Abstract
This study achieves a better operational simplicity for the phycoremediation of reverse osmosis (RO) concentrate using Scenedesmus quadricauda microalgae. Under continuous illumination with CO2 supplementation, algal growth in the RO concentrate resulted in a conversion of polymeric organic matter (a mixture of humic substances and polysaccharides) to biodegradable fractions and their prompt removal along with inorganic nutrients (NO3− and PO43−). The algae-induced degradation of humic-like substances which are typically refractory to microbial decomposition was demonstrated indirectly. In this study, we also investigated the effects of algae treatment on the growth of Escherichia coli and removal of trace organic compounds (TOrCs) from the RO concentrate. Our results indicate that algae treatment using aeration with 10% (v/v) CO2 under continuous illumination is highly feasible as a safe and inexpensive technology to remove non- or slowly-biodegradable organics, reduce enteric bacteria, and attenuate TOrCs in wastewater. However, the results should be not generalized but critically discussed due to limitations of using the synthetic RO concentrate in evaluating the performance of wastewater remediation with microalgae.
Keywords: Reverse osmosis concentrate, Scenedesmus quadricauda, Escherichia coli, Refractory organic matter, Trace organic compounds
1. Introduction
Reverse osmosis (RO) is increasingly used for the tertiary treatment and indirect reuse of the secondary effluent from urban wastewater. The concentrate resulting from the filtration processes commonly contains high concentrations of dissolved salts, recalcitrant organics, and trace organic compounds (TOrCs) (Sun et al., 2014; Westerhoff et al., 2009). In particular, the strength and recalcitrance of effluent organic matter (EfOM) can be affected by both the characteristics of the raw sewage and the performance of the reclamation processes typically involved in biological treatment processes. The EfOM consists of a heterogeneous mixture of refractory organic compounds with diverse structures and varying origin, including dissolved natural organic matter, soluble microbial products, endocrine disrupting compounds, pharmaceuticals and personal care products residues, disinfection by-products, metabolites/transformation products and others (Michael-Kordatou et al., 2015). Characterization of EfOM and diagnostic work involving EfOM have extensively been conducted during the last decade (Guo et al., 2011; Kim and Dempsey, 2012). Vakondios et al. (2014) classified the EfOM into two major groups and determined their compositional distribution using a spectrophotometric technique, i.e., biomass associated products (e.g., polysaccharides and proteins) and humic substances. Some studies have reported the preferential removal of non-humic components of EfOM through soil aquifer treatment when compared to humic components that are required longer travel times/distances for sustainable biodegradation (Amy and Drewes, 2007; Maeng et al., 2008). The humic substances constitute the major fraction of the EfOM and due to their recalcitrance to microbial degradation, it is necessary to utilize complementary technologies to degrade and mineralize them accumulated in the RO concentrate.
Various integrated treatment methods have been suggested considering the cost and efficiency in treating the RO concentrate, but these are still in their infancy. Such combined systems involve biological stabilization, physicochemical solute separation, and advanced oxidation processes (AOPs), all of which have been comprehensively reviewed (Joo and Tansel, 2015; Pérez-González et al., 2012). Lee et al. (2009a; 2009b) explored the effect of pretreatment with biological activated carbon (BAC) on the performance during deionization, and they also combined ozonation with BAC to improve the biodegradability of organic matter in the RO concentrate. Some studies have reported that electrochemical oxidation can improve the degradation of dissolved organics in RO concentrate produced from municipal wastewater (Pérez et al., 2010; Radjenovic et al., 2011). The combination of UV-based photochemical and electrochemical processes was also investigated (Hurwitz et al., 2014). Similarly, a couple of different oxidation processes and their combinations have been investigated at the bench scale (Zhou et al., 2011). UV/H2O2 treatment has potential to degrade organic pollutants (Liu et al., 2012), and most recently, Umar et al. (2016a; 2016b) examined coagulation followed by AOPs and also suggested a sequence consisting of coagulation, UV/H2O2, and BAC treatment to remove organic matter from a highly saline RO concentrate.
Alternatively, algae-mediated treatment is effective in removing nutrients and heavy metals, reducing chemical and biochemical oxygen demand, and degrading xenobiotic compounds and other contaminants (Rawat et al., 2016). Wang et al. (2016) successfully cultivated two strains of microalgae in RO concentrate, which resulted in concurrent removal of nutrients and hardness. Ahmed et al. (2017) reported that microalgae treatment could remove almost all types of TOrCs to some extent when compared to other biological processes that were found less effective in TOrCs removal from urban wastewater. Despite previous demonstrations of algae as useful mediators for wastewater remediation, limited research has been carried out in treating RO concentrate to date. This paper addresses the usefulness of microalgae-mediated treatment. It also proposes a cost-effective treatment strategy to achieve the biodegradation of refractory organic matter along while also removing nutrients from RO concentrate in a single bioreactor.
We have demonstrated that microalgae-based treatment is very effective at removing organic and inorganic components from a wide range of recalcitrant wastewaters (Kim et al., 2016a; Kim et al., 2014a; Kim et al., 2014b). We recently noticed a notable increase in biodegradability of organic compounds can be achieved with the growth of Scenedesmus quadricauda in highly saline RO concentrate under continuous illumination without CO2 supplementation (submitted to the other journal for publication). In this latest work, we investigated the mechanisms for algae-induced degradation by measuring the reactive oxygen species (ROS) that are capable of transforming or decomposing organic chemicals, although the extent of the degradation mechanism is not yet fully understood. ROS are inevitable byproducts of photosynthesis and the generation of ROS is accelerated by excess light in conjunction with other stressors such as nutrient limitation (Borowitzka, 2016). Algae are capable to biotransform and biodegrade aromatic pollutants commonly found in nature and waste waters (Semple et al., 1999). Many microalgae can take up and utilize organic compounds for growth in the light, which is known as mixotrophy. Scenedesmus is a dominant genus of algae commonly found in wastewater ponds (Lyon et al., 2015), and we previously determined that S. quadricauda can engage in mixotrophic growth in addition to the common photoautotrophic growth when using CO2 as the sole carbon source. Our prior observations motivated us to use S. quadricauda to sequentially degrade and assimilate color-causing refractory organic matter from RO concentrate in a single biological reactor, resulting in operational simplicity and adaptability to existing water reuse facilities. To the best of our knowledge, it has not yet been reported that S. quadricauda microalga can be used as both a disintegrator and an assimilator in a single compartment system with continuing light. Thus, the main hypothesis to be tested was that algae treatment that could increase the biodegradability of color-causing refractory organics would also promptly utilize the degraded organic fractions when treating RO concentrate under continuous illumination. In this study, different phycoremediation modes were evaluated, and we also investigated their impacts on the growth of enteric bacteria and the attenuation of TOrCs accumulated in the RO concentrate.
