Abstract
Purpose:
CD38 has emerged as a high-impact therapeutic target in multiple myeloma, with the approval of daratumumab (anti-CD38 monoclonal antibody). The clinical importance of CD38 in chronic lymphocytic leukemia (CLL) patients has been known for over two decades, though it’s relevance as a therapeutic target in CLL remains understudied.
Experimental Design:
We investigated the biological effects and anti-tumor mechanisms engaged by daratumumab in primary CLL cells. Besides its known immune-effector mechanisms (ADCC, CDC and ADCP), we also measured direct apoptotic effects of daratumumab alone or in combination with ibrutinib. In vivo anti-leukemic activity was assessed in a partially-humanized xenograft model. The influence of CD38 on BCR signaling was measured via immunoblotting of Lyn, Syk, BTK, PLCγ2, ERK1/2 and AKT.
Results:
In addition to immune-effector mechanisms; daratumumab also induced direct apoptosis of primary CLL cells, which was partially dependent on FcγR crosslinking. For the first time, we demonstrated the influence of CD38 on BCR signaling where interference of CD38 downregulated Syk, BTK, PLCγ2, ERK1/2 and AKT; effects that were further enhanced by addition of ibrutinib. In comparison to single agent treatment, the combination of ibrutinib and daratumumab resulted in significantly enhanced anti-CLL activity in vitro and significantly decreased tumor growth and prolonged survival in the in vivo CLL xenograft model.
Conclusions:
Overall, our data demonstrate the anti-tumor mechanisms of daratumumab in CLL; furthermore, we show how co-targeting BTK and CD38 lead to a robust anti-CLL effect, which has clinical implications.
Keywords: CD38, CLL, Ibrutinib, Daratumumab
Statement of Translational Relevance
Increased CD38 expression on chronic lymphocytic leukemia (CLL) cells is linked to aggressive disease features and poor clinical outcome. Biologically, CD38 promotes CLL cell proliferation through association with multiple cell surface receptors, including the B-cell receptor (BCR). As therapeutic opportunities to disrupt CD38 function become increasingly available, we investigated the anti-tumor activity of daratumumab (anti-CD38 human monoclonal antibody) in patient-derived CLL cells. Daratumumab was noted to promote immune-effector mediated cytolysis as well as direct apoptosis of CLL cells. Inhibition of CD38 (with daratumumab or a small molecule inhibitor) decreased activation of BCR signaling proteins and this effect was enhanced by ibrutinib. In vivo, the combination of ibrutinib and daratumumab significantly delayed tumor growth in B-cell leukemia-bearing mice and prolonged their survival. Altogether, our results suggest that combination ibrutinib and daratumumab yields greater anti-CLL activity than either agent alone and support clinical evaluation of this regimen in CLL patients.
Introduction
CD38 is a highly conserved transmembrane type II glycoprotein; expressed on B-lymphocytes and other hematopoietic cells.1 Physiologically, CD38 functions as an ectoenzyme and a co-receptor; the latter depending on its spatiotemporal association with other cell surface (and cell-type specific) antigens. On B-lymphocytes, CD38 associates with the B-cell receptor (BCR) complex [(BCR)/CD81/CD19/CD21] and amplifies signal intensity transmitted through the complex to drive cell proliferation.2 CLL patients with a higher proportion of CLL cells expressing CD38 (≥30%) have a shorter time to symptomatic disease and a more aggressive clinical course; with inferior survival vs. patients who <30% of CD38+ CLL cells,3,4 thus establishing CD38 expression as a marker of poor prognosis.5,6 Despite its known association with an aggressive CLL phenotype, the role of CD38 as a therapeutic target remains unclear.
Daratumumab is a first-in-class anti-CD38 therapeutic monoclonal antibody (mAb) approved for the treatment of relapsed/refractory multiple myeloma (MM).7 It comprises a fully human IgG1κ mAb, which binds the C-terminus of CD38 at an epitope composed of ß-strand–containing amino acids 233–246 and 267–280.8 A report by Matas-Cespedes et al demonstrated the anti-leukemic effects of single agent daratumumab in ex vivo and in vivo CLL models.9 The cytotoxicity reported was modest; with partial insight into the direct killing mechanism of daratumumab in CLL cells. We hypothesized that CD38 is a high value target in CLL and blocking of its receptorial function can be translated into a clinically beneficial therapeutic strategy through improved understanding of the mechanism(s) that link CD38 to CLL cell survival. Here, we provide evidence that CD38 engagement by daratumumab modulates BCR signaling and enhances the anti-CLL activity of ibrutinib. Our observations provide important preclinical evidence for clinical exploitation of CD38 as a target for treatment of CLL.
Materials and Methods
Written informed consent was obtained from all patients whose samples were used in this study, approved by the Mayo Clinic Jacksonville Institutional Review Board and in accordance with the Declaration of Helsinki. Peripheral blood was collected from patients with a confirmed diagnosis of CLL who were not on active anti-CLL treatment or those off anti-CLL therapy for ≥1 month. This was followed by isolation of CD19+/CD5+ B-cells (primary CLL cells). Peripheral blood mononuclear cells (PBMCs) from human donors were used in antibody-dependent cell mediated cytotoxicity (ADCC) assays, 10% human serum was used in complement-dependent death (CDC) assays and human macrophages were used in antibody-dependent cellular phagocytosis (ADCP) assays, as described by de Weers et al.8 Apoptosis, mitochondrial transmembrane permeability and western blotting assays were conducted per prior methods.10–13 All cells were cultured in AIM-V media under conditions previously reported by us.10,11 CD38 receptor density on CLL cells was quantified as MFI and cell surface antibody bound/cell (sAbc). For certain experiments, PBMCs were isolated from Patients 4, 18, 19, 28 and 31 and CD19/CD5+ CLL cells were selected out using magnetic beads, followed by flow sorting with an anti-CD38 APC antibody for separation of CD19+/CD38hi and CD19+/CD38lo purified cells. Cells were then treated with trypsin-EDTA for 10 min and washed twice followed by culture in AIM-V serum-free media for ≥24h. CD38 expression in purified cells was again reassessed using a multi-epitope FITC conjugated anti-CD38 antibody (Cytognos CD38 multi-epitope-FITC antibody). Our sorting strategy is presented in Supplemental Figures 1 and 2. JVM13 (CD38+) and MEC1 (CD38-) cell lines were also used in experiments. An in vivo model of disseminated disease14,15 was established using luciferase labeled JVM13 (JVM13-Luc) cells, injected via tail vein I.V. into 6 – 8 week old NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice, following a protocol approved by the Mayo Clinic IACUC. Ibrutinib and kuromanin were purchased from Selleckchem (Houston, TX, USA). Daratumumab was acquired through the Mayo Clinic pharmacy and came pre-dissolved/diluted. Statistical analyses were performed using R Statistical Software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria); further detailed in figure legends. Data are represented as mean ± standard error of the mean (SEM), unless otherwise stated in the figure legend.
A full description of all assays is presented in Supplemental Materials & Methods.
Results
Patient and sample characteristics.
