Abstract
Cancer development and progression occurs in concert with alterations in the surrounding stroma. Cancer cells can functionally sculpt their microenvironment through the secretion of various cytokines, chemokines, and other factors. This results in a reprogramming of the surrounding cells, enabling them to play a determinative role in tumor survival and progression. Immune cells are important constituents of the tumor stroma and critically take part in this process. Growing evidence suggests that the innate immune cells (macrophages, neutrophils, dendritic cells, innate lymphoid cells, myeloid-derived suppressor cells, and NK cells) as well as adaptive immune cells (T cells and B cells) contribute to tumor progression when present in the tumor microenvironment (TME). Crosstalk between cancer cells and the proximal immune cells ultimately results in an environment that fosters tumor growth and metastasis. Understanding the nature of this dialog will allow for improved therapeutics that simultaneously target multiple components of the TME, increasing the likelihood of favorable patient outcomes.
Keywords: cancer, tumor microenvironment, innate immunity, cancer therapy
Introduction
The tumor microenvironment (TME) is complex and continuously evolving. In addition to stromal cells, fibroblasts, and endothelial cells, the TME comprises innate and adaptive immune cells. Previous studies have focused predominantly on adaptive immune cells in the context of cancer. T lymphocytes in particular, have been a target of interest for their potent cytotoxic capabilities, so much so that their differentiation status became a model for other cell types and was coined the “Th1/Th2 paradigm” [1]. This dichotomy posits that T cells orchestrate pathogen-dependent immune responses by differential production of cytokines: Th1 cells govern a pro-inflammatory phenotype and Th2 cells orchestrate an immune suppressive phenotype. Current TME-targeted treatments have focused predominantly on T cells; prime examples include checkpoint blockade and chimeric antigen receptor (CAR) T cell therapies. With an expansion of the literature regarding the TME, it is now evident that the innate immune response not only indirectly influences the TME by controlling T cell fate, but also critically sculpts the TME. These innate immune cell types include macrophages (Mφs), dendritic cells (DCs), neutrophils, myeloid derived suppressor cells (MDSCs), natural killer cells (NKs), and innate lymphoid cells (ILCs). Mechanistically, cytokines within the TME manipulate immune functions that culminate in muted immune responses that guide tumor progression. It is essential to develop a comprehensive understanding of the innate immune cells and extend this knowledge to current therapies that target dysfunctional cells in the TME. In this review, we summarize the current knowledge on the ability of the TME to co-opt innate immune cells for cancer promotion and clinical strategies targeting these innate immune responses in the context of cancer.
Macrophages
Of all of the innate immune cells, monocyte derived macrophages (Mφs) best reflect the Th1/Th2 paradigm. Simplistically, Mφs can be polarized into inflammatory M1 (classically activated) or immune-suppressive M2 Mφs (alternatively activated) [2]. Mφs modulate immune responses through pathogen phagocytosis and antigen presentation, and also function in wound healing and tissue repair, thus necessitating them for immune homeostasis [3]. Mφs are tissue-specific and ubiquitous; they contribute to all stages of wound healing, tissue formation, coagulation, inflammation, and tissue reorganization [4]. Mφs first appear in the yolk sac on embryonic day 7, and from there they disseminate to peripheral tissues to establish tissue resident Mφs, although a majority of adult tissue Mφ populations (including the spleen, lung, and skin), originate in the fetal liver, indicating that the Mφs established by the yolk sac are replaced by those that originate in the fetal liver. Specifically, hematopoietic stem cells colonizing the fetal liver give rise to all hematopoietic lineages, including monocytes [5]. In the context of cancer, one form of Mφ recruitment includes recruitment from the bone marrow as monocytes by chemokines (CCL1, CCL2, CCL3, CCL4, CCL5, CCL7, CXCL1, CXCL2, CXCL4, CX3CL1), leading to Mφ differentiation in response to cytokines (CSF-1) that are secreted by many different cell types, including tumor cells, osteoblasts, and uterine epithelial cells [4, 6].
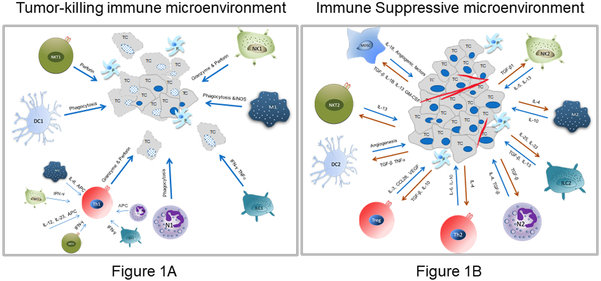
The TME potentiates immune suppressive M2 Mφs through the secretion of cytokines such as IL-4 (Figure 1A, B). Cumulatively, this enables tumor growth and progression as Mφs can make up to 50% of tumor mass [7]. High Mφ infiltration of most tumor types including breast cancer, gastric cancer, lung cancer, hepatoma, and other malignancies correlated with a negative prognosis, further establishing their role in cancer progression [8-10]. Also, an aspect of their normal tissue remodeling abilities includes regulation of epithelial cell movement. This function of Mφs is co-opted by tumor cells within the TME; Mφs release factors (e.g., EGF) that promote the movement and invasion of cancer cells [11, 12].
Figure 1. Cross Talk in the Tumor Microenvironment.
A. Depicts the impact of inflammatory or tumor suppressive immune cells on tumor cells in the TME. The bold arrows show the impact that immune cells ideally have on tumor cells (TC). The interactions between NKTs, DCs, T cells, Neutrophils, ILCs, Mφ, and NKs and tumor cells are depicted. Fibroblasts are denoted with the letter ‘F’.
B. Depicts the cross talk between immune cells in the TME that have been polarized to an immune suppressive type, and the cytokines secreted by the TCs that contribute to this Th2-like polarization.
While the M1/M2 classification is a simplified understanding of Mφ phenotype and function, in reality Mφs are plastic in nature and exist in a continuum of functional states [7]. M2 Mφs can further be classified into M2a, M2b, M2c, and M2d subsets (Table 1). These subsets are defined based on their different inducers namely: IFN-γ, and LPS for M1; IL-4, IL-10, IL-13 for M2a; TLR agonists for M2b; IL-10, TNF-α, and glucocorticoids for M2c; and TLR and adenosine A2A receptor for M2d [13]. Furthermore, these differential Mφ subtypes have different functional roles as outlined in Table 1. Therefore, it is unsurprising that Mφs that exhibit properties of both M1 and M2 exist in distinct proportions in the TME, depending on the tumor type, although the M2 phenotype is typically favored. This poses a conundrum since Mφ-mediated killing of cancer cells is virtually non-existent in the TME of tumors with high proportions of M2 Mφs. The wound healing phenotype of M2 Mφs established by the TME enables tumor growth, proliferation, angiogenesis, and epithelial-mesenchymal transition (EMT) [14]. There are many aspects of the TME, including cytokines and hypoxia, that orchestrate Mφ polarization and function (Table 1) [15]. IL-4, commonly present in the TME, initiates STAT6 signaling in Mφs, launching a transcriptional program that directs alternative polarization of Mφs. In a recent publication, Hanna et al have identified that tumor cells engage in a dialog with Mφs via secreted Hedgehog ligands [16]. This kindles a feed-forward loop that sustains alternatively polarized Mφs within the TME. Interfering with this crosstalk re-programmed the TME to be immune reactive and diminished the occurrence of metastasis. Given their role in inducing a pre-metastatic niche (“a favorable microenvironment for survival and outgrowth of tumor cells induced at distal sites by tumors” [17]), aiding in extravasation of circulating cancer cells, and promoting metastasis [18], Mφs present as prime candidates for therapeutic intervention.
