Significance
GABA not only plays critical roles in the central nervous system but also is involved in various peripheral tissues, such as intestine, stomach, etc. Here, we tried to identity the function of GABA signaling in hematopoietic progenitors. We found GABRR1 is the only GABA receptor expressed in subsets of both human and mouse hematopoietic stem cells and megakaryocyte progenitors. Further studies showed inhibition of GABRR1 with a knockout mouse model, CRISPR-mediated deletion, or GABA antagonist treatment inhibited megakaryocyte and platelet differentiation, while activation of GABRR1 through lentivirus-mediated overexpression or GABA agonist treatment promoted platelet generation. Thus, our work identifies a link between this neural receptor and the hematopoietic system and potentially uncovers a strategy to efficiently generate megakaryocytes and platelets.
Keywords: GABA, GABRR1, hematopoietic stem cell, megakaryocyte progenitors
Abstract
GABRR1 is a rho subunit receptor of GABA, the major inhibitory neurotransmitter in the mammalian brain. While most investigations of its function focused on the nervous system, its regulatory role in hematopoiesis has not been reported. In this study, we found GABRR1 is mainly expressed on subsets of human and mouse hematopoietic stem cells (HSCs) and megakaryocyte progenitors (MkPs). GABRR1-negative (GR−) HSCs led to higher donor-derived hematopoietic chimerism than GABRR1-positive (GR+) HSCs. GR+ but not GR− HSCs and MkPs respond to GABA in patch clamp studies. Inhibition of GABRR1 via genetic knockout or antagonists inhibited MkP differentiation and reduced platelet numbers in blood. Overexpression of GABRR1 or treatment with agonists significantly promoted MkP generation and megakaryocyte colonies. Thus, this study identifies a link between the neural and hematopoietic systems and opens up the possibility of manipulating GABA signaling for platelet-required clinical applications.
γ-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the vertebrate central nervous system and plays a role in neurogenesis (1–5). In addition, GABA is involved in various peripheral tissues and organs, such as the intestine and stomach (6, 7), and in embryonic stem cells (8). However, to date, the cell-type-specific expression of GABA receptors and their anatomical distribution and functional properties in hematopoietic stem and progenitor cells (HSPCs) have not been reported.
Hematopoietic stem cells (HSCs) are capable of generating multiple different cell types in a stepwise way (9). Megakaryocyte–erythroid progenitors (MEPs), derived from HSCs, are bipotent progenitors, which can differentiate into either megakaryocyte progenitors (MkPs; which give rise to platelets) or erythroid progenitors (EPs; which give rise to erythrocytes) (10, 11). Supplementing MkPs or platelets is a promising strategy to overcome thrombocytopenia for rapid recovery of blood-clotting function in patients (12, 13) from trauma and surgery, chemotherapy or radiation-induced thrombocytopenia, sepsis, and other indications. Recently, attempts have been made for the induction of differentiation of platelets from various sources, including HSPCs, pluripotent stem cells, and even other lineage cells (14–17), and therefore, identification of the regulators that facilitate MkP generation and differentiation during normal hematopoiesis has become an important topic.
Gene Expression Commons (GEXC, https://gexc.riken.jp/), designed by us to perform probeset meta-analysis for a particular microarray platform and profile absolute expression of any gene on the microarray (18), has established both human and mouse hematopoiesis models. In a previous study, we analyzed transcription factors expressed differentially in MkP cells and verified their function in HSCs and MkPs by gene knockout or overexpression, which provides a method to discover the regulatory network, and these identified genes could be part of a diagram of megakaryocyte development (19). In this study, we first discovered in GEXC that GABRR1 was expressed predominantly in MkP and therefore determined the expression of all GABA receptors in GEXC “mouse hematopoiesis.” While GABRR1 is selectively expressed in MkPs, the other GABA receptors were not expressed in any of the HSPC populations. Further analysis by real-time PCR and flow cytometry demonstrated that a subset of HSCs and MkPs express GABRR1. Transplantation experiments showed GABRR1-negative (GR−) HSCs led to higher donor-derived multilineage hematopoietic chimerism than GABRR1-positive (GR+) HSCs. Reduction of the GABRR1 signal by genetic knockout or specific antagonists in mouse or human cells significantly decreased megakaryocyte differentiation and platelet generation, while GABRR1 overexpression or agonist treatment increased megakaryocyte and platelet development. Thus, GABA signaling through its receptor GABRR1 may play a role in the regulation of HSCs and MkPs.
Results
The Expression of Gabrr1 in Mouse HSCs and MkPs.