CLL patients (n=36) representing all clinical and genetic subsets were included in the study (clinical characteristics in Supplemental Table S1, S2). Relatively consistent with prior reports, we noted 27.7% (n=10) of patients had CD38+ disease (using standard 30% cutoff);16,17 whereas the remaining patients (n=26, 72.2%) had CD38- disease. Notably, 16.6% (n=6) of patients carried a deletion in chromosome 17p positive (Del17p+).
Daratumumab induces immune-mediated cytotoxicity in CLL cells.
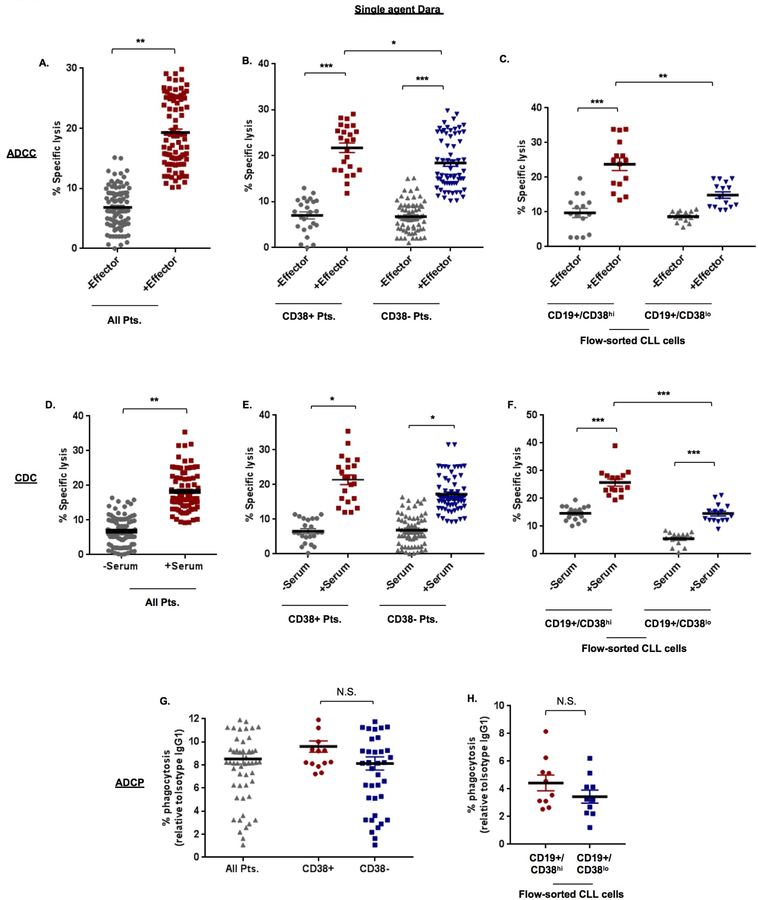
We first investigated the ability of daratumumab to induce CLL-specific lysis through immune-effector mechanisms (ADCC, CDC, ADCP). Mean specific lysis from ADCC was 17.59±1.30% (Figure 1A). Subset analysis in CLL cells from patients defined as having CD38+ and CD38- disease showed significantly greater ADCC (p=0.023) in CD38+ cases (24.63±3.46%) vs. CD38- cases (14.11±1.11%; Figure 1B). In flow-sorted CD19+/CD38hi clones treated with daratumumab, ADCC was noted in 22.45±2.36% of cells. And in CD19+/CD38lo clones, ADCC was noted in 16.47±1.03% of cells (Figure 1C). We then assessed cell death via CDC in all CLL cells (n=30) and noted that mean specific lysis, was in the order of 14.88±0.92% (Figure 1D). Marginally higher levels were observed in CLL cells from CD38+ patients (18.22±2.41%) vs. CD38- patients (13.58±0.95%) (Figure 1E). In flow-sorted cells, CDC was noted in 19.10±1.98% and 9.23±1.81% of CD19+/CD38hi clones and CD19+/CD38lo clones, respectively. (Figure 1F). Comparative analysis in JVM13 and MEC1 cells did not show significant induction of ADCC or CDC with daratumumab alone (Supplemental Figures 3A – C). Cell death through ADCP has been previously reported with daratumumab in myeloma cells.18 We noted ADCP in 8.52±0.44% of CLL cells (Figure 1G); with little difference observed between CLL cells from CD38+ (9.37±0.77%) vs. CD38- patients (8.79±0.92%). Similar findings were seen in flow-sorted CD19+/CD38hi or CD19+/CD38lo purified cells. (Figure 1H). Comparative analysis in the cell lines showed higher ADCP in JVM13 (18.01±1.0%) vs. MEC1 (3.55±0.14%) (Supplemental Figure 3D). Correlation between ADCP results, CD38 MFI (r=0.49, p=0.006) and CD38 sAbc (r=0.41, p=0.021) was significant (Supplemental Figure 4A, B). Similarly, correlation between CDC results and % of CD38 expressing cells was also significant (r=0.49, p=0.006) (Supplemental Figure 4C). ADCC cell death assay results did not show any significant correlation with either CD38 MFI/sAbc or % of CD38 expressing cells (Supplementary Table S3).
Figure 1. Daratumumab induces CLL cell death through immune effector-mediated mechanisms.
A. Antibody-dependent cell-mediated cytotoxicity (ADCC) induced by single agent daratumumab (Dara, 0.1µg/mL) was determined in Calcein-AM labeled primary CLL cells (target) from 30 patients, ex vivo, in the absence or presence of effector (peripheral blood mononuclear) cells from healthy human donors at an E:T ratio of 50:1 for 6hr. Specific lysis was calculated as described in Supplemental Materials & Methods. Spontaneous release was determined using a non-specific IgG1-b12 isotype antibody at 0.1μg/mL. B. ADCC in primary CLL cells from CD38+ (n=8) and CD38- (n=22) Pts. was assessed as a subset analysis and showed significantly higher specific lysis in cells from CD38+ patients. C. ADCC was also assessed in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells from 5 patients (Pts. 4, 18, 19, 28 and 31) and revealed a similar trend. D. Specific lysis from complement-dependent cytotoxicity (CDC) was measured in CLL cells in the presence of 10% human serum from a single healthy donor for 1hr. E. Subset analysis of specific lysis from CDC induced in CLL cells from CD38+ (n=8) vs. CD38- (n=22) Pts. was also determined. F. Similarly, CDC was determined in flow-purified CD19+/CD38hi and CD19+/CD38lo CLL cells. G. Cell death through phagocytosis was assessed in Calcein-AM labeled primary CLL cells (n=30), with subset analysis in CD38+ vs. CD38- cases and separately in flow-purified CD19+/CD38hi and CD19+/CD38lo CLL cells (H.) using CD11b+ macrophages differentiated from healthy human donor monocytes at an E:T ratio of 2:1 for 6hr incubation period. % phagocytosis was determined as described in Supplementary Materials & Methods. Results are expressed as mean ± SEM. Comparative significance analyses between the groups (brackets) show p-values. * p≤0.05, **p<0.01, ***p<0.001
Daratumumab can directly induce apoptosis of CLL cells.