Table 1.
Innate Immune Cells in the Tumor Microenvironment
| Cell Type | Normal Functions | Stimulatory Cytokines in the TME |
Cytokine/ Chemokine Secretion |
Human Markers | Mouse Markers | Effect | Source (s) |
|---|---|---|---|---|---|---|---|
| MACROPHAGES | |||||||
| M1 | Activate Th1 responses, phagocytosis, Type 4 hypersensitivity | IFN-γ | IL-12, IL-23, IL-1β, IL-6, IL-12, IL-23, CCL10, CCL11, CCL2-5, CCL8, CCL9 | CD64, IDO, SOCS1, CXCL10, CD80, CD86, CD68, MHC-II, IL-1R, SOCS3 | CXCL9, CXCL10, CXCL11, NOS2 | Anti-tumor | [2, 13] [93] |
| M2a | Activate Th2 responses, wound healing, allergy | IL-4, IL-10, IL-13, CSF1, CCL2, CCL3, CCL14 | IL-4, L-arginine, PGE2, IL-10, TGF-β, IL-1ra, CCL17, CCL22, CCL24 | MRC1, TGM2, CD23, CCL22, CD163, IL-1R II | Mrc1, Tgm2, Fizz1, Ym1/2, Arg1, MHC-II, IL-1ra | Pro-tumor | [2, 13] [93] |
| M2b | Th2 activation, immunoregulation | TLR agonists, Immunocomplex | IL-1, IL-6, IL-10, TNF-α, CCL1 | CD86, MHC-II | CD86, MHC-II | Pro-tumor | [13] [93] |
| M2c | Tissue repair, immunoregulation, matrix remodeling | IL-10, TNF-α, Glucocorticoids | TGF-β, IL-10, CCR2 | CD163, Mrc2 | CD163, Mrc1 | Pro-tumor | [13] [93] |
| M2d | Angiogenesis, clearance of apoptotic tissues | TLR, adenosine A2A receptor | TNF-α, TGF-β, VEGF-A, IL-10, IL-12, CCL5, CXCL10, CXCL16 | VEGF | VEGF | Pro-tumor | [13] [93] |
| DENDRITIC CELLS | |||||||
| Immature DCs | Recognize antigens, migrate to secondary lymphoid organs, phagocytosis, minimal APC, induce T cell anergy and promote Th2 and T-reg responses | N/A | N/A | CD11c, HLA-DR, FLT3L | Cd11c, MHCII, FLT3L, CD45 | Depends on tumor type | [20, 94] |
| cDC1 | APC to CD8 T cells, cross presentation, secretion of IL-12 | IL-12, TNF-α, IFN-γ | CD11c, CD141, XCR1, HLA-DR, Necl2, CLEC9A, CD80, CD86, CD40, CCR7, FLT3, TLR3, CD103, CADM1, CD26, BTLA, CD226, CD13 CD33, CXCR3, CXCR4 CLEC9A | CD11c, CD8α (lymphoid), MHCII, Clec9A, CD103 (Non-Lymphoid), DEC205, XCR1, CD80, CD86, CADM1, CD26, CD24 | Depends on tumor type | [19, 95-97] | |
| cDC2 | APC to CD4 T cells | TGF-β, IL-6, IL-8, IL-1, IL-12, IL-23, IL-10, TNF-α | CD11c, HLA-DR, CD1c, CD11b, CD80, CD86, FLT3, CLEC7A, CLEC6A, Dectin 1&2, CD40, CADM1, CD172a, CD2, SIRPA, FceR1, DCIR, CD62L, MHCII, ILT1 | CD11c, CD11b, MHCII, CD4 +/−, Sirpa+, CD80, CD86, CD172a, CD26 | Depends on tumor type | [19, 95-97] | |
| pDCs | Abundant secretory activity (IFN type 1), respond to viral infections | Type 1 IFN, TNF, IL-6 | CD11c, HLA-DR, CD304, CD303, CD123, FLT3, B220, PDCA1, FceR1, ILT3, ILT7, DR6, CD300A, BTLA, CD62L, CD45RA | CD11c, B220, CD45, Siglec H, CD317, Gr-1, Ly6C | Depends on tumor type | [20, 95-97] | |
| MoDCs | Produce high levels of the pro-inflammatory cytokines TNF, IL-6, and IL-12 | TNF-α, IL-1, IL-12, IL-23 | CD11c, CD14, Factor XIIIA, HLA-DR, CD62L, CXCR3, CD209, CD1c, CD80, CD86, CD64, MAR-1 | CD11c, MHC-II, CD11b, F4/80, Ly6C, CD206, CD115, CD107b, FcεRI, CD80, CD86 | Anti-tumor | [19, 94, 96, 98, 99] | |
| Tolerogenic DCs | Diminished APC, stimulate Th2 responses and Tregs to induce tolerance | PGE2, TGF-β, VEGF, IL-10, TNF-α | TGF-β | C1QA, C3AR1, CD163, CD300LF, CFH, CSGALNACT1, FcyR11A, FcyR11B, P2RY14, ZBT16 | SLAM, PDL1, PDL2, DEC205, IDO | Pro-tumor | [98-100] |
| NEUTROPHILS | |||||||
| N1 | Phagocytosis; release of NETs, inflammatory cytokines, toxin and ROS; respiratory burst, promotion of tumor cell apoptosis | N/A | TNF-α, IL-1, IFNs, MMP-8, Defensins, Along with toxic substances and reactive oxygen species. | TNF-α, I-CAM1, FAS, ROS | TNF-α, I-CAM1, FAS, ROS | Anti-tumor | [23, 30, 33] |
| N2 | Support angiogenesis, cancer cell migration and invasion, immune surveillance, and metastasis as well as secrete chemokines, cytokines and ROS/RNS | TGF-β, Angiotensin II | Oncostatin-M, MMP-9, CXCL1, CXCL8, CCL-3, Neutrophil elastase (NE), CXCL6, Collagenase IV, Heparanase, TGF-β, PGE2 | Arginase, CCL2, CCL5 | Arginase, CCL2, CCL5 | Pro-tumor | [23, 30, 33] |
| MYELOID DERIVED SUPPRESSOR CELLS | |||||||
| M-MDSCs | Suppress innate and adaptive immune responses | CSF-1, CCL2, CCL7, HIf1α, CXCL1 | NO, CCL3, CCL4, CCL5, Arg1, PGE2, IL-4 | CD11b+, HLADRlo/−, CD14+ | Cd11b+. Ly6Chi, CD49d+ | Pro-tumor | [41-43] |
| PMN-MDSCs | Suppress innate and adaptive immune responses | ROS, Arg1, PGE2, IL-4 | CD11b+, HLADR−, CD15+, CD14− | Cd11b+, Gr-1hi, Ly6G+, Ly6Clo | Pro-tumor | [41-43] | |
| eMDSCs | Suppress innate and adaptive immune responses | N/A | CD33+, Lin−, CD13−, CD14− CD3−, CD6− | Not well characterized | Pro-tumor | [42] | |
| NATURAL KILLER CELLS | |||||||
| CD56hi CD16+/− NKs |
Produce inflammatory cytokines | TGF-β, PGE2, IDO, IL-10 | IFN-γ, TNF-α | CD16+/−, CD56, NKG2A, CCR7, CXCR, CXCR3 | NKp46, NK1.1, CD122 | Depends on tumor type | [48, 51] |
| CD56lo CD16hi NKs |
Promote antibody dependent cellular cytotoxicity, high perforin production, enhanced killing | TGF-β, PGE2, IDO, IL-10 | IL-22, IL-10 | CD16hi, perforinhi | Not well characterized | Depends on tumor type | [48, 51] |
| INNATE LYMPHOID CELLS | |||||||
| ILC1 NK Cells | Cytotoxicity, macrophage activation, chronic inflammation, CD8 T cell activation | N/A | IFN-γ, TNF-α | CD56, NKp46, NKp44, IL/12RB2, DNAM1 | CD56, NKp46, NKp44, IL/12RB2, CD161, TIGIT, CTLA-4, CD96, NKG2A | Anti-tumor | [57, 62] |
| ILC1 Non-NK | Macrophage activation, chronic inflammation | N/A | IFN-γ, TNF-α | ICOS, IL-1R, IL/12RB2, CCR6 | ICOS, IL-1R IL/12RB2 | Anti-tumor | [57, 62] |