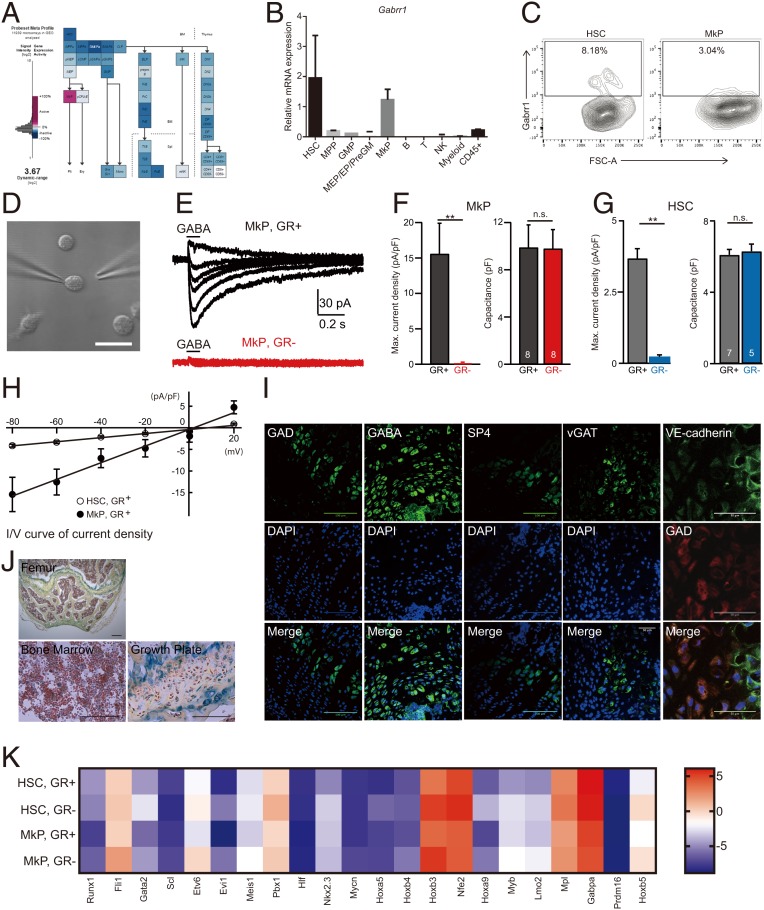
In this study, we first determined the expression of all GABA receptors (α1 to α6, β1 to β3, γ1 to γ3, δ, ε, θ, π, ρ1 to ρ3) in GEXC mouse hematopoiesis and found that MkP cell populations were selectively GABRR1+ and other GABA receptors were not expressed in any of the HSPC populations (Fig. 1A and SI Appendix, Fig. S1A). RT-PCR of hematopoietic cell populations confirmed its expression pattern (Fig. 1B). The GABAA-ρ receptors (Gabrr1-3) are ligand-gated ion channels that play physiological roles in the retina, spinal cord, and brain (4, 20, 21). Gabrr1 can be composed entirely of homo- or heteropentamer ρ subunits (22). Because only Gabrr1 is expressed, we expect that our hematopoietic cells only could form homo-oligomers.
Fig. 1.
Gabrr1 expression and function in mouse hematopoietic system. (A) Expression of Gabrr1 in different mouse hematopoietic populations in Gene Expression Commons. (B) Gene expression analysis of Gabrr1 in different mouse HSPC populations by real-time PCR. Data shown are mean ± SD from 3 replicates of the same group and are representative of at least 3 independent experiments. (C) Gabrr1 expression analysis in mouse HSPCs from bone marrow by multicolor flow cytometry. Numbers represent the percentage of Gabrr1+ cells. Data shown are representative of n = 6 mice. (D) A representative image of a GR+ MkP for patch-clamp recording of GABA-evoked currents. (Scale bar, 20 uM.) (E) The representative current traces induced by application of 1 mM GABA in GR+ and GR− MkPs held at various membrane potentials from −80 mV at a step of 20 mV. (F and G) Summary graph of the maximum current density and cell capacitance of GR+ and GR− MkPs (F) and GR+ and GR− HSCs (G). (H) The I-V curves of peak current densities of GR+ MkPs and GR+ HSCs (the x axis shows the holding voltages; the y axis shows the current densities [peak current/cell capacitance]). (I) Immunostaining analysis shows expression of GAD65+GAD67, GABA, vGAT, VE-cadherin, and synaptophysin (SP4) in the growth plate/epiphysis. (Scale bar, 50 uM, Right 2 panels; 100 uM, Left 3 panels.) (J) Mice sections in different sites stained with pentachrome. (Scale bar, 100 uM, Lower; 200 uM, Upper.) (K) The HSC- and MkP-associated genes were analyzed in purified mouse GR+ and GR− HSCs and MkPs by real-time PCR. Data shown in F and G are mean ± SEM. The number of cells analyzed is indicated in the bars. **P < 0.01; n.s., not significant; preGM, pre granulocyte-macrophage progenitor; NK, natural killer; FSC, forward scatter.
Further analysis of mouse bone marrow by flow cytometry revealed that Gabrr1 is mainly expressed on a subset of HSCs (8.18 ± 1.53%) and MkPs (3.04 ± 0.7%) (Fig. 1C and SI Appendix, Fig. S1B) (23). Other blood lineage (CD45+) cells were negative (SI Appendix, Fig. S1C). Gabrr1 expression in immunophenotypically defined HSCs (pHSCs, CD34-Flk2-CD150+KLS), multipotent progenitors subset A (MPPa) (CD34+Flk2- CD150+KLS), and multipotent progenitors subset B (MPPb) (CD34+Flk2-CD150-KLS) (24) showed 1–3% of those early stem and progenitors cells expressed Gabrr1 (SI Appendix, Fig. S1D). In addition, Gabrr1 expression was detected at a higher level in platelet-biased HSCs (SI Appendix, Fig. S1E) (25).
GR+ and GR− HSCs or MkPs cells were then purified and tested by electrophysiological recording using patch-clamp techniques. The clamped cell was held at various membrane potentials and incubated with GABA (Fig. 1D). We observed a prominent GABA-induced inward current in GR+ MkP cells but not in GR− cells (Fig. 1 E and F). Similarly, significant currents were induced by GABA application to GR+ HSCs, although the amplitude appears to be smaller than that in GR+ MkP cells (Fig. 1 G and H). Taken together, these results suggested that Gabrr1 expressed in HSCs and MkPs is functional as an ion channel.
