Significance
Early maturing rice cultivars usually have a low yield, suffering from a shortening maturity duration in comparison with late-maturing ones. Here we demonstrate that shortening of maturity duration with no yield penalty of rice can be achieved by the quantitative trait locus Early flowering-completely dominant (Ef-cd). Ef-cd is a long noncoding RNA transcribed from the antisense strand of the flowering activator OsSOC1 locus, which can positively regulate the expression of OsSOC1. Physiological analysis demonstrated that Ef-cd could facilitate nitrogen utilization and also improve the photosynthesis rate. This study provides breeders with a valuable genetic resource that is useful for balancing grain yield with maturity duration for further elevating food production in the world.
Keywords: lncRNA, rice, heading date, yield
Abstract
The contradiction between “high yielding” and “early maturing” hampers further improvement of annual rice yield. Here we report the positional cloning of a major maturity duration regulatory gene, Early flowering-completely dominant (Ef-cd), and demonstrate that natural variation in Ef-cd could be used to overcome the above contradictory. The Ef-cd locus gives rise to a long noncoding RNA (lncRNA) antisense transcript overlapping the OsSOC1 gene. Ef-cd lncRNA expression positively correlates with the expression of OsSOC1 and H3K36me3 deposition. Field test comparisons of early maturing Ef-cd near-isogenic lines with their wild types as well as of the derivative early maturing hybrids with their wild-type hybrids conducted under different latitudes determined that the early maturing Ef-cd allele shortens maturity duration (ranging from 7 to 20 d) without a concomitant yield penalty. Ef-cd facilitates nitrogen utilization and also improves the photosynthesis rate. Analysis of 1,439 elite hybrid rice varieties revealed that the 16 homozygotes and 299 heterozygotes possessing Ef-cd matured significantly earlier. Therefore, Ef-cd could be a vital contributor of elite early maturing hybrid varieties in balancing grain yield with maturity duration.
The growing world population calls for continued increases in food production (1). Elevating grain yield per unit area is one of the most effective ways to increase food production because urbanization continues to decrease the area available for growing crops (2). Normally, a crop plant which produces higher yield needs a longer growth period because the production rate of carbohydrate via photosynthesis is certainly restricted (3, 4). In other words, early maturing cultivars usually have a low yield, suffering from shortening maturity duration in comparison with late-maturing cultivars.
The first generation of green revolution semi-dwarf varieties, such as IR8, IR20, IR24, and IR26, successfully provided large yield increases; however, these varieties required up to 160 d or longer for maturing or harvesting (5, 6). Likewise, Shanyou1 and Shanyou2, the first generation of hybrid cultivars commercialized in the late 1970s in China, also offer notable yield increase, but are again characterized as having a slow harvest turnaround time (5, 7, 8). In rice breeding, “high yielding” and “early maturing” remains a long-standing paradox, and the development of early maturing and high-yielding cultivars has been a great challenge.
Rice breeders have invested much effort to develop early maturing cultivars that allow for multiple crops per year. Ce64 is such an early maturing restorer line, and its derivative hybrids, Weiyou64 and Shanyou64, were the first 2 hybrid cultivars that could produce 2 crops a year (9). Subsequently, another early maturing restorer line, Minghui77, has played a leading role, demonstrated by 7,446,700 ha covered by its derivative hybrids from 1991 to 2010 in China (10). Similarly, the International Rice Research Institute (IRRI) released the earlier maturing IR36 with 110 d of maturity in 1976 and subsequently released IR50 with 105 d and IR58 with 100 d (6). Until now, although many related genes have been cloned and functionally characterized (11, 12), it is still difficult to overcome the negative association that causes early maturing cultivars to suffer a yield penalty from shortened vegetative growth periods. Here, we addressed this by cloning the dominant Early flowering-completely dominant (Ef-cd) gene and found that the Ef-cd locus significantly shortens maturity durations to around 7 to 20 d in near-isogenic lines (NILs) and hybrid cultivars without causing a yield penalty.
Ef-cd, first discovered from the male sterile line 6442S-7 as a dominant locus for earliness, has been mapped to the short arm on chromosome 3 (13, 14). Then Ef-cd was introgressed into 6 late-maturing cultivars, creating a set of 6 high-generation NILs for Ef-cd (ranging from BC5 to BC13, and F9 to F14) (SI Appendix, Fig. S1A). When tested under 3 different photoperiod environments across China, the NILs headed consistently earlier than their recurrent parents, flowering on average 8.9 to 19.7 d earlier depending on geographical location (SI Appendix, Fig. S1B).