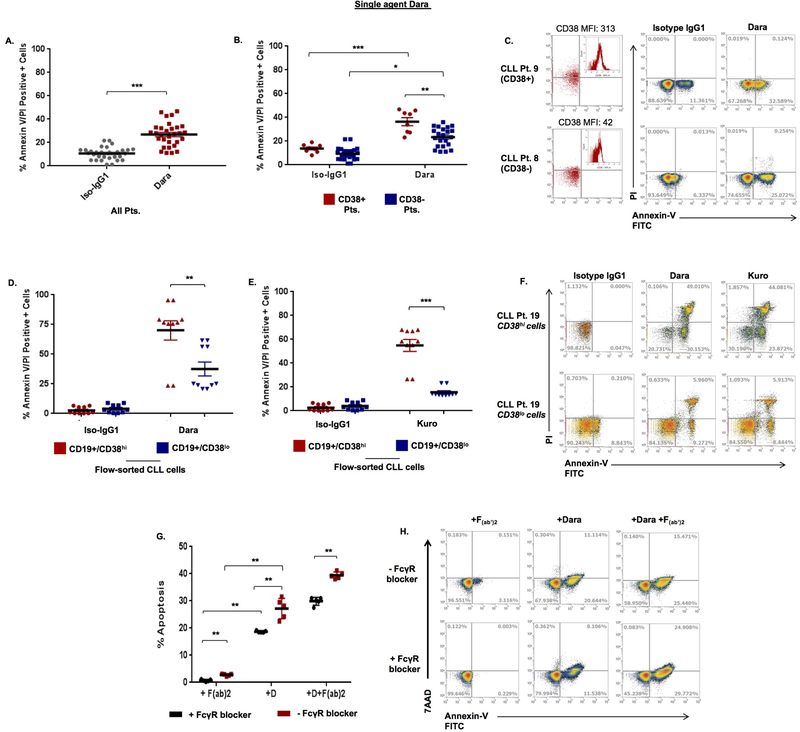
Prior studies in MM cells suggest that daratumumab can directly induce apoptosis.19 In CLL cells treated with daratumumab, we noted 31.33±2.56% annexin-V/PI positivity overall (Figure 2A). Subset analysis of CD38+ and CD38- cases showed greater annexin-V/PI positivity in CLL cells from CD38+ patients (36.41±3.34%) vs. CD38- patients (23.39±1.70%) (p=0.002) (Figures 2B, C); with similar effects in JVM13 (37.58±1.88%) vs. MEC1 cells (14.82±1.37%) (Supplemental Figure 5). In unsorted CLL cells from patients with either CD38+ or CD38- disease, a significant correlation was observed between % apoptosis with CD38 MFI (r=0.39, p=0.036), CD38 sAbc (r=0.53, p=0.003) and % CD38 positivity of CLL cells (r=0.45, p=0.012) (Supplemental Table S3). Interestingly, in flow-sorted CLL cells, a significant degree of apoptosis (p<0.001) was noted in CD19+/CD38hi cells (70.10±12.18%); with comparatively lower apoptosis in CD19+/CD38lo cells (31.57±11.22%) (Figure 2D, F). A significant correlation between apoptosis results with % CD38+ CLL cells (r=0.45, p=0.012), CD38 MFI (r=0.39, p=0.036) and CD38 sAbc (r=0.53, p=0.003) was observed (Supplemental Figures 4D – F). Other than association between degree of CDC induction and patient age (p=0.036), no other significant correlation was observed between daratumumab-mediated cell death and clinical characteristic of patients (Supplemental Table S4)
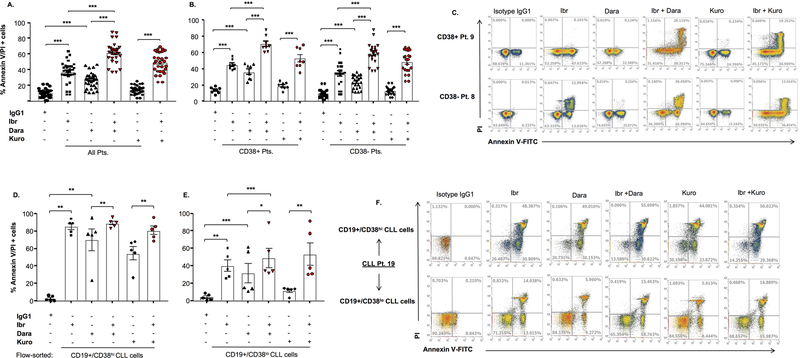
Figure 2. Daratumumab induces apoptosis in CLL cells, which is partially dependent on FcγR crosslinking.
A. Apoptosis was assessed in CLL cells treated with daratumumab or IgG1-b12 isotype antibody (0.1µg/mL) for 24h, followed by staining with annexin-V/propidium iodide (PI) and flow cytometry analysis. B. Subset analysis was also performed in CD38+ (n=8) and CD38- (n=22) CLL cases. C. Annexin-V/PI+ cell scatter plots from a representative CD38+ CLL patient (Pt. 9, 38% CD38+ cells) and a CD38- patient (Pt. 8, 7.54% CD38+ cells) are shown. D. Flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells (n=5 patients) were treated with daratumumab (0.1 µg/mL) or (E.) kuromanin (Kuro, 10µM, flavonoid small molecule CD38-enzymatic inhibitor) and showed significantly more apoptosis in the CD38hi population relative to the CD38lo fraction of CLL cells (representative scatterplot from a single patient shown in panel F). G. From a subset of patients (n=7, primarily CD38- cases), CLL cells were treated with daratumumab, F(ab’)2 (control) or the combination of daratumumab + F(ab’)2 in the presence or absence of a pan-FcγR blocker (Human TruStain FcX). F(ab’)2 alone triggered no apoptosis and while daratumumab treatment of CLL cells +/− F(ab’)2 without FcγR blocker showed significant cell death (red bars), the addition of an FcγR blocker significantly decreased the degree of apoptosis (black bars). H. A representative patients, scatterplot showing apoptosis. Data are presented as mean ± SEM. * p≤0.05, **p<0.01, ***p<0.001
To test whether small molecule-based inhibition of CD38 could induce apoptosis, we used kuromanin, a flavonoid inhibitor of CD38 enzymatic activity,20,21 which has been previously used to interrogate CD38 biology.22 In flow-sorted CLL cells, apoptosis induced by kuromanin was significantly greater (p<0.001) in CD19+/CD38hi vs. CD19+/CD38lo cells (54.97±4.99 vs. 15.60±1.28, respectively, Figures 2E, F).
In a subset of CLL patient cells, we also examined whether daratumumab-induced apoptosis was due to FC-gamma receptor (FcγR) crosslinking. CLL cells were noted to express FcγRI (CD64, 73.67±3.37%), FcγRII (CD32, 95.18±1.85%) and FcγRIII (CD16, 19.31±3.89%) (Supplemental Figure 6). When treated with daratumumab + an FcγR blocker, the percentage of apoptotic CLL cells was significantly lower (18.7±0.15%) than cells treated with daratumumab alone (27.21±1.67%) (p=0.007). Addition of an F(ab’)2 fragment to daratumumab-treated CLL cells triggered further apoptosis (39.43±0.56%), however, a decrease was noted when FcγR blocker was added (29.91±1.3% apoptosis, p=0.0079) (Figures 2G, H). Altogether, these results indicated to us that daratumumab-mediated apoptosis in CLL cells is partially-dependent on FcγR crosslinking.
Targeting CD38 modulates proteins associated with BCR signaling.
