Significance
Type 2 diabetes (T2D) has emerged as a major threat to human health worldwide. In T2D, pancreatic β cells are unable to release sufficient amounts of insulin to maintain physiological blood glucose levels. Acetylcholine (ACh) stimulates insulin release from mouse and human pancreatic β cells by activating a cell surface receptor known as M3 muscarinic ACh receptor (M3R). In the present study, we show that a drug that binds to an allosteric site on the M3R (a site distinct from the ACh-binding site) greatly enhances ACh-induced, M3R-mediated insulin release and improves glucose homeostasis in obese, glucose-intolerant mice. This proof-of-concept study strongly suggests that positive allosteric modulators of M3R function may prove beneficial for the treatment of T2D.
Keywords: G protein-coupled receptor, acetylcholine, insulin release, muscarinic receptor, allosteric modulator
Abstract
Given the global epidemic in type 2 diabetes, novel antidiabetic drugs with increased efficacy and reduced side effects are urgently needed. Previous work has shown that M3 muscarinic acetylcholine (ACh) receptors (M3Rs) expressed by pancreatic β cells play key roles in stimulating insulin secretion and maintaining physiological blood glucose levels. In the present study, we tested the hypothesis that a positive allosteric modulator (PAM) of M3R function can improve glucose homeostasis in mice by promoting insulin release. One major advantage of this approach is that allosteric agents respect the ACh-dependent spatiotemporal control of M3R activity. In this study, we first demonstrated that VU0119498, a drug known to act as a PAM at M3Rs, significantly augmented ACh-induced insulin release from cultured β cells and mouse and human pancreatic islets. This stimulatory effect was absent in islets prepared from mice lacking M3Rs, indicative of the involvement of M3Rs. VU0119498 treatment of wild-type mice caused a significant increase in plasma insulin levels, accompanied by a striking improvement in glucose tolerance. These effects were mediated by β-cell M3Rs, since they were absent in mutant mice selectively lacking M3Rs in β cells. Moreover, acute VU0119498 treatment of obese, glucose-intolerant mice triggered enhanced insulin release and restored normal glucose tolerance. Interestingly, doses of VU0119498 that led to pronounced improvements in glucose homeostasis did not cause any significant side effects due to activation of M3Rs expressed by other peripheral cell types. Taken together, the data from this proof-of-concept study strongly suggest that M3R PAMs may become clinically useful as novel antidiabetic agents.
G protein-coupled receptors (GPCRs) play key roles in regulating the function of pancreatic β cells, including the release of insulin (1, 2). For this reason, various β-cell GPCRs are considered attractive targets to stimulate insulin secretion from pancreatic β cells for the treatment of type 2 diabetes (T2D) (1, 2). However, the only GPCR-based drugs in current clinical use as antidiabetic drugs are glucagon-like peptide-1 (GLP-1) receptor agonists, such as exendin-4, and dipeptidyl peptidase-4 inhibitors, which slow the breakdown of endogenous GLP-1 (3, 4).
Like GLP-1, the neurotransmitter acetylcholine (ACh) greatly facilitates insulin secretion when blood glucose levels are elevated in both experimental animals and humans (5, 6). Following its release from parasympathetic nerve terminals, ACh stimulates β-cell M3 muscarinic ACh receptors (M3Rs), which in turn leads to the activation of Gq-type G proteins (Gq/11) (7–9). Stimulation of Gq/11 triggers the activation of PKC and raises intracellular calcium levels, resulting in enhanced insulin release from β cells in the presence of high glucose concentrations (5, 6).
We previously showed that deletion of β-cell M3Rs causes severe impairments in glucose tolerance and insulin secretion in mice, while in contrast, overexpression of M3Rs in β cells leads to improved glucose tolerance and enhanced insulin release in lean and obese mice (8, 10). These observations suggest that β-cell M3Rs may represent a potential drug target for the treatment of T2D.
M3Rs are expressed not only by pancreatic β cells, but also by various other peripheral cell types, including smooth muscle and glandular cells. It is well known that activation of these receptors triggers smooth muscle contraction and stimulates glandular secretion (11). For this reason, it is likely that the potential use of orthosteric M3R agonists (which do not currently exist) as insulinomimetics would cause severe side effects.
During the past 10 to 15 y, novel drugs have been developed that can modulate the function of the M3R and other muscarinic ACh receptor (mAChR) subtypes by binding to a receptor site distinct from the conventional (orthosteric) ACh-binding site (12–15). This so-called “allosteric site” is located on the extracellular receptor surface and shows significant sequence variation among the 5 mAChR subtypes (16, 17).