2. Experimental methods
2.1. Synthetic RO concentrate
Stock solutions of inorganic constituents were individually prepared by dissolving NaHCO3, Na2SO4, CaCl2·2H2O, KCl, NaNO3, MgCl2·6H2O, and NaH2PO4 in deionized water. Synthetic RO concentrate was prepared by adding stock solutions in commercially available mineral water (Samdasu, Korea) to achieve the following characteristics: HCO3− 488 mg L−1, SO42− 250 mg L−1, NO3− 133 mg L−1, Ca2+ 100 mg L−1, K+ 70 mg L−1, PO43− 31 mg L−1, Mg2+ 20 mg L−1. The chloride concentration in synthetic RO concentrate was adjusted to 1000 mg L−1 using the above chemicals and NaCl.
Synthetic RO concentrate was autoclaved for 30 min and was cooled to room temperature prior to adding organic compounds. Dextran (15‒25 kDa) from Leuconostoc spp. and humic acid sodium salt were used to identify the extent of biodegradation during algae treatment. Dextran has been frequently used as a good surrogate for polysaccharide-like substances present in secondary effluent (Contreras et al., 2009), and humic-like substances are typically refractory to microbial degradation, biogenic, and yellow-colored organic acids. Aldrich humic acid was purified to remove inorganic impurities using the method previously proposed by Hur and Schlautman (2003). Unless specified otherwise, all experiments have used synthetic wastewater prepared by blending 60% dextran and 40% purified humic acid. The organic concentration was shown as COD and/or dissolved organic carbon (DOC).
For some experiments, either E. coli or TOrCs were added to synthetic RO concentrate. The E. coli ATCC 8739 was inoculated at 4.0±2.1×105 mL−1 in the RO concentrate before its use. The bacterial culture was prepared in Luria-Bertani broth (Jantama et al., 2008) and incubated for 24 h at 37 °C on an orbital shaker. The bacterial pellet harvested by centrifugation was re-suspended and washed three times with synthetic RO concentrate prior to inoculation. Stock solutions of the ten TOrCs (i.e., bezafibrate, carbamazepine, caffeine, diclofenac, ibuprofen, fenoprofen, gemfibrozil, ketoprofen, naproxen, and pentoxifylline) were individually prepared in methanol using each of the TOrCs. Their diluted mixtures were used as a working solution and spiked at 41±3 μg L−1 per each chemical into the RO concentrate. The physicochemical properties of the selected compounds have been reported in detail elsewhere (Kim et al., 2015a).
All chemicals used to prepare synthetic RO concentrate were of analytical grade and were supplied by Sigma-Aldrich (St. Louis, MO). Unless specified otherwise, the COD, TN, TP, DOC, UV absorbance at 254 nm (UV254), and chromaticity in the mixture ranged from 96 to 123 mg L−1, 33 to 39 mg L−1, 10 to 11 mg L−1, 31 to 36 mg L−1, 1.05 to 1.26 cm−1, and 522 to 572 mg Pt-Co L−1, respectively. The pH of the mixture ranged between 7.6 and 7.8. The final concentrations of organic and inorganic components in the synthetic RO concentrate were selected based on the previously reported observations with real RO concentrate (Badruzzaman et al., 2009; Hurwitz et al., 2014; Lee et al., 2009a; Umar et al., 2016b; Zhou et al., 2011).
2.2. Strain, pre-cultivation, and batch experiments
The S. quadricauda strain (AG 10003) was obtained from the Korea Collection for Type Culture in the Korea Research Institute of Bioscience and Biotechnology (Jeongeup, Korea). The stock culture of S. quadricauda was grown in 2 L flasks containing 1.5 L of sterilized BG-11 medium (Rippka et al., 1979) in air enriched with 5% CO2 under continuous white fluorescent light illumination (75 μmol photons m‒2 s‒1) at 25 °C. A quarter of the culture was transferred to new medium every 10 days before the start of the stationary growth phase.
Batch experiments were conducted twice in duplicate to determine the treatability of the RO concentrate with S. quadricauda. The algal cells in the exponential growth phase were collected via centrifugation (3000 rpm, 10 min), washed by mineral bottled water, and inoculated at 200 mg dry weight L−1 (corresponding to 5.1±0.6×105 cells per mL) into flasks containing 800 mL of synthetic wastewater (see Fig. 1). The flasks were incubated under continuous illumination with a light intensity of 150 μmol photons m‒2 s‒1 at 25 °C while shaking at 130 rpm for 48 h. For some experiments, aeration was optionally applied to the flasks with a gas mixture of 90% air and 10% CO2 at a flow rate of 40‒50 mL min−1. During incubation, 50 mL of mixed liquor was collected from each flask at 24 and 48 h to measure the removal of organic and inorganic constituents. The cell growth was monitored by determining the optical density of mixed liquor at 680 nm (OD680), of which the correlation was verified for the algal cell concentration using fluorescence-based flow cytometry (see Supporting Information Fig. S1). Extracellular H2O2 content in non-aerated cultures was measured using the Pierce™ Quantitative Peroxide Assay Kits (Thermo Scientific, Rockford, IL) and a UV/Vis spectrophotometer (DR/5000, Hach). The algae-induced degradation of dissolved organic matter was also characterized by conducting a 12-h cultivation of S. quadricauda in the light under non-aerated conditions; for this purpose, three different combinations of organic matter were applied in preparing RO concentrate in which a mixture of humic acid (40%) and dextran (60%), dextran only, and no organic matter were separately added for each cultivation.
Fig. 1.
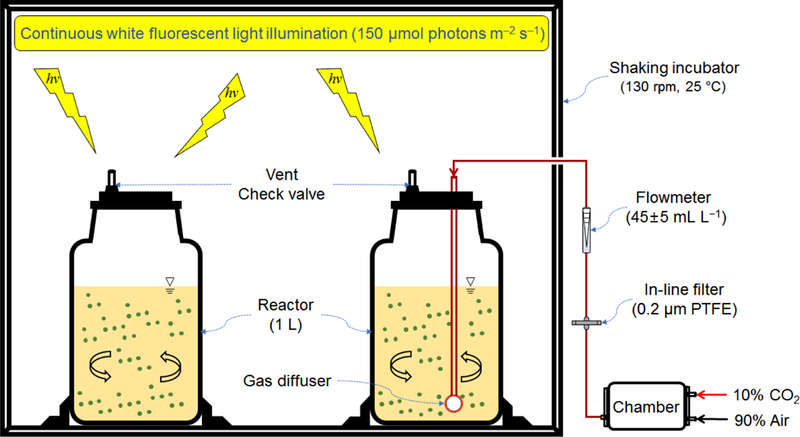
Experimental set-up for treatment of synthetic RO concentrate with S. quadricauda microalga.
Separate batch tests were performed twice in duplicate to identify the heterotrophic assimilation of organic carbons in S. quadricauda microalga and evaluate their assimilability depending on the complexity of carbonaceous organic matter. Glucose was used as organic matter for this comparative examination in parallel with the polymeric organics consisting of humic acid and dextran. Tests were conducted using an identical apparatus as that described above, but with aeration (air only) applied to the flasks under dark while shaking at 130 rpm for 48 h. The mixed liquor was collected every 24 h from each flask to determine the treatability of the RO concentrate.