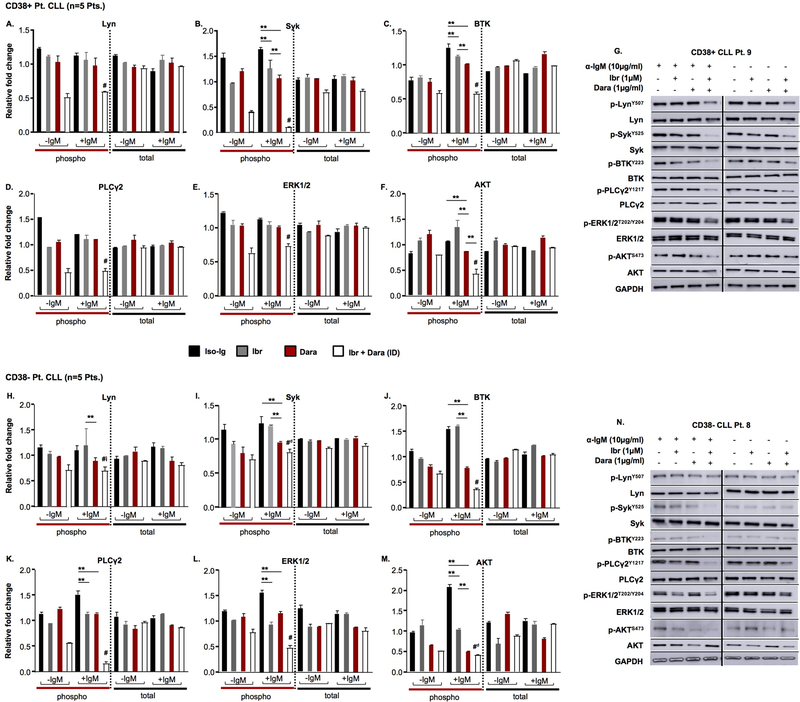
CD38 co-localizes with CD19 and CD81 in the lipid rafts at the cell membrane and this results in amplification of BCR and collateral signaling events.23 CLL cells with high proliferative potential inherently depend on BCR signaling and consequently are also reliant on CD38 co-receptorial function.24–26 Indeed, prior studies have demonstrated an increase in ERK activity (one of the terminal effectors of BCR signaling) upon CD38 agonistic ligation.21–23,27 Given our interest in targeting CD38; its established role in BCR signal amplification23,27 and the high clinical relevance of BCR-targeting agents in CLL,28 we questioned if therapeutic interference of CD38 would modulate the BCR pathway. Basal expression of BCR signaling components: (p) p-Lyn, p-Syk, p-BTK, p-PLCγ2, p-ERK1/2 and p-AKT in CLL cells from CD38+ versus CD38- patients is shown in Supplemental Figure 7A – F. We treated (IgM-stimulated) CLL cells from both CD38+ and CD38- patients with daratumumab, ex vivo, and noted a significant decrease in p-Syk, p-BTK, p-ERK1/2 and p-AKT (Figures 3A – G for CD38+ CLL patient cells and Figures 3H – N for CD38- CLL patient cells; compare red bars with black bars; p<0.05). As expected though, comparative analysis between CLL cells from the CD38+ vs. CD38- patients, revealed the % decrease in proximal BCR signaling proteins (p-Lyn, p-BTK and p-PLCγ2) elicited by daratumumab was significantly more notable in CLL cells from CD38+ patients (p<0.05). Intriguingly, we observed the opposite for phospho-ERK and phospho-AKT; which decreased more significantly so in daratumumab-treated CLL cells from CD38- patients (relative to CLL cells from CD38+ patients) (Supplemental Figures 8A – F). Next, to test whether changes in BCR proteins could also be elicited by small molecule inhibition of CD38, we treated CLL cells with kuromanin and observed relatively analogous changes in BCR signaling proteins, albeit, compared to daratumumab, the intensity of inhibition was less (Supplemental Figures 9A – G). This observation is critical as it demonstrates that targeting CD38 by either an antagonistic mAb or a small molecule, leads to reduction in the signaling capabilities of the BCR complex. Given the biological functions of CD38 (particularly in regulation of BCR signaling), we reasoned that targeting both CD38 (with daratumumab/kuromanin) and BTK (with ibrutinib), would lead to synergistic reduction in phosphorylated BCR proteins. As anticipated, in tumor cells from CD38+ CLL patients, the combined effect of ibrutinib + daratumumab (ID) resulted in a significantly more pronounced decrease in p-Lyn, p-BTK, p-PLCγ2, p-ERK1/2 and p-AKT levels compared to either of the agents alone (Figures 3A – G, combination treatment highlighted by open bars). In CLL cell lysates from CD38- patients treated with the ID combination, only p-BTK, p-PLCγ2 and p-ERK1/2 were significantly downregulated compared to either agent alone (Figures 3H – N). Representative immunoblots are shown in Figures 3G and 3N. Likewise, in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells, we probed for p-BTK and p-PLCγ2. CD38hi clones appeared to have higher BTK expression vs. CD38lo clones. In addition, all drugs (including the ID combination) appeared to more effectively downregulate p-BTK as well as p-PLCγ2 in CD38hi clones (Supplemental Figure 9H). Altogether, these results allowed us to conclude that blocking either the receptor function (daratumumab) or the enzymatic properties (kuromanin) of CD38 leads to downregulation of BCR-associated proteins in CLL cells and which can be further amplified through simultaneous inhibition of BTK with ibrutinib.
Figure 3. Targeting CD38 results in downregulation of B-cell receptor (BCR) signaling proteins, which is further augmented by ibrutinib treatment.
Phosphorylated (p-) and total protein levels for Lyn, Syk, BTK, PLCγ2, ERK1/2 and AKT were probed for by western blot in CLL cell lysates (−/+ BCR stimulation with anti-IgM for 1hr), from CD38+ patients (A – G, Pts. 11, 16, 26, 33 and 34) and CD38- patients (H – N, Pts. 8, 14, 28, 35 and 36). Primary CLL cells were treated for 2hr with isotype IgG1-b12 Ab (control, 0.1µg/mL), ibrutinib (Ibr, 1µM), daratumumab (Dara, 0.1µg/mL) or the combination of Ibr + Dara before lysate preperation. G, N. Representative western blots from a CD38+ and CD38- CLL Pt. are shown. Results shown are mean ± standard deviation (SD). *p≤0.05; **p<0.01; # indicates statistically significant (p<0.05) difference compared to control or single agent-treated cells. #d indicates significant (p<0.05) compared to all single agent or control treated cells, except daratumumab-treated cells. #i indicates significant (p≤0.05) compared to all single agent or control treated cells, except ibrutinib-treated cells.
Ibrutinib significantly augments the cytotoxic activity of daratumumab.