Over the past few years, considerable progress has been made in developing subtype-selective mAChR modulators (12–15). Interestingly, these efforts have led to the identification of VU0119498, a positive allosteric modulator (PAM) that facilitates ACh signaling through M3Rs expressed by cultured CHO cells (18, 19). VU0119498 also enhances ACh-dependent signaling through the Gq-coupled M1 and M5 mAChRs, but it has no activity at the Gi/o-coupled M2 and M4 mAChRs (18, 19).
In this proof-of-concept study, we examined whether a PAM that can act on β-cell M3Rs has therapeutic potential for the treatment of T2D. We found that VU0119498 treatment of wild-type (WT) mice led to enhanced glucose-stimulated insulin secretion (GSIS) and greatly improved glucose tolerance in lean and obese glucose-intolerant mice. These effects were mediated by β-cell M3Rs, as studied in mutant mice lacking M3Rs selectively in their β cells. VU0119498 also significantly potentiated ACh-induced insulin secretion from human islets. Our data support the concept that selective M3R PAMs may prove beneficial as novel antidiabetic agents.
Results
VU0119498 Augments ACh-Mediated Increases in Insulin Secretion and Intracellular Calcium Levels in MIN6-K8 Cells.
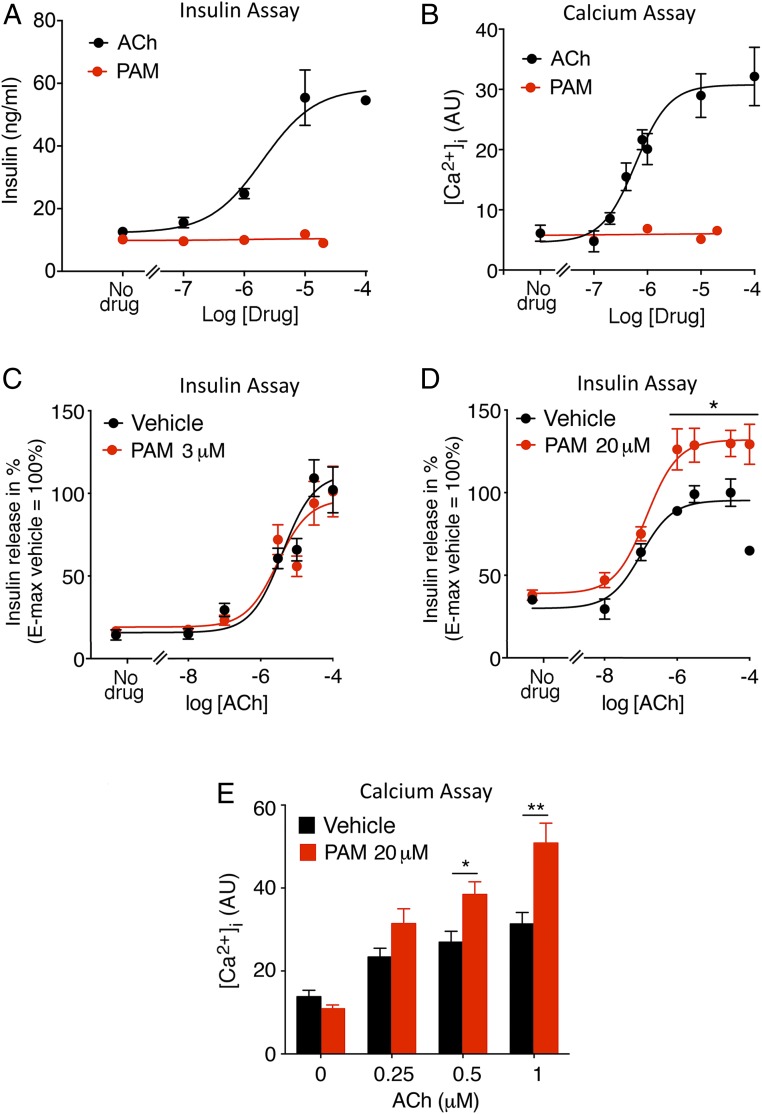
To test the hypothesis that the PAM VU0119498 can promote M3R-mediated insulin secretion, we initially carried out in vitro studies using the MIN6-K8 cell line derived from mouse β cells (20). We previously demonstrated that MIN6 cells predominantly express the M3R subtype (21). As shown in Fig. 1 A and B, ACh treatment of MIN6 cells caused concentration-dependent increases in insulin secretion and intracellular calcium levels ([Ca2+]i). In contrast, PAM treatment alone had no effect on insulin release and [Ca2+]i levels (Fig. 1 A and B). We next treated MIN6-K8 cells with increasing concentrations of ACh in the presence of 2 different concentrations of PAM, 3 and 20 μM. While 3 μM PAM did not affect ACh-induced insulin release (Fig. 1C), treatment with 20 μM PAM led to a significant augmentation of ACh-induced insulin secretion (Fig. 1D). Consistent with this observation, ACh-mediated increases in [Ca2+]i were also significantly elevated in the presence of 20 μM PAM (Fig. 1E).