| ILC2 | Stimulate T cell responses through Th2 related cytokines, promotes skin inflammation | IL-33, IL-25 | IL-5, IL-13, | CD117, CD127, ICOS, CD294, IL-1R, ST2, IL-17RB, CD161, NKp30, PD1, CRTH2 | CD127, ICOS, CD294, IL-1R, ST2, IL-17RB, Sca1, PD1, CRTH2 | Pro-tumor and anti-tumor | [57, 62] |
| ILC3 | Chronic inflammation, intestinal homeostasis, lymphoid development bacterial immunity, | IL-23, IL-1β | IL-22, IL-17, GM-CSF | CD127, CD117, CD25, IL-1R, ICOS, IL-23R, MHCII, CCR6, NKp44, NKp30, NKp46, CD161 | CD127, CD117, CD25, IL-1R, ICOS, IL-23R, Sca1, MHCII, NKp46, CD161 | Pro-tumor | [57, 62] |
N/A: Not Applicable
Dendritic Cells
Dendritic cells (DCs) bridge the gap between the adaptive and innate immune systems. They initiate pathogen-specific T cell responses and are therefore important for bolstering protective immunity. It is important to note that B cells and Mφs also perform antigen presentation, albeit with lower activity then that of DCs. To effectively stimulate the adaptive immune response, DCs must recognize, capture, and present antigens, up-regulate co-stimulatory molecules, produce inflammatory cytokines, and then travel to secondary lymphoid organs for antigen presentation to T cells. The inability of DCs to perform these functions greatly hampers the immune response to pathogens, viruses, and tumors. DCs are functionally classified into different subtypes such as classical DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte derived inflammatory DCs (moDCs). cDCs can be further divided into cDC1 and cDC2. cDC1s develop under the control of the transcription factors IRF8, ID2, and BATF3, and cDC2s develop under the control of transcription factors IRF4, ID2, ZEB, and Notch2/KLF4 [19]. These subsets are also functionally distinct: cDC1s are capable of cross presentation and thus are able to present both endogenous and exogenous antigens, whereas cDC2s only present exogenous antigens and do not typically perform cross presentation. cDCs and pDCs are present and active during steady state conditions, while moDCs tend to only arise during inflammation. DCs specialize in different functions dependent on their stage of maturation and differentiation (Table 1). DCs can localize and acclimate to different tissues such as skin, lung, intestine, and liver and efficiently respond to environmental stimuli [20].
Analogous to Mφs, DCs are plastic in nature and can be stratified into specific subtypes. In the context of cancer, DCs are broadly referred to as tumor infiltrating dendritic cells (TIDC), which will be the predominant focus of this section. TIDCs can be immunogenic or tolerogenic dependent upon environmental signals. Examples of DCs that contribute to immune suppression include CD5hi cDC2s which stimulate Th2, Th17, and T regulatory responses [19]. It is important to note that each of the subtypes referred to in Table 1 can make up TIDCs which often adopt an immune suppressive phenotype due to the suppressive nature of the TME.
Tumors classically reprogram their microenvironment to support their survival. In the context of DCs, they do so by secreting cytokines to upregulate transcriptional and metabolic pathways that promote a tolerogenic phenotype, such as those that involve IDO, Arg1, iNOS, and STAT3 [21]. These pathways trigger alterations in DC metabolism, metabolite production, energetic shifts, and/or alterations of chromatin accessibility [22]. These modifications impact every aspect of DC functionality, including their abilities to secrete inflammatory cytokines and to prime effector T cells. Generally, DCs patrolling the TME encounter immune suppressive factors such as vascular endothelial growth factor (VEGF), IL-10, transforming growth factor beta (TGF-β), prostaglandin E2 (PGE2), and other cytokines (seen in Figure 1B) that inhibit DC maturation into immunogenic cells and promote their development into a tolerogenic phenotype, not only stunting their Th1 priming capacities, but also affording them the ability to promote Th2 and T regulatory responses [20]. Once removed from the TME, these DCs regain their ability to effectively process antigen and prime T cells [23], demonstrating that stimulating DC inflammatory functions in the TME may be an effective therapeutic strategy.
Further complexity regarding DC plasticity arises when considering different tumor types. DCs have been reported to be tumor-promoting in some TMEs, and tumor-suppressive in others. For example, TIDCs correlate with a positive prognosis in endometrial carcinoma but not in breast cancer [24, 25]. This could be indicative of a tumor stage-dependent phenomenon, i.e. DCs are tumor suppressive in early stages and become tumor promoting as the tumor progresses. Furthermore, infiltrating TIDC percentages differ among tumor types, suggesting that TMEs vary in their capacities to potently polarize TIDCs to tolerogenic dendritic cells [26]. Adding to this complexity, there are discrepancies among DC phenotypes between subtypes of the same tumor type. For example, transcriptomics of triple negative breast cancers reveals upregulated interferon pathways for all DC subtypes, whereas this is not the case in luminal breast cancer [27]. As such, the DC composition and functionality is tremendously influenced by the tumor type or the tumor subtype and its unique TME.