2.3. Flow cytometric analysis
The algal cellular suspension was diluted with cultivation medium to a cell density of approximately 5.6±1.3×105 cells mL−1 prior to flow cytometric analysis. The analysis was immediately carried out using a Partec CyFlow® Cube6 flow cytometer (Partec GmbH, Görlitz, Germany) equipped with a 20 mW blue diode pumped solid-state laser emitting at 488 nm. The autoclaved cellular suspension was also analyzed for negative control to differentiate intact cells from non-viable cells and non-algal particles. The flow cytometer operated to monitor the cell density using volumetric counting hardware measuring the number of fluorescent particles in 200 μL of the sample. The cell size and granularity were relatively determined using light scattering properties of the biomass in the forward scatter (FSC) and side scatter (SSC) channels, and chlorophyll red fluorescence was also collected with a 620 nm long band pass filter (FL3). All collected fluorescence data was processed using the FCS Express 4 Cytometry software (De Novo Software, Glendale, CA); the electronic gating application of the software was used to isolate positive signals from the algal cells and to exclude noise from the non-algal particles or instrument itself. The algal populations were distinguishable from other events such as inorganic particles and bacteria due to the size (FSC), granularity (SSC), and chlorophyll content (FL3) (see Supporting Information Fig. S2).
The collected mixed liquor was immediately stained with a mixture of SYBR® Green I and propidium iodide in order to differentiate enteric bacteria with intact cellular membranes from the disrupted microbial cells and microalgae. Where necessary, each stained sample was diluted with deionized water to 10% (v/v) of the initial concentrations, prior to flow cytometric analysis (Vital et al., 2012). The cytometric cell count was determined and the density plots were collected in comparison with negative control using an identical cytometer and software as those described above. Intact cell counting was conducted as previously reported (Park et al., 2016) and the procedures have been described in detail (Berney et al., 2008).
2.4. Characterization of dissolved organic matter
Fluorescence spectra were collected using a Shimadzu RF-5301PC fluorescence spectrometer with a 150W xenon lamp source. Samples were micro-filtered and diluted with carbon-free electrolyte solution to a concentration of 1 mg C L−1 prior to spectroscopic analysis. Three dimensional spectra were obtained by repeatedly measuring the emission (Em) spectra within a range of 280–600 nm, with excitation (Ex) wavelengths from 200 to 400 nm spaced at 10 nm intervals in the excitation domain. The spectra were then concatenated into an excitation-emission matrix (EEM) (Park et al., 2016). Dissolved organic matter was also characterized by liquid chromatography with online organic carbon detection (LC-OCD) (DOC-Labor, Germany). Detailed information regarding the LC-OCD system is provided in an earlier report (Huber et al., 2011).
2.5. Analytical methods
The water samples were manually filtered with 0.45 μm polyethersulfone microfilters prior to measuring the water quality. Dichromate, persulfate digestion, and acid persulfate digestion methods were employed to measure the COD, TN, and TP in the water samples using a DR/5000 spectrophotometer. An identical spectrophotometer was also used to measure the chromaticity, UV254, and OD680. The DOC was determined (TOC-V CPN, Shimadzu, Japan) to obtain the specific UV absorbance (SUVA) value which is calculated from the UV254 divided by the DOC of the water sample. The pH was also measured using a pH meter (Orion Star A215, Thermo Scientific). Visual MINTEQ (model 3.0) was used to calculate the expected theoretical percent removal for phosphate at a given pH of the RO concentrate. Each measurement was carried out in triplicate, and the average values were reported.
TOrCs tested in this study were analyzed using an Agilent 1200 high-performance liquid chromatography. The chromatographic separation was carried out on a Zorbax Eclipse Plus C18 column (2.1 × 150 mm, 3.5 μm particle size) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in methanol). The elution rate was set at 0.35 mL min−1 and injection volume was 3 μL. Mass spectrometry detection was performed on an Agilent 6460 triple-quadrupole mass spectrometer equipped with a dual jet stream electrospray ionization source. Quantitative and qualitative analysis for TOrCs was performed by multiple reaction monitoring (MRM). MRM precursor and product ion pairs were selected at the highest peak intensity with different fragment and collision energy (see Supporting Information Table S3).
3. Results and discussion
The growth of S. quadricauda resulted in a significant increase in the pH of RO concentrate over the course of the experiment (see Fig. 2a). It is well known that phototrophs consume CO2 and concurrently produce OH ions during photosynthesis, which leads to a raise in pH and a subsequent increase in the saturation of carbonate minerals (Power et al., 2011). This could allow for precipitation or complexation by carbonates, bicarbonates, or hydroxides of Ca and Mg, suggesting that a steep decrease in TP was partially associated with the complexation of the cations and phosphate ions in the aqueous phase. For the first 24 h of cultivation, TP was removed by 88% (determined after microfiltration), but a notable co-precipitation of organic matter did not occur (see Fig. 2b). Under continuous illumination without CO2 supplementation, the growth of S. quadricauda in RO concentrate resulted in dramatic decreases in both color and UV254, while a negligible change in DOC was found upon algae treatment. True color depends exclusively on the dissolved components in wastewater, and it is typically linked to the presence of humic-like substances that are usually derived from transformations of wastewater organic matter in biological treatment processes. Moreover, UV254 is strongly correlated with the extent of electron-rich sites such as aromatic functional groups and double-bonded carbon groups in an organic molecule. Interestingly, the reduction in color and UV254 was mostly achieved during the first 12 h of cultivation but was not a consequence of pH increase (see Supporting Information Fig. S4). Slight decreases in the chromaticity and UV absorbance of organic matter were found in a separate experiment to increase the pH by adding an alkaline solution, suggesting that unidentified mechanisms are involved in the degradation and/or deformation of condensed organic matter upon algae treatment.
Fig. 2.