Fig. 1.
VU0119498 (PAM) promotes ACh-mediated insulin secretion and intracellular calcium mobilization in MIN6-K8 cells. (A and B) Insulin secretion and calcium assays. ACh, but not PAM, causes concentration-dependent increases in insulin release (A) and intracellular calcium ([Ca2+]i) levels (B). (C and D) ACh-induced insulin secretion in the presence of PAM. While 3 μM PAM had no significant effect on ACh-stimulated insulin release (C), the presence of 20 μM PAM augmented ACh-induced insulin secretion (D). (E) Enhancement of ACh-mediated increases in [Ca2+]i in the presence of 20 μM PAM. Changes in [Ca2+]i were determined via FLIPR (AU, arbitrary units). Data are given as mean ± SEM of at least 3 independent experiments. *P < 0.05; **P < 0.01 (C and D, 2-way ANOVA followed by Bonferroni’s post hoc test; E, 2-way ANOVA followed by Tukey’s post hoc test).
VU0119498 Enhances ACh-Induced Insulin Release in Mouse and Human Pancreatic Islets.
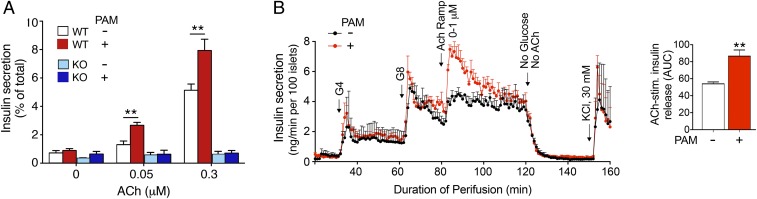
Mouse pancreatic islets virtually exclusively express the M3R subtype (7). ACh and other orthosteric muscarinic agonists are known to greatly increase insulin secretion in the presence of elevated glucose concentrations (7–9). As expected, this effect has been shown to be mediated by β-cell M3Rs (8, 9). Studies with pancreatic islets prepared from male WT mice confirmed that ACh stimulated insulin secretion in a concentration-dependent fashion (glucose concentration in the incubation mixture, 16.7 mM; Fig. 2A). This response was absent in pancreatic islets prepared from mice lacking M3Rs (M3R KO mice) (22), indicative of the involvement of M3Rs. In agreement with the results obtained with cultured MIN6 cells, ACh (0.05 and 0.3 μM)-induced insulin secretion responses were significantly enhanced in the presence of 20 μM PAM (Fig. 2A). VU0119498 alone had no significant effect on insulin release in either WT or M3R KO islets (Fig. 2A).
Fig. 2.
VU0119498 (PAM) promotes ACh-stimulated insulin secretion in isolated mouse and human islets in an M3R-dependent fashion. (A) Insulin secretion studies with isolated islets from WT mice and whole-body M3R KO mice. All assays were carried out in the presence of 16.7 mM glucose. ACh-induced increases in insulin release were studied in the absence or presence of 20 μM PAM. The amount of insulin secreted into the medium during the 1-h incubation period was normalized to the total insulin content of each well (islets plus medium). Data are given as mean ± SEM of at least 3 independent experiments, each carried out in duplicate or triplicate. **P < 0.01, 2-way ANOVA followed by Tukey’s post hoc test. (B) Insulin release studies with perifused human islets. Perifused human islets were incubated with of 4 mM and 8 mM glucose (G4 and G8, respectively) in either the absence or presence of 5 μM PAM. During the 8G perifusion period, islets were incubated with increasing concentrations of ACh (0 to 1 μM). The bar diagram to the right demonstrates the stimulatory effect of PAM on ACh-induced insulin release at 8G (AUC, area under the curve). Experimental details are provided in SI Appendix, Methods. Each curve represents the mean ± SEM of 3 independent perfusion experiments (180 human islets per group and perifusion). **P < 0.001, 2-tailed Student’s t test.
We also studied ACh-mediated insulin release using pancreatic islets prepared from female WT mice. As observed with islets from male WT mice, ACh (0.3 μM)-induced insulin secretion was significantly enhanced in the presence of 20 μM PAM (SI Appendix, Fig. S1). This effect was not observed when the ACh concentration was reduced to 0.05 μM (SI Appendix, Fig. S1), perhaps due to lower M3R numbers or less efficient M3R-stimulus coupling in β-cells from female mice.