Neutrophils
Neutrophils account for up to 70% of circulating leukocytes and are the first line of defense against pathogens [28]. These cells are typically short-lived, persisting up to five days in circulation [29]. Upon tissue damage or infection, epithelial cells secrete neutrophil homing chemokines, compelling them to extravasate from circulation and enter the damaged tissue where they secrete inflammatory cytokines, release neutrophil extracellular traps (NETs), and phagocytose invading microorganisms [30]. NETs are composed of a chromatin backbone as a vehicle for antimicrobial peptides and toxins and are released as a further method of attack, although to the detriment of the neutrophil [31, 32]. In the context of cancer, tumor associated neutrophils (TANs) also follow the Th1/Th2 paradigm and exhibit an N1 (tumor-suppressive) or N2 (tumor-promoting) phenotype (Table 1). The phenotype of neutrophils in the TME depends on the tumor type and the stage of disease progression. Neutrophils are inflammatory during early tumor stages, but as the tumor progresses, they adopt an immunosuppressive phenotype [33]. Neutrophils modulate inflammation via production of reactive intermediates (ROS/RNS). They also re-configure the extracellular matrix through secretion of neutrophil elastase (NE) and matrix metalloproteinases (MMP8/9) in the TME and promote angiogenesis (Oncostatin-M), tumor progression (PGE2), and invasion (through the release of ROS/RNS, NE, MMP-9). NETs are comprised of MMPs, cathepsin G, and NE [34, 35]. These proteases degrade pro-inflammatory cytokines and re-position the TME to enhance tumor progression and aid in metastasis [36].
The plasticity of circulating neutrophils is an important feature in cancer patients. These neutrophils, called high-density neutrophils (HDNs) or low-density neutrophils (LDNs), correspond to N1 and N2 phenotypes, respectively. In many cancer types, LDNs, which exhibit a more immature phenotype, predominate in the circulation and may contribute to cancer progression and metastasis [29]. A detailed understanding of neutrophils and signals that pivot neutrophils to become immune suppressive holds much promise towards re-programming the TME. This is important given that they are present in the tumor in large numbers. The unique mechanism of NET-osis (NET formation) may prove to be a promising therapeutic target. While pre-clinical models demonstrate effectiveness of NET targeting, evidence on the clinical front is awaited.
Myeloid-derived Suppressor Cells
Another cell type that can be found in the TME includes myeloid derived suppressor cells (MDSCs). Some argue that MDSCs are a subtype of neutrophils [33], as there are several overlapping markers between MDSCs and TANs that make distinguishing between these cell types challenging. It is still debated if MDSCs represent a separate lineage of cells or are polarized immature neutrophils [37]. Despite this quandary, MDSCs are defined as, “a heterogeneous population of cells of myeloid origin that comprise myeloid progenitor cells and immature macrophages, immature granulocytes and immature dendritic cells” [38]. Accordingly, MDSCs and TANs clearly differentiate into distinct cell types even though they both stem from myeloid progenitor cells. Other than being hypodense, MDSCs are divergent from neutrophils in several ways, including reduced expression of CD16 and CD62L, and increased expression of Arg1, CD66B, and CD11b [39, 40]. MDSCs can be further categorized into subsets: monocytic MDSCs (M-MDSCs), which are distinguished by a CD11b hi, LY6C hi, and LY6G lo phenotype, polymorphonuclear MDSCs (PMN-MDSCs), which display a CD11b hi, LY6C lo, and LY6G hi phenotype, and early stage MDSCs (eMDSCs) which are CD13- and CD14-, and CD33+ in humans [41, 42]. It is noteworthy that both M-MDSCs and PMN-MDSCs present within the TME have an enhanced suppressive phenotype when compared with MDSCs present within peripheral lymphoid organs, due to increased expression of suppressive molecules by MDSCs in the TME [43].
MDSCs present in the TME contribute to immunosuppression, including T cell suppression and innate immune regulation, through various mechanisms (Table 1) [43]. Furthermore, MDSCs sculpt the primary TME and also initiate formation of the pre-metastatic niche. In particular, MDSCs enhance tumor cell stemness, increase angiogenesis, and advance the metastatic process by promoting EMT though IL-6 secretion [44, 45]. MDSCs also are influenced by the TME (Figure 1B) which further perpetuates their inherent immunosuppressive functions. For example, HIF-1α, a key player in the hypoxic tumor microenvironment, aids in MDSC differentiation to tumor promoting TAMs [46]. Also, factors in the TME can alter the metabolism of MDSCs toward fatty acid oxidation, prompting an upregulation of Arg1 and NOS2 production [47]. The critical role of MDSCs in tumorigenesis, growth, the establishment of the pre-metastatic niche, and metastatic outgrowth warrants the need to effectively target them by depletion or blockade. Although their critical role in the survival and advancement of tumors is well known, there are currently no FDA approved drugs or therapies that directly target MDSCs.
Natural Killer Cells and Natural Killer T Cells
Natural Killer cells (NKs) are circulatory, innate lymphoid cells recognized for their cytotoxic effector functions. Classically, there are two subsets of NKs defined by their expression of CD16 and CD56 levels: namely, CD56hi CD16+/− and CD56lo CD16hi [48]. CD56hi CD16 +/− NKs secrete inflammatory cytokines whereas CD56lo CD16hi NKs specialize in cytotoxic functions and cell mediated killing. Within the cancer framework, these cells are extremely efficient in eliminating malignant cells and limiting tumor metastases [49]. Their significance in tumor surveillance is illustrated by a correlation between low NK cell activity and increased cancer risk [50]. NKs employ death receptor mediated apoptosis and perforin/granzyme-mediated cytotoxicity to target tumor cells and limit primary tumor growth [51]. While NKs characteristically destroy circulating tumor cells, they are much less efficient at cell killing within the TME. Tumors deploy many mechanisms to evade destruction by NKs, including coating themselves in collagen to engage inhibitory NK receptors and utilizing platelets as a shield to avoid NK detection [52]. Within the TME, both NK subsets exhibit reduced inflammatory cytokine production and reduced or no cytotoxicity and both subsets will be referred to collectively as tumor infiltrating natural killer cells (TINKs). Many cytokines commonly present in the TME diminish NK effector functions (Table 1). These cytokines can stunt the cytotoxicity of TINKs (Figure 1B), which not only display diminished cytotoxicity, but also contribute to arresting the proliferation and expansion of T cells, enhancing their immune suppressive properties (these cells are often referred to as NKregs as well). Future efforts for developing therapeutic approaches could consider augmentation of cytotoxic NKs and/or targeting of TINKs. It is tempting to speculate that administration of NKs may enable a cancer preventative approach, or at the very least, a metastasis preventative approach as NKs are extremely efficient at targeting circulating cancer cells.