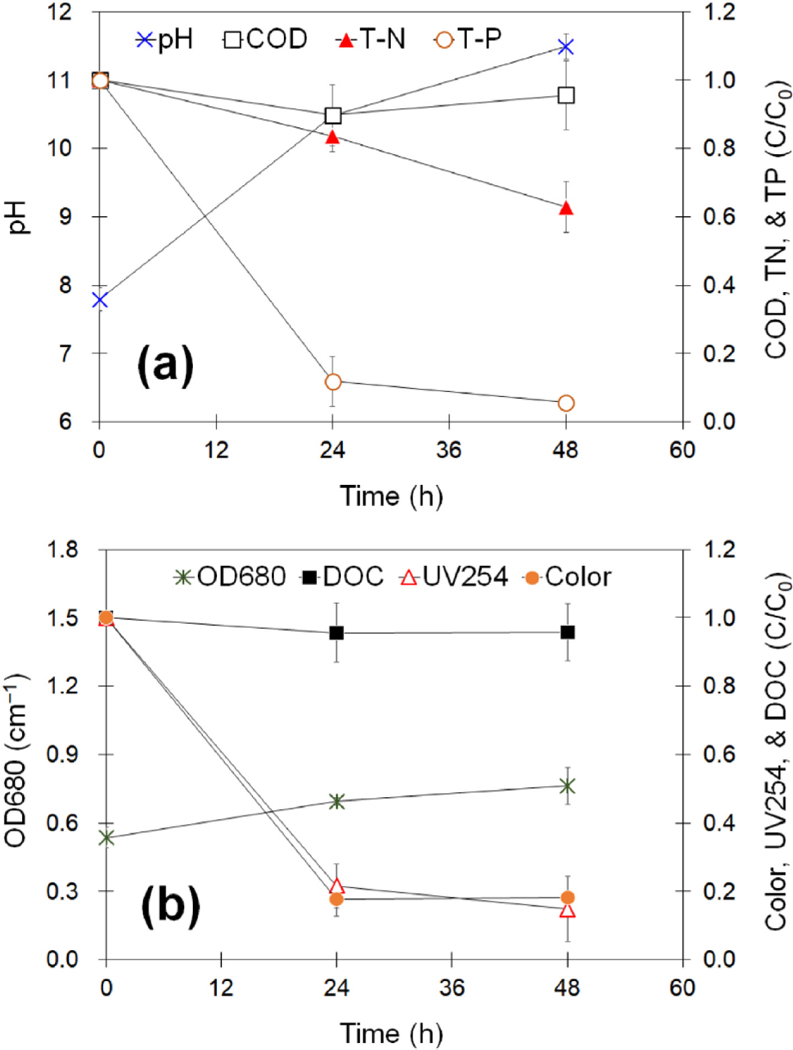
Variations in water quality during algal growth in synthetic RO concentrate under continuous illumination without aeration for 48 h.
In photosynthetic organisms, ROS are continuously produced as byproducts through various metabolic pathways localized in mitochondria, peroxisomes, chloroplast, and even at the plasma membrane (Pérez-Pérez et al., 2012). S. quadricauda can also produce ROS under experimental conditions (see Supporting Information Fig. S5) and H2O2 is the only molecule that is membrane-permeable and can be readily released out of algal cells (Kawano et al., 2010). The H2O2 reaction chemistry is complex, and it is potentially capable of degrading a wide range of organic compounds depending on the conditions. Direct oxidation has typically been viewed to not play a major role in the degradation of organic contaminants by H2O2, but for the majority of applications it necessarily requires activation in some way or another (Petri et al., 2011). A high pH close to a pKa of 11.6 is thought to catalyze the activation of H2O2, but oxidative peroxide bleaching has been observed even at a lower pH. Considering the exoelectrogenic activity of eukaryotic phototrophs (McCormick et al., 2011), the activation of H2O2 can also be catalyzed by electrons transferred from the cell surface across the plasma membrane in which many redox enzymes are located. O2 as well as H2O2 may generate a wide variety of free radicals and other reactive species that are capable of transforming or decomposing organic chemicals. In addition, humic substances contain a variety of redox-active functional groups that are capable of accepting and donating electrons, which can result in increased organic radical contents (Aeschbacher et al., 2010). In summary, it appears that one or more chemical processes should be involved in increasing the degradability of such condensed organic matter, although an integrated understanding of the mechanisms related to electron movement from the algal cell surfaces to the specific sites on organic molecules is still lacking.
Oxidative bleaching substantially decreases the light absorption due to a split in the aromatic rings, which is consistent with the results shown for the fluorescence and SUVA measurements of this study (see Fig. 3). The algal growth resulted in a significant decrease in the SUVA of organic components, indicating a decrease in the condensation degree of the organic structures (Kim et al., 2015b; Sillanpää et al., 2015). Fig. 3 also shows that algae-induced degradation resulted in a considerable change in the fluorescent properties of organic matter, similar to the SUVA results. We have previously reported that biological treatment processes can remove tryptophan-like components (T1 and T2) to a much greater extent than humic-like components (A and C) (Kim et al., 2016a; Kim et al., 2015a). During the cultivation of S. quadricauda in the RO concentrate without added humic acid under non-aerated conditions, both T1 and T2 did not appear and also a negligible change was found in the DOC (see Supporting Information Fig. S6), implying that the microalga under the given conditions is able to transform humic-like substances into more biodegradable forms.
Fig. 3.
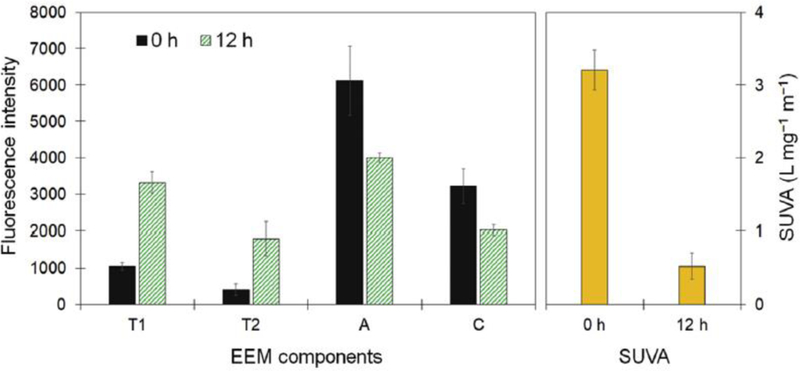
Changes in the fluorescence and absorption properties of dissolved organic matter after the first 12 h cultivation of S. quadricauda in the light under non-aerated conditions. T1: Ex/Em 220‒240/330‒360 nm, T2: Ex/Em 270‒280/330‒360 nm, A: Ex/Em 230‒260/400‒450 nm, and C: Ex/Em 300‒340/400‒450 nm.
In overall, our result indicates that relatively condensed, and thus stable, organic matter was degraded but not completely decomposed by algal cells under continuous illumination. Speculating on the light-dependent mechanisms for algae-induced degradation of refractory organic matter, the cultivation of S. quadricauda under aerated conditions should also be feasible in converting non- or slowly-biodegradable organic compounds into easily-assimilable ones and their prompt uptake with inorganic nutrients for growth in the light. Fig. 4 shows a couple of positive results in the context of operational simplicity when treating RO concentrate using a single bioreactor. S. quadricauda successfully removed carbonaceous organics consisting of humic substances (40%) and polysaccharides (60%), i.e., more than 70% of DOC was removed after the first 24 h cultivation using aeration with 10% (v/v) CO2 under continuous illumination. Likewise, the UV254 decreased by 78% of the initial UV absorbance after the 24 h cultivation and further notable decrease was not found for an additional 24 h of cultivation. A near-identical pattern of decrement in color was also found in aerated cultivation of microalgae. The decreases in the UV254 and color for the aerated culture were similar to those observed for the non-aerated culture which showed a negligible change in the DOC over the course of the experiment (see Fig. 2b). The humic substances would not be completely removed due to higher concentration of slowly biodegradable matter (see Supporting Information Fig. S7). The humic substances are dark colored and polyfunctional polymers formed through the synthesis of products by chemical and biological processes, known also as the browning reaction transformations in wastewater treatment plants. The recalcitrant characteristics of UV-absorbing humic substances against the utilization by microalgae has been reported in our previous studies (Kim et al., 2016a; 2016b). These previous observations are very suggestive, because the heterotrophic algae treatment without lighting did not remove the UV-absorbing humic substances which are linked to the presence of slowly biodegradable matter accumulated in biologically treated piggery wastewater. In this study, results of the RO concentrate treatment under dark with aeration (air only) also showed the recalcitrant characteristics of organic mixture toward S. quadricauda when compared to the utilization of glucose by the microalga (see Supporting Information Fig. S8).