We then sought to identify the source of GABA in bone marrow. Since glutamic acid decarboxylase 1 (GAD1) and GAD2 synthesize GABA from glutamate, we searched for the bone marrow cell source of GABA by examining the expression of GADs by real-time PCR. Among all cells tested, including bone marrow cell mixtures, HSPC populations, mature blood cells, skeletal lineage (Tie2- AlphaV+) cells, nonskeletal and nonendothelial (Tie2- AlphaV-) cells, and all Tie2+ cells (26), only Tie2+ cells from the bone marrow cell suspension fraction showed expression of GADs by real-time PCR analysis (SI Appendix, Fig. S1F). Tie2 marks rare HSCs, early progenitors of and mature endothelial cells, and perhaps other cells not yet placed in a lineage (27, 28).
Because cell suspensions can exclude some sessile cells, we sectioned mouse bones and performed in situ immunofluorescence staining for GADs using antisera commonly used to detect potential GABAergic cells. GAD-positive cells appear in highest concentration at the growth plate and the femoral epiphysis (Fig. 1 I and J) (29, 30). More staining showed that GABA and vesicular GABA transporter (vGAT) could also be detected in the same region. However, costaining of GADs with endothelial cell surface markers, including CD31 and vascular endothelial cadherin (VE-cadherin), shows that there are only very rare double-positive cells (Fig. 1I and SI Appendix, Fig. S1G), so the Tie2+ cells are not endothelial cells in general. The GAD+ cells in the epiphysis resemble cartilage progenitors. Synaptophysin can be detected with antisera to SP4 (Fig. 1I), and here we detect some positive cells also in the epiphysis, but they do not have the morphology of neurons. These results suggested that nonneural cells in the bone and bone marrow are candidates for GABA production and release but do not definitively show which cells are GABAergic.
Gabrr1 Expression Distinguishes Mouse HSC and MkP Populations.
We isolated GR+ and GR− HSCs and MkPs and examined the gene expression patterns by real-time PCR. We checked the expression of HSC and MkP shared transcripts and HSC, MEP, MkP, myeloid, erythroid, lymphoid and platelet lineage-associated genes (25, 31) and found that both GR+ and GR− HSC and MkP cells expressed corresponding cell lineage–specific genes (Fig. 1K and SI Appendix, Fig. S2A). GR− HSC and GR− MkP populations maintained higher expression levels of multipotency genes than GR+ populations, while GR+ populations exhibited higher myeloid, platelet, and erythroid genes, and none of them expressed lymphoid genes (Fig. 1K and SI Appendix, Fig. S2A). After in vitro differentiation of GR+ and GR− HSCs, flow cytometry analysis showed that GR− cells contained more progenitor cells, which is consistent with gene expression analysis results (SI Appendix, Fig. S2B).
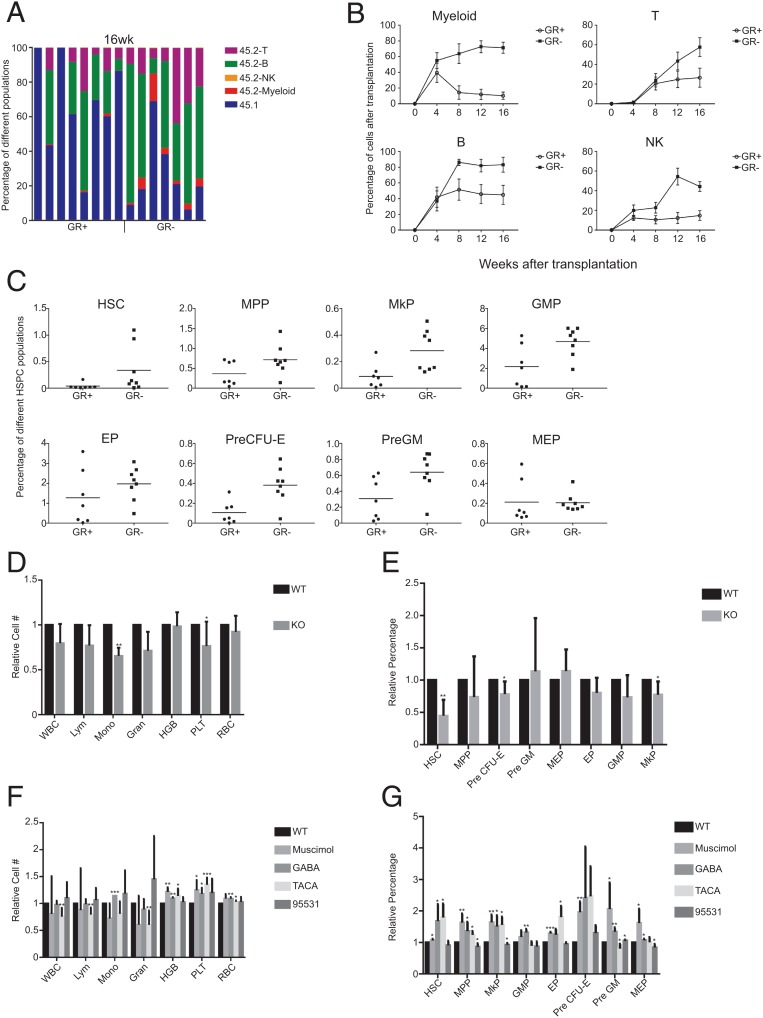
We then characterized GABRR1-expressing and GABRR1-negative cells by HSC transplantation. GR+ and GR− HSCs from CD45.2 C57BL/6 mice were transplanted with supporting CD45.1 bone marrow cells into irradiated CD45.1 mouse recipients. The results, presented in Fig. 2 A and B and SI Appendix, Fig. S2C, showed GR− HSCs have higher full multilineage reconstitution than GR+ HSCs. Twenty weeks after transplantation, GR− HSC transplanted mice had higher frequencies of HSCs, MPPs, MkPs, granulocyte-macrophage progenitors (GMPs), and EPs (Fig. 2C). Secondary transplantation showed GR− HSCs have the capacity of robust multilineage chimerism, suggesting they are long-term HSCs, while GR+ cells contain fewer long-term HSCs than the GR− population (SI Appendix, Fig. S2 D and E).