Also prevalent in the TME are natural killer T cells (NKTs), which are CD1d restricted, innate-like T lymphocytes that, like T cells, possess a T cell receptor, and like NKs, respond quickly to antigenic exposure [53]. Also, like T cells, overstimulation of NKTs can render them anergic. There are two major types of NKTs- Type 1 NKTs (NKTI) and Type II (NKTII) cells- which are characterized by their distinct T cell repertoires. While NKTIs express the Va14Ja18 invariant TCR alpha chain, the T cell repertoire of NKTIIs is less defined [54]. Both types can be dissected into further subsets that reflect the T cell subsets that play inflammatory or immune suppressive roles in the context of the TME. Specifically, NKTIs can be divided into Th1-like, Th2-like, Th17-like, Treg-like, and T follicular helper (TFH)-like NKTs; and NKTIIs can be divided into Th1-like and Th2-like NKTs. Furthermore, NKTs are reported to switch back and forth between inflammatory and immune suppressive subsets in response to their environment. In particular, NKTIs are typically anti-tumor, whereas NKTIIs are predominantly pro-tumor. NKTIs have been reported to prevent metastatic breast cancer [55] in mouse models. However, NKTIIs have been reported to support MDSCs in a B cell lymphoma mouse model [54, 56]. As such, targeting NKTIIs and supplementation with NKTIs may provide an exciting therapeutic approach.
Innate Lymphoid Cells
Another crucial component of the TME are the innate lymphoid cells (ILCs) which have characteristics similar to those of NK cells. ILCs share a common lymphoid progenitor with B and T cells, but lack B and T cell receptors and are thus classified as innate immune cells [57]. ILCs contribute to T cell polarization through antigen presentation and cytokine secretion [58]. There are three types of ILCs (ILC1, ILC2, and ILC3) classified on the basis of their production of Th1, Th2, and Th17-based cytokines and distinct transcription factors. [59]. ILC1s tend to exhibit anti-tumor functions through cytokine production (mainly IFN-γ). Furthermore, ILC1s can be divided into NK ILC1s and non-NK ILC1s based on their expression or lack thereof of the NK specific transcription factor, Eomesodermin. Importantly, NK ILCs can be distinguished from conventional NKs by differences in transcriptional regulation, phenotype, and localization as described by Seillet et al [60]. While ILC2s can functionally either promote or antagonize tumor growth depending on the tumor type (Figure 1), ILC3s are classically pro-tumorigenic. ILC polarization is determined by the composition of each specific TME (Table 1). As such, ILCs are differentially associated with different tumor types, likely because different tumor types have distinct TME compositions; for example, ILC2s are typically found in the TME of breast and gastric cancer, ILC3s are implicated in colon cancer [61, 62], and ILC1s prevent melanoma growth through the production of inflammatory cytokines [63, 64]. ILC3s may differentiate into ILC1s upon IL-12 stimulation, and ILC1s may differentiate into ILC3s upon stimulation by retinoic acid and IL-23 [62]. The conversion of ILC1 to ILC3 stunts their ability to aggressively target the tumor. This plasticity offers an attractive opportunity for therapeutically reprogramming ILC3s to ILC1s.
Immune Cells and Other Components of the Microenvironment
While the importance of direct interactions between tumor cells and immune cells is clear, it is also noteworthy to mention that immune cell interactions with other components in the TME can impact tumor fate. For example, it has been reported that the extracellular matrix (ECM) can play both supportive and inhibitory roles to the adaptive immune response by providing migratory pathways that allow T cells to invade the tissue or by directly inhibiting T cell proliferation, respectively [65]. Also, lymphatic vessels can regulate the immune microenvironment. Lymphatic vessels have been linked to providing nutrients to tumors through increased angiogenesis. They may also serve as migratory highways for immune cells [66], and lymphatic endothelial cells have also been reported to directly regulate DC activation [67]. Immune cells also interact with stromal cells, including cancer associated fibroblasts (CAFs). CAFs exhibit wound healing properties and have been implicated as contributors to tumor proliferation, invasion, and metastasis. CAFs may secrete immune suppressive cytokines that polarize Mφs to the M2 phenotype and contribute to CD8+ T cell exhaustion and deletion [68].
These observations indicate a complex series of interactions between immune cell types and non-tumor cells within the TME that clearly impact tumor progression, invasion, and metastasis. Therefore, not only should therapy designs consider tumor-immune cell cross talk and tumor-stromal cross talk, but also stromal-immune cell cross talk as it contributes significantly to tumor development.
Current and Future Therapeutics
The tumor masterfully controls its surrounding environment to promote its establishment, growth, survival, and spread. One of the chief ways it does this is through reprogramming innate immune cells to foster tumor growth and survival, leaving the patient with a weakened defense and often a worse prognosis. This is a potential Achilles heel of the tumor; as such, re-programming the innate immune system is a potentially important approach to improve patient outcomes.
Macrophage Therapies
Previous clinical trials targeting Mφs in the TME have been unsuccessful. Many prior trials involved the activation and injection of Mφs into cancer patients using various activation methods such as IFN-γ, mifamurtide, and LPS, but none of these methods were therapeutically efficacious [69-71]. There have been some promising clinical trials utilizing anti-M-CSFR antibodies. One such example includes the administration of RG7155, an anti-M-CSFR antibody, to diffuse-type giant cell tumor (Dt-GCT) patients. This strategy led to decreased TAM infiltration and overall positive patient responses [72]. It is noteworthy that anti-M-CSFR antibodies have yet to be successful in glioblastoma models, and there is still work to be done on this front. Ongoing clinical trials that target Mφ receptor, CSF-1R, and the CCL2-CCR2 signaling axis ablate tumor infiltrating Mφs and show promise in advanced solid tumors [73]. Moreover, the efficacy of CSF-1R inhibition is vastly improved when combined with receptor tyrosine kinase inhibitors. In addition to targeting CSF-1R and the CCL2-CCR2 signaling axis, there are ongoing clinical trials targeting CXCL12/CXCR4, CD40, and angiopoietin1/2 [74]. Treatment with IFN-α has yielded favorable outcomes in melanoma patients. IFN-α promotes an inflammatory environment, stimulates Mφs towards an M1 type, and has been demonstrated to reduce tumor growth and diminish metastasis [75].
Dendritic Cell Therapies
Targeting DC activation via DC vaccination is another therapeutic option. An important consideration in using DC vaccinations as cancer treatment is the method of priming DCs with tumor antigen. Options including priming with whole tumor cells, tumor cell lysate, apoptotic bodies, exosomes, or DNA or RNA need to be considered when designing an effective DC vaccine [76-78]. Thus far, whole cell vaccines seem to be the most promising. Several DC vaccination trials are currently ongoing (clinicaltrials.gov). One trial () involves enrichment of DC from glioma patients, pulsation with tumor lysate, and autologous intradermal injection. In their phase I clinical trial, Hus et al primed DCs from B-cell chronic lymphocytic lymphoma patients with tumor lysates and autologously vaccinated patients with these primed DC. This strategy resulted in an increase in cytotoxic T cell response. An example of a successful DC based therapy for prostate cancer is Provenge®. The regimen for Provenge® therapy involves harvesting monocytes from prostate cancer patients, differentiating and activating them in vivo with PAP antigen, and introducing them back into the patient. This therapy has achieved significant success marked by diminished tumor burden in prostate cancer patients. A new DC vaccine targeting glioblastoma is DCVax®-L which includes autologous DCs loaded with glioblastoma tumor lysate. This vaccine has been tested in a phase III clinical trial for glioblastoma, and overall patient survival was shown to increase by 6 months [79].
