenz J. P., Waterbury J. B., Eglinton T. I., Summons R. E., Hopanoids in marine cyanobacteria: Probing their phylogenetic distribution and biological role. Geobiology 10, 311–319 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Bandyopadhyay A., Elvitigala T., Liberton M., Pakrasi H. B., Variations in the rhythms of respiration and nitrogen fixation in members of the unicellular diazotrophic cyanobacterial genus Cyanothece. Plant Physiol. 161, 1334–1346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sáenz J. P., Hopanoid enrichment in a detergent resistant membrane fraction of Crocosphaera watsonii: Implications for bacterial lipid raft formation. Org. Geochem. 41, 853–856 (2010). [Google Scholar]
- 26.Angle J. C., et al. , Methanogenesis in oxygenated soils is a substantial fraction of wetland methane emissions. Nat. Commun. 8, 1567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogard M. J., et al. , Oxic water column methanogenesis as a major component of aquatic CH4 fluxes. Nat. Commun. 5, 5350 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Welander P. V., Summons R. E., Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production. Proc. Natl. Acad. Sci. U.S.A. 109, 12905–12910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]