Fig. 2.
The effects of activation or inactivation of Gabrr1 on HSPC differentiation in mice. (A) Percentage of chimerism at 16 wk after transplanting GR+ HSCs (n = 8 mice) or GR− HSCs (n = 7 mice) into primary recipients. Each column represents an individual mouse. (B) Average donor lineage contributions of myeloid cells, B cells, T cells, and NK cells in primary transplants. Error bars denote SD. (C) Frequencies of HSPC populations from donor mice contributed in recipients. (D) The complete blood cell counts in peripheral blood of B6; 129S4-Gabrr1tm1Llu/J mice and their approximate control wild-type (WT) B6129SF2/J mice. For each cell count, the number from the WT mice was set to 1, and the number from Gabrr1 knockout (KO) mice was normalized to that. (E) The blood cell counts in peripheral blood of mice treated with agonists or antagonists of Gabrr1. WBC, white blood cell; Mono, monocyte; HGB, hemoglobin; Gran, granulocyte; Lym, lymphocyte; PLT, platelets. For each cell count, the number from the WT mice was set to 1, and the number from mice by other treatments was normalized to that. (F) Summary of mouse HSPCs from bone marrow of mice treated with agonists or antagonists of Gabrr1. For each HSPC population, the number from the WT mice was set to 1, and the number from mice by other treatments was normalized to that. Data shown in D, E, and F are mean ± SD of individual mice groups (n = 6 for each group) within the same experiment. *P < 0.05, **P < 0.01, ***P < 0.001.
Function of Gabrr1 in Mouse Hematopoiesis.
To further address how Gabrr1 is involved in the regulation of hematopoiesis, we used Gabrr1 knockout mice B6; 129S4-Gabrr1tm1Llu/J (GR−/− mice) (21) and used B6129SF2/J hybrid mice as controls (SI Appendix, Fig. S3A). GR−/− mice had significantly lower levels of blood platelets (87.7 ± 4.92%), and reduction of monocyte was also observed, while white blood cells, lymphocytes, HGB, and red blood cells (RBCs) in GR−/− mice were not significantly changed (Fig. 2D). Among c-Kit-enriched HSPCs, HSCs were decreased in GR−/− mice to 50% the level of these control mice, while MkPs present in the c-Kit-enriched marrow cells were reduced by 13 ± 2.6% compared with controls (Fig. 2E and SI Appendix, Fig. S3B).
We then examined the effects of agonists and antagonists of Gabrr1 in C57BL/6J mice, including the agonists GABA, trans-4-aminocrotonic acid (TACA), Muscimol, and antagonist SR95531. After 7 d of treatment, we found that GABA treatment increased platelet numbers by 17.7 ± 11.2%, and treatment by Muscimol and TACA showed increases of 35.0 ± 13 and 24.6 ± 19.0%, respectively (Fig. 2F). RBC numbers were slightly increased with these treatments. SR95531 did not affect platelet number significantly (Fig. 2F). Bone marrow MkPs were increased by several different agonist treatments, including GABA by 63.7 ± 24.4%, Muscimol by 55.0 ± 26.7%, and TACA by 23.3 ± 31.1%. Interestingly, EP and pre colony forming unit-erythroid (CFU-E) were also increased, consistent with the RBC increase in peripheral blood (Fig. 2G and SI Appendix, Fig. S3C).
The Expression and Function of GABRR1 in Human HSPCs.