Fig. 4.
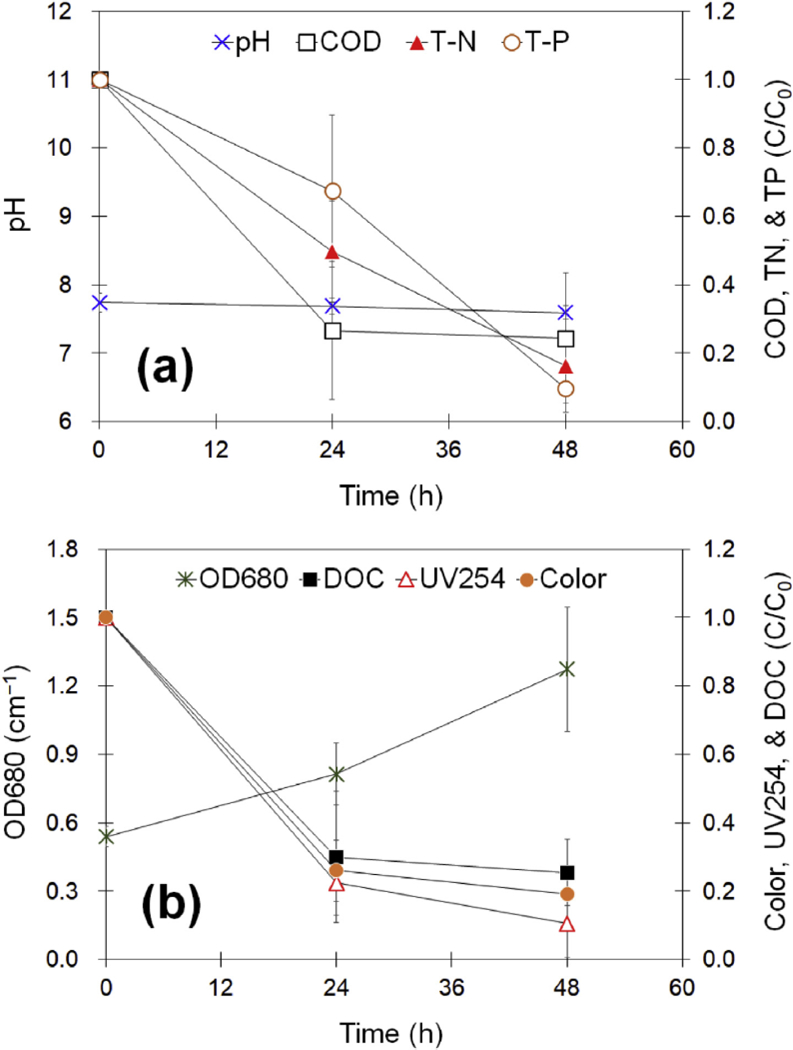
Variations in water quality during algal growth in synthetic RO concentrate using aeration with 10% (v/v) CO2 under continuous illumination for 48 h.
Fig. 4 also shows that the algae treatment using aeration with 10% (v/v) CO2 achieved 84% removal of TN at the end of the test, which was greater than that observed for the non-aerated culture examined in this study (see Fig. 2a). The growth of microalgae under the aerated conditions can alternatively proceed by directly incorporating an organic substrate in the oxidative assimilation process for storage material production, which offers the possibility of greatly increased cell productivity and thus is more desirable for the better removal of nutrients. Likewise, the TP removal was comparable to that achieved under non-aerated conditions, but it was mostly attributed to biotic utilization at a neutral pH. The removal of organic and inorganic components should be associated not only with the biomass yield, but also with the biosynthesis and accumulation of biomolecules in algal cells (see Supporting Information Fig. S9).
The results of the batch experiment with RO concentrate using two different cultivation strategies are shown in Fig. 5 (see Supporting Information Fig. S10), where the levels of E. coli after the given period of time are displayed in comparison with the control batch. The cultivation of S. quadricauda using aeration with 10% (v/v) CO2 was carried out at near-neutral conditions (pH 7.7±0.2). This resulted in almost complete growth inhibition of E. coli when compared to that observed for control batch, possibly due to competitions between them for assimilable organic carbon and/or other nutrients from the RO concentrate. Likewise, the growth inhibition of E. coli was also found after the 48 h cultivation without aeration, but it was more likely due to abiotic inactivation of E. coli at an extreme pH condition (> pH 11) (see Fig. 2). Parhad and Rao (1974) showed that the increasing pH of wastewater resulting from algal growth was responsible for the gradual reduction and eventual elimination of E. coli. A similar conclusion using E. coli placed in dialysis tubes in a eutrophic lake was drawn by Ansa et al. (2011). For the first 24 h cultivation, the E. coli yield was much greater for non-aerated culture than that observed for aerated culture (even for control culture), likely because organic matter was deformed (and/or degraded) and subsequently allowed for consumption by E. coli under the oxic conditions provided by algal photosynthesis. This observation supports that the S. quadricauda microalga is capable of converting refractory organics to biodegradable ones using continuous lighting, coincided with the results of the spectroscopic measurements in this study. E. coli decay was originally expected regardless of solution pH values when grown in association with algae, since the algae treatment without aeration that could degrade organic compounds would also damage the extracellular structures of E. coli. Further investigations are needed to ascertain whether other factors such as superoxide dismutase are protecting E. coli from algae-induced oxidation. Our examination revealed that algae treatment can alleviate the burden of post-treatment processes to comply with discharge permits that limit total coliform bacteria in effluent. Conventional disinfection methods such as UV-irradiation, chlorination, and ozonation typically require an additional pre- or post-treatment and/or a high level of energy to generate oxidants and produce undesirable by-products.
Fig. 5.