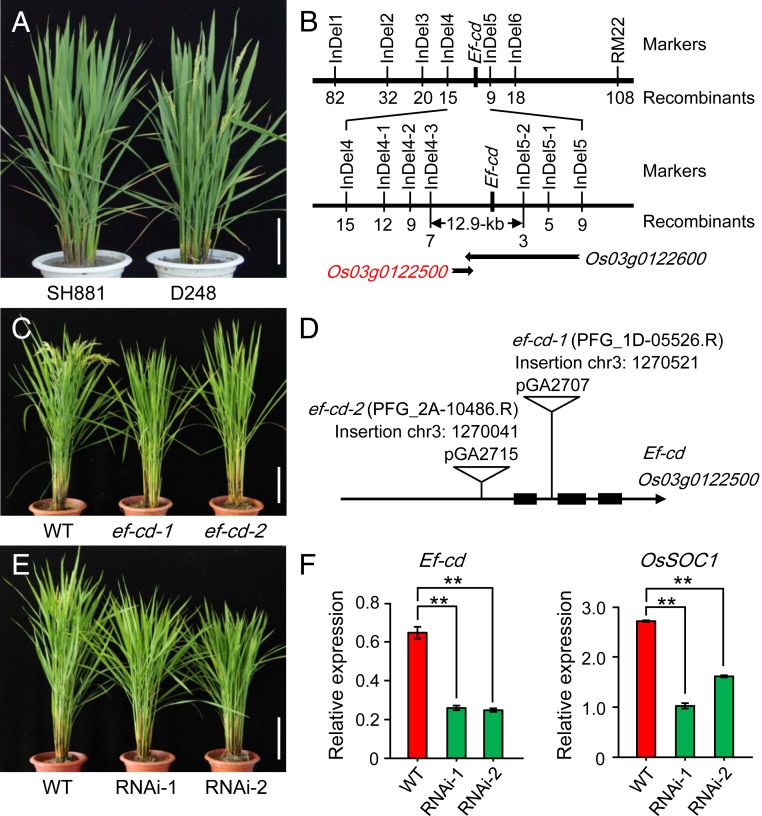
To identify the underlying gene for Ef-cd, we conducted high-resolution mapping using 4,800 F2 late-maturing plants derived from a cross between early maturing NIL D248 (BC5F11) and its recurrent parent Shuhui881 (SH881) (Fig. 1A). Finally, we fine-mapped Ef-cd in a 12.9-kb interval flanked by InDel4-3 and InDel5-2 (Fig. 1B). The marker InDel5-2 was located in the first intron of Os03g0122600, which indicated that Os03g0122500 and part of Os03g0122600 was located in the mapped region (SI Appendix, Fig. S2). Os03g0122600, also known as OsSOC1/OsMADS50/DTH3, was previously reported as a flowering activator (15, 16). Os03g0122500 is transcribed from the antisense strand of Os03g0122600, and its transcription is supported by a full-length cDNA AK242050 that is annotated as a long noncoding RNA (lncRNA) (https://rapdb.dna.affrc.go.jp). Compared with SH881, D248 harbors a number of genetic variations in the promoter region, as well as 1 insertion in the first intron and 1 single-nucleotide polymorphism in the second intron of Os03g0122500 (SI Appendix, Fig. S2).
Fig. 1.
Os03g0122500 was the causal gene of Ef-cd. (A) NIL D248 headed earlier than its recurrent parent Shuhui881 (SH881). (B) Ef-cd was mapped in a 12.9-kb region. (C) Two T-DNA insertion mutants of Os03g0122500 headed later than the wild-type Hwayoung. (D) The insertion occurred in the first intron of Os03g0122500 in mutant ef-cd-1 and in the promoter region of Os03g0122500 in mutant ef-cd-2. The triangle represents the T-DNA insertion. The types of the binary vectors used for constructing the T-DNA insertion mutants are above the triangles. (E) Two RNAi lines of Os03g0122500 headed later than their wild-type Nipponbare. (F) Expression levels of Ef-cd and OsSOC1 were significantly decreased in the RNAi lines compared to their wild type. The P value was calculated using Student’s t test. **P < 0.01. (Scale bars, 20 cm.)