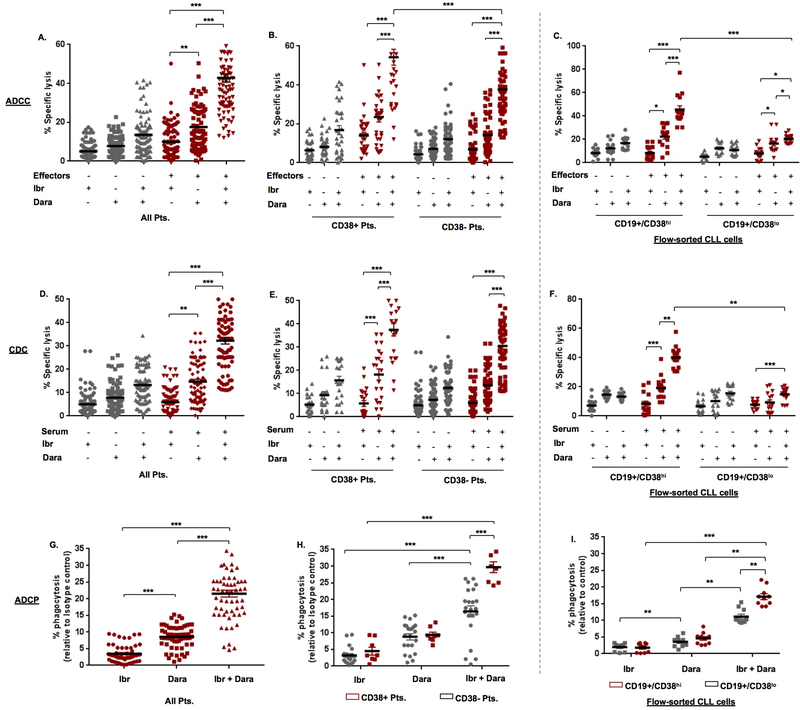
Previous studies have shown that inhibition of BTK can downregulate cell surface antigens on CLL cells.29 Thus, we first examined if ibrutinib modulates CD38 expression and noted that ibrutinib did not downregulate CD38 on CLL cells (24h exposure) (Supplemental Figure 10). As such, we proceeded towards anti-tumor testing of the ID combination in primary CLL cells. Cytolysis from ADCC in ibrutinib-treated CLL cells was 9.92±0.88%, which significantly (p<0.001) increased to 42.81±1.12%, in ID-treated cells (Figure 4A). This effect was more pronounced in cells from CD38+ (63.73±4.43%) vs. CD38- (35.21±1.61%) CLL patients (Figure 4B). In flow-sorted CD19+/CD38hi clones treated with the ID combination, ADCC was 45.53±3.19% and in CD19+/CD38lo cells was 20.29±0.95% (p<0.001) (Figure 4C). When we examined CDC, lysis induced by ibrutinib alone was 6.17±0.56% and this was significantly (p<0.001) higher in cells treated with ID combination (32.23±1.47%) (Figure 4D). Although the trend in specific lysis was higher in cells from CD38+ (40.25±2.82%) vs. CD38- patients (29.56±1.60%), the difference was not significant (Figure 4E). In flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells, specific lysis from ID treatment was 44.13±2.59% and 14.86±1.18%, respectively (p<0.01) (Figure 4F). We then determined the effect on ADCP and noted that ibrutinib alone induced negligible phagocytosis (3.47±0.32%), whereas in ID-treated cells, ADCP increased to 21.50±1.02% (Figure 4G). And the difference in ADCP was significantly appreciable between ID-treated CLL cells from CD38+ (29.96±1.41%) vs. CD38- patients (18.42±0.94%) (p<0.001; Figure 4H). Examination in flow-sorted CD19+/CD38hi cells, ADCP was noted in 17.35±0.90% of ID-treated clones vs. 10.64±0.88% in CD19+/CD38lo cells (p<0.01; Figure 4I). Of note, comparative analyses (for ADCC, CDC, ADCP) in JVM13 and MEC1 cell lines was also performed and showed similar results as in primary CLL cells, with some expected variations (Supplemental Figure 3). A significant correlation between ADCC, CDC and ADCP in ID-treated CLL cells with % CD38+ CLL cells, CD38 MFI and CD38 sAbc was noted (p<0.05; Supplementary Table S3). A significant correlation between degree of immune-mediated cytolysis (ADCC, CDC and ADCP) and CD38 expression (% CD38+ cells, MFI/sAbc) was observed in ID-treated CLL cells. Interestingly, significantly higher ADCC as well as CDC were noted in ID-treated CLL cells from Del17p+ patients (p=0.021 and p=0.036, Supplementary Table S5). These data demonstrate that co-targeting CD38 and BTK results in a significant increase in immune-directed CLL cell killing (vs. either daratumumab or ibrutinib alone) and while this effect was perceptible in both CLL cells from CD38- and CD38+ patients, it was more pronounced in the latter.
Figure 4. The immune-mediated cytolytic activity of daratumumab is significantly enhanced by ibrutinib.
A. ADCC was examined in Calcein-AM labeled primary CLL cells (n=30 Pts.) treated with either IgG1-b12 isotype Ab (0.1µg/mL), ibrutinib (Ibr, 1µM), daratumumab (Dara, 0.1µg/mL) or the combination of Ibr + Dara with or without effector cells at an E:T ratio of 50:1 for 6hr. Specific lysis was calculated as described in Supplementary Materials & Methods and in the same manner as Figure 1. B. ADCC in cells from CD38+ (n=8) and CD38- (n=22) patients treated with Ibr, Dara or Ibr + Dara was analyzed. C. Similarly, ADCC was determined in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells from 5 patients and showed a similar trend. D, E. CDC was measured in Calcein-AM labeled CLL cells incubated with human serum (10%) from a healthy donor for 1hr; effect of Ibr, Dara or combination treatment in CLL cells from CD38+ (n=8) vs. CD38- (n=22) patients. F. CDC in a similar manner was examined in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells (n=5 patients). G. ADCP was assessed in Calcein-AM labeled CLL cells treated with either Ibr, Dara or Ibr + Dara by incubating target tumor cells with human monocyte-derived macrophages (effectors) from a healthy donor at an E:T ratio of 2:1. Flow cytometry analysis to detect CD11b+ macrophage engulfment of Calcein-AM labeled tumor cells was used to calculate % phagoctyosis. H. ADCP levels were separately analyzed for CD38+ and CD38- CLL patients. I. ADCP was also determined in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells from 5 patients. Data are presented as mean ± SEM. * p≤0.05, **p<0.01, ***p<0.001
We also examined apoptosis and observed 36.86±2.56% annexin-V/PI positivity in CLL cells treated with ibrutinib, which increased to 61.90±2.41% in ID-treated cells (Figure 5A). Subset analysis of cells from CD38+ vs. CD38- CLL patients revealed 45.26±2.11% vs. 33.81±3.18% apoptosis in ibrutinib-treated and 70.71±3.44% vs. 58.7±2.77% apoptosis in ID-treated CLL clones, respectively (Figure 5B). Scatter plots from 2 representative patients are shown in Figure 5C. Using a different combination of probes (7AAD/annexin-V), we noted a similar trend (56.6±4.92%; 7AAD/annexin-V positivity) in ID-treated CLL cells from 7 patients (5 of who had CD38- disease) (Supplementary Figure 11). In flow-purified CD19+/CD38hi and CD19+/CD38lo cells, ID combination treatment resulted in apoptosis of 89.53±2.16% and 49.27±10.97% of cells, respectively (Figure 5D and E). Scatter plots from a representative experiment are shown in Figure 5F. Akin to primary CLL cells, similar results were seen in ID-treated CLL cell lines (JVM13: 70.88±4.23% and MEC1: 41.03±1.62, Supplementary Figure 5). We also assessed apoptosis in CLL cells exposed to kuromanin +/− ibrutinib, where the combination of these two agents showed significantly greater annexin-V/PI positivity (49.53±2.39%, Figure 5A), compared to either kuromanin or ibrutinib alone. This validates the fact that the cytotoxicity of ibrutinib is enhanced with concurrent targeting of CD38 and this effect is independent of whether a mAb or an anti-CD38 small molecule is used. Apoptosis was mirrored by a loss of mitochondrial membrane permeability by 69.22±2.49% in CLL cells (n=30 patients); the (%) change of which was marginally higher in cells from patients that were CD38- (Dilc15 MFI 1100±193.7 vs. 4340±608.2 in control cells) compared to those who were clinically categorized as CD38+ (Dilc15 MFI 687.3±111.11 vs. 2145±289.8 in controls) (Supplementary Figures 12A – C). In flow-sorted CD19+/CD38hi and CD19+/CD38lo cells, a similar trend was noted (Supplementary Figure 12D – G).