We next examined whether similar results could be obtained with human islets. We perifused human islets with increasing concentrations of glucose (4 and 8 mM) in either the absence or presence of PAM (5 μM). VU0119498 alone had no significant effect on the increases in insulin secretion caused by 4 and 8 mM glucose (Fig. 2B). During the perifusion period with 8 mM glucose, we exposed islets to increasing concentrations of ACh (0 to 1 μM) in the presence of neostigmine (10 µM), a cholinesterase inhibitor. Fig. 2B shows that ACh treatment caused a significant increase in insulin secretion; ACh was present during the 80- to 120-min perifusion period. This effect declined over time, possibly due to desensitization of the M3R and/or downstream signaling molecules and the depletion of the readily releasable insulin granule pool (6). Strikingly, ACh-stimulated insulin release was greatly augmented in the presence of 5 μM PAM (Fig. 2B). This observation clearly indicates that VU0119498 also promotes signaling through M3Rs expressed by human β cells (note that human β-cells almost exclusively express the M3R subtype) (23).
VU0119498 Improves Glucose Tolerance and Insulin Secretion in Mice in a β-Cell M3R-Dependent Fashion.
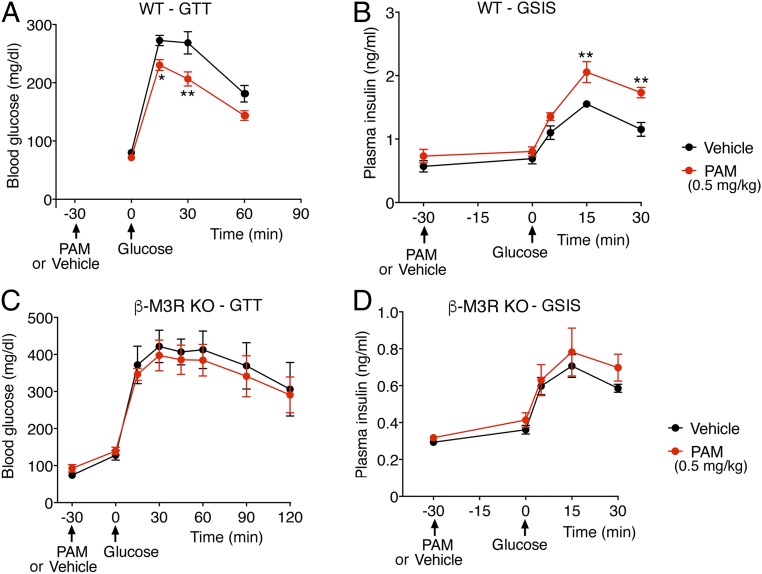
We next examined whether VU0119498 treatment of WT mice affected glucose tolerance. After an overnight fast, we injected WT mice with either vehicle or PAM (0.1, 0.5, or 2 mg/kg i.p). Thirty minutes later, the mice received a bolus of glucose via oral gavage (2 g/kg), followed by monitoring of blood glucose levels. It is well known that oral administration of glucose stimulates the activity of cholinergic, parasympathetic nerves and ultimately promotes the release of ACh in pancreatic islets (5, 6). The lowest PAM dose used (0.1 mg/kg i.p.) had no significant effect in this oral glucose tolerance test (OGTT) (SI Appendix, Fig. S2). In contrast, a 5-fold higher PAM dose (0.5 mg/kg i.p.) caused a significant improvement in glucose tolerance (Fig. 3A). The same PAM dose also significantly augmented GSIS (Fig. 3B). The highest PAM dose used (2 mg/kg i.p.) caused similar improvements in the OGTT and GSIS (SI Appendix, Fig. S3 A and B).
Fig. 3.
VU0119498 (PAM)-dependent improvements in glucose tolerance and insulin secretion in WT mice requires the presence of β-cell M3Rs. Male WT mice maintained on regular chow were fasted overnight and then injected with PAM (0.5 mg/kg i.p) or vehicle. Thirty minutes later, the mice received glucose via oral gavage (2 g/kg). (A) OGTT. (B) GSIS after oral glucose administration. (C) OGTT with mice lacking M3Rs selectively in pancreatic β cells (β-M3R-KO mice). (D) GSIS with β-M3R-KO mice. Note that the beneficial metabolic effects displayed by PAM-treated WT mice were absent in PAM-treated β-M3R-KO mice. Blood samples were collected from the tail vein. All experiments were carried out with 10- to 20-wk-old male littermates. Data are given as mean ± SEM (n = 5–8 per group). *P < 0.05, **P < 0.01, 2-way ANOVA followed by Bonferroni’s post hoc test.