It is a common challenge to disentangle the individual effects of sense and antisense transcripts, as altered antisense transcript may simultaneously affect the expression of sense transcript (17–19). Similar to the Ef-cd transcribed from the antisense strand of the OsSOC1 locus, in humans, a lncRNA HOTTIP is transcribed from the HOXA locus, and ectopic expression of HOTTIP RNA failed to activate expression of the distal HOXA gene, suggesting that the precise genomic distance between lncRNA and its target gene is critical for the colinear activation (20). Since the entire OsSOC1 spans nearly 30 kb in the rice genome, and also the early maturing NILs and their recurrent parents are indica varieties which are recalcitrant to transform, it is difficult to perform complementation experiments with constructs containing Ef-cd or OsSOC1. To verify that Os03g0122500 but not OsSOC1 was Ef-cd, we screened T-DNA libraries and luckily found 2 ingenious T-DNA insertion mutant lines, one (ef-cd-1) with a insertion in the first intron of Os03g0122500 and the other (ef-cd-2) with an insertion in the promoter region of Os03g0122500 but away from the 3′UTR of OsSOC1. As a result of the lesions in Os03g0122500, both T-DNA lines headed later than the wild type (Fig. 1 C and D). We also generated a mutant harboring a 158-bp deletion in the promoter of Os03g0122500 by using CRISPR/Cas9 technology, and the mutant also showed an expected later flowering phenotype (SI Appendix, Fig. S3 A–C). Furthermore, a fragment in the first exon of Os03g0122500 was used to generate customized RNA interference (RNAi) transgenic plants for Os03g0122500. All of the RNAi transgenic lines with reduced expression of Os03g0122500 showed a later flowering phenotype (Fig. 1 E and F). As there was not any variant in the Os03g0122500-coding region between NIL D248 and SH881, we hypothesized that the variants in its promoter region might affect its transcription. To this end, we fused the promoter fragments from NIL D248 and its recurrent parent SH881, respectively, with the firefly luciferase-coding sequence and assayed the activity in vitro. The luciferase activity of pD248::LUC was 7.34 times higher than that of pSH881::LUC (SI Appendix, Fig. S3D), indicating that the promoter of Os03g0122500 in D248 has much higher activity. All of the above results cumulatively indicate that Os03g0122500 is Ef-cd, as nucleotide variants in the promoter of Os03g0122500 modulated its transcriptional activity and contributed to the phenotypic consequences.
Because the floral transition of wild-type plants (SH881) generally occurred at about the 60th day after germination in both long-day and short-day conditions, we checked Ef-cd and OsSOC1 expression levels before this stage. The results showed stronger expression of Ef-cd and OsSOC1 in the early maturing NIL D248 than in its wild type, and the expression levels of Ef-cd and OsSOC1 were positively correlated (SI Appendix, Fig. S4 A and B). Furthermore, the OsSOC1 expression levels were significantly reduced in the ef-cd T-DNA mutants (SI Appendix, Fig. S4 C and D). In contrast, the Ef-cd expression level was not significantly reduced in the Ossoc1 mutant plants (SI Appendix, Fig. S4 E and F). These results suggest that Ef-cd regulates the expression of OsSOC1, but not vice versa. In addition, the expression levels of both Hd3a and RFT1 (2 rice florigen genes) were significantly higher in D248 than those in SH881 in both short-day and long-day conditions (SI Appendix, Fig. S4 G and H).
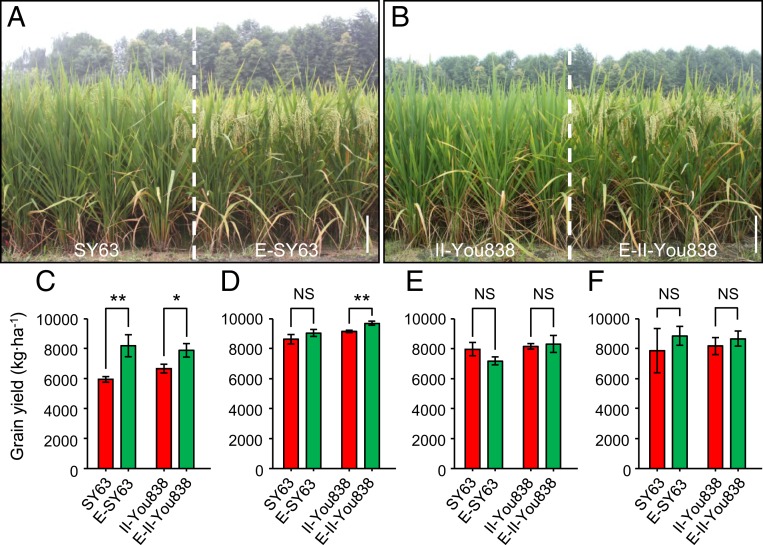
Field tests revealed that, except for plant height, there were no statistical differences between early maturing NILs and their late-maturing recurrent parents for the yield-related agronomic traits, demonstrating no yield penalty from Ef-cd (Dataset S1). Shanyou63 (SY63) is a hybrid cultivar with the largest planting area in China, as is II-You838 in southern China. SY63 is derived from the cross between the male-sterile line Zhenshan97A (ZS97A) and the restorer line Minghui63 (MH63), and II-You838 is derived from the cross between the male-sterile line II-32A and the restorer line Fuhui838 (FH838). Using NILs containing the early maturing Ef-cd allele in the MH63 (named D330) or II-32A (named E-II-32A) background, we