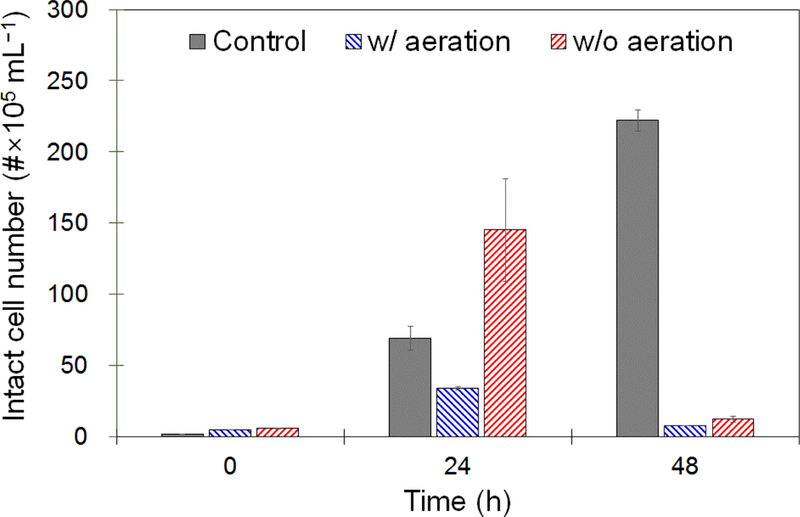
Effects of culture conditions on the yield of E. coli grown in association with S. quadricauda in synthetic RO concentrate. The intact cell concentration was determined with the microbial viability based on the cell membrane integrity.
The algae treatment notably removed some of the examined TOrCs especially under continuous illumination with aeration, and the extent of attenuated TOrCs is shown in Fig. 6a. One high (>30 μg L−1), two moderate (>10 μg L−1), and four low (<10 μg L−1) decrements were found, and the other three TOrCs (fenoprofen, ketoprofen, and naproxen) were hardly removed during algae treatment regardless of the cultivation methods (data not shown). The highest removal variation among the examined TOrCs was recorded for caffeine (>86%) after the 48 h cultivation under continuous lighting with aeration. A high biodegradability of caffeine has been reported in previous studies (Ahmed et al., 2017; Garcia-Rodríguez et al., 2014). Likewise, the algae treatment was effective at reducing bezafibrate (>28%), ibuprofen (>29%), and diclofenac (>18%) from wastewater, which was consistent with previously reported work (Matamoros et al., 2015). In contrast to others, the changes in levels of fenoprofen, ketoprofen, and naproxen were negligible, but other investigators reported the reverse conclusion. The removal of TOrCs from urban wastewater was investigated by Matamoros et al. (2015) using pilot-scale microalgae-based treatment system, and they reported that ketoprofen and naproxen were removed successfully. Inconsistent results could be caused by photoinduced degradation, longer retention time, algal-microbial consortium, and their combinations in the pilot test. Carbamazepine is known as recalcitrant toward to biological degradation such as slow sand filtration (Kim et al., 2015a), and it was removed in algae reactors to some extent. Under continuous lighting with aeration, S. quadricauda removed carbamazepine by 2.4 μg L−1 for the first 24 h and no change was found with further cultivation continued up to 48 h. This suggests that the removal of carbamazepine was likely attributed to abiotic processes, correlated with its non-polar hydrophobicity. Our results indicated that the extent of removed TOrCs during algal growth was independent on their characteristics, e.g., among the six hydrophilic ionic TOrCs which have high pKa values ranging between 3.73 and 4.53, bezafibrate, ibuprofen, and diclofenac were substantially removed, but the others were not changed. Likewise, two hydrophilic neutral TOrCs (caffeine and pentoxifyline) were also contrary to each other in terms of their treatability by the algae treatment.
Fig. 6.
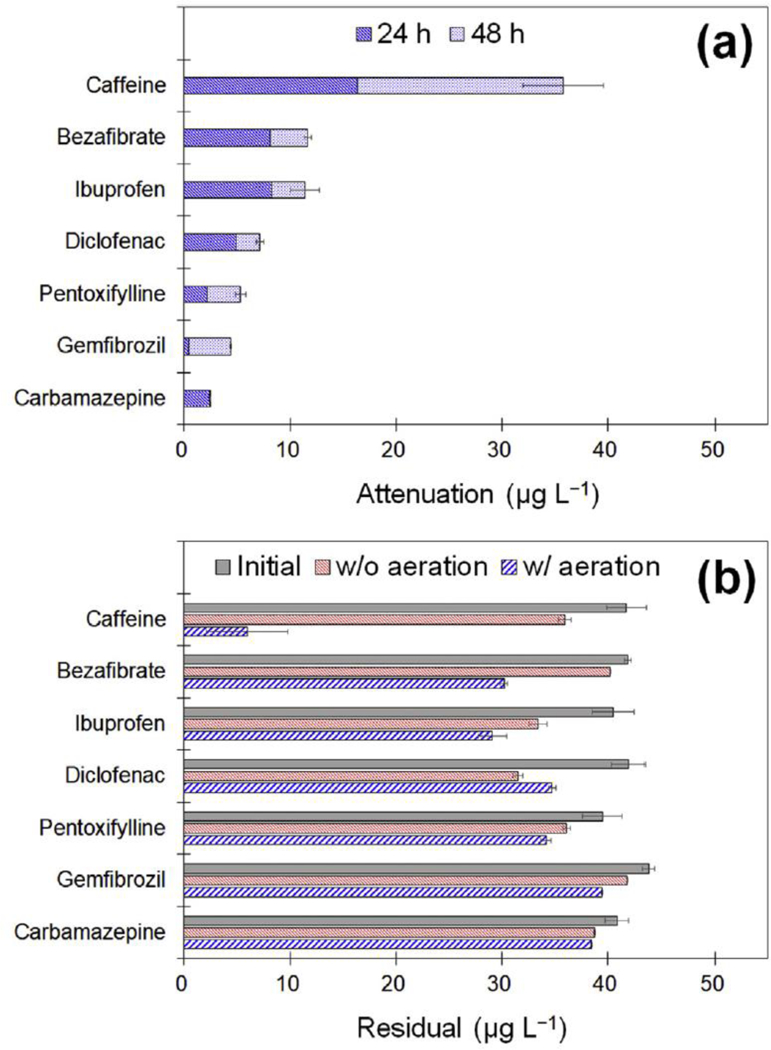
Removal of trace organic chemicals during the growth of S. quadricauda in synthetic RO concentrate (a) under continuous lighting with aeration and (b) under aerated and non-aerated conditions for 48 h.
The cultivation of S. quadricauda under continuous illumination with aeration was more effective at removing the TOrCs from RO concentrate when compared to using non-aeration algae treatment except diclofenac (>26%) (see Fig. 6b). The growth of algae under non-aerated conditions significantly changes both water chemistry and algal physiological properties, readily affecting the removal of certain compounds in algae treatment. Therefore, further research is needed to assess the overall mechanisms (e.g., co-metabolism, biosorption, and so forth) depending on the cultivation method and ways in which it could be enhanced through phycoremediation system design.