To examine whether the PAM-dependent improvements in glucose tolerance and GSIS observed with WT mice in vivo were due to PAM’s action on β-cell M3Rs, we used a conditional gene deletion strategy to selectively inactivate the M3R gene in β cells of adult mice. Previous work has demonstrated that tamoxifen (TMX) induces Cre activity in Pdx1-Cre-ER transgenic mice selectively in pancreatic β cells (24, 25). Therefore, we crossed Pdx1-Cre-ER mice with homozygous floxed M3R mice (8). Subsequent matings led to the generation of fl/fl M3R-Pdx1-Cre-ER mice. TMX treatment of fl/fl M3R-Pdx1-Cre-ER resulted in the selective reduction of M3R expression in islets/β cells (SI Appendix, Fig. S4). In what follows, we refer to the TMX-treated fl/fl M3R-Pdx1-Cre-ER mice as “β-M3R-KO mice.”
We subjected β-M3R-KO mice to the same in vivo metabolic tests as WT mice (i.e., OGTT and GSIS). In contrast to the striking metabolic improvements that we observed with WT mice, PAM treatment (0.5 mg/kg i.p.) of β-M3R-KO mice had no significant effect on OGTT and GSIS results (Fig. 3 C and D). Taken together, these findings strongly suggest that the beneficial metabolic effects observed after PAM treatment of WT mice are caused by the ability of VU0119498 to promote signaling via β-cell M3Rs.
VU0119498 Improves Glucose Tolerance and Insulin Secretion in Obese, Glucose-Intolerant Mice.
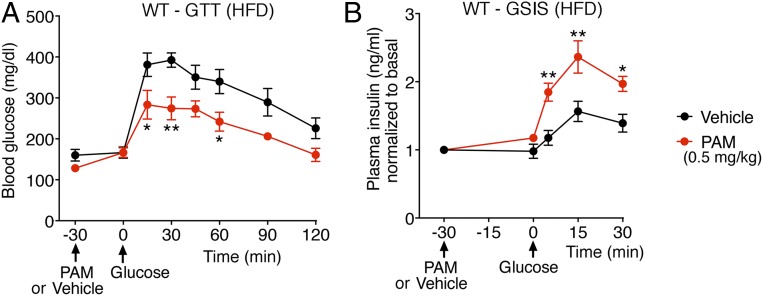
To explore the potential usefulness of VU0119498 or compounds with similar pharmacologic properties as antidiabetic drugs, we maintained male WT mice on a high-fat diet (HFD) for at least 8 wk. This calorie-rich diet caused significant weight gain (obesity) and glucose intolerance (Fig. 4A), a key feature of T2D. Strikingly, PAM treatment (0.5 mg/kg i.p.) of the HFD WT mice resulted in significantly improved glucose tolerance and GSIS (Fig. 4 A and B), indicative of the therapeutic potential of M3R PAMs.
Fig. 4.
VU0119498 (PAM) improves glucose tolerance and insulin secretion in obese, glucose-intolerant WT mice. Male WT mice that had been maintained on an HFD for 8 to 10 wk were fasted overnight and then injected with PAM (0.5 mg/kg i.p.) or vehicle. Thirty minutes later, the mice received glucose via oral gavage (1 g/kg). (A) OGTT. (B) GSIS after oral glucose administration. Blood samples were collected from the tail vein. All experiments were carried out with 10- to 20-wk-old male littermates. Data are given as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01, 2-way ANOVA followed by Bonferroni’s post hoc test.