developed F1 hybrids derived from crosses between ZS97A and D330 (named E-SY63) and from crosses between E-II-32A and FH838 (named E-II-You838). Then we conducted pairwise field testing of E-SY63 (ZS97A/D330) with SY63 (ZS97A/MH63) and of E-II-You838 (E-II-32A/FH838) with II-You838 (II-32A/FH838) in 4 different locations across China. Both of the Ef-cd NIL hybrids matured earlier than their corresponding late-maturing hybrids in all 4 locations (Fig. 2 A and B). More interestingly, the 2 NIL hybrids produced greater yields than their corresponding hybrids in Beijing (Fig. 2C), and 1 NIL hybrid produced a greater yield in Jiaxing (Fig. 2D), while yields did not differ in the other 2 locations (Fig. 2 E and F). These results strongly demonstrate no yield penalty in hybrids that matured earlier due to introgression of the Ef-cd locus into the parental male-sterile lines and/or restorer lines of hybrid rice. It should be noted that the 2 NIL hybrids headed earlier in a range from 6.9 to 15.9 d in 4 different locations, suggesting that Ef-cd might also coordinate with the environmental factors, such as temperature and day length, in addition to genetic background.
Fig. 2.
Analysis of heading date and grain yield for the effect of Ef-cd in 4 different latitudes including Beijing (39°54′ N, Beijing City), Jiaxing (30°75′ N, Zhejiang Province), Chengdu (30°42′ N, Sichuan Province), and Fuzhou (26°08′ N, Fujian Province). (A) Ef-cd NIL hybrid E-SY63 (ZS97A/D330) headed and matured earlier than the corresponding hybrid SY63 (ZS97A/MH63) in the 4 different latitudes. (B) Ef-cd NIL hybrid E-II-You838 (E-II-32A/FH838) headed and matured earlier than the corresponding hybrid II-You838 (II-32A/FH838) in the 4 different latitudes. Statistical comparison of the grain yields between SY63 and E-SY63 and II-You838 and E-II-You838 in (C) Beijing (sowing date was May 8, 2015), (D) Jiaxing (sowing date was June 30, 2015), (E) Chengdu (sowing date was May 15, 2015), and (F) Fuzhou (sowing date was June 18, 2015). The P value was calculated using Student’s t test. *P < 0.05; **P < 0.01; NS, not significant. (Scale bars, 20 cm.)
To explore its physiological basis, we conducted 15N-nitrate and 15N-ammonium feeding experiments with the early maturing NIL and its recurrent parent and found that the acquisition of nitrate and ammonium increased significantly more in the early maturing NIL than in the recurrent parent (SI Appendix, Fig. S5). In addition, we also measured the physiological features of E-SY63 and SY63, and the results showed that the leaf length, leaf width, leaf area index, chlorophyll concentration, and light-saturated photosynthetic rate were all significantly increased in E-SY63 compared with those in SY63 (SI Appendix, Fig. S6). Furthermore, the strand-specific RNA sequencing (ssRNA-seq) was carried out, and differentially expressed genes (DEGs) between the early maturing NIL D248 and its recurrent parent SH881 were analyzed. In total, 2,075 DEGs were identified, and 782 of them showed up-regulation and 1,293 of them showed down-regulation in the early maturing NIL (Dataset S2). According to Gene Ontology (GO) enrichment analysis, the “cellular nitrogen compound metabolic process” and the “chlorophyll metabolic process” were in the top 5 enriched GO terms of the up-regulated genes (SI Appendix, Fig. S7A), so we investigated the DEGs which related to nitrogen metabolism, chlorophyll metabolism, and photosynthesis. We found 16 DEGs related to nitrogen metabolism, and 11 of them showed up-regulation in the early maturing NIL (SI Appendix, Fig. S7B). In addition, 18 DEGs were related to chlorophyll metabolism and photosynthesis, and 15 of them showed up-regulation in the early maturing NIL (SI Appendix, Fig. S7C). Recently, yield potential of crops has been considered to be limited by photosynthesis (21, 22) and photosynthetic capacity closely related to leaf N content in C3 plants (23, 24). In our study, many of the DEGs had been reported to facilitate nitrogen utilization (25–31) and improve photosynthesis (32–35). These results provided further supportive evidence of the regulatory role of Ef-cd in shortening rice maturity duration without yield penalty. Of course, plant life span is complicated, and additional factors may be involved.