Figure 5. Co-targeting CD38 and BTK leads to significantly greater apoptosis of primary CLL cells than compared to singular targeting of either CD38 or BTK.
A. Apoptosis was assessed in primary CLL cells treated with daratumumab (Dara, 0.1µg/mL), kuromanin (Kuro, 10µM) +/− ibrutinib (Ibr, 1µM), Ibr alone or IgG1-b12 isotype antibody for 24h, followed by staining with annexin-V/propidium iodide (PI) and flow cytometry analysis. B. Subset analysis was also performed in primary CLL cells from CD38+ (n=8) and CD38- (n=22) patients. C. Annexin-V/PI+ cell scatter plots from a representative CD38+ CLL case (Pt. 9) and a CD38- case (Pt. 8) are shown. D, E. Apoptosis was also assessed separately in flow-sorted CD19+/CD38hi and CD19+/CD38lo CLL cells (n=5 patients). F. Annexin-V/PI+ cell scatter plots from a representative patient’s (Pt. 19.) CD38hi CLL clones and CD38lo CLL clones are shown. Results expressed as mean ± SEM. * p≤0.05, **p<0.01, ***p<0.001
The combination of daratumumab and ibrutinib reduces tumor burden in a mouse model of CLL.
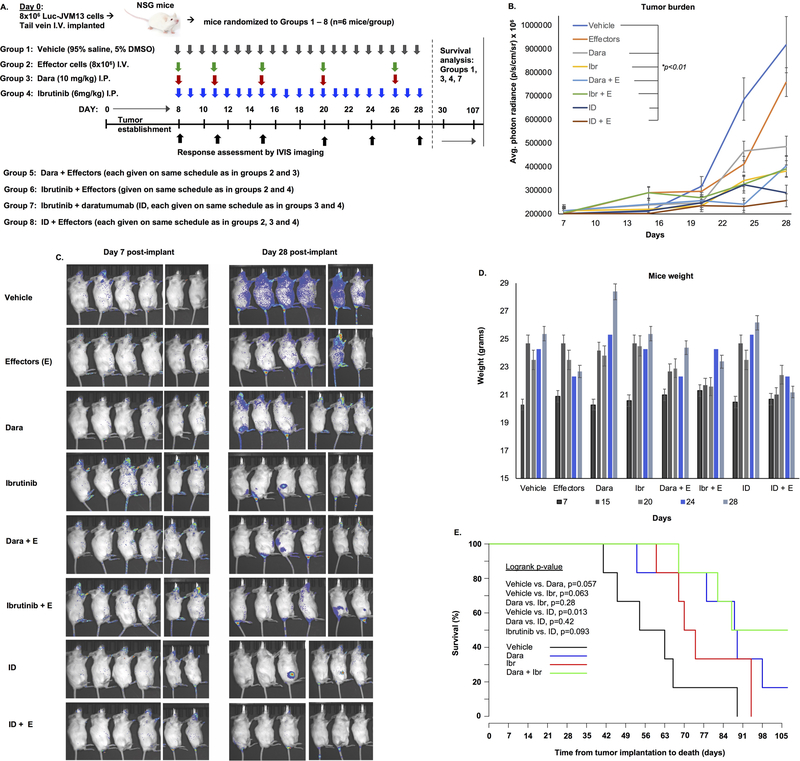
We tested the anti-CLL activity of daratumumab +/− ibrutinib in an in vivo disseminated disease model system. NSG mice were injected with JVM13-Luc cells via tail vein injection and disease burden was monitored by bioluminescent signal. On Day 7 post-implantation, mice were randomly divided into 8 groups (6 mice/ group) to receive either; (1) vehicle, (2) Effector cells only on the days that daratumumab was given, (3) Daratumumab alone, (4) Ibrutinib, (5) daratumumab + effector cells, (6) Ibrutinib + effector cells, (7) ID combination and (8) ID combination + effector cells (Figure 6A). On post-implantation Day 28, treatment was concluded in all groups before onset of any signs/symptoms of xeno-GVHD (typically occurring at week 4, post-implantation of human effector cells)15 and final anti-tumor response was assessed. Compared to vehicle or effector-cell only groups, all other groups showed significantly reduced tumor burden (p<0.01). Tumor burden in mice treated with daratumumab + effector cells vs. ibrutinib + effector cells was not significantly different (p=0.063). However, in mice treated with ID therapy (+/− effector cells), significantly lower disease burden was noted; ~ 3.5 and 2.8-fold lower than that observed in vehicle (p<0.01) or effector cell alone-treated (p<0.01) mice, respectively (Figure 6B, C). No significant changes in weight were noted in any of the treatment groups (Figure 6D). Mice in groups 2, 5, 6 and 8, which were administered effector cells, were sacrificed on Day 30; whereas mice remaining in the other groups (that did not receive effector cells) were followed up to 107 days for survival analysis (Figure 6E). A trend for longer survival in the daratumumab monotherapy vs. vehicle treated cohort was noted (median OS 89 vs. 59 days, respectively, p=0.057). By Day 107, 50% of the mice in the ID combination treated arm were alive and healthy.
Figure 6. Daratumumab and ibrutinib significantly delay tumor growth and extend survival of leukemia-bearing mice.
A. NSG mice were implanted with JVM13-Luc (8×106) cells via I.V. tail vein injection and post-implantation day 7, mice were randomized in 8 groups to receive either 1. vehicle (control, I.P.), 2. Effector cells only (healthy donor PBMCs, 8×106 cells, I.V.), 3. Daratumumab alone on a weekly schedule (20/10/10/10/10 mg/kg, I.P.), 4. ibrutinib (6mg/kg, I.P.), 5. daratumumab + effector cells, 6. Ibrutinib + effectors, 7. Ibrutinib and daratumumab (ID) combination and 8. ID combination + effectors. B, C. By Day 28, tumor burden (average photon radiance, p/s/cm/sr x 105) in vehicle-treated mice reached a median of 9.18 vs. 7.59 in effector-only treated mice, 4.87 in daratumumab-treated mice, 4.03 in daratumumab + effector-treated mice, 3.81 in ibrutinib-treated mice, 3.90 in ibrutinib + effector-treated mice, 2.89 in ID-treated mice and 2.57 in ID + effector-treated mice. D. Minor variances in weight of mice over duration of treatment were noted, however these differences were not significant. E. Survival study was performed in mice from treatment groups 1, 3, 4 and 7 (that did not receive effector cells). Pairwise comparison of survival is shown. Notably, ID-treated mice had significantly longer survival compared to vehicle treated mice.