4. Conclusions
Under continuous illumination with CO2 supplementation, S. quadricauda successfully removed color-causing refractory organic matter along with inorganic nutrients from synthetic RO concentrate. The algae-induced degradation of the refractory organics followed by a prompt assimilation of the biodegradable fractions for growth in the light under aerated conditions. The algae-induced decolorization and degradation has an advantage over electrochemical oxidation technologies in that the photo sensitive living organisms are capable of self-repair, reproduction, and nutrient uptake. The cultivation of S. quadricauda with aeration also resulted in a substantial inhibition of E. coli growth in the RO concentrate, which could consequently lower uses of energy and/or chemicals required for wastewater disinfection processes for both reuse and discharge to surface waters. It is worthwhile to note that our results are limited to the synthetic RO concentrate. More work should be thus performed using real RO concentrates to document that the use of algal treatment is highly feasible as a safe and inexpensive technology to remove non- or slowly-biodegradable organics, reduce enteric bacteria, and attenuate TOrCs in wastewater.
Supplementary Material
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2015R1A2A2A03005744). Additional support was provided by a grant (17CTAP-C129744-01) from the Technology Advancement Research Program funded by the Ministry of Land, Infrastructure and Transport of Korean government. This work has been subjected to the U.S. Environmental Protection Agency’s administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the agency; therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Aeschbacher M, Sander M, Schwarzenbach RP, 2010. Novel Electrochemical Approach to Assess the Redox Properties of Humic Substances. Environ. Sci. Technol. 44 (1), 87–93. [DOI] [PubMed] [Google Scholar]
- Ahmed MB, Zhou JL, Ngo HH, Guo W, Thomaidis NS, Xu J, 2017. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J Hazard Mater 323, Part A, 274–298. [DOI] [PubMed] [Google Scholar]
- Amy G, Drewes J, 2007. Soil Aquifer Treatment (SAT) as a Natural and Sustainable Wastewater Reclamation/Reuse Technology: Fate of Wastewater Effluent Organic Matter (EfOM) and Trace Organic Compounds. Environmental Monitoring and Assessment 129 (1), 19–26. [DOI] [PubMed] [Google Scholar]
- Ansa EDO, Lubberding HJ, Ampofo JA, Gijzen HJ, 2011. The role of algae in the removal of Escherichia coli in a tropical eutrophic lake. Ecol. Eng. 37 (2), 317–324. [Google Scholar]
- Badruzzaman M, Oppenheimer J, Adham S, Kumar M, 2009. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Membr. Sci. 326 (2), 392–399. [Google Scholar]
- Berney M, Vital M, Hülshoff I, Weilenmann H-U, Egli T, Hammes F, 2008. Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res. 42 (14), 4010–4018. [DOI] [PubMed] [Google Scholar]
- Borowitzka MA, 2016. The Physiology of Microalgae. Borowitzka MA, Beardall J, Raven JA (eds), pp. 601–652, Springer International Publishing, Cham. [Google Scholar]
- Contreras AE, Kim A, Li Q, 2009. Combined fouling of nanofiltration membranes: Mechanisms and effect of organic matter. J. Membr. Sci. 327 (1–2), 87–95. [Google Scholar]
- Garcia-Rodríguez A, Matamoros V, Fontàs C, Salvadó V, 2014. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—a review. Environmental Science and Pollution Research 21 (20), 11708–11728. [DOI] [PubMed] [Google Scholar]
- Guo J, Peng Y, Guo J, Ma J, Wang W, Wang B, 2011. Dissolved organic matter in biologically treated sewage effluent (BTSE): Characteristics and comparison. Desalination 278 (1–3), 365–372. [Google Scholar]
- Huber SA, Balz A, Abert M, Pronk W, 2011. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatography – organic carbon detection – organic nitrogen detection (LC-OCD-OND). Water Res. 45 (2), 879–885. [DOI] [PubMed] [Google Scholar]
- Hur J, Schlautman MA, 2003. Using Selected Operational Descriptors to Examine the Heterogeneity within a Bulk Humic Substance. Environ. Sci. Technol. 37 (5), 880–887. [DOI] [PubMed] [Google Scholar]
- Hurwitz G, Hoek EMV, Liu K, Fan L, Roddick FA, 2014. Photo-assisted electrochemical treatment of municipal wastewater reverse osmosis concentrate. Chem Eng J 249, 180–188. [Google Scholar]
- Jantama K, Zhang X, Moore JC, Shanmugam KT, Svoronos SA, Ingram LO, 2008. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 101 (5), 881–893. [DOI] [PubMed] [Google Scholar]
- Joo SH, Tansel B, 2015. Novel technologies for reverse osmosis concentrate treatment: A review. Journal of Environmental Management 150, 322–335. [DOI] [PubMed] [Google Scholar]
- Kawano T, Irie K, Kadono T, 2010. Symbioses and Stress: Joint Ventures in Biology. Seckbach J, Grube M (eds), pp. 177–195, Springer Netherlands, Dordrecht. [Google Scholar]
- Kim H-C, Choi WJ, Chae AN, Park J, Kim HJ, Song KG, 2016a. Evaluating integrated strategies for robust treatment of high saline piggery wastewater. Water Res. 89, 222–231. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Choi WJ, Chae AN, Park J, Kim HJ, Song KG, 2016b. Treating high-strength saline piggery wastewater using the heterotrophic cultivation of Acutodesmus obliquus. Biochem Eng J 110, 51–58. [Google Scholar]
- Kim H-C, Choi WJ, Maeng SK, Kim HJ, Kim HS, Song KG, 2014a. Ozonation of piggery wastewater for enhanced removal of contaminants by S. quadricauda and the impact on organic characteristics. Bioresour. Technol. 159 (0), 128–135. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Choi WJ, Ryu JH, Maeng SK, Kim HS, Lee B-C, Song KG, 2014b. Optimizing Cultivation Strategies for Robust Algal Growth and Consequent Removal of Inorganic Nutrients in Pretreated Livestock Effluent. Appl. Biochem. Biotechnol. 174 (4), 1668–1682. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Dempsey BA, 2012. Comparison of two fractionation strategies for characterization of wastewater effluent organic matter and diagnosis of membrane fouling. Water Res. 46 (11), 3714–3722. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Noh JH, Chae S-R, Choi J, Lee Y, Maeng SK, 2015a. A multi-parametric approach assessing microbial viability and organic matter characteristics during managed aquifer recharge. Sci. Total Environ. 524–525, 290–299. [DOI] [PubMed] [Google Scholar]