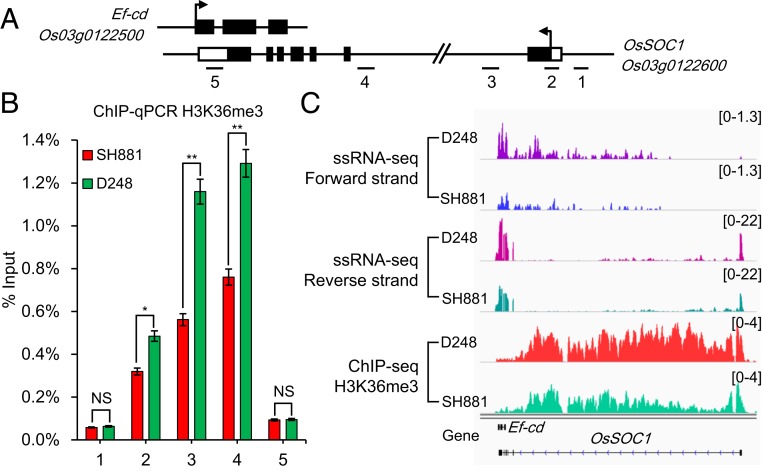
Natural antisense transcripts can exert their regulatory functions by acting as epigenetic regulators of gene expression and chromatin modifications (20, 36–39). Epigenome data showed that the chromatin modifications were obvious around the Ef-cd-OsSOC1 locus (http://structuralbiology.cau.edu.cn/cgi-bin/hgTracks) (SI Appendix, Fig. S8A). Histone Lys methylation is a well-studied epigenetic modification with both activating and repressing roles in gene expression (40). In our previous study, the level of H3K36me2/3 in the OsSOC1 chromatin region was decreased in the late-flowering mutant lvp1/sdg724 (41). In this study, we compared 3 mutants with their wild types for the expression levels of both Ef-cd and OsSOC1: the sdg724 mutant with a modification of H3K36me2/3 (41); lc2 as a component of the PRC2 complex mutant with a modification of H3K27me2/3 (42); and chr729 as a subunit of the chromatin-remodeling complexes with a modification of H3K4me2/3 (43). Except for lc2 mutant, the expressions of Ef-cd and OsSOC1 decreased significantly in both late-flowering mutants chr729 and sdg724 (SI Appendix, Fig. S8 B–D). These results indicated that the Ef-cd-OsSOC1 locus had complex chromatin modifications. Meanwhile, using SH881 and NIL D248, we investigated the H3K36me3 level at 5 loci within the OsSOC1 gene (Fig. 3A) and found that the level of H3K36me3 increased in D248 at the second, third, and fourth loci (Fig. 3B). Additionally, The results of chromatin immunoprecipitation sequencing (ChIP-seq) and ssRNA-seq further confirmed that D248 had a higher level of H3K36me3 and a higher expression level surrounding the OsSOC1 locus compared with SH881 (Fig. 3C). Recently, an increasing number of studies have revealed that the internal transcription initiation occurs frequently in the genes of eukaryotes (44, 45). In our study, the results of ssRNA-seq forward strand showed that other natural antisense transcripts (NATs) could exist in the long intron of OsSOC1 (Fig. 3C). Transcript structure analysis by StringTie also demonstrated that, in addition to Os03g0122500-1, other isoforms may exist for Ef-cd (SI Appendix, Fig. S9A). Many isoforms of Ef-cd with polyadenylated tails were also detected by a 3′ rapid amplification of cDNA ends (3′-RACE) assay (SI Appendix, Fig. S9 B and C). Moreover, the DNaseI track revealed an obvious DNaseI peak in the long intron of OsSOC1, which implied that alternative transcript isoforms of OsSOC1 could exist and initiate near this intronic DNaseI hypersensitive site (SI Appendix, Fig. S8A). These NATs and alternative transcript isoforms of OsSOC1 and Ef-cd might also play important roles in phenotypic determination. These features of Ef-cd were quite similar to the lncRNA COOLAIR described previously (36, 46), and a recent study reported MAS, a NAT-lncRNA produced from the MAF4 locus (47). MAS activates MAF4 by interacting with WDR5a, one core component of the COMPASS-like complexes, and recruiting WDR5a to MAF4 to enhance H3K4me3 (47). Based on our data, we speculated that Ef-cd could recruit an undefined complex that may contain SDG724, which leads to an increase of the H3K36me3 level in the OsSOC1 locus and promotes the expression of OsSOC1 (SI Appendix, Fig. S10). Further efforts to identify Ef-cd–interacting proteins will be of particular importance to obtain new insight into its regulatory mechanism.