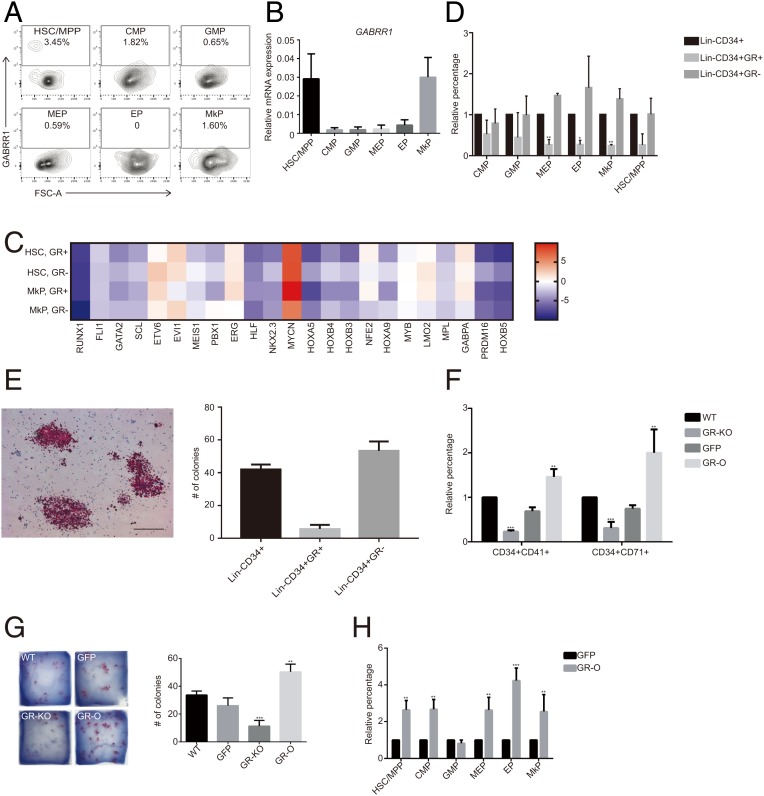
To investigate the role of GABRR1 in human hematopoiesis, we checked GABRR1 cell surface protein expression by fluorescence activated cell sorting (FACS). GABRR1 is mainly expressed in human HSC/multipotent progenitor (MPP) (3.45 ± 1.0%), common myeloid progenitor (CMP) (1.82 ± 0.34%), and MkP (1.60 ± 0.16%) (Fig. 3A). RT-PCR analysis confirmed the result (Fig. 3B). HSC or MkP gene expression analyses by RT-PCR in GR+ and GR− HSC/MPP or MkP cells showed patterns similar to those in mouse HSPCs, with higher multipotent gene expression in GR− cells (Fig. 3C). Next, we differentiated Lin-CD34+GR+ and Lin-CD34+GR− cells in vitro. The results showed Lin-CD34+GR− cells included more progenitor cells (Fig. 3D). Functional megakaryocyte colony-forming assay showed GR− Lin-CD34+ cells generated more MK colonies than GR+ Lin-CD34+ cells (Fig. 3E). Those results indicate that both in mice and in humans, GABRR1 influenced HSC multipotency and megakaryocyte differentiation.
Fig. 3.
GABRR1 expression and the consequence of GABRR1 ablation or overexpression in the human hematopoietic system. (A) GABRR1 expression analysis in human HSPCs from bone marrow by multicolor flow cytometry. Numbers represent the percentage of GABRR1+ cells. (B) Expression of GABRR1 in human HSPC populations by real-time PCR analysis. Data shown are mean ± SD. (C) The HSC- and MkP-associated genes were analyzed in purified GR+ HSCs, GR− HSCs, GR+ MkPs, and GR− MkPs by real-time PCR. (D) Flow cytometry analysis of the HSPC subpopulations after differentiation in vitro from human Lin-CD34+, Lin-CD34+GR+, and Lin-CD34+GR− cells. (E) CFU-MK colonies generated from Lin-CD34+, Lin-CD34+GR+, or Lin-CD34+GR− cells. (Scale bar, 100 uM.) (F) The changes of CD34+CD41+ cells (MkP) and CD34+CD71+ cells (EP) after 7 d of differentiation from purified CD34+ cells with GABRR1 knockout or overexpression. The percentage of each HSPC population from the nontreated WT cells was set to 1, and the percentage from other treatments was normalized to that. (G) CFU-MK colonies generated from CD34+ bone marrow cells with GABRR1 knockout or overexpression. GR-KO, GABRR1 knockout; GR-O, GABRR1 overexpression. (H) Quantification of human HSPC subpopulation changes by multicolor flow cytometry after 7 d of differentiation from bone marrow CD34+ cells with GABRR1 overexpression. The percentage of each HSPC population from the nontreated cells was set to 1, and the percentage from GABRR1-overexpressing cells was normalized to that. Data shown are representative of at least 3 independent experiments. Error bars indicate SD. *P < 0.05, **P < 0.01, ***P < 0.001.
We then genetically manipulated GABRR1 expression levels through lentivirus-mediated gene knockout and overexpression. First, CRISPR/Cas9-mediated gene knockout was used to eliminate GABRR1 expression (32). PCR analysis confirmed its expression level was reduced in CD34+ cells (SI Appendix, Fig. S4C). Then, CD34+ cells were cultured and differentiated by supplementing cytokines TPO, hSCF, hIL3, hIL6, and Flt3 in vitro (SI Appendix, Fig. S4A). Both CD34+CD41+ (selective MkP/megakaryocyte markers) (33, 34) and CD34+CD71+ (selective EP/erythrocyte markers) (35) cells were reduced by 30 to 40% (Fig. 3F and SI Appendix, Fig. S4B).
Overexpression of GABRR1 in human CD34+ cells led to a significant increase of CD34+CD41+ and CD34+CD71+ populations by ∼3- to 4-fold (Fig. 3F and SI Appendix, Fig. S4B). Using RT-PCR analysis, we analyzed gene expression levels of megakaryocyte-related genes, erythroid genes, and genes of both lineages in both GABRR1 knockout and overexpression cells (11). The results showed the RNA expression of those genes was significantly enhanced in GABRR1-overexpressing cells but reduced in GABRR1 knockout cells (SI Appendix, Fig. S4 C and D). Starting with 10,000 CD34+ cells, we obtained 52 megakaryocytic colonies from GABRR1-overexpressing cells, 8 from GABRR1 knockout cells, 30 colonies from nonvirus-treated control, and 20 from the vector control transduced group (Fig. 3G).