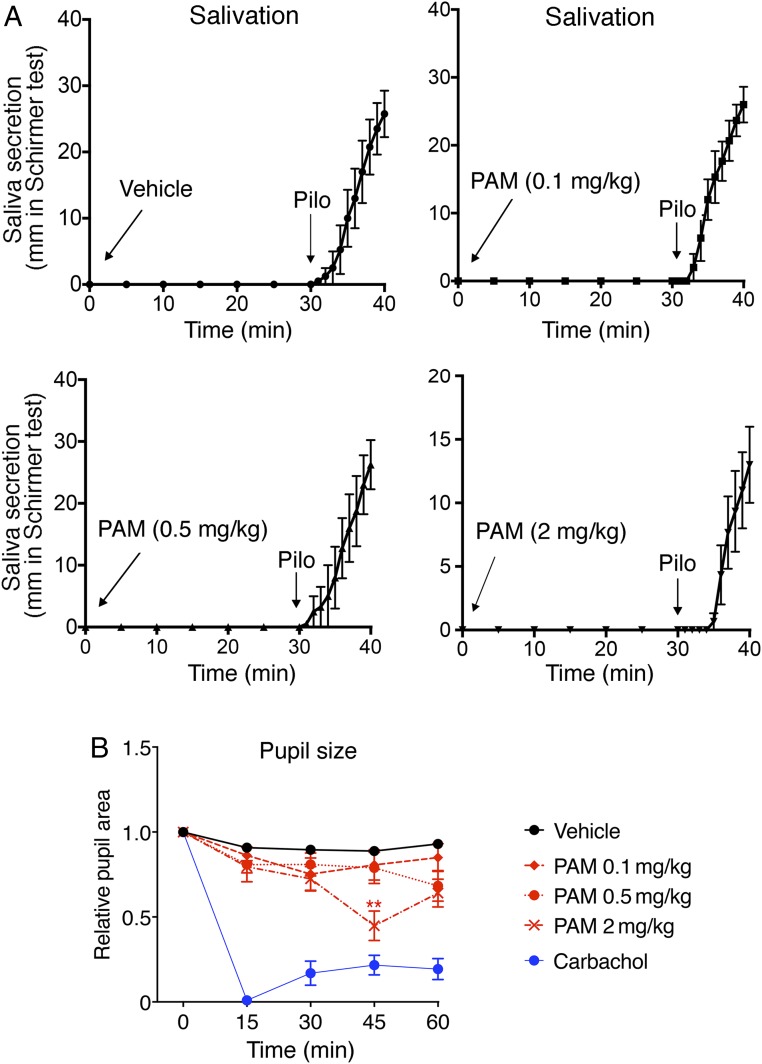
VU0119498 Has Little or No Effect on Smooth Muscle Activity or Salivary Secretion In Vivo.
M3Rs not only are expressed by pancreatic β cells, but are also present in several other peripheral tissues, including smooth muscle and exocrine glands (11). Systemic treatment of experimental animals with orthosteric muscarinic agonists was found to stimulate insulin release (21, 26, 27) but to also cause other, unwanted M3R-mediated effects. such as enhanced smooth muscle activity and salivary secretion (11). For this reason, we examined whether VU0119498 treatment of WT mice led to enhanced smooth muscle activity and increased salivary secretion (potential side effects).
Studies with M3R KO mice have shown that M3Rs play a key role in mediating ACh-induced salivation (28). Treatment of male WT mice with increasing doses of VU0119498 (0.1, 0.5, and 2 mg/kg i.p.) had no effect on salivary secretion over the 30-min observation period (Fig. 5A). In contrast, administration of pilocarpine (1 mg/kg i.p.), an orthosteric muscarinic agonist, triggered copious salivary secretion in all experimental animals (Fig. 5A).
Fig. 5.
VU0119498 (PAM) treatment has little or no effect on salivation and pupil diameter in WT mice. (A) Saliva secretion test. WT mice (10-wk-old males) were treated i.p. with either vehicle or with increasing doses of PAM (0.1, 0.5, and 2 mg/kg). Note that the PAM was inactive at all doses tested. In contrast, pilocarpine (Pilo; 1 mg/kg i.p.), an orthosteric muscarinic agonist, consistently stimulated salivary section in all mice and all experiments (positive control) (n = 3 or 4 per group). (B) Pupil constriction test. WT mice were treated with either vehicle or increasing doses of VU0119498 (0.1, 0.5, and 2 mg/kg i.p.). Carbachol (1 to 2 μL of a 100 μM solution) was applied topically to 1 eye as a positive control. (Carbachol is an orthosteric muscarinic agonist.) Saliva secretion and pupil constriction tests were performed as described in SI Appendix, Methods. Data are given as mean ± SEM (n = 9 or 10 per group). **P < 0.01 vs. vehicle, 2-way ANOVA followed by Tukey’s post hoc test.
Since activation of M3Rs in smooth muscles of the eye causes pupillary constriction (28), we carried out a series of pupil constriction tests. We injected WT mice with either vehicle or increasing doses of VU0119498 (0.1, 0.5, and 2 mg/kg i.p.) and then monitored pupil size over a 60-min period using video analysis software. In general, PAM treatment had little or no effect on pupil diameter (Fig. 5B). Only the highest PAM dose used (2 mg/kg) caused a significant reduction in pupil size at the 45-min time point (Fig. 5B). Topical application of carbachol eye drops (1 to 2 μL of a 100 μM solution) caused maximum pupillary constriction in all mice in which it was applied (Fig. 5B).
Discussion
Given the worldwide diabetes epidemic, there is an urgent need to develop novel classes of antidiabetic agents endowed with increased long-term efficacy and reduced side effects. Several studies have shown that activation of β-cell M3Rs by muscarinic agonists results in striking increases in insulin release in both human and mouse islets (7–9). Since this effect is only observed in the presence of high glucose concentrations, β-cell M3Rs represent an attractive target to stimulate insulin release for therapeutic purposes. Since the amino acids lining the orthosteric binding pocket of the different mAChR subtypes are nearly identical (15, 29), attempts to develop M3R-selective orthosteric muscarinic agonists have been unsuccessful thus far.