Fig. 3.
Analysis of H3K36me3 level and mRNA expression level. (A) Genomic structure of the Ef-cd and OsSOC1 locus. Black solid boxes indicate exons, and white boxes indicate untranslated regions. The number under each line corresponds to the number on the x axis in B for the H3K36me3 level in the region. (B) The results of ChIP-qPCR analysis to confirm H3K36me3-binding sites in SH881 and D248 surrounding the OsSOC1 locus. The percentage of ChIP’ed DNA to input DNA (% input) was detected using qPCR of ChIP samples. (C) Genomic tracks display gene expression change and H3K36me3 ChIP-seq change in D248 and SH881 surrounding the Ef-cd and OsSOC1 locus. The top 2 tracks are normalized Ef-cd intensity; the third and fourth tracks are normalized OsSOC1 gene intensity; the bottom 2 tracks are normalized genomic coverage of H3K36me3 in D248 and SH881. The Ef-cd and OsSOC1 genomic tracks are shown below the profiles. The P value was calculated using Student’s t test. *P < 0.05; **P < 0.01; NS, not significant.
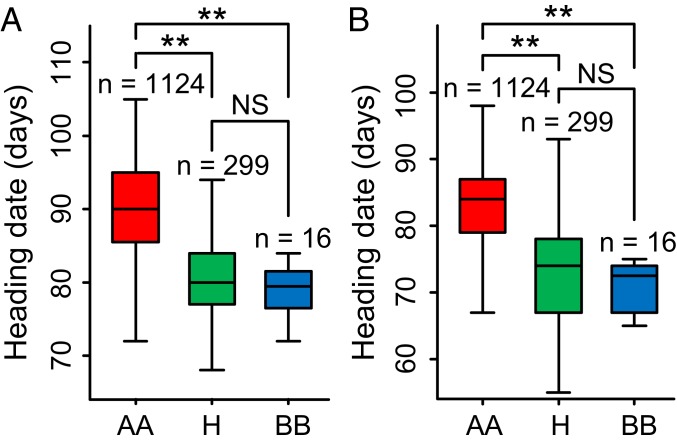
According to the pedigree information, the Ef-cd locus of D248 was derived from the IRRI variety IR9761-19 (SI Appendix, Fig. S11A). Therefore, we checked the frequency of the Ef-cd locus in rice cultivars using the 122500InDel-1 marker developed by the 36-bp insertion-deletion polymorphism in the promoter sequence of Ef-cd between D248 and SH881. All of the early maturing cultivars (Ce64, IR30, IR36, IR50, IR58, IR64, and IR74) were found to contain the Ef-cd allele (SI Appendix, Fig. S11B). Interestingly, the Ef-cd locus is common to some Chinese leading early maturing restorer cultivars such as Ce64-7, Minghui77, R402, and To463, but is absent in the leading late-maturing Chinese restorer cultivars such as MH63, 9311, SH881, and Fuhui838 (SI Appendix, Fig. S11C). These results indicate that Ef-cd had clearly been unintentionally exploited in early maturing hybrid rice breeding programs in China. Given the popularity of these restorer lines, the genotypic variation at the Ef-cd locus might have contributed to the variance in maturation times among hybrid cultivars. A recent survey among 1,495 elite hybrid rice cultivars via genomic analysis concluded that the Ef-cd-OsSOC1 locus is the major QTL underlying heading variation in both Sanya and Hangzhou (48). We analyzed 1,439 elite hybrid rice cultivars using the 122500InDel-1 and sf0301285586 markers (49) that were completely linked with the Ef-cd-OsSOC1 locus (Dataset S3). All of the hybrid cultivars with homozygous and heterozygous Ef-cd genotype matured significantly earlier than those with homozygotes of ef-cd in both Sanya and Hangzhou (Fig. 4 A and B). These elite early maturing hybrids have been widely applied for many years in China, demonstrating the vital contribution of the Ef-cd-OsSOC1 locus to hybrid rice production.
Fig. 4.
Analysis of genotype and phenotype interaction of Ef-cd among elite hybrid cultivars. Hybrid rice varieties with early maturing NIL D248 homozygous (BB) and heterozygous (H) genotype headed significantly earlier than those with its wild type SH881 genotype (AA) in Sanya (A) and Hangzhou (B). The P value was calculated using Student’s t test. **P < 0.01; NS, not significant.