Despite success with DC vaccinations, there are challenges associated with them, including high cost, the absence of universal vaccine, the need for massive amounts of DCs, and issues with polarizing conventional DCs in vitro. Previous attempts at DC vaccinations focused on moDCs which are rare and do not functionally resemble cross-presenting DCs in vivo [80]. It is now recognized that cDCs comprise the DC subtype that is most likely to come into contact with cancer cells in the TME and mount the ensuing immune response. While cDCs are challenging to isolate, a cDC vaccine for melanoma has been reported to elicit a cytotoxic T cell response making them functionally more relevant [81]. Further work is required to standardize methods to effectively isolate cDCs for antigen loading and DC vaccination. A new focus for DC therapy involves directly targeting them in vivo. In vivo delivery of antibodies to cDC1 receptors conjugated to tumor antigens results in better DC activity and a higher rate of primed T cells. This is expected to reduce treatment costs due to the universality of the therapy and improve therapeutic effectiveness since DCs in vivo are already at the tumor site (in contrast to direct tumor injections which are not always possible or effective depending on tumor type). Combining this approach with immune checkpoint inhibitor blockade therapy will allow for rapid, effective T cell priming without T cell exhaustion.
Neutrophil Therapies
There are ongoing efforts to target neutrophils in the TME. Preclinical models have yielded optimistic success in reducing neutrophil number by squelching G-MCSF from the TME. Reparixin is a noncompetitive allosteric inhibitor of CXCR1 and CXCR2 [82] and targets neutrophil maturation to inhibit the immunosuppressive impact of tumor-induced N2 neutrophils. Reparixin is currently in one phase I and two phase II clinical trials for metastatic breast cancer. Targeting neutrophil polarization is another enticing therapeutic option through TGF-β inhibitors [83]. While there are currently many clinical trials that use TGF-β inhibitors, off target effects and cytotoxicity have been reported [84].
Myeloid derived suppressor cell therapies
There are currently several ongoing clinical trials that target MDSCs in different cancer types including leukemia, melanoma, glioblastoma, and breast cancer [85]. These trials utilize different mechanisms of indirectly impacting MDSC function, including targeting Arg1, iNOS, and STAT3 activities, metabolism through CD36, and trafficking through CXCR2 [85]. MDSC depletion is another tested avenue for cancer therapeutics. There has been some success in triggering MDSC apoptosis with gemcitabine and 5-fluorouracil, correlating with diminished tumor growth. Docetaxel, doxorubicin, paclitaxel, and tyrosine kinase inhibitors have also been demonstrated to reduce the numbers and effectiveness of MDSCs in the TME [85]. There also are therapies targeting MDSCs in combination with immune checkpoint inhibitors. A phase I/II clinical trial in renal cell carcinoma patients using atezolizumab and a histone-deacetylase inhibitor shows promise (). Also, a phase II clinical trial in melanoma patients combines ipilimumab and ATRA, which blocks retinoic acid signal transduction, leading to the differentiation of MDSCs into Mφs and DCs (). ATRA alone also leads to a reduction of MDSC frequencies in small cell lung cancer [86, 87]. While these trials show moderate yet encouraging success, off-target effects of these drugs may contribute to diminished therapeutic efficacy.
Natural killer cell therapies
Multiple enduring clinical trials aim to stimulate the immune system with NK cell therapy. For example, there is a phase I trial targeting advanced biliary tract cancer via allogeneic NK injection (). Yang et al. pioneered allogeneic NK cell therapy by activating allogeneic NKs with IL-2, followed by administration to advanced lymphoma patients [88]. The results revealed diminished T-reg and MDSC populations and increased expression of NKG2D on cytotoxic T cells [89]. NK cell therapy in combination with chemotherapy for small cell lung cancer () is also an effective strategy [90]. Also, the use of CAR-NK cells, genetically engineered cells that directly target tumor specific antigens in an HLA-unrestricted manner, has shown favorable outcomes in pre-clinical studies for B cell malignancies, ovarian, breast, prostate, and colon cancers [91]. All of these approaches have exhibited varying degrees of positive outcomes, but they also are limited by toxicity and detrimental side effects, high cost, and low efficacy [51, 92]. In contrast, there have been few successful clinical trials for ILC therapy in cancer.
Conclusion
Each of the therapeutic approaches discussed in this review has focused on targeting one aspect of the immune system. While some of these treatments yield positive outcomes, a more definitive and likely more effective approach involves altering multiple facets of the TME through a strong inflammatory response by promoting the inflammatory innate immune cells. There are multiple strategies that target immune suppressive cells, but unfortunately many of these responses are important for self-tolerance mechanisms and aid in protection against autoimmunity. Targeting immune suppressive cells cannot focus on a global depletion of all innate cells in the TME as this could cause dire effects in the host. The solution must be an intricate combination that involves selective inhibition or depletion of robust tumor suppressive cytokines and cell types in addition to bolstering the inflammatory phenotype of immune cells.
Acknowledgements
We acknowledge funding from the Department of Defense (W81XWH-14–1-0516 and W81XWH-18–1-0036), NCI R01CA169202, and The Breast Cancer Research Foundation of Alabama (BCRFA) to L.A.S. We would also like to acknowledge Will Jackson who contributed to figure development and conceptualization of this work, Dr. Rajeev Samant, Dr. Ann Hanna, Brandon Metge, and Sarah Bailey for editorial suggestions, and the UAB O’Neal Comprehensive Cancer Center (P30CA013148).
Abbreviations:
- Arg1
Arginase 1
- CAF
Cancer Associated Fibroblast
- CAR
Chimeric Antigen Receptor
- CD
Cluster of Differentiation
- cDC1
Conventional Dendritic Cell 1
- cDC2
Conventional Dendritic Cell 2
- DC
Dendritic Cell
- ECM
Extracellular Matrix
- eMDSC
Early stage MDSC
- EMT
Epithelial-Mesenchymal Transition
- HDN
High Density Neutrophil
- IDO
Indole amine 2,3 dioxygenase
- IFN- γ
Interferon Gamma
- iNOS
Inducible Nitric Oxide Synthase
- IL
Interleukin
- ILC
Innate Lymphoid Cell
- LDN
Low Density Neutrophil
- Mφ
Macrophage
- MDSC
Myeloid-derived Suppressor Cell
- M-MDSC
Monocytic Myeloid-derived Suppressor Cell
- MMP
Matrix Metalloproteinase
- MoDC
Monocyte Derived Dendritic Cell
- NE
Neutrophil Elastase
- NET
Neutrophil Extracellular Traps
- NK
Natural Killer Cell
- NKT
Natural Killer T Cell
- NKTI
Type I Natural Killer T Cell
- NKTII
Type II Natural Killer T Cell
- pDC
Plasmacytoid Dendritic Cell
- PGE2
Prostaglandin E2
- PMN-MDSC
Polymorphonuclear Myeloid-derived Suppressor Cell
- ROS
Reactive Oxygen Species
- RNS
Reactive Nitrogen Species
- STAT
Signal Transducer and Activator of Transcription
- TAN
Tumor Associated Neutrophil
- TFH
T Follicular Helper Cells
- TGF-β
Transforming Growth Factor Beta
- Th1
Type I helper T cell
- Th2
Type II helper T cell
- TIDC
Tumor Infiltrating Dendritic Cell
- TINK
Tumor Infiltrating Natural Killer Cell
- TME
Tumor Microenvironment
- TNF-α
Tumor Necrosis Factor Alpha
- VEGF
Vascular Endothelial Growth Factor
Footnotes
Conflict of Interest:
The authors have no conflict of interest to disclose.