Over the past decade, considerable attention has been focused on the development of novel classes of drugs that can regulate GPCR activity by binding to allosteric receptor sites that are generally less well conserved. As has been discussed in detail elsewhere, the potential clinical use of either negative or positive allosteric modulators of GPCR function (NAMs and PAMs, respectively) offers several advantages over orthosteric GPCR agonists (30, 31). Most importantly, the potential therapeutic use of allosteric agents respects the spatiotemporal control of receptor signaling; that is, NAMs or PAMs modulate receptor activity only in the presence of an endogenous agonist.
Interestingly, recent attempts to develop novel mAChR subtype-selective allosteric agents have led to the identification of VU0119498, a PAM that facilitates ACh-mediated activation of M3Rs stably expressed in CHO cells (18, 19). In this proof-of-concept study, we provide convincing evidence that VU0119498 greatly augments ACh-dependent, M3R-mediated insulin release from cultured pancreatic β cells as well as mouse and human pancreatic islets (Figs. 1 and 2). Moreover, systemic administration of the PAM also led to a robust stimulation of GSIS and improved glucose homeostasis in vivo (Figs. 3 and 4). These beneficial metabolic effects were absent in mice selectively lacking M3Rs in β cells (Fig. 3), providing clear evidence that VU0119498 acts on β-cell M3Rs. Moreover, acute treatment of obese, glucose-intolerant mice with a single dose of the PAM (0.5 m/kg i.p.) led to greatly improved glucose tolerance and enhanced GSIS (Fig. 4), indicative of the potential clinical usefulness of M3R PAMs.
VU0119498 not only acts as a PAM at M3Rs, but also enhances ACh-dependent signaling through M1 and M5 mAChRs, which share considerable sequence homology with M3Rs (18, 19). In contrast to M3Rs, the M1 and M5 mAChRs are predominantly expressed in the central nervous system, where they regulate various behavioral processes (11). For this reason, muscarinic PAMs that have a similar pharmacologic profile as VU0119498 but do not penetrate the blood-brain barrier are unlikely to cause significant central nervous system side effects. At present, muscarinic PAMs that selectively facilitate ACh signaling through M3Rs are not available; however, given the rapid advances in the structural analysis of PAM/GPCR complexes (32), it is likely that such agents will be identified in the not-so-distant future via structure-guided drug design.
As discussed above, VU0119498 augmented the ability of ACh to stimulate insulin secretion by enhancing the action of ACh at β-cell M3Rs. Since M3Rs are widely expressed by smooth muscle tissues and various exocrine and endocrine glands, we anticipated that VU0119498 would also affect other peripheral functions regulated by the activity of M3Rs. For example, phenotypic analysis of M3R KO mice has shown that M3Rs play key roles in promoting salivary secretion and smooth muscle contractility (28, 33). As a result, M3R KO mice exhibit reduced muscarinic agonist-induced salivary secretion and impaired pupillary constriction, among other deficits (28, 33). To our surprise, acute VU0119498 treatment had no significant effect on salivary secretion and pupil diameter at doses that caused pronounced increases in insulin secretion in vivo in WT mice (Fig. 5). One possible explanation for this observation is that PAM activity depends on the actual concentrations of ACh in the different M3R-expressing tissues. It is also possible that the efficacy of M3R-response coupling differs between tissues and may be responsible for the ability of VU0119498 to selectively enhance ACh signaling in β cells. Finally, VU0119498, due to its specific physicochemical and pharmacokinetic properties, may become enriched in islets and most likely in other tissues as well. Clearly, more detailed studies are needed to distinguish between these different possibilities.
It should also be noted that muscarinic antagonists with preference for M3Rs are used in the treatment of chronic obstructive lung disease and overactive bladder (11). Clearly, more detailed studies are required to explore potential adverse effects of VU0119498 on airway resistance and bladder function, as well as other physiological processes in which M3Rs are involved.
In conclusion, our data suggest a new strategy for the development of novel antidiabetic drugs. Since most GPCRs are predicted to feature allosteric binding sites, the approach described here also may be applicable to other classes of GPCRs that regulate β-cell function (1, 2).