Recently, in China, a breakthrough in yield potential has been achieved through the development of indica-japonica intersubspecies rice hybrids. In general, most of these hybrids mature late, which thus limits their cultivated adaptability. We believe that the deployment of the Ef-cd locus which makes maturity occur earlier without yield penalty can improve these hybrids to be adapted to new ecological areas and production seasons, leading to more extensive cultivation.
Materials and Methods
During all stages of developing early maturing NILs using 6 late-maturing recurrent parents and the early maturing donor line 6442S-7 (SI Appendix, Fig. S1A), the genotypes of offspring plants were analyzed for the Ef-cd locus using markers RM231, RM22, C515, and InDel1 (14) (Dataset S4), and the homozygotes were selected for continuous backcrosses and self-pollinations until the NILs of Ef-cd became genetically stable. Two recurrent parents of SH881 and MH63 and their 2 early maturing NILs D248 and D330 were selected for phenotyping the major agronomic traits of Beijing in 2009. Details on the following are available in SI Appendix, SI Materials and Methods: material preparation and growth conditions, fine mapping of Ef-cd, vector construction and plant transformation, RNA isolation and quantitative RT-PCR, RACE, LUC assays, labeling with 15N-nitrate or 15N-ammonium for determination of 15N accumulation, photosynthesis-related parameter measurements, ChIP assay, strand-specific RNA sequencing and ChIP-seq data analysis, analysis of differentially expressed genes, and accession numbers. The primers used in this study are listed in Dataset S4.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Shannon R. M. Pinson (US Department of Agriculture, Agricultural Research Service, Dale Bumpers National Rice Research Center) for discussions and comments on the manuscript. We thank Dr. Daoxiu Zhou (Huazhong Agricultural University) and Dr. Qian Qian (China National Rice Research Institute, Chinese Academy of Agricultural Sciences) for kindly providing chr729 and lc2 mutants and their corresponding wild types, respectively. This work was supported by grants from the National Natural Science Foundation of China (31371588, 91335107, and 30771314), the National Key Research and Development Program (2016YFD0101801, 2017YFD0100501), and the National Transgenic Major Project of China (2018ZX0800912B).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GEO) and are accessible through GEO Series accession nos. GSE133746 (strand-specific RNA-seq data) and GSE134021 (ChIP-seq data).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815030116/-/DCSupplemental.
References
- 1.Crist E., Mora C., Engelman R., The interaction of human population, food production, and biodiversity protection. Science 356, 260–264 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Bren d’Amour C., et al. , Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. U.S.A. 114, 8939–8944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans J. R., Improving photosynthesis. Plant Physiol. 162, 1780–1793 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rossi M., Bermudez L., Carrari F., Crop yield: Challenges from a metabolic perspective. Curr. Opin. Plant Biol. 25, 79–89 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Virmani S. S., Aquino R. C., Khush G. S., Heterosis breeding in rice (Oryza sativa L.). Theor. Appl. Genet. 63, 373–380 (1982). [DOI] [PubMed] [Google Scholar]
- 6.Khush G. S., Rice improvement at IRRI: An example of international collaboration. Breed. Res. 3, 281–286 (2001). [Google Scholar]
- 7.Xie W., et al. , Breeding signatures of rice improvement revealed by a genomic variation map from a large germplasm collection. Proc. Natl. Acad. Sci. U.S.A. 112, E5411–E5419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J. M., Xin Y. Y., Yuan L. P., Hybrid Rice Technology Development: Ensuring China’s Food Security (International Food Policy Research Institute, Washington, DC, 2009). [Google Scholar]
- 9.Xiao J., et al. , Identification of trait-improving quantitative trait loci alleles from a wild rice relative, Oryza rufipogon. Genetics 150, 899–909 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F. X., Zheng J. T., Xie H. A., Application of indica hybrid rice restorer line Minghui 77. J. Fujian Agric. Sci. 27, 773–779 (2012). [Google Scholar]
- 11.Matsubara K., Hori K., Ogiso-Tanaka E., Yano M., Cloning of quantitative trait genes from rice reveals conservation and divergence of photoperiod flowering pathways in Arabidopsis and rice. Front. Plant Sci. 5, 193 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori K., et al. , Genetic architecture of variation in heading date among Asian rice accessions. BMC Plant Biol. 15, 115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong C. L., Sun Y. Y., Wang P. R., Huang X. Q., Deng X. J., Gene effect analysis and evaluation of application potential of rice dominant earliness gene Ef-cd. Zuo Wu Xue Bao 33, 384–388 (2007). [Google Scholar]
- 14.Deng X. J., et al. , Identification and gene mapping of completely dominant earliness in rice. Agric. Sci. China 1, 11–18 (2002). [Google Scholar]