References
- 1.McGuirk P and Mills KH, Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol, 2002. 23(9): p. 450–5. [DOI] [PubMed] [Google Scholar]
- 2.Yang L and Zhang Y, Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol, 2017. 10(1): p. 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H, et al. , Macrophage peroxisome proliferator-activated receptor gamma deficiency delays skin wound healing through impairing apoptotic cell clearance in mice. Cell Death Dis, 2015. 6: p. e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peiseler M and Kubes P, Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg, 2018. 44(3): p. 335–349. [DOI] [PubMed] [Google Scholar]
- 5.Varol C, Mildner A, and Jung S, Macrophages: development and tissue specialization. Annu Rev Immunol, 2015. 33: p. 643–75. [DOI] [PubMed] [Google Scholar]
- 6.Qin L, et al. , NCOA1 Directly Targets M-CSF1 Expression to Promote Breast Cancer Metastasis. Cancer Res, 2014. 74(13): p. 3477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J and Bae JS, Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm, 2016. 2016: p. 6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso AP, et al. , Macrophages stimulate gastric and colorectal cancer invasion through EGFR Y(1086), c-Src, Erk1/2 and Akt phosphorylation and smallGTPase activity. Oncogene, 2014. 33(16): p. 2123–33. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, et al. , CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-kappaB/SOX4 signaling. Cell Death Dis, 2018. 9(9): p. 880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, et al. , Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget, 2017. 8(18): p. 30576–30586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami S, et al. , Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res, 2005. 65(12): p. 5278–83. [DOI] [PubMed] [Google Scholar]
- 12.Wyckoff J, et al. , A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res, 2004. 64(19): p. 7022–9. [DOI] [PubMed] [Google Scholar]
- 13.Funes SC, et al. , Implications of macrophage polarization in autoimmunity. Immunology, 2018. 154(2): p. 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng X, et al. , Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. Oncotarget, 2017. 8(29): p. 48436–48452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrova V, et al. , The hypoxic tumour microenvironment. Oncogenesis, 2018. 7(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna A, et al. , Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. Oncoimmunology, 2019. 8(3): p. 1548241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peinado H, et al. , Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer, 2017. 17(5): p. 302–317. [DOI] [PubMed] [Google Scholar]
- 18.Doak GR, Schwertfeger KL, and Wood DK, Distant Relations: Macrophage Functions in the Metastatic Niche. Trends Cancer, 2018. 4(6): p. 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collin M and Bigley V, Human dendritic cell subsets: an update. Immunology, 2018. 154(1): p. 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motta JM and Rumjanek VM, Sensitivity of Dendritic Cells to Microenvironment Signals. J Immunol Res, 2016. 2016: p. 4753607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumpter TL, et al. , Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol, 2012. 189(8): p. 3848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basit F, et al. , Human Dendritic Cell Subsets Undergo Distinct Metabolic Reprogramming for Immune Response. Front Immunol, 2018. 9: p. 2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridlender ZG, et al. , Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 2009. 16(3): p. 183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaput N, et al. , The Janus face of dendritic cells in cancer. Oncogene, 2008. 27(45): p. 5920–31. [DOI] [PubMed] [Google Scholar]
- 25.Aspord C, et al. , Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med, 2007. 204(5): p. 1037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran Janco JM, et al. , Tumor-infiltrating dendritic cells in cancer pathogenesis. J Immunol, 2015. 194(7): p. 2985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michea P, et al. , Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol, 2018. 19(8): p. 885–897. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, et al. , The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med, 2014. 12: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deniset JF and Kubes P, Recent advances in understanding neutrophils. F1000Res, 2016. 5: p. 2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uribe-Querol E and Rosales C, Neutrophils in Cancer: Two Sides of the Same Coin. J Immunol Res, 2015. 2015: p. 983698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cools-Lartigue J, et al. , Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci, 2014. 71(21): p. 4179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin W, et al. , Tumor-Infiltrating NETs Predict Postsurgical Survival in Patients with Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Fridlender ZG and Albelda SM, Tumor-associated neutrophils: friend or foe? Carcinogenesis, 2012. 33(5): p. 949–55. [DOI] [PubMed] [Google Scholar]
- 34.Demers M, et al. , Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology, 2016. 5(5): p. e1134073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He M, et al. , Peritumoral stromal neutrophils are essential for c-Met-elicited metastasis in human hepatocellular carcinoma. Oncoimmunology, 2016. 5(10): p. e1219828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schauer C, et al. , Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med, 2014. 20(5): p. 511–7. [DOI] [PubMed] [Google Scholar]
- 37.Pillay J, et al. , Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci, 2013. 70(20): p. 3813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI and Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol, 2009. 9(3): p. 162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez PC, et al. , Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res, 2009. 69(4): p. 1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youn JI, et al. , Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol, 2012. 91(1): p. 167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Ostrand-Rosenberg S, and Bronte V, Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol, 2012. 12(4): p. 253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bronte V, et al. , Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun, 2016. 7: p. 12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar V, et al. , The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol, 2016. 37(3): p. 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Condamine T, et al. , Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med, 2015. 66: p. 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Condamine T, et al. , Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol, 2016. 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corzo CA, et al. , HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med, 2010. 207(11): p. 2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hossain F, et al. , Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res, 2015. 3(11): p. 1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stabile H, et al. , Role of Distinct Natural Killer Cell Subsets in Anticancer Response. Front Immunol, 2017. 8: p. 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glasner A, et al. , NKp46 Receptor-Mediated Interferon-gamma Production by Natural Killer Cells Increases Fibronectin 1 to Alter Tumor Architecture and Control Metastasis. Immunity, 2018. 48(2): p. 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imai K, et al. , Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet, 2000. 356(9244): p. 1795–9. [DOI] [PubMed] [Google Scholar]