Since HSC differentiation into MkPs involves several steps (9), we next determined at which stage GABRR1 functions. By analyzing the frequencies of HSPCs populations in GABRR1-overexpressing cells (Fig. 3H and SI Appendix, Fig. S4E), we found that HSC/MPP increased to 263.6 ± 51.6% and almost all downstream progenies also increased (CMP to 267.3 ± 53.6%, MEP to 263.3 ± 68.7%, MkPs to 254.5 ± 92.6%, EPs to 423.2 ± 67.7%). Those results indicated GABRR1 affects MkP generation at the early stage of differentiation.
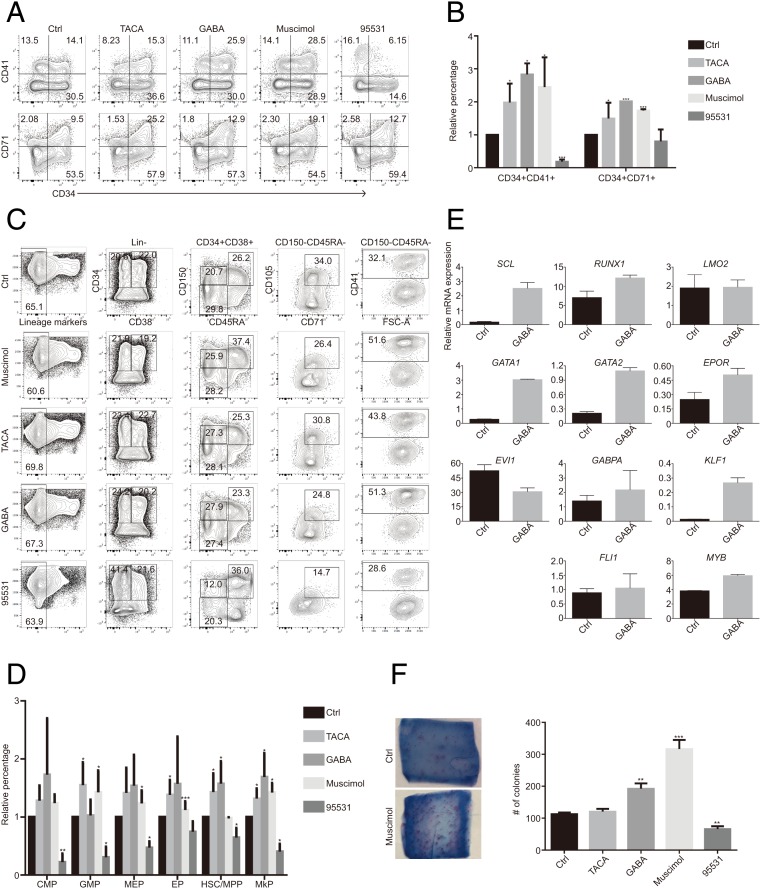
We treated human CD34+ cells with different agonists and antagonists of GABRR1. The CD34+CD41+ and CD34+CD71+ populations increased ∼2- and 4-fold, with the treatment of GABRR1 agonists (GABA, TACA, and Muscimol) and decreased dramatically when treated with GABRR1 antagonist SR95531 (Fig. 4 A and B). GABA was the most effective treatment that increased MkPs, to 168.9 ± 41.0%. The other agonists also produce 1.5- to 2-fold increases in the MkP frequency. The antagonist SR95531 decreased the frequency of all HSPCs tested, especially the MkPs, which were decreased to 40.3 ± 13.6% (Fig. 4 C and D). Real-time PCR analysis demonstrated that GABA significantly increased the megakaryocyte-related genes (Fig. 4E), while the other agonists showed similar effects. The CFU-MK assay showed that GABA, Muscimol, and TACA generated 1 to 3-fold increases in megakaryocyte colonies, while SR 95531 decreased them by 40% (Fig. 4F). These results indicated that GABRR1-mediated GABA signaling could regulate human hematopoiesis.
Fig. 4.
The effects of GABRR1 agonists and antagonists on human HSPC differentiation. (A) Flow cytometry analysis of CD34+CD41+ (MkP) and CD34+CD71+ (EP) percentage in 7-d differentiated CD34+ cells treated with agonists or antagonists of GABRR1. (B) Quantification of CD34+CD41+ (MkP) and CD34+CD71+ (EP) percentage in 7-d differentiated CD34+ cells treated with agonists or antagonists of GABRR1. The percentage of each HSPC population from the nontreated cells was set to 1, and the percentage from other treatments was normalized to that. (C and D) Flow cytometry (C) and quantification (D) of human HSPC subpopulation changes by multicolor flow cytometry after 7 d of differentiation from CD34+ bone marrow cells treated with agonists or antagonists of GABRR1. The percentage of each HSPC population from the nontreated cells was set to 1, and the percentage from other treatments was normalized to that. (E) Gene expression analysis of megakaryocytic and/or erythroid cell-associated genes by real-time PCR in 7-d differentiated cells from CD34+ bone marrow cells treated with GABA. (F) CFU-Mk colonies generated from CD34+ bone marrow cells treated with agonists or antagonists of GABRR1. Data shown in B, D, E, and F are mean ± SD from at least 3 independent experiments *P < 0.05, **P < 0.01, ***P < 0.001. Here, “Ctrl” refers to the vehicle-alone (H2O) group.
Discussion
To date, the precise control of HSC differentiation to MkPs is largely unknown, and there is currently no efficient way to produce MkPs from HSCs for clinical applications. In our study, we have identified a potential regulator of MkPs both in mice and in humans. We found that GABRR1 is expressed in subsets of HSCs and MkPs (Figs. 1 B and C and 3 A and B). Inhibition of GABRR1 signaling by genetic knockout or antagonists significantly decreased megakaryocyte and platelet differentiation, while overexpression of GABRR1 or agonist treatment increased megakaryocytic lineage development (Figs. 2 D–G, 3 G–H, and 4 A–C). Although all of the in vitro studies clearly support the function of GABRR1 in megakaryocytic lineage differentiation, it would be interesting to see in the future in an in vivo circumstance whether GABRR1 has a similar function, for example, to assess the content of platelets in GABRR1 transgenic mice in normal conditions or to check the functional role of GABRR1 in platelet production under challenging conditions, such as major blood loss or chemically induced thrombocytopenia, or in mouse models with increased platelet counts.
In the present study, we found GABRR1 is expressed only in subsets of HSCs and MkPs but not other hematopoietic progenitor populations. Previous studies identified many common features between HSCs and MkPs, such as the requirement for TPO, shared cell surface markers, and specific transcription factors (31). Our study adds more evidence to these shared features. Furthermore, our gene expression analysis and in vivo study clearly demonstrated the differences between GABRR1+ and GABRR1− HSCs, suggesting that there might be several different HSC subtypes, as suggested in another study (25), and warrant further study to identify more surface markers to elucidate these subtypes.
Interestingly, our study showed GABRR1+ cells produce fewer megakaryocyte colonies than GABRR1− progenitors, while GABRR1 knockout or antagonist also decreased megakaryocyte differentiation. We think there is a cellular hierarchy in the megakaryocyte lineage, with GABRR1− cells at the apex. GABRR1− cells in the HSC phenotype transplant better than GABRR1+ cells, and the increased colony counts could be due to the self-renewal potential of HSC versus the multipotent but poorly self-renewing GABRR1+ cells. Therefore, the GABRR1− population self-renews and also produces GABRR1+ cells, which could further generate megakaryocyte colonies. However, working out the kinetics of these responses will be complicated, as the 2 populations differ in self-renewal.
Our in vitro studies of human hematopoietic progenitors showed significant depletion of platelets with antagonist and augmentation with agonists. Similarly, the in vivo treatments in mice showed the agonists increased platelets, while the antagonist did not affect platelet numbers as significantly as the agonists. There are many possible reasons, including a species-specific effect and the half-life of the reagents in vivo; we dosed mice with the published effective concentration. It is also possible that the effect of blockade of GABRR1 with its antagonist could be compensated (to some extent) by other signal factors such as TPO.
Regulation of hematopoiesis by the nervous system has been reported and is an active area of research (36). In 2001, our laboratory, by taking advantage of microarray on purified cells, demonstrated an overlap between the genetic programs of hematopoietic and neural stem cells, indicating a relationship between the genes expressed in the hematopoietic and nervous systems (37). Another report showed that HSC migration out of the bone marrow depends critically on signals from the sympathetic nervous system, as uridine diphosphate-galactose ceramide galactosyltransferase-deficient (Cgt−/−) mice display virtually no HSPC egress from bone marrow following granulocyte colony-stimulating factor (G-CSF) or fucoidan administration (38). Studies of Drosophila larva showed that the peripheral nervous system supports blood cell homing and survival (39). Interestingly, in Drosophila, olfactory stimulation could induce the secretion of GABA from a small set of neurosecretory cells. The GABA levels in the circulation promote blood cell maintenance (40). Here in our study, we identified a conserved link between the neural product GABA and hematopoietic systems in mice and humans that may provide a strategy for producing MkPs and then platelets by manipulating GABRR1-mediated GABA signaling.
Materials and Methods
Cell isolation and culture, transplantations and peripheral blood analyses, virus production and transduction, colony-forming unit assay, flow cytometry, RNA isolation and real-time PCR, electrophysiology, gene expression commons analysis, and immunohistochemistry were done as described in SI Appendix.
Mice.
C57BL/6J, B6.SJL-Ptprca Pepcb/BoyJ, B6; 129S4-Gabrr1tm1Llu/J, and B6129SF2/J mice were purchased from the Jackson Laboratory and were bred at our animal facility according to NIH guidelines. Male mice of similar ages (6–10 wk) were used in the experiments. All animal protocols were approved by the Stanford University Administrative Panel on Laboratory Animal Care.
Plasmids.
The LentiCRISPR V2 plasmid was purchase from Addgene. The single-guide ribonucleic acid of GABRR1 was designed and cloned into the all-in-one CRISPR lentiviral vector. The pCDH-MSCV-MCS-EF1-GFP+Puro cDNA cloning and expression vector (CD713B-1) was purchased from SBI. GABRR1 cDNA (NM_001256703.1) was cloned from pDONR223, which was purchased from DNASU and inserted under the murine stem cell virus promoter. The same empty vector without GABRR1 cDNA was used as the vehicle control.
Supplementary Material
Acknowledgments
We thank Thomas C. Südhof and Yasuo Mori for scientific discussion and technical assistance, Tal Raveh for help in editing this manuscript, Libuse Jerabek and Terry Storm for laboratory management, Aaron McCarty and Charlene Wang for help with animal care and experiments, and FACS core at Stanford Institute for Stem Cell Biology and Regenerative Medicine for help with flow cytometry. This work is supported by National Institute of Health (NIH) Grants U01-HL099999 and R01-CA086065 (to I.L.W.). F.Z. is a Seibel Scholar of the Seibel Stem Cell Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906251116/-/DCSupplemental.
References
- 1.Awapara J., Landua A. J., Fuerst R., Seale B., Free gamma-aminobutyric acid in brain. J. Biol. Chem. 187, 35–39 (1950). [PubMed] [Google Scholar]
- 2.Dietl M. M., Cortés R., Palacios J. M., Neurotransmitter receptors in the avian brain. III. GABA-benzodiazepine receptors. Brain Res. 439, 366–371 (1988). [DOI] [PubMed] [Google Scholar]
- 3.Ge S., et al. , GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature 439, 589–593 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagiolini M., et al. , Specific GABAA circuits for visual cortical plasticity. Science 303, 1681–1683 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Liu X., Wang Q., Haydar T. F., Bordey A., Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8, 1179–1187 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang Y. Y., et al. , A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat. Med. 13, 862–867 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Erdö S. L., Wolff J. R., gamma-Aminobutyric acid outside the mammalian brain. J. Neurochem. 54, 363–372 (1990). [DOI] [PubMed] [Google Scholar]
- 8.Andäng M., et al. , Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 451, 460–464 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Seita J., Weissman I. L., Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 640–653 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakorn T. N., Miyamoto T., Weissman I. L., Characterization of mouse clonogenic megakaryocyte progenitors. Proc. Natl. Acad. Sci. U.S.A. 100, 205–210 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klimchenko O., et al. , A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 114, 1506–1517 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Stroncek D. F., Rebulla P., Platelet transfusions. Lancet 370, 427–438 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., et al. , Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: A cautionary tale. Blood 125, 3627–3636 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takizawa H., et al. , Lnk regulates integrin alphaIIbbeta3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J. Clin. Invest. 120, 179–190 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takayama N., Eto K., In vitro generation of megakaryocytes and platelets from human embryonic stem cells and induced pluripotent stem cells. Methods Mol. Biol. 788, 205–217 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Nakamura S., et al. , Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 14, 535–548 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Tozawa K., et al. , Megakaryocytes and platelets from a novel human adipose tissue-derived mesenchymal stem cell line. Blood 133, 633–643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seita J., et al. , Gene expression commons: An open platform for absolute gene expression profiling. PLoS One 7, e40321 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu F., et al. , Screening for genes that regulate the differentiation of human megakaryocytic lineage cells. Proc. Natl. Acad. Sci. U.S.A. 115, E9308–E9316 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen R. W., Sieghart W., International union of pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 60, 243–260 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng W., et al. , Function of gamma-aminobutyric acid receptor/channel rho 1 subunits in spinal cord. J. Biol. Chem. 278, 48321–48329 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Torres V. I., Weiss D. S., Identification of a tyrosine in the agonist binding site of the homomeric rho1 gamma-aminobutyric acid (GABA) receptor that, when mutated, produces spontaneous opening. J. Biol. Chem. 277, 43741–43748 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Pronk C. J., et al. , Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1, 428–442 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Chen J. Y., et al. , Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanjuan-Pla A., et al. , Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502, 232–236 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Chan C. K., et al. , Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc. Natl. Acad. Sci. U.S.A. 110, 12643–12648 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiel M. J., et al. , SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Chan C. K., et al. , Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan C. K., et al. , Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worthley D. L., et al. , Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160, 269–284 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H., Cantor A. B., Common features of megakaryocytes and hematopoietic stem cells: What’s the connection? J. Cell. Biochem. 107, 857–864 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shalem O., et al. , Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boitano A. E., de Lichtervelde L., Snead J. L., Cooke M. P., Schultz P. G., An image-based screen identifies a small molecule regulator of megakaryopoiesis. Proc. Natl. Acad. Sci. U.S.A. 109, 14019–14023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berridge M. V., Ralph S. J., Tan A. S., Cell-lineage antigens of the stem cell-megakaryocyte-platelet lineage are associated with the platelet IIb-IIIa glycoprotein complex. Blood 66, 76–85 (1985). [PubMed] [Google Scholar]
- 35.Mori Y., Chen J. Y., Pluvinage J. V., Seita J., Weissman I. L., Prospective isolation of human erythroid lineage-committed progenitors. Proc. Natl. Acad. Sci. U.S.A. 112, 9638–9643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanoun M., Maryanovich M., Arnal-Estapé A., Frenette P. S., Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 86, 360–373 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terskikh A. V., et al. , From hematopoiesis to neuropoiesis: Evidence of overlapping genetic programs. Proc. Natl. Acad. Sci. U.S.A. 98, 7934–7939 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katayama Y., et al. , Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Makhijani K., Alexander B., Tanaka T., Rulifson E., Brückner K., The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development 138, 5379–5391 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shim J., et al. , Olfactory control of blood progenitor maintenance. Cell 155, 1141–1153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.