- 15.Lee S., Kim J., Han J. J., Han M. J., An G., Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. Plant J. 38, 754–764 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Bian X. F., et al. , Heading date gene, dth3 controlled late flowering in O. Glaberrima Steud. by down-regulating Ehd1. Plant Cell Rep. 30, 2243–2254 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Goff L. A., Rinn J. L., Linking RNA biology to lncRNAs. Genome Res. 25, 1456–1465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassett A. R., et al. , Considerations when investigating lncRNA function in vivo. eLife 3, e03058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ard R., Allshire R. C., Marquardt S., Emerging properties and functional consequences of noncoding transcription. Genetics 207, 357–367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K. C., et al. , A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long S. P., Marshall-Colon A., Zhu X. G., Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161, 56–66 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Zhu X. G., Long S. P., Ort D. R., Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Evans J. R., Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78, 9–19 (1989). [DOI] [PubMed] [Google Scholar]
- 24.Makino A., Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 155, 125–129 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J., et al. , pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants. Plant Biotechnol. J. 15, 1273–1283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J., et al. , Agronomic nitrogen-use efficiency of rice can be increased by driving OsNRT2.1 expression with the OsNAR2.1 promoter. Plant Biotechnol. J. 14, 1705–1715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng H., et al. , Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 62, 2319–2332 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Liu C., et al. , Ornithine δ-aminotransferase is critical for floret development and seed setting through mediating nitrogen reutilization in rice. Plant J. 96, 842–854 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Liu X., et al. , Identification and functional assay of the interaction motifs in the partner protein OsNAR2.1 of the two-component system for high-affinity nitrate transport. New Phytol. 204, 74–80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma X., et al. , OsARG encodes an arginase that plays critical roles in panicle development and grain production in rice. Plant J. 73, 190–200 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Wang W., et al. , Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 30, 638–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higuchi-Takeuchi M., et al. , Functional analysis of two isoforms of leaf-type ferredoxin-NADP(+)-oxidoreductase in rice using the heterologous expression system of Arabidopsis. Plant Physiol. 157, 96–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kong W., et al. , The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol. Biol. 92, 177–191 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Sade N., et al. , Delaying chloroplast turnover increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. J. Exp. Bot. 69, 867–878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakuraba Y., et al. , The rice faded green leaf locus encodes protochlorophyllide oxidoreductase B and is essential for chlorophyll synthesis under high light conditions. Plant J. 74, 122–133 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Swiezewski S., Liu F., Magusin A., Dean C., Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature 462, 799–802 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Ietswaart R., Wu Z., Dean C., Flowering time control: Another window to the connection between antisense RNA and chromatin. Trends Genet. 28, 445–453 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Chekanova J. A., Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 27, 207–216 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Heo J. B., Lee Y. S., Sung S., Epigenetic regulation by long noncoding RNAs in plants. Chromosome Res. 21, 685–693 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahrez W., et al. , H3K36ac is an evolutionary conserved plant histone modification that marks active genes. Plant Physiol. 170, 1566–1577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun C., et al. , The histone methyltransferase SDG724 mediates H3K36me2/3 deposition at MADS50 and RFT1 and promotes flowering in rice. Plant Cell 24, 3235–3247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J., Hu J., Qian Q., Xue H. W., LC2 and OsVIL2 promote rice flowering by photoperoid-induced epigenetic silencing of OsLF. Mol. Plant 6, 514–527 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Hu Y., et al. , CHD3 protein recognizes and regulates methylated histone H3 lysines 4 and 27 over a subset of targets in the rice genome. Proc. Natl. Acad. Sci. U.S.A. 109, 5773–5778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKnight K., Liu H., Wang Y., Replicative stress induces intragenic transcription of the ASE1 gene that negatively regulates Ase1 activity. Curr. Biol. 24, 1101–1106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen M., et al. , Transcription-driven chromatin repression of Intragenic transcription start sites. PLoS Genet. 15, e1007969 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Csorba T., Questa J. I., Sun Q., Dean C., Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc. Natl. Acad. Sci. U.S.A. 111, 16160–16165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao X., et al. , Global identification of Arabidopsis lncRNAs reveals the regulation of MAF4 by a natural antisense RNA. Nat. Commun. 9, 5056 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X., et al. , Genomic analysis of hybrid rice varieties reveals numerous superior alleles that contribute to heterosis. Nat. Commun. 6, 6258 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao H., et al. , RiceVarMap: A comprehensive database of rice genomic variations. Nucleic Acids Res. 43, D1018–D1022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.