- 51.Langers I, et al. , Natural killer cells: role in local tumor growth and metastasis. Biologics, 2012. 6: p. 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer S, et al. , Platelet-mediated shedding of NKG2D ligands impairs NK cell immune-surveillance of tumor cells. Oncoimmunology, 2018. 7(2): p. e1364827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair S and Dhodapkar MV, Natural Killer T Cells in Cancer Immunotherapy. Front Immunol, 2017. 8: p. 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krijgsman D, Hokland M, and Kuppen PJK, The Role of Natural Killer T Cells in Cancer-A Phenotypical and Functional Approach. Front Immunol, 2018. 9: p. 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gebremeskel S, et al. , Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology, 2015. 4(3): p. e995562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renukaradhya GJ, et al. , Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood, 2008. 111(12): p. 5637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flores-Borja F, et al. , Crosstalk between Innate Lymphoid Cells and Other Immune Cells in the Tumor Microenvironment. J Immunol Res, 2016. 2016: p. 7803091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Burg N, et al. , Activated group 3 innate lymphoid cells promote T-cell-mediated immune responses. Proc Natl Acad Sci U S A, 2014. 111(35): p. 12835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyoizumi S, et al. , Fate Decision Between Group 3 Innate Lymphoid and Conventional NK Cell Lineages by Notch Signaling in Human Circulating Hematopoietic Progenitors. J Immunol, 2017. 199(8): p. 2777–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seillet C, Belz GT, and Huntington ND, Development, Homeostasis, and Heterogeneity of NK Cells and ILC1. Curr Top Microbiol Immunol, 2016. 395: p. 37–61. [DOI] [PubMed] [Google Scholar]
- 61.Jovanovic IP, et al. , Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer, 2014. 134(7): p. 1669–82. [DOI] [PubMed] [Google Scholar]
- 62.van Beek JJP, et al. , Innate Lymphoid Cells in Tumor Immunity. Biomedicines, 2016. 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuchs A, et al. , Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity, 2013. 38(4): p. 769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mattner J and Wirtz S, Friend or Foe? The Ambiguous Role of Innate Lymphoid Cells in Cancer Development. Trends Immunol, 2017. 38(1): p. 29–38. [DOI] [PubMed] [Google Scholar]
- 65.Pickup MW, Mouw JK, and Weaver VM, The extracellular matrix modulates the hallmarks of cancer. EMBO Rep, 2014. 15(12): p. 1243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lund AW, et al. , Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest, 2016. 126(9): p. 3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Podgrabinska S, et al. , Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol, 2009. 183(3): p. 1767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lakins MA, et al. , Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun, 2018. 9(1): p. 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faradji A, et al. , Phase I study of liposomal MTP-PE-activated autologous monocytes administered intraperitoneally to patients with peritoneal carcinomatosis. J Clin Oncol, 1991. 9(7): p. 1251–60. [DOI] [PubMed] [Google Scholar]
- 70.Hennemann B, et al. , Phase I trial of adoptive immunotherapy of cancer patients using monocyte-derived macrophages activated with interferon gamma and lipopolysaccharide. Cancer Immunol Immunother, 1998. 45(5): p. 250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevenson HC, et al. , Fate of gamma-interferon-activated killer blood monocytes adoptively transferred into the abdominal cavity of patients with peritoneal carcinomatosis. Cancer Res, 1987. 47(22): p. 6100–3. [PubMed] [Google Scholar]
- 72.Ries CH, et al. , Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell, 2014. 25(6): p. 846–59. [DOI] [PubMed] [Google Scholar]
- 73.Ostuni R, et al. , Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol, 2015. 36(4): p. 229–39. [DOI] [PubMed] [Google Scholar]
- 74.Quail DF and Joyce JA, Molecular Pathways: Deciphering Mechanisms of Resistance to Macrophage-Targeted Therapies. Clin Cancer Res, 2017. 23(4): p. 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Palma M, et al. , Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell, 2008. 14(4): p. 299–311. [DOI] [PubMed] [Google Scholar]
- 76.Boczkowski D, et al. , Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med, 1996. 184(2): p. 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao J, et al. , DNA vaccines targeting the encoded antigens to dendritic cells induce potent antitumor immunity in mice. BMC Immunol, 2013. 14: p. 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hamilton DH, et al. , Immunological targeting of tumor cells undergoing an epithelial-mesenchymal transition via a recombinant brachyury-yeast vaccine. Oncotarget, 2013. 4(10): p. 1777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Willigen WW, et al. , Dendritic Cell Cancer Therapy: Vaccinating the Right Patient at the Right Time. Front Immunol, 2018. 9: p. 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saxena M and Bhardwaj N, Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer, 2018. 4(2): p. 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lesterhuis WJ, et al. , Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol, 2008. 66(2): p. 118–34. [DOI] [PubMed] [Google Scholar]
- 82.Ocana A, et al. , Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer, 2017. 16(1): p. 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colak S and Ten Dijke P, Targeting TGF-beta Signaling in Cancer. Trends Cancer, 2017. 3(1): p. 56–71. [DOI] [PubMed] [Google Scholar]
- 84.Connolly EC, Freimuth J, and Akhurst RJ, Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci, 2012. 8(7): p. 964–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fleming V, et al. , Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol, 2018. 9: p. 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iclozan C, et al. , Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother, 2013. 62(5): p. 909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weber R, et al. , Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front Immunol, 2018. 9: p. 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Y, et al. , Phase I Study of Random Healthy Donor-Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors. Cancer Immunol Res, 2016. 4(3): p. 215–24. [DOI] [PubMed] [Google Scholar]
- 89.Veluchamy JP, et al. , The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments. Front Immunol, 2017. 8: p. 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang X, et al. , First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res, 2018. 8(6): p. 1083–1089. [PMC free article] [PubMed] [Google Scholar]
- 91.Rezvani K, et al. , Engineering Natural Killer Cells for Cancer Immunotherapy. Mol Ther, 2017. 25(8): p. 1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bolourian A and Mojtahedi Z, Possible damage to immune-privileged sites in natural killer cell therapy in cancer patients: side effects of natural killer cell therapy. Immunotherapy, 2017. 9(3): p. 281–288. [DOI] [PubMed] [Google Scholar]
- 93.Roszer T, Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm, 2015. 2015: p. 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Steinman RM, Hawiger D, and Nussenzweig MC, Tolerogenic dendritic cells. Annu Rev Immunol, 2003. 21: p. 685–711. [DOI] [PubMed] [Google Scholar]
- 95.Ma DY and Clark EA, The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol, 2009. 21(5): p. 265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conejo-Garcia JR, Rutkowski MR, and Cubillos-Ruiz JR, State-of-the-art of regulatory dendritic cells in cancer. Pharmacol Ther, 2016. 164: p. 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guilliams M, et al. , Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity, 2016. 45(3): p. 669–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Collin M, McGovern N, and Haniffa M, Human dendritic cell subsets. Immunology, 2013. 140(1): p. 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen K, et al. , Tissue-resident dendritic cells and diseases involving dendritic cell malfunction. Int Immunopharmacol, 2016. 34: p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gueguen C, et al. , Changes in markers associated with dendritic cells driving the differentiation of either TH2 cells or regulatory T cells correlate with clinical benefit during allergen immunotherapy. J Allergy Clin Immunol, 2016. 137(2): p. 545–58. [DOI] [PubMed] [Google Scholar]