Methods
All animal experiments were conducted in accordance with US National Institutes of Health Guidelines for Animal Research and were approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Animal Care and Use Committee. Detailed information on the materials and methods used in this study, including in vitro and in vivo insulin release studies using MIN6 cells as well as mouse and human pancreatic islets, the generation of mutant mice lacking M3Rs selectively in pancreatic β cells, calcium measurements, and various in vivo metabolic tests, is provided in SI Appendix, Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jaroslawna Meister (National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) for the analysis of human gene expression data and Yinghong Cui (NIDDK) for excellent technical assistance. This work was supported by the NIDDK’s Intramural Research Program (L.Z., M.R., A.C., J.P., H.Z., D.D., D.H.A., and J.W.), the National Institute of Dental and Craniofacial Research (Grant 1-ZIA-DE000738, to T.M. and J.E.M.), and the National Institute of Mental Health (J.L.L. and S.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.N.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904943116/-/DCSupplemental.
References
- 1.Ahrén B., Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 8, 369–385 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Amisten S., Salehi A., Rorsman P., Jones P. M., Persaud S. J., An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol. Ther. 139, 359–391 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Ahren B., Glucagon-like peptide-1 receptor agonists for type 2 diabetes: A rational drug development. J. Diabetes Investig. 10, 196–201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drucker D. J., Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Ahrén B., Autonomic regulation of islet hormone secretion–Implications for health and disease. Diabetologia 43, 393–410 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Gilon P., Henquin J. C., Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr. Rev. 22, 565–604 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Duttaroy A., et al. , Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes 53, 1714–1720 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Gautam D., et al. , A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 3, 449–461 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Zawalich W. S., et al. , Effects of muscarinic receptor type 3 knockout on mouse islet secretory responses. Biochem. Biophys. Res. Commun. 315, 872–876 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Ruiz de Azua I., Gautam D., Guettier J. M., Wess J., Novel insights into the function of β-cell M3 muscarinic acetylcholine receptors: Therapeutic implications. Trends Endocrinol. Metab. 22, 74–80 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wess J., Eglen R. M., Gautam D., Muscarinic acetylcholine receptors: Mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 6, 721–733 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Bock A., Schrage R., Mohr K., Allosteric modulators targeting CNS muscarinic receptors. Neuropharmacology 136, 427–437 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Conn P. J., Jones C. K., Lindsley C. W., Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol. Sci. 30, 148–155 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger W. A. C., Sexton P. M., Christopoulos A., Thal D. M., Toward an understanding of the structural basis of allostery in muscarinic acetylcholine receptors. J. Gen. Physiol. 150, 1360–1372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse A. C., et al. , Muscarinic acetylcholine receptors: Novel opportunities for drug development. Nat. Rev. Drug Discov. 13, 549–560 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruse A. C., et al. , Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504, 101–106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dror R. O., et al. , Structural basis for modulation of a G-protein-coupled receptor by allosteric drugs. Nature 503, 295–299 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Bridges T. M., et al. , Chemical lead optimization of a pan G(q) mAChR M(1), M(3), M(5) positive allosteric modulator (PAM) lead. Part I: Development of the first highly selective M(5) PAM. Bioorg. Med. Chem. Lett. 20, 558–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marlo J. E., et al. , Discovery and characterization of novel allosteric potentiators of M1 muscarinic receptors reveals multiple modes of activity. Mol. Pharmacol. 75, 577–588 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki M., et al. , Establishment of new clonal pancreatic β-cell lines (MIN6-K) useful for study of incretin/cyclic adenosine monophosphate signaling. J. Diabetes Investig. 1, 137–142 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruiz de Azua I., et al. , RGS4 is a negative regulator of insulin release from pancreatic beta-cells in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 107, 7999–8004 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada M., et al. , Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature 410, 207–212 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Xin Y., et al. , RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metab. 24, 608–615 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Gu G., Dubauskaite J., Melton D. A., Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Fu A., et al. , Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab. 10, 285–295 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Fukudo S., et al. , Muscarinic stimulation and antagonism and glucoregulation in nondiabetic and obese hyperglycemic mice. Diabetes 38, 1433–1438 (1989). [DOI] [PubMed] [Google Scholar]
- 27.Ahrén B., Sauerberg P., Thomsen C., Increased insulin secretion and normalization of glucose tolerance by cholinergic agonism in high fat-fed mice. Am. J. Physiol. 277, E93–E102 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Matsui M., et al. , Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc. Natl. Acad. Sci. U.S.A. 97, 9579–9584 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thal D. M., et al. , Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531, 335–340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langmead C. J., Christopoulos A., Functional and structural perspectives on allosteric modulation of GPCRs. Curr. Opin. Cell Biol. 27, 94–101 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Thal D. M., Glukhova A., Sexton P. M., Christopoulos A., Structural insights into G-protein-coupled receptor allostery. Nature 559, 45–53 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Munk C., et al. , An online resource for GPCR structure determination and analysis. Nat. Methods 16, 151–162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T., et al. , M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J. Physiol. 558, 561–575 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.