Abstract
There is no evidence-based treatment for heart failure with preserved ejection fraction (HFpEF). While lower heart rates (HR) provide an unequivocal benefit for patients with HF with reduced EF, higher HR might convey important hemodynamic and substrate-modifying benefits in patients with diastolic dysfunction. In a prospective study of 20 stable outpatients with diastolic dysfunction and pacemakers, we evaluated the effects of a 4-week increase in the lower pacemaker rate to 80 beats per minute (bpm) followed by reversal to the previous lower HR setting from weeks 4 to 6. We assessed quality of life (MLHFQ), six-minute walk test (6MWT) and NT-proBNP levels. Pacing at 80bpm significantly improved quality of life and the 6MWT (p≤0.05). There was a strong positive correlation between the pacing-induced changes in NT-proBNP and baseline QRS intervals (r2=0.31, p<0.01). Stratification by QRS duration revealed that pacing at 80bpm led to −21±26% reduction in NT-proBNP in patients with QRS≤150ms, whereas QRS>150ms was associated with a 26±35% increase in NT-proBNP (p<0.01). Patients physiologically paced from the conduction system had a −46±26% reduction in NT-proBNP at 80bpm as compared to 4±26% and 13±26% change with pacing from the right atrial appendage and right ventricular apical septum (pinteraction=0.04). In conclusion, increasing the lower rate setting of pacemakers to 80bpm in patients with diastolic dysfunction improves quality of life, functional capacity and NT-proBNP for those patients with a baseline QRS≤150ms. These findings suggest that higher HRs may provide meaningful benefits to patients with left ventricular diastolic dysfunction and HFpEF.
Keywords: diastolic dysfunction, heart rate, pacing
INTRODUCTION
About half of the patients with heart failure have a normal or preserved ejection fraction (HFpEF) for which there is no evidence-based treatment.1-2 Because pharmacological heart rate (HR) lowering is beneficial in HF with a reduced ejection fraction (HFrEF), it is assumed that lower HRs also provide a benefit to patients with HFpEF.1,3 However, recent reports in other patient-populations with a normal EF suggest that pharmacological HR-lowering is associated with adverse outcomes such as an increased risk of atrial fibrillation, heart failure and stroke.4-8 These counterintuitive outcomes are explained by reflected systemic pressure waves, which at lower HRs result in higher central blood pressures9. Also at lower HRs ventricular filling time is prolonged leading to increased ventricular filling pressures and wall stress.10 Meanwhile, pacing above the resting HR acutely lowers left ventricular filling pressures in HFpEF patients.11,12 Elevations in HR may also induce a beneficial myocardial substrate remodeling.13-15 For these reasons, we hypothesized that patients with HFpEF may derive a significant benefit from higher resting HRs which would be reflected in an improvement in symptoms, quality of life, functional capacity and lower NT-proBNP levels. In a clinical exploration of this concept we evaluated the effects of a temporary elevation in the resting HR in patients with pacemakers and evidence of left ventricular diastolic dysfunction.
METHODS
This study was conducted at the University of Vermont Medical Center (UVMMC) in Burlington, Vermont between 2017 and 2018. The protocol was approved by the UVM institutional review board. 1531 patients scheduled at the UVMMC Pacemaker Clinic were screened for the following inclusion criteria: (1) a DDD(R) mode pacemaker; (2) dyspnea on exertion; (3) echocardiogram that reported a left ventricular (LV) EF ≥50%; LV end-diastolic volume <80mL/m2, LV hypertrophy and left atrial dilation; (4) ability to perform a six-minute walk test (6MWT); (5) stable on current medications without heart failure hospitalizations over 6 months and ≥18 years of age. Exclusion criteria: (1) Blood pressure >160/100mmHg; (2) creