Significance
l-Dopa–induced dyskinesia (LID) is a major complication of dopaminergic therapy in Parkinson’s disease. These involuntary movements have been associated with several molecular changes in the striatum, including increased levels of ΔFosB. However, whether ΔFosB upregulation plays a major role in the development of the primate LID or is an epiphenomenon of chronic l-Dopa treatment remains unknown. Using transgenic expression of ΔFosB in the striatum of parkinsonian primates, we show that elevated levels of ΔFosB lead to LID development in the absence of chronic l-Dopa exposure. The physiological and molecular changes associated with LID are also replicated in this model. These findings suggest that ΔFosB elevation is responsible for LID generation and that repressing its expression can have therapeutic value.
Keywords: ΔFosB, l-Dopa–induced dyskinesia, striatal projection neurons, Parkinson’s disease, nonhuman primate models
Abstract
Long-term dopamine (DA) replacement therapy in Parkinson’s disease (PD) leads to the development of abnormal involuntary movements known as l-Dopa–induced dyskinesia (LID). The transcription factor ΔFosB that is highly up-regulated in the striatum following chronic l-Dopa exposure may participate in the mechanisms of altered neuronal responses to DA generating LID. To identify intrinsic effects of elevated ΔFosB on l-Dopa responses, we induced transgenic ΔFosB overexpression in the striatum of parkinsonian nonhuman primates kept naïve of l-Dopa treatment. Elevated ΔFosB levels led to consistent appearance of LID since the initial acute l-Dopa tests. In line with this motor response, striatal projection neurons (SPNs) responded to DA with changes in firing frequency that reversed at the peak of the motor response, and these unstable SPN activity changes in response to DA are typically associated with the emergence of LID. Transgenic ΔFosB overexpression also induced up-regulation of other molecular markers of LID. These results support an autonomous role of striatal ΔFosB in the adaptive mechanisms altering motor responses to chronic DA replacement in PD.
Levodopa-induced dyskinesia (LID) in Parkinson’s disease (PD) is a disabling complication of dopamine (DA) replacement therapy commonly generated in the context of extensive nigrostriatal DA loss and chronic drug exposure. LID development has been associated with dysregulated mechanisms in striatal microcircuits altering the function of striatal projection neurons (SPNs) (1–3). These neurons undergo progressive morphological and excitability changes that largely affect their response to DA modulation (3, 4). In dyskinetic parkinsonian primates with pathological SPN hyperactivity, the neuronal responses to dopaminergic stimulation are typically unstable. DA induces firing rate changes that are reversed during the peak of the response in a large number of neurons, thereby causing an imbalance of striatal discharges and a reversal of parkinsonism complicated by dyskinesias (5, 6). The underpinnings of these physiological changes are not clear, but several signaling molecules have been identified to play a role in LID mechanisms. Among the most established markers are phosphorylated ERK1/2 (extracellular signal-regulated kinase) and DARPP-32 (DA- and cAMP-regulated phosphoprotein, 32 kDa), modified H3 and H4 (Ser10-histone H3), and overexpressed ΔFosB (7–12). These molecules participate in regulating transcription and translation that can lead to persistent plasticity changes in striatal neurons. p-ERK1/2 activates mTORC1, the mammalian target of rapamycin complex 1, and thus participates in the regulation of mRNA translation for various cellular responses, including synaptic plasticity (13, 14). p-DARPP-32 activates protein phosphatase 1 (pp-1), which in turn regulates a number of downstream signaling molecules, including the phosphorylation of glutamate receptor subunits (15, 16). Of particular interest are the posttranslational modifications of H3/H4 and up-regulation of the transcription factor ΔFosB, which highly impact the expression of multiple genes (10, 17, 18).
ΔFosB (the truncated splice variant of FosB) is up-regulated in models of drug addiction and other chronic stimuli (19). A key element in LID development is also chronic drug exposure, i.e., the nonphysiologic, pulsatile l-Dopa administration. The striatal ΔFosB content is significantly elevated across rodent and primate models and correlates with the severity of LID (11, 20). This Fos family member that is highly stable (18, 21) can induce the long-lasting gene regulation underlying chronic plasticity changes in striatal neurons. Continuous recordings of SPNs during l-Dopa administration in primates reveal profoundly altered SPN responses to DA (5, 22). The large and steady ΔFosB accumulation in SPNs may thus be a primary driver in the molecular cascade of LID mechanisms.
To determine whether ΔFosB may trigger LID development, we induced transgenic overexpression of ΔFosB in the striatum of parkinsonian (macaque) primates with extensive DA depletion but naïve of chronic l-Dopa treatment. Behavioral and electrophysiological analyses showed that striatal ΔFosB overexpression directly leads to abnormal SPN responses to dopaminergic stimulation and LID expression. In addition, elevated ΔFosB levels induced molecular changes typically associated with LID such as p-DARPP32 increase, indicating a primary role of this transcription factor in LID development.
Results
Transgenic ΔFosB Overexpression in the Striatum Generates Dyskinetic Responses to l-Dopa in Parkinsonian Primates.
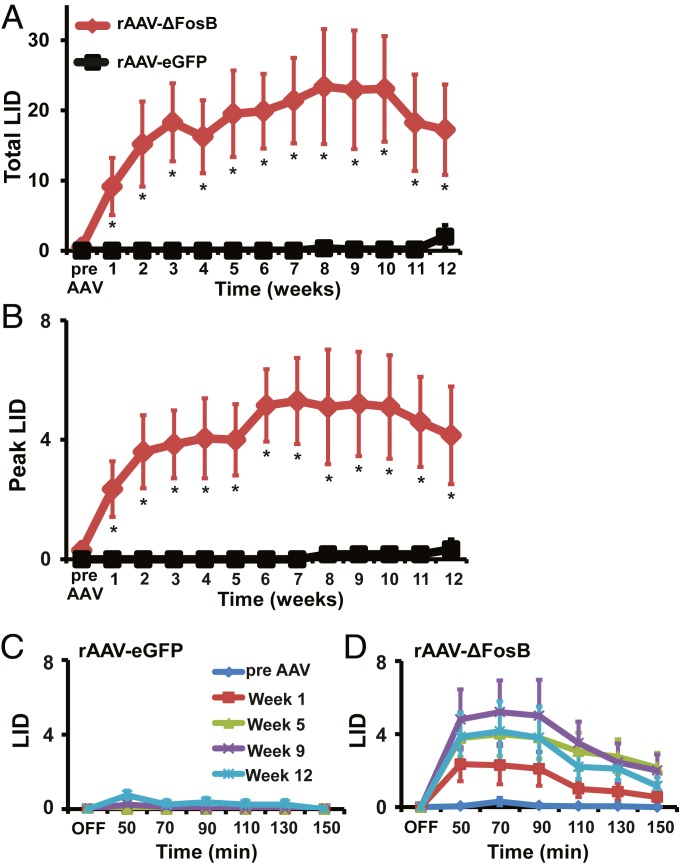
To determine the behavioral effects of transgenic ΔFosB overexpression, 9 primates with stable parkinsonism (see Methods for description of the animal model developed after a series of systemic MPTP [1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine] injections and SI Appendix, Table S1 for demographics and clinical characteristics) and naïve of chronic dopaminergic drug exposure received bilateral striatal injections of rAAV-ΔFosB (n = 5) or rAAV-eGFP (n = 4) as control virus. During the month prior to virus injection, 3 tests of subcutaneous (s.c.) l-Dopa injections at intervals of 7 d provided the baseline (previrus) response, confirming the lack of LID in the absence of chronic l-Dopa treatment in all animals. In 2 animals of each virus group, vectors were injected into the striatum through surgically implanted recording chambers after electrophysiologic mapping (animals prepared to analyze effects on neuronal activity). In the remaining animals, vectors were injected surgically with stereotaxic guidance. Beginning at 4 wk post viral vector injections, the response to the predetermined s.c. l-Dopa dose was tested at weekly intervals for a total of 3 mo (see timeline and location of virus injections in Fig. 1). Striatal rAAV-ΔFosB injection induced LID from the first l-Dopa test post virus injection, and LID progressed in successive weeks, reaching maximal scores between weeks 6 and 8, followed by a slight decline between weeks 9 and 12 (P < 0.001; Fig. 2 A, B, and D). LID was not expressed in the control group injected with rAAV-eGFP during the entire assessment period of 12 wk with the exception of 1 animal that began to develop LID by the ninth week (P > 0.05; Fig. 2 A–C). rAAV-ΔFosB–induced LID was mild to moderate choreodystonic movements variably expressed in the face, neck, and limbs with the usual predominance in lower limbs, all features indistinguishable from typical LID in the primate model (SI Appendix, Fig. S1 A–C). The time course of l-Dopa response shows a single peak of dyskinesias reaching the highest scores between 50 and 90 min post s.c. injection (Fig. 2D and SI Appendix, Fig. S1 D–F), which parallels the monophasic peak-dose LID also commonly seen in the primate. Striatal rAAV-ΔFosB injection did not induce dyskinesias without l-Dopa administration, and no animal in either rAAV group showed additional motor abnormalities or other adverse reactions post virus injection. Results in control animals exposed to the same schedule of discrete (weekly) l-Dopa tests pre and post rAAV-eGFP virus injection showing mild dyskinesias starting by week 9 indicate that LID development requires a higher level of exposure, as typically occurs with chronic daily l-Dopa treatment. In contrast, the rapid LID development in animals naïve of chronic l-Dopa exposure but injected with rAAV-ΔFosB indicates a primary role of striatal ΔFosB in LID mechanisms.
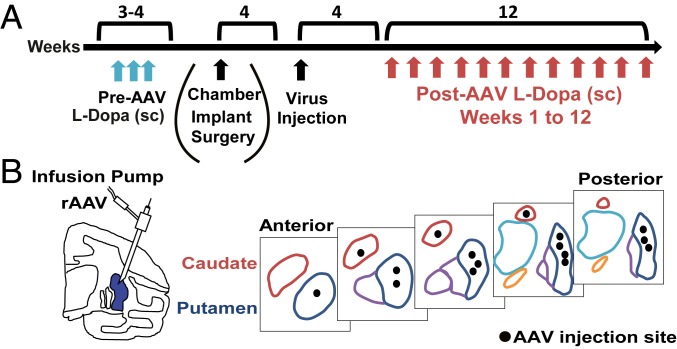
Fig. 1.
Timeline and design of experiments. (A) Timeline. Studies began for all MPTP-treated primates with assessment of the response to s.c. l-Dopa injections (blue arrows) during 3 to 4 wk in order to determine effective “on” response of the selected dose and the absence of LID in acute tests. At week 4, recording chambers were implanted surgically (only in a subgroup of 4 animals used for physiology), and, 4 wk later, viral vectors (rAAV-ΔFosB or rAAV-eGFP) were infused into the striatum accessed through the chambers (SI Appendix, Table S1). In the remaining 5 animals, viral vectors were infused under stereotaxic surgery. In all animals, 4 wk after virus infusion, weekly acute l-Dopa tests began and continued until the 12th week (red arrows). (B) Schematic drawing of the virus injection sites illustrating the injectrode locations in the putamen and the caudate and putamen planes from anterior to posterior regions.
Fig. 2.
Striatal overexpression of ΔFosB leads to appearance of l-Dopa–induced dyskinesias. (A and B) Total (A) and peak (B) LID scores following s.c. injection of l-Dopa are shown before (pre AAV) and after striatal infusions of rAAV-ΔFosB (n = 5) or rAAV-eGFP (n = 4). Total LID scores are calculated as the sum of scores from all postinjection time points, and peak scores are the highest LID scores taken at any one interval (usually 50 to 70 min post injection). Animals infused with rAAV-ΔFosB developed LID from the first s.c. l-Dopa test (week 1) after virus injection and continued to show higher LID scores during the successive 12 wk (red), while only 2 animals infused with control virus developed mild LID starting between 9 and 10 wk after virus injection (black). (C and D) Time course of absolute LID scores after s.c. injection of l-Dopa in animals infused with rAAV-eGFP (C) or rAAV-ΔFosB (D) showing LID development. Scores before rAAV injection (pre-AAV, blue) and following rAAV injection at weeks 1 (red), 5 (green), 9 (purple), and 12 (light blue) are compared. At each week after rAAV-ΔFosB injection, animals showed LID progressing to the maximal level at 9 wk after virus injection. After control rAAV-eGFP injection, mild LID developed (small peak between 50 and 90 min) in 2 animals by the end of the testing period (weeks 9 and 12). OFF, before s.c. l-Dopa injection. Data are means ± SEM (*P < 0.05, 2-way ANOVA for repeated measures followed by Fisher’s PLSD test).
Striatal rAAV-ΔFosB injection did not induce changes in the parkinsonian “off” state (basal motor disability scores, MDS), which remained the same throughout the 12-wk assessment period (SI Appendix, Fig. S2A). More importantly, striatal rAAV-ΔFosB injection had no impact on the antiparkinsonian action of l-Dopa measured by MDS in the “on” state (reversal of parkinsonian motor disability), which remained unchanged from pre to post virus l-Dopa responses and were not different between the 2 animal groups (SI Appendix, Fig. S2 B–D). Thus, striatal rAAV-ΔFosB injection did not lead to overall sensitization to DA with augmented l-Dopa responses that could accelerate LID development but had a specific impact on the molecular events leading to LID.
Striatal ΔFosB Overexpression Induces the Pathological Responses of Striatal Projection Neurons to DA That Are Associated with Dyskinesias.
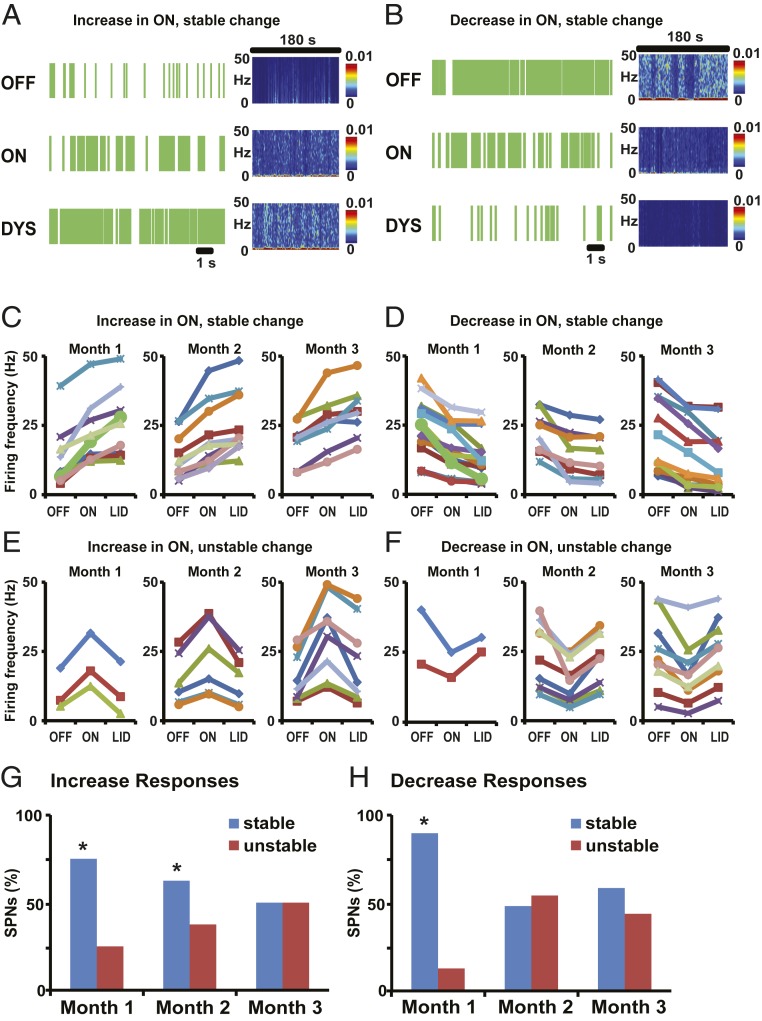
To determine the effect of ΔFosB overexpression on SPN responses to DA, we analyzed striatal recordings performed once weekly postvirus injections (see Fig. 3 for the timeline of continuous recordings while the animal transitioned to motor states induced by l-Dopa administration). In the control rAAV-eGFP group, the “on” state was characterized by a large predominance of stable firing changes in SPNs with either a D1-like response (increases in 75% units) or D2-like response (decreases in 88% units) during the first month of recordings (see Methods for definition of stable and unstable responses; Fig. 4 A–F). Increasing l-Dopa exposure with weekly injections led to reduction of the stable responses and increase of unstable responses in the second and third months, ending in a similar percentage of SPNs with stable and unstable responses by the ninth week when dyskinesias began to express in this group, i.e., 50% unstable D1-like responses and 43% unstable D2-like responses (Fig. 4 G and H; P < 0.01 for differences at months 1 and 2).
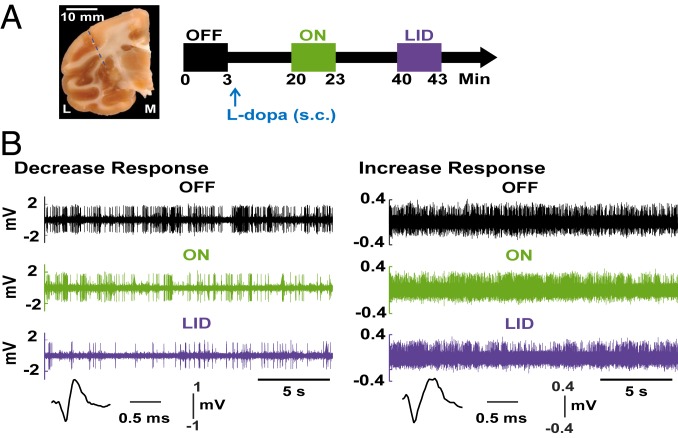
Fig. 3.
Timeline of recordings and typical SPN responses. (A, Left) A coronal section of the primate brain with a small scar at the site of guide cannula penetration. The dashed blue line represents the injectrode trajectory. L, lateral; M, medial. (A, Right) Timeline of the continuous single-cell recording showing data storage before s.c. injection of l-Dopa (“OFF” state, black box), after transitioning to the “ON” state (green box) 20 min following l-Dopa injection, and the subsequent dyskinesia (LID) state (purple box) at the peak-dose l-Dopa effect. (B) Examples of typical activity changes in SPNs through the transition to motor states. Spiking activity recorded in the putamen continuously while the monkey passed from the “OFF” state (black) to the “ON” (green) and to ON-with-dyskinesias (“LID”, purple) states are shown. The 2 examples depict spiking raw data in each motor state with stable changes of frequency (sustained decreased [Left] or increased [Right] activity throughout the ON-LID state). The analysis after spike sorting confirmed the observed activity changes corresponding to single SPNs.
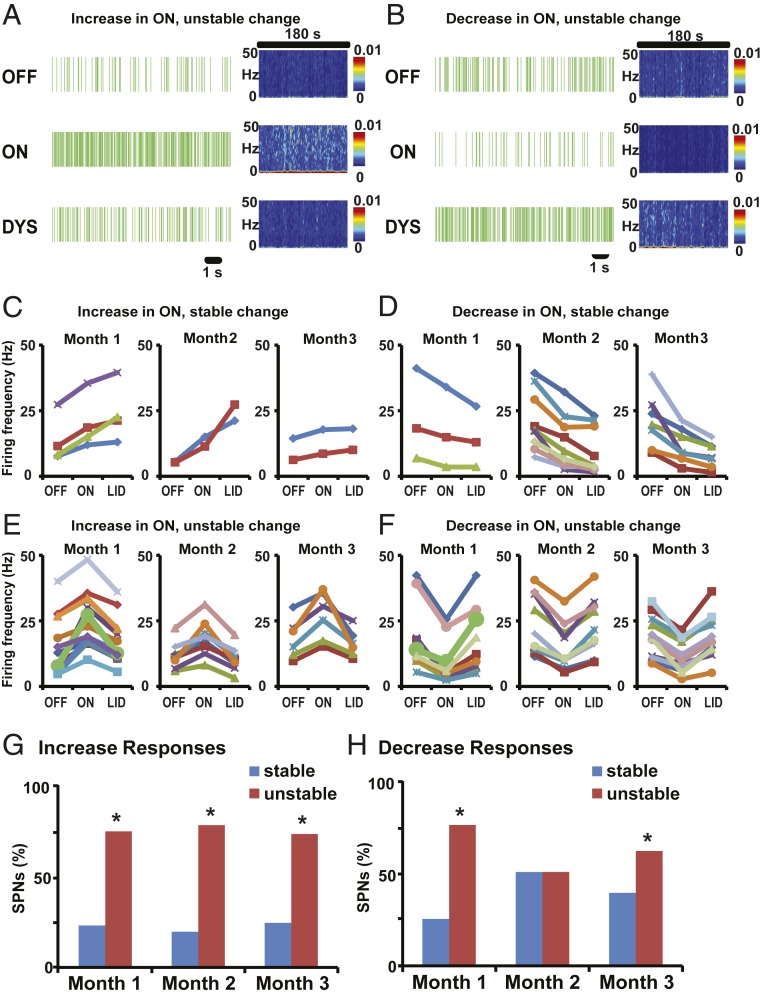
Fig. 4.
SPNs predominantly show stable responses to DA in control animals infused with rAAV-eGFP. (A and B) Examples of SPN stable activity changes in response to s.c. l-Dopa injection in animals infused with rAAV-eGFP. Short (12 s) raster plots and spectrograms for the whole segment duration (180 s) are shown for each segment (OFF, ON, and DYS). Firing rates of SPNs were increased (A) or decreased (B) from the OFF to the ON state. In DYS state, SPNs with increase or decrease in ON continued to change their firing rates in the same direction, ie, increase (A) or decrease (B). (C–F) Changes of SPN firing frequency (in Hertz) across units grouped by the type of response: stable and unstable increase in ON-LID transition (C and E, respectively) or stable and unstable decrease in ON-LID transition (D and F, respectively). In each of the 4 types of response, units recorded weekly in each month are presented in separate graphs. In each graph, each curve represents an individual SPN. Responses were classified as increase or decrease depending on significant firing frequency change from OFF to ON and as stable or unstable by significant firing frequency change in the same or opposite direction in the transition from ON to LID, all taken at P < 0.01. No significant change from ON to LID was also taken as stable. SPN firing rasters in A and B are derived from SPNs shown as a thick green curve in month 1 of C and D, respectively. (G and H) The proportion of SPNs with stable (blue bars) or unstable (red bars) responses through the recordings of each month. In the group of SPNs with increased activity in the ON state (G), a progression to reduced stable responses in the transition to LID was found from month 1 to month 2 and to month 3 (75%, 62.5%, and 50% of SPNs in each month). In the group of SPNs with decreased activity in the ON state (H), more rapid reduction of stable responses in the transition to LID developed after month 1 (87.5%, 47%, and 57% of SPNs in each month; *P < 0.05 versus SPNs with unstable changes in the same month).
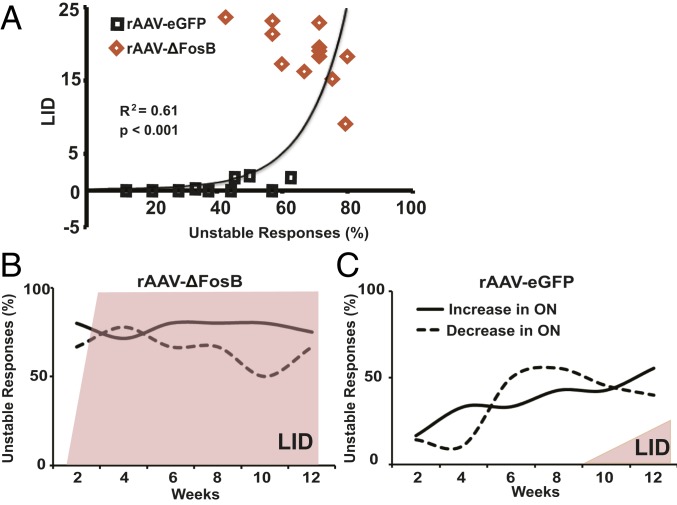
Notably, in the rAAV-ΔFosB group, unstable responses largely predominated from the first month of recordings post virus injections (Fig. 5 A–F). The proportion of SPNs with unstable responses was significantly higher in most recordings from the first to the 12th week (Fig. 5 G and H; P < 0.01; see details in SI Appendix, Table S2). In the first month, such pathological instability of DA responses developed in 77% of the total SPNs with D1-like response and 75% of the total SPNs with D2-like response. Usually, unstable responses develop in ∼50% of units in each SPN subset (D1- or D2-like response) following chronic daily l-Dopa treatment (5). The effects of ΔFosB overexpression observed in SPNs with either D1- or D2-like responses were maintained throughout the 3 mo of the testing period, oscillating between 50% and 75% unstable firing changes (Fig. 5 G and H). Taken together, these data show the correlative development of LID and unstable SPN responses to DA (Fig. 6A), with over 50% SPNs in both subsets exhibiting unstable responses to DA, but with different timing, i.e., early in the rAAV-ΔFosB group and late in the control rAAV-eGFP group (Fig. 6 B and C). This physiological correlate indicates engagement of ΔFosB in the molecular pathways controlling responses to DA extensively across SPNs, and thereby generating dyskinesias.
Fig. 5.
Striatal overexpression of ΔFosB induces rapid development of unstable SPN responses to DA. (A and B) Examples of SPN unstable activity changes in response to s.c. l-Dopa injection in animals infused with rAAV-ΔFosB. Short (12 s) raster plots and spectrograms for the whole segment duration (180 s) are shown for each segment (OFF, ON, and DYS). Firing rates of SPNs were increased (A) or decreased (B) from the OFF to the ON state. In DYS state, SPNs with increase (A) or decrease (B) in ON changed their firing rates in the opposite direction (inverted response), creating unstable firing rate changes during the whole ON state (pre and post LID occurrence). (C–F) Changes of SPN firing frequency (in Hertz) across units grouped by the type of response: stable and unstable increase in ON-LID transition (C and E, respectively) or stable and unstable decrease in ON-LID transition (D and F, respectively). Other details of graphs are shown in the homologous panels of Fig. 4. SPN firing rasters in A and B are derived from SPNs shown as a thick green curve in month 1 of E and F, respectively. (G and H) The proportion of SPNs with stable (blue bars) or unstable (red bars) responses through the recordings of each month. In the group of SPNs with increased activity in the ON state (G), unstable responses in the transition to LID fully developed in month 1 and remained high in subsequent months 2 and 3 (76.5%, 80%, and 75% of SPNs in each month). In the group of SPNs with decreased activity in the ON state (H), unstable responses in the transition to LID also developed rapidly in month 1 and remained high but more variable in months 2 and 3 (75%, 50%, and 61% of SPNs in each month; *P < 0.05 versus SPNs with unstable changes in the same month).
Fig. 6.
Correlation between unstable SPN responses to DA and the development and severity of LID. (A) The correlation between unstable SPN responses to DA and LID scores shows a strong relationship with the appearance of mild LID following the development of ∼50% of unstable SPN responses. The regression analysis (exponential model) used averages of total LID scores paired with the percentages of SPNs with unstable responses per week in each group (control, black squares; transgenic ΔFosB, orange diamonds). (B and C) Proportions of SPNs with unstable responses to DA as recorded every week and grouped by the firing frequency change in the ON state (increase or decrease depicted as plain or dashed lines, respectively) after striatal infusion of rAAV-ΔFosB (B) and rAAV-eGFP (C). After rAAV-ΔFosB infusion (B), unstable responses to DA highly predominated in both types of SPN responders: ON-increase (D1-like SPNs) and ON-decrease (D2-like SPNs). LID (shaded area) also developed rapidly and persisted at a high level during the 12-wk assessment period, while unstable responses to DA of either type remained above 50%. After rAAV-eGFP infusion (C), unstable responses to DA progressed to reach 50% in both types of SPN responders toward the end of the testing period. Note also the size difference in LID shaded area that indicates mild and partial LID development (2 of 4 animals) in the control group. Percentages of unstable responses were calculated from all SPNs recorded every 2 wk for each group of virus infusion.
Analysis of pools of neurons in the “off” state showed similar SPN firing frequencies in the 2 viral vector groups and no significant differences in successive weeks (SI Appendix, Fig. S3), indicating no effect of the transgenes (ΔFosB or eGFP) on basal SPN activity.
Increased FosB Immunopositive Neurons and ΔFosB Proteins in the Striatum Confirms Transgenic Overexpression.
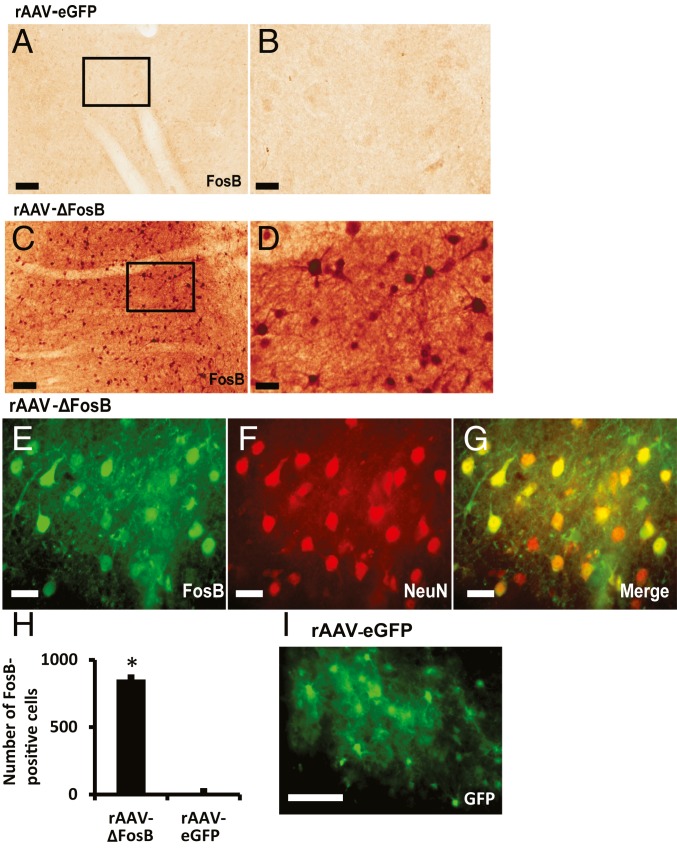
High FosB expression was detected in striatal neurons of animals injected with rAAV-ΔFosB in comparison with control animals injected with rAAV-eGFP. The antibody used for immunostaining reacts with the N terminus of FosB/ΔFosB, and since the virus plasmid was constructed with ΔFosB cDNA, the protein highly expressed in the rAAV-ΔFosB group is presumed to be largely ΔFosB (Fig. 7 A–D). Therefore, the robust staining of FosB proteins in the rAAV-ΔFosB group was produced by the specific transgene expression. Double staining with antibodies against FosB and the neuronal marker NeuN showed that 86% of FosB immunopositive cells costained for NeuN (Fig. 7 E–G). These findings indicate that a large majority of recorded neurons likely expressed the transgene. Cell counting in both virus groups showed a large difference in FosB expression (Fig. 7H), and the efficiency of rAAV-eGFP infection in control animals was also confirmed by eGFP immunostaining (Fig. 7I).
Fig. 7.
Transgenic expression of ΔFosB in the primate striatum. (A–D) FosB staining in the striatum of monkeys that were infused with rAAV-eGFP (A and B) or rAAV-ΔFosB (C and D). Animals were euthanized 16 wk after virus infusion. B and D are high magnifications of the squares denoted in A and C, respectively. Images show a large difference in FosB expression between animals infused with rAAV-ΔFosB (C and D) and those infused with rAAV-eGFP (A and B). (E–G) Double immunofluorescence with FosB and NeuN staining in the striatum of animals infused with rAAV-ΔFosB. FosB is expressed in striatal neurons that are also positive for NeuN (merge in G). (H) Cell counting of neurons positive for FosB in the striatum of animals infused with rAAV-ΔFosB (n = 3) or rAAV-eGFP (n = 3). Striatal neurons positive for FosB were counted in 10 microscopic fields in each monkey. (I) eGFP immunofluorescence in the striatum of animals infused with rAAV-eGFP (*P < 0.01 versus animals infused with rAAV-eGFP). (Scale bars: A and C, 100 µm; B, D and E–G, 25 µm; and I, 50 µm.)
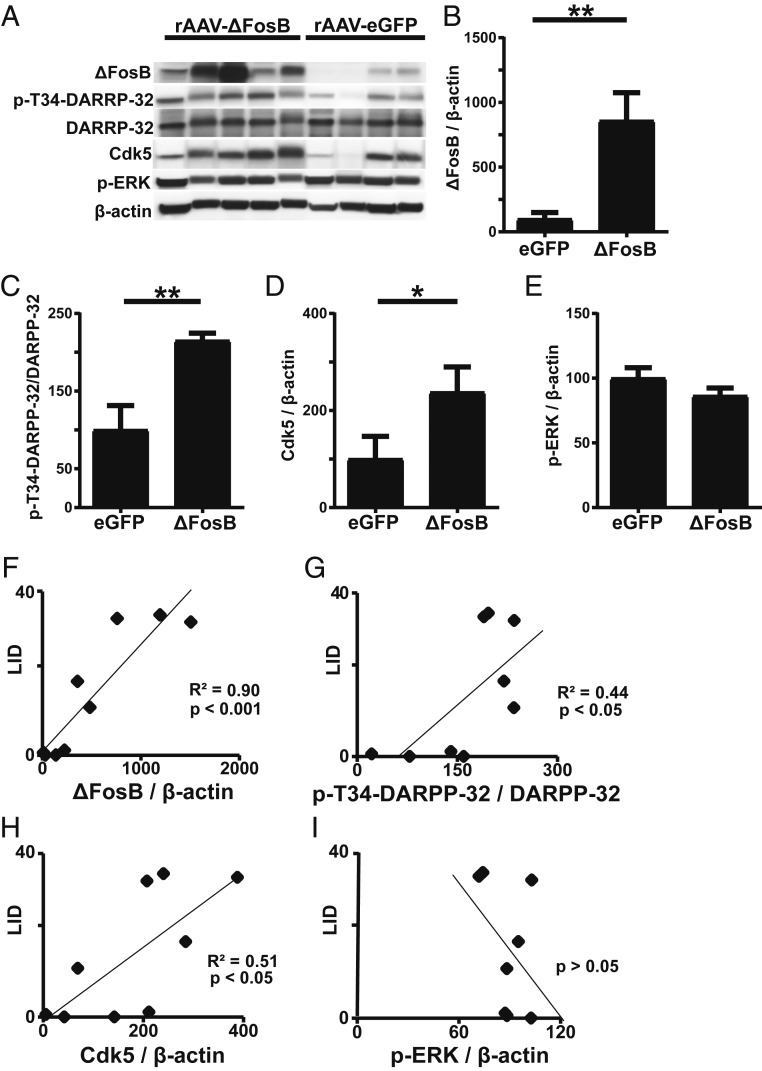
Increased ΔFosB expression was confirmed by Western blotting as well. While some variability was seen in the degree of overexpression among animals injected with rAAV-ΔFosB, a clear difference with an 8-fold increase in mean ΔFosB protein content was detected in comparison with the control rAAV-eGFP injected group (Fig. 8 A and B).
Fig. 8.
Regulation of molecular markers of LID induced by ΔFosB overexpression. (A) Western blot analyses of striatal tissue lysates for the indicated proteins. Each lane represents striatal tissue lysate from a separate animal. (B–E) Quantification of indicated band intensities relative to β-actin in A (B, D, and E) or quantification of p-Thr34-DARPP-32 band intensity relative to total DARPP-32 expression in A (C). Data represent means ± SEM (*P < 0.05 and **P < 0.001, unpaired t tests). (F–I) Correlations between protein levels and LID scores. For linear regression analyses, the total LID scores taken at the ninth week (highest levels) of the 12 post-AAV assessments were paired with the level of protein (relative band intensity as expressed in B–E) in each animal (n = 9).
Impact of ΔFosB Overexpression on Molecular Markers of LID.
Comparison of striatal tissue samples between animals injected with rAAV-ΔFosB versus rAAV-eGFP showed significant differences in additional molecular markers associated with LID (Fig. 8A). Phosphorylation of DARPP-32 at Thr34, which is augmented in LID (7), doubled in the rAAV-ΔFosB group compared with the control group (Fig. 8C). No significant difference was detected in total DARPP-32 level. Similarly, the expression of cyclin-dependent kinase 5 (Cdk5), another protein increased in LID (23), was more than double in the ΔFosB group compared with the rAAV-eGFP group (Fig. 8D). Similar to ΔFosB, the levels of these markers also correlated with the severity of LID (Fig. 8 F–H). Another kinase, ERK, which is activated during priming rather than maintenance of dyskinesia (24), was not different between the 2 groups (Fig. 8 E and I). These molecular changes suggest that elevated striatal levels of ΔFosB in parkinsonian primates can mimic the molecular microenvironment of LID.
Discussion
Elevated striatal levels of ΔFosB by transgenic induction, instead of the typical chronic exposure to l-Dopa, sufficed to generate LID in the parkinsonian primate. Following regular l-Dopa therapy, there are various molecular changes in striatal neurons leading to the pathogenetic cascade underlying altered neuronal responses to dopaminergic stimulation and LID development (1, 2). Previous rodent studies have strongly supported a causal relationship between ΔFosB up-regulation and abnormal involuntary movement (AIMs) (7–11), particularly using transgenic models (25). This relationship could not be predicted using standard animal models of LID in the context of multiple interacting changes induced by long-term l-Dopa treatment. In the present study, LID developed in response to acute l-Dopa administration in primates with transgenic overexpression of striatal ΔFosB but lacking the effects of “chronic” l-Dopa treatment, which likely induces additional molecular changes. Control animals (rAAV-eGFP) had a similar degree of parkinsonism and identical exposure to acute l-Dopa injections, but did not exhibit LID. The induced ΔFosB overexpression acted as a primary molecular mediator of the dyskinesias induced by l-Dopa, and the high ΔFosB levels attained by the transgene were similar to the consistent up-regulation induced by chronic l-Dopa treatment (25). Therefore, these results support an autonomous role of ΔFosB in the pathogenetic signaling cascades underlying the dyskinesias induced by chronic l-Dopa treatment. Furthermore, the induced ΔFosB up-regulation was associated with typical abnormal SPN responses to DA. A rapid development of “unstable” firing changes in the “on” state (a correlate of LID in primates exposed to chronic l-Dopa therapy [5, 6, 22]) was present in animals with ΔFosB overexpression. In addition, up-regulation of other striatal signaling molecules also known as LID markers correlated with higher ΔFosB levels. Together, the behavioral, physiological, and molecular correlates of increased ΔFosB levels validate its leading role in LID development.
Differences in motor responses to DA between the ΔFosB transgene and control groups were robust, and Western blot analysis confirmed large differences in ΔFosB levels. In 2 rAAV-eGFP–injected control animals, LID began to appear between the 11th and 12th week of weekly l-Dopa exposure. This was accompanied by an increase in ΔFosB expression as determined after euthanasia by Western blot analysis of striatal tissue at the end of assessments (12th week). Nevertheless, ΔFosB levels by the 12th week were significantly lower in the control group. Previous studies had shown AIMs induced by transgenic ΔFosB expression in rats naïve of l-Dopa treatment (25). Here, we used the primate model of PD that fully reproduces the motor phenotype including pathological l-Dopa responses in humans (26), and these results are therefore translatable to the LID development that occurs in patients (2). There are key points in the experimental design that validate the results of the present primate study. To assess differences induced mainly by ΔFosB overexpression, the dopaminergic stimulation in both animal groups was limited to a minimum pre- and post-AAV injections (3 l-Dopa tests at baseline to select doses for equivalent effects in each subject and 12 injections after the virus, all exposures at weekly intervals). In fact, only by the end of the treatment period did 2 control animals begin to develop LID. To create a similar propensity to developing LID among subjects and across the 2 groups, an advanced parkinsonism at the level of moderate motor disability scores was reached in all subjects. This level of disability was selected to ensure survival without regular dopaminergic therapy, but having sufficient DA lesion to develop LID. Also, the attained degree of disability prevented spontaneous recovery (26), which can introduce variable responses to l-Dopa and interfere with LID development. Critically, all animals in ΔFosB and control virus groups had similar predisposing conditions to develop LID, but differ only in one factor: striatal ΔFosB levels. After transgenic overexpression of ΔFosB, LID develops with the same topographic distribution as observed following long-term l-Dopa therapy (27) (SI Appendix, Table S3), suggesting a common pathophysiology in both conditions. Finally, the lack of changes in motor disability scores over the course of weekly tests in both groups excludes the possibility of motor changes due to nonspecific effects of viral injections. Striatal injection of rAAV-ΔFosB induced a single behavioral effect, i.e., the early and rapid generation of LID, indicating the specificity of this transcription factor for mechanisms related to dyskinetic responses to DA stimulation in PD.
Striatal ΔFosB overexpression was also associated with rapid development of altered SPN responses to DA. Firing rate changes in the “on” state were unstable in a large proportion of SPNs very early in primates with elevated striatal ΔFosB levels and correlated with early development of dyskinetic responses to acute l-Dopa administration. Increased or decreased SPN activity in response to DA is sustained throughout the peak-dose effect (stable SPN responses) in the absence of dyskinesias, but the activity changes are typically reversed (unstable SPN response) in ∼50% of SPNs in dyskinetic responses to l-Dopa (5, 22). This creates imbalance of outputs between and within striatal circuits. While SPN responses to DA in the control group progressed slowly to the unstable type in 50% of units by the third month of testing, unstable responses in the transgenic ΔFosB group were already developed in more than 75% of SPNs during the first month. The different evolution of SPN responses between the 2 groups correlated with the timing of LID development, indicating an association between ΔFosB expression and the unstable SPN responses to DA leading to LID emergence.
The mechanisms responsible for altered SPN responses to DA are unclear (6). Unstable SPN responses in the “on” state can be produced by interrupting DA modulation of cell excitability at the molecular pathways transducing D1R and D2R signaling. However, nondopaminergic signaling mechanisms may interfere with DA modulation, reversing the induced “on” response. In particular, glutamatergic and cholinergic signals that are highly dysregulated in advanced stages of PD may play a role in altered responses to DA (15, 28, 29). ΔFosB can function as a transcriptional activator or a repressor for a variety of genes that likely induce molecular changes affecting various signaling mechanisms in SPNs. Using candidate gene approach, some target genes of ΔFosB have been identified in the striatum in models of stress and other disorders (18). Of interest, target genes include the AMPA receptor subunit GluR2 and the Ca2+/calmodulin-dependent protein kinase II (CaMKII), whose regulation may have a significant impact on SPN excitability (30, 31). While the present data do not address the molecular changes resulting from striatal ΔFosB up-regulation in PD, the strong association between elevated ΔFosB levels and development of unstable SPN responses to DA suggests that genes regulated by ΔFosB are involved in the cellular/circuit signaling generating LID.
The molecular changes detected in striatal lysates following transgenic overexpression of ΔFosB reflect some of the alterations seen in the context of chronic l-Dopa exposure. Increased phosphorylation and activation of the key regulator of striatal neuronal signaling DARPP-32 at Thr34 as well as up-regulation of Cdk5 were present in this gene delivery model. Our molecular analyses in transgenic ΔFosB versus control animals suggest that overexpression of the transcription factor induces upstream gene regulation likely involving key kinases and phosphatases, which can mediate the increase in p-DARPP-32 (12, 32). Cdk5 is a direct target of the transcriptional activity of ΔFosB (33). Changes in both of these markers are typically present in models of dyskinesia (23) induced by chronic l-Dopa treatment in both primate and rat models, and can be reversed by antidyskinetic drugs (34). Additionally, p-ERK, which is activated primarily following acute l-Dopa administration in rodent models of LID (7) and diminishes with chronic l-Dopa therapy in parkinsonian primates (24), is not altered with sustained ΔFosB overexpression. The link between these molecular changes and altered neuronal activity in response to dopamine necessitates further investigation. Nevertheless, these findings collectively support that ΔFosB overexpression per se can lead to the molecular and physiological changes forming the LID substrate (SI Appendix, Fig. S4). In contrast, such an autonomous role cannot be attributed to other molecular markers of LID that are also regulated by chronic exposure to l-Dopa but remain to be examined further.
In conclusion, the results of transgenic ΔFosB overexpression in the striatum of parkinsonian primates implicate a primary, leading role of this transcription factor in the mechanisms of LID development. Accordingly, ΔFosB or its regulated genes may be candidate targets for gene therapy strategies to control disabling LID in patients with advanced PD. This premise also underscores the importance of profiling the striatal genes regulated by this transcription factor in the context of PD. Furthermore, understanding the molecular substrates of ΔFosB and its associated activator protein-1 (AP-1) underlying altered SPN responses to DA may also help identify pharmacological targets to treat LID. In addition to previously identified genes (e.g., MAPK-related genes, Rgs16, somatostatin/G-coupled receptor genes, Nurr1, Trh) that are significantly regulated in LID models (32), AP-1–mediated transcriptional changes may also lead to aberrant regulation of glutamate receptor subunits through increased expression or changes in membrane trafficking and phosphorylation states, which are known to occur after long-term DA depletion and replacement (15, 35). Overall, the present data encourage developing strategies for targeting ΔFosB or its regulated genes as a therapy for LID.
Materials and Methods
Detailed information on the experimental methods and materials used are provided in SI Appendix, Materials and Methods.
Animal Preparation.
Studies in nonhuman primates (9 adult macaques) were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All animals received systemic weekly administration of MPTP (0.2 to 0.4 mg/kg i.v.) until stabilization of moderate parkinsonism. The baseline motor response to l-Dopa (s.c.) was assessed acutely previrus injection. Animals were maintained without regular l-Dopa treatment.
Overexpression of ΔFosB.
rAAV-∆FosB and rAAV-eGFP vectors were constructed as described before (25). Plasmids were constructed with a cassette containing chicken β-actin-promoter/cytomegalovirus enhancer (CAG promoter), which yields high expression in striatal cells. The virus was injected either under stereotaxic surgery (animals used only for behavioral assessment) or through the recording chamber after electrophysiologic mapping (animals used also for neural recordings) (5). Both viruses were injected at equal particle doses (1 × 1012 GC/mL) into the striatum bilaterally. A total of 104 µL was injected in each hemisphere between the putamen (90 µL) and the caudate nucleus (14 µL), and this volume was distributed in 16 sites using 1 or 2 depths per injection track (total injection tracks: 12). The volume was estimated to cover entirely the posterolateral putamen and posterior portion of the caudate body, plus a large precommissural portion of both nuclei (see Fig. 1B).
Behavioral Assessment.
l-Dopa responses were assessed at baseline and post virus injection using the standardized Motor Disability Scale for MPTP-treated nonhuman primates, Part I-MDS and Part II-LID (27, 36). The l-Dopa dose for each animal was predetermined before AAV injection during baseline assessments (Fig. 1A), and the individual dose (14 to 21 mg/kg, s.c.) remained unchanged in all 12 postvirus assessments. In recording experiments, animals were scored while freely moving in the cage at the end of the recording. All tests were videotaped for blinded scoring.
Electrophysiology.
Two animals from each group (rAAV-ΔFosB and rAAV-eGFP) were used for single-cell recordings every week at the same time as the behavioral assessments of weekly l-Dopa responses to avoid additional drug exposure for collection of neural data. After behavioral assessment and recordings in the “off” state, the animal received the predetermined s.c. l-Dopa dose, and the recording of neuronal activity continued during the transition to l-Dopa–induced motor states, collecting data at the onset of the “on” state and at the time corresponding to LID peak, whether or not dyskinesias were observed (see timeline of recordings in Fig. 3A). As animals were not exposed to regular daily l-Dopa treatment, LID was generally not present in the rAAV-eGFP group. In the absence of LID, data were stored at the equivalent time frame of LID in the other group. Subsequently, the animal was rapidly transferred to the cage for complete behavioral assessment during the “on” state as proceeded in the other animals. Single-cell recordings in the striatum were performed using tungsten microelectrodes and standard techniques (15, 22) for isolation and continuous recording of SPNs. The final classification of units was always performed with offline analysis. During “off” state, 624 SPNs were analyzed from week 1 to week 12.
As reported previously in continuous recordings of individual neurons during the transition to motor states, the SPN activity in the “on” state increased or decreased, showing a D1- or D2-like response to DA, respectively (Fig. 3B). A total of 98 (rAAV-eGFP group) and 83 (rAAV-ΔFosB group) SPNs were recorded continuously from “off” to “on” and to dyskinesia states throughout the 12 wk of recordings post virus injection. D2-like responses slightly predominated in both virus groups (54 of 98 in eGFP group and 48 of 83 in ΔFosB group; SI Appendix, Table S2), likely due to better isolation of units with higher level of hyperactivity. Nevertheless, a considerable number of SPNs with D1-like response was recorded in each virus group (44 and 35 in eGFP and ΔFosB groups, respectively). In dyskinetic animals, the increase or decrease in SPN activity developed initially in the “on” state usually reverses at the peak of the motor response in a large number of neurons, typically 50% and above. This reversal of the SPN activity changes by a significant difference (P < 0.01 in each neuron) during the “on” state, which is called “unstable” response, reveals the inability to sustain the response to DA modulation (5, 15, 22). Such widespread pathological instability of SPN responses is distinctively associated with the expression of dyskinesias. By contrast, a “stable” response to DA is characterized by an increase or decrease in SPN firing frequency that does not significantly change during the peak-dose effect.
Immunohistochemistry and Immunoblotting.
Brain tissues from a total of 6 randomly selected animals, 3 monkeys injected with rAAV-ΔFosB and 3 monkeys injected with rAAV-eGFP, were used for IHC. Samples of dissected striatum from each animal in both viral groups were used for IB (antibodies used are listed in SI Appendix).
Statistics.
All data composed continuous variables (including noninteger individual MDS and LID values) and were analyzed with ANOVA for repeated measures and post hoc Fisher’s test (behavioral data) or Bonferroni’s test (firing frequency data) if the ANOVA F value was significant. The obtained sorted and classified spiking data were then minimally postprocessed using Matlab (MathWorks). All units with statistically significant changes in the “on” state were used to classify SPN responses to DA (increase or decrease firing rate). Significant firing rate changes in the “dyskinesia” state in the opposite direction to the changes developed in the onset of the “on” state were classified as unstable responses.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants NS073994 (S.M.P. and M.M.M.) and NS045962, RR000165, and OD011132 (S.M.P.). Additionally, M.M.M. is the William Dow Lovett Professor of Neurology and is supported by the Michael J. Fox Foundation for Parkinson’s Research, the American Parkinson Disease Association, the New Jersey Health Foundation, and by NIH Grants AT006868, NS096032, and NS101134. E.S.P. is supported by NIH Grants NS070898 and NS095003 and by the State of New Jersey. We thank the veterinary department of the Yerkes Primate Research Center and Bhagya Laxmi Dyavar Shetty in Dr. Papa’s laboratory for assistance in the maintenance and care of the primates used to model advanced PD.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1907810116/-/DCSupplemental.
References
- 1.Calabresi P., Di Filippo M., Ghiglieri V., Tambasco N., Picconi B., Levodopa-induced dyskinesias in patients with Parkinson’s disease: Filling the bench-to-bedside gap. Lancet Neurol. 9, 1106–1117 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Jenner P., Molecular mechanisms of L-DOPA-induced dyskinesia. Nat. Rev. Neurosci. 9, 665–677 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Fieblinger T., et al. , Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun. 5, 5316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villalba R. M., Smith Y., Loss and remodeling of striatal dendritic spines in Parkinson’s disease: From homeostasis to maladaptive plasticity? J. Neural Transm. (Vienna) 125, 431–447 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang L., DeLong M. R., Papa S. M., Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J. Neurosci. 28, 7537–7547 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck G., Singh A., Papa S. M., Dysregulation of striatal projection neurons in Parkinson’s disease. J. Neural Transm. (Vienna) 125, 449–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santini E., et al. , Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 27, 6995–7005 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santini E., et al. , L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J. Neurochem. 108, 621–633 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Du H., et al. , Levetiracetam ameliorates L-DOPA-induced dyskinesia in hemiparkinsonian rats inducing critical molecular changes in the striatum. Parkinsons Dis. 2015, 253878 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholas A. P., et al. , Striatal histone modifications in models of levodopa-induced dyskinesia. J. Neurochem. 106, 486–494 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Andersson M., Hilbertson A., Cenci M. A., Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson’s disease. Neurobiol. Dis. 6, 461–474 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Picconi B., et al. , Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat. Neurosci. 6, 501–506 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Santini E., Heiman M., Greengard P., Valjent E., Fisone G., Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci. Signal. 2, ra36 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Kelleher R. J., 3rd, Govindarajan A., Jung H. Y., Kang H., Tonegawa S., Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell 116, 467–479 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Singh A., et al. , Glutamatergic tuning of hyperactive striatal projection neurons controls the motor response to dopamine replacement in parkinsonian primates. Cell Rep. 22, 941–952 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder G. L., Fienberg A. A., Huganir R. L., Greengard P., A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J. Neurosci. 18, 10297–10303 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nestler E. J., Barrot M., Self D. W., DeltaFosB: A sustained molecular switch for addiction. Proc. Natl. Acad. Sci. U.S.A. 98, 11042–11046 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler E. J., ∆FosB: A transcriptional regulator of stress and antidepressant responses. Eur. J. Pharmacol. 753, 66–72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renthal W., et al. , Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J. Neurosci. 28, 7344–7349 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valastro B., Andersson M., Lindgren H. S., Cenci M. A., Expression pattern of JunD after acute or chronic L-DOPA treatment: Comparison with deltaFosB. Neuroscience 144, 198–207 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Ulery P. G., Rudenko G., Nestler E. J., Regulation of DeltaFosB stability by phosphorylation. J. Neurosci. 26, 5131–5142 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A., Liang L., Kaneoke Y., Cao X., Papa S. M., Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J. Neurophysiol. 113, 1533–1544 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aubert I., et al. , Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann. Neurol. 57, 17–26 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Santini E., et al. , Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in L-DOPA-induced dyskinesia. PLoS One 5, e12322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao X., et al. , Striatal overexpression of DeltaFosB reproduces chronic levodopa-induced involuntary movements. J. Neurosci. 30, 7335–7343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potts L. F., et al. , Modeling Parkinson’s disease in monkeys for translational studies, a critical analysis. Exp. Neurol. 256, 133–143 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck G., Maehara S., Chang P. L., Papa S. M., A selective phosphodiesterase 10A inhibitor reduces L-Dopa-induced dyskinesias in parkinsonian monkeys. Mov. Disord. 33, 805–814 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han C., et al. , Intrastriatal injection of ionomycin profoundly changes motor response to l-DOPA and its underlying molecular mechanisms. Neuroscience 340, 23–33 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Ding Y., et al. , Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc. Natl. Acad. Sci. U.S.A. 108, 840–845 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vialou V., et al. , DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 13, 745–752 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robison A. J., et al. , Behavioral and structural responses to chronic cocaine require a feedforward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J. Neurosci. 33, 4295–4307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiman M., et al. , Molecular adaptations of striatal spiny projection neurons during levodopa-induced dyskinesia. Proc. Natl. Acad. Sci. U.S.A. 111, 4578–4583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J., et al. , Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: Role of [Delta]FosB. J. Neurosci. 20, 8965–8971 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potts L. F., et al. , Dual κ-agonist/μ-antagonist opioid receptor modulation reduces levodopa-induced dyskinesia and corrects dysregulated striatal changes in the nonhuman primate model of Parkinson disease. Ann. Neurol. 77, 930–941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G., et al. , Antidyskinetic effects of MEK inhibitor are associated with multiple neurochemical alterations in the striatum of hemiparkinsonian rats. Front. Neurosci. 11, 112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papa S. M., Chase T. N., Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann. Neurol. 39, 574–578 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.