Discussion
Targeting CD38 for therapeutic purposes has been largely examined in multiple myeloma (MM).30 In CLL, preclinical proof-of-concept for disrupting CD38 function was first reported by Vaisitti et al where inhibition of its enzymatic activity with the flavonoid kuromanin slowed CLL cell homing and adhesion in vitro and in a murine model.22 Subsequently, Matas-Cespedes and colleagues reported on the anti-CLL activity of daratumumab showing its mechanism of action to be through ADCC and ADCP, with a trend for higher cytolytic activity in CLL cells from CD38+ patients.9 Our results herein support these findings and show that in addition to immune-effector mediated cell kill, daratumumab induces apoptosis in CLL cells; partially dependent on FcγR-mediated cross-linking and which is actualized through destabilization of the mitochondria.
While the enzymatic functions of CD38 and its inhibition in CLL cells have been described previously,1 the receptorial properties of CD38 and particularly their role in signal transmission through the BCR complex are less understood. Studies by Deaglio and Malavasi et al have provided significant insight on localization of CD38/BCR complexs in cell membrane lipid rafts. Indeed, CD38 ligation with an agonistic anti-CD38 mAb (IB4) results in calcium flux and increased ERK1/2 activity,22,27 however, antagonism of CD38 and its effects on BCR signaling have not been previously reported. We show for the first time that mAb-based engagement of CD38 (with daratumumab, which minimally inhibits CD38 ecto-enzymatic function) or small molecule-based targeting of CD38 (with kuromanin, which primarily inhibits enzymatic activity of CD38) results in significant downregulation of proximal (Syk, BTK), terminal (PLCγ2, ERK) and collateral (AKT) proteins involved in BCR signaling. Our observation that CD38 enzymatic inhibition can to a large extent mimic CD38-receptorial block in terms of apoptosis induction and BCR signaling attenuation (mostly in CD38+/ CD38hi CLL cells) opens avenues of investigation for the use of highly-specific small molecule inhibitors of CD38 such as 78c, reported by Tarrago et al.31 Moreover, highly specific inhibition of CD38 (receptor and NADase activity) may also be able to shift the T-cell repertoire from a pro- to anti-tumor disposition as eloquently shown by Chatterjee et al using CD38 KO mice.32
The effects of individually targeting CD38 and BTK yielded modest downregulation of BCR components. Thus, we considered whether disrupting CD38 and BTK simultaneously could further decrease the aforementioned proteins; translating to enhanced lethality in CLL cells. As expected, co-targeting of CD38 (with daratumumab/kuromanin) and BTK (ibrutinib) significantly reduced most of the phosphorylated BCR signaling proteins and was associated with not only increased apoptosis and mitochondrial disturbance, but also significantly greater immune-effector mediated cell death. In the case of ADCC, this is not entirely surprising as ibrutinib shifts Th1, Th2 and CD8+ T-cell populations towards an overall anti-tumor disposition.33,34 This immunomodulation in turn potentially synergizes with the T-cell modulating properties of daratumumab in both the MM35 and CLL microenvironment.36 Although this may explain enhanced ADCC, it does not explain improved CDC or ADCP. Further studies on complement inhibitor protein expression changes as well as the effects of BTK +/− CD38 inhibition on macrophages are being conducted by us under both ex vivo and in vivo conditions. Altogether, the overall anti-tumor activity of daratumumab is significantly enhanced when partnered with ibrutinib and this is reflected by increased CLL-cytolysis in every underlying assay: ADCC, CDC, ADCP, apoptosis (Supplementary Table S6).
To more accurately gauge CLL cell sensitivity toward daratumumab, kuromanin and/or ibrutinib based on CD38 expression/receptor density, we used FACS-purified CD38hi and CD38lo CD19+/CD5+ CLL cells in our workflow. While results measuring ADCC, CDC and ADCP showed a similar trend as seen in unsorted CD19+/CD5+ CLL cells from CD38+ and CD38- CLL patients, the results from the apoptosis assays were more noteworthy. In CD38hi CLL clones, we detected significant apoptosis from ibrutinib alone, which did not increase with the addition of daratumumab. Contrastingly, albeit lower overall compared to CD38hi cells, the magnitude of apoptosis induced by ID therapy was more prominent in CD38lo clones. The significance of these findings is unclear, however, overall our data suggest that CD38 receptor levels and survival dependency on BCR signaling are intricately linked and associated with response to BCR/CD38-targeting agents. These associations however, should be cautiously interpreted as they were determined in ex vivo assays, whereas the effect of ibrutinib or daratumumab in humans is remarkably enhanced through engagement and reshaping of the innate and adaptive immune environment.35,37 Additionally, as CD38 status has not been reported to be a determinant of clinical response to ibrutinib, it is plausible that CD38+ and CD38- CLL patients alike would demonstrate equivalent response to ibrutinib + daratumumab combination treatment.
As daratumumab does not bind murine CD38 (thus precluding use of transgenic Eu-TCL1 mice), we established a disseminated disease xenograft model to study the anti-CLL activity profile of daratumumab (+/− ibrutinib). In this short course study, the goal was to measure time to tumor growth before onset of xeno-GVHD could impact the results. We noted significant activity of daratumumab relative to mice treated with effector-cells only (serving as a control for all mice groups that received drug + effectors). Survival analysis was only performed on mice that received drug without effector cells. Although underpowered, this analysis suggested that the ID combination was superior in prolonging the survival of mice compared to vehicle-treated mice (p=0.013). Although the median survival of mice treated with single agent daratumumab or ibrutinib was lower than mice that received combination ID treatment, the differences were not statistically significant. Further experiments that can incorporate the immune-effector activity of daratumumab in an appropriate mouse model system are needed to comprehensively evaluate the survival advantage conferred with use of daratumumab +/− ibrutinib.
In summary, our data highlights that: 1.) Daratumumab induces cell death in primary CLL cells through various mechanisms ex vivo (ADCC, CDC, ADCP and apoptosis); 2.) statistically significant correlations between CD38 receptor density (MFI or sAbc) with ADCC, ADCP and apoptosis were observed; 3.) targeting CD38 with either daratumumab or kuromanin can significantly modulate BCR-associated and these effects are more prominent in CD38+ cases and 4.) the combination of daratumumab and ibrutinib induces significantly more immune-effector mediated and direct apoptosis of CLL cells from CD38+ and CD38- patients alike. Based on our results, while single agent daratumumab may be more effective in CLL patients with CD38+ disease, the combination of daratumumab and ibrutinib may be highly effective in all treatment requiring patients irrespective of CD38 expression status. Our data provide the framework for future clinical investigations entailing therapeutic strategies targeting CD38 and BTK together.
Supplementary Material
Acknowledgements
The experiments and analysis carried out in this study were supported in part by the Daniel Foundation of Alabama (AC-K), the Predolin Foundation (AC-K), the University of Iowa/Mayo Clinic Lymphoma SPORE Developmental Research program (P50 CA097274, AP) and the Mayo Clinic Cancer Center (CA015083, AC-K).