- Kim H-C, Shin J, Won S, Lee J-Y, Maeng SK, Song KG, 2015b. Membrane distillation combined with an anaerobic moving bed biofilm reactor for treating municipal wastewater. Water Res. 71, 97–106. [DOI] [PubMed] [Google Scholar]
- Lee LY, Ng HY, Ong SL, Hu JY, Tao G, Kekre K, Viswanath B, Lay W, Seah H, 2009a. Ozone-biological activated carbon as a pretreatment process for reverse osmosis brine treatment and recovery. Water Res. 43 (16), 3948–3955. [DOI] [PubMed] [Google Scholar]
- Lee LY, Ng HY, Ong SL, Tao G, Kekre K, Viswanath B, Lay W, Seah H, 2009b. Integrated pretreatment with capacitive deionization for reverse osmosis reject recovery from water reclamation plant. Water Res. 43 (18), 4769–4777. [DOI] [PubMed] [Google Scholar]
- Liu K, Roddick FA, Fan L, 2012. Impact of salinity and pH on the UVC/H2O2 treatment of reverse osmosis concentrate produced from municipal wastewater reclamation. Water Res. 46 (10), 3229–3239. [DOI] [PubMed] [Google Scholar]
- Lyon SR, Ahmadzadeh H, Murry MA, 2015. Biomass and Biofuels from Microalgae: Advances in Engineering and Biology. Moheimani RN, McHenry PM, de Boer K, Bahri AP (eds), pp. 95–115, Springer International Publishing, Cham. [Google Scholar]
- Maeng SK, Sharma SK, Magic-Knezev A, Amy G, 2008. Fate of effluent organic matter (EfOM) and natural organic matter (NOM) through riverbank filtration. Water Sci. and Technol. 57 (12), 1999–2007. [DOI] [PubMed] [Google Scholar]
- Matamoros V, Gutiérrez R, Ferrer I, García J, Bayona JM, 2015. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J Hazard Mater 288, 34–42. [DOI] [PubMed] [Google Scholar]
- McCormick AJ, Bombelli P, Scott AM, Philips AJ, Smith AG, Fisher AC, Howe CJ, 2011. Photosynthetic biofilms in pure culture harness solar energy in a mediatorless bio-photovoltaic cell (BPV) system. Energy & Environmental Science 4 (11), 4699–4709. [Google Scholar]
- Michael-Kordatou I, Michael C, Duan X, He X, Dionysiou DD, Mills MA, Fatta-Kassinos D, 2015. Dissolved effluent organic matter: Characteristics and potential implications in wastewater treatment and reuse applications. Water Res. 77, 213–248. [DOI] [PubMed] [Google Scholar]
- Pérez-González A, Urtiaga AM, Ibáñez R, Ortiz I, 2012. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 46 (2), 267–283. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez ME, Lemaire SD, Crespo JL, 2012. Reactive Oxygen Species and Autophagy in Plants and Algae. Plant Physiol. 160 (1), 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez G, Fernández-Alba AR, Urtiaga AM, Ortiz I, 2010. Electro-oxidation of reverse osmosis concentrates generated in tertiary water treatment. Water Res. 44 (9), 2763–2772. [DOI] [PubMed] [Google Scholar]
- Parhad NM, Rao NU, 1974. Effect of pH on Survival of Escherichia coli. Journal (Water Pollution Control Federation) 46 (5), 980–986. [Google Scholar]
- Park JW, Kim H-C, Meyer AS, Kim S, Maeng SK, 2016. Influences of NOM composition and bacteriological characteristics on biological stability in a full-scale drinking water treatment plant. Chemosphere 160, 189–198. [DOI] [PubMed] [Google Scholar]
- Petri BG, Watts RJ, Teel AL, Huling SG, Brown RA, 2011. In Situ Chemical Oxidation for Groundwater Remediation. Siegrist LR, Crimi M, Simpkin JT (eds), pp. 33–88, Springer New York, New York, NY. [Google Scholar]
- Power IM, Wilson SA, Small DP, Dipple GM, Wan W, Southam G, 2011. Microbially Mediated Mineral Carbonation: Roles of Phototrophy and Heterotrophy. Environ. Sci. Technol. 45 (20), 9061–9068. [DOI] [PubMed] [Google Scholar]
- Radjenovic J, Bagastyo A, Rozendal RA, Mu Y, Keller J, Rabaey K, 2011. Electrochemical oxidation of trace organic contaminants in reverse osmosis concentrate using RuO2/IrO2-coated titanium anodes. Water Res. 45 (4), 1579–1586. [DOI] [PubMed] [Google Scholar]
- Rawat I, Gupta SK, Shriwastav A, Singh P, Kumari S, Bux F, 2016. Algae Biotechnology: Products and Processes. Bux F, Chisti Y (eds), pp. 249–268, Springer International Publishing, Cham. [Google Scholar]
- Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY, 1979. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 111 (1), 1–61. [Google Scholar]
- Semple KT, Cain RB, Schmidt S, 1999. Biodegradation of aromatic compounds by microalgae. FEMS Microbiol. Lett. 170 (2), 291–300. [Google Scholar]
- Sillanpää M, Matilainen A, Lahtinen T, 2015. Natural Organic Matter in Water, pp. 17–53, Butterworth-Heinemann, Oxford. [Google Scholar]
- Sun Y-X, Gao Y, Hu H-Y, Tang F, Yang Z, 2014. Characterization and biotoxicity assessment of dissolved organic matter in RO concentrate from a municipal wastewater reclamation reverse osmosis system. Chemosphere 117, 545–551. [DOI] [PubMed] [Google Scholar]
- Umar M, Roddick F, Fan L, 2016a. Comparison of coagulation efficiency of aluminium and ferric-based coagulants as pre-treatment for UVC/H2O2 treatment of wastewater RO concentrate. Chem Eng J 284, 841–849. [Google Scholar]
- Umar M, Roddick F, Fan L, 2016b. Impact of coagulation as a pre-treatment for UVC/H2O2-biological activated carbon treatment of a municipal wastewater reverse osmosis concentrate. Water Res. 88, 12–19. [DOI] [PubMed] [Google Scholar]
- Vakondios N, Koukouraki EE, Diamadopoulos E, 2014. Effluent organic matter (EfOM) characterization by simultaneous measurement of proteins and humic matter. Water Res. 63, 62–70. [DOI] [PubMed] [Google Scholar]
- Vital M, Dignum M, Magic-Knezev A, Ross P, Rietveld L, Hammes F, 2012. Flow cytometry and adenosine tri-phosphate analysis: Alternative possibilities to evaluate major bacteriological changes in drinking water treatment and distribution systems. Water Res. 46 (15), 4665–4676. [DOI] [PubMed] [Google Scholar]
- Wang X-X, Wu Y-H, Zhang T-Y, Xu X-Q, Dao G-H, Hu H-Y, 2016. Simultaneous nitrogen, phosphorous, and hardness removal from reverse osmosis concentrate by microalgae cultivation. Water Res. 94, 215–224. [DOI] [PubMed] [Google Scholar]
- Westerhoff P, Moon H, Minakata D, Crittenden J, 2009. Oxidation of organics in retentates from reverse osmosis wastewater reuse facilities. Water Res. 43 (16), 3992–3998. [DOI] [PubMed] [Google Scholar]
- Zhou T, Lim T-T, Chin S-S, Fane AG, 2011. Treatment of organics in reverse osmosis concentrate from a municipal wastewater reclamation plant: Feasibility test of advanced oxidation processes with/without pretreatment. Chem Eng J 166 (3), 932–939. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