Footnotes
Conflict of Interest: Fabio Malavasi has received research support from Janssen Pharmaceuticals, Celgene, Tusk Therapeutics and Centrose, and serves on Advisory Boards for Centrose and Tusk Therapeutics.
Supplementary information is available online.
References
- 1.Malavasi F, Deaglio S, Funaro A, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008;88(3):841–886. [DOI] [PubMed] [Google Scholar]
- 2.Funaro A, De Monte LB, Dianzani U, Forni M, Malavasi F. Human CD38 is associated to distinct molecules which mediate transmembrane signaling in different lineages. Eur J Immunol 1993;23(10):2407–2411. [DOI] [PubMed] [Google Scholar]
- 3.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 4.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol 2001;115(4):854–861. [DOI] [PubMed] [Google Scholar]
- 5.Morabito F, Mangiola M, Stelitano C, Deaglio S, Callea V, Malavasi F. Peripheral blood CD38 expression predicts time to progression in B-cell chronic lymphocytic leukemia after first-line therapy with high-dose chlorambucil. Haematologica 2002;87(2):217–218. [PubMed] [Google Scholar]
- 6.Durig J, Naschar M, Schmucker U, et al. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia 2002;16(1):30–35. [DOI] [PubMed] [Google Scholar]
- 7.Costello C An update on the role of daratumumab in the treatment of multiple myeloma. Therapeutic Advances in Hematology 2017;8(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Weers M, Tai Y-T, van der Veer MS, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. The Journal of Immunology 2011;186(3):1840–1848. [DOI] [PubMed] [Google Scholar]
- 9.Matas-Céspedes A, Vidal-Crespo A, Rodriguez V, et al. The Human CD38 Monoclonal Antibody Daratumumab Shows Antitumor Activity and Hampers Leukemia–Microenvironment Interactions in Chronic Lymphocytic Leukemia. Clinical Cancer Research 2017;23(6):1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitta KS, Khan AN, Ersing N, et al. Neem leaf extract induces cell death by apoptosis and autophagy in B-chronic lymphocytic leukemia cells. Leuk Lymphoma 2014;55(3):652–661. [DOI] [PubMed] [Google Scholar]
- 11.Paulus A, Masood A, Miller KC, et al. The investigational agent MLN2238 induces apoptosis and is cytotoxic to CLL cells in vitro, as a single agent and in combination with other drugs. Br J Haematol 2014;165(1):78–88. [DOI] [PubMed] [Google Scholar]
- 12.Paulus A, Akhtar S, Caulfield TR, et al. Coinhibition of the deubiquitinating enzymes, USP14 and UCHL5, with VLX1570 is lethal to ibrutinib- or bortezomib-resistant Waldenstrom macroglobulinemia tumor cells. Blood Cancer J 2016;6(11):e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulus A, Akhtar S, Yousaf H, et al. Waldenstrom macroglobulinemia cells devoid of BTKC481S or CXCR4WHIM-like mutations acquire resistance to ibrutinib through upregulation of Bcl-2 and AKT resulting in vulnerability towards venetoclax or MK2206 treatment. Blood Cancer J 2017;7(5):e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matas-Cespedes A, Vidal-Crespo A, Rodriguez V, et al. The Human CD38 Monoclonal Antibody Daratumumab Shows Antitumor Activity and Hampers Leukemia-Microenvironment Interactions in Chronic Lymphocytic Leukemia. Clin Cancer Res 2017;23(6):1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herman SE, Wiestner A. Preclinical modeling of novel therapeutics in chronic lymphocytic leukemia: the tools of the trade. Semin Oncol 2016;43(2):222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 17.Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 2002;99(3):1023–1029. [DOI] [PubMed] [Google Scholar]
- 18.Overdijk MB, Verploegen S, Bogels M, et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 2015;7(2):311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overdijk MB, Jansen JH, Nederend M, et al. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcgamma Receptor-Mediated Cross-Linking. J Immunol 2016;197(3):807–813. [DOI] [PubMed] [Google Scholar]
- 20.Kellenberger E, Kuhn I, Schuber F, Muller-Steffner H. Flavonoids as inhibitors of human CD38. Bioorg Med Chem Lett 2011;21(13):3939–3942. [DOI] [PubMed] [Google Scholar]
- 21.Vaisitti T, Aydin S, Rossi D, et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010;24(5):958–969. [DOI] [PubMed] [Google Scholar]
- 22.Vaisitti T, Audrito V, Serra S, et al. The enzymatic activities of CD38 enhance CLL growth and trafficking: implications for therapeutic targeting. Leukemia 2015;29(2):356–368. [DOI] [PubMed] [Google Scholar]
- 23.Deaglio S, Capobianco A, Bergui L, et al. CD38 is a signaling molecule in B-cell chronic lymphocytic leukemia cells. Blood 2003;102(6):2146–2155. [DOI] [PubMed] [Google Scholar]
- 24.Zupo S, Isnardi L, Megna M, et al. CD38 expression distinguishes two groups of B-cell chronic lymphocytic leukemias with different responses to anti-IgM antibodies and propensity to apoptosis. Blood 1996;88(4):1365–1374. [PubMed] [Google Scholar]
- 25.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood 2003;101(3):1087–1093. [DOI] [PubMed] [Google Scholar]
- 26.Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and chronic lymphocytic leukemia: a decade later. Blood 2011;118(13):3470–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deaglio S, Vaisitti T, Billington R, Bergui L, Genazzani AA, Malavasi F. CD38/CD19: a lipid raft–dependent signaling complex in human B cells. Blood 2007;109(12):5390–5398. [DOI] [PubMed] [Google Scholar]
- 28.Jeyakumar D, O’Brien S. B cell receptor inhibition as a target for CLL therapy. Best Pract Res Clin Haematol 2016;29(1):2–14. [DOI] [PubMed] [Google Scholar]
- 29.Pavlasova G, Borsky M, Seda V, et al. Ibrutinib inhibits CD20 upregulation on CLL B cells mediated by the CXCR4/SDF-1 axis. Blood 2016;128(12):1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van de Donk NW, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev 2016;270(1):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarrago MG, Chini CCS, Kanamori KS, et al. A Potent and Specific CD38 Inhibitor Ameliorates Age-Related Metabolic Dysfunction by Reversing Tissue NAD(+) Decline. Cell Metab 2018;27(5):1081–1095 e1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee S, Daenthanasanmak A, Chakraborty P, et al. CD38-NAD(+)Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab 2018;27(1):85–100 e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122(15):2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Q, Sivina M, Robins H, et al. Ibrutinib Therapy Increases T Cell Repertoire Diversity in Patients with Chronic Lymphocytic Leukemia. J Immunol 2017;198(4):1740–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128(3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manna A, Lewis-Tuffin LJ, Ailawadhi S, Chanan-Khan AA, Paulus A. Using anti-CD38 immunotherapy to enhance anti-tumor T-cell immunity in chronic lymphocytic leukemia (CLL). The Journal of Immunology 2018;200(1 Supplement):58.17–58.17. [Google Scholar]
- 37.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017;127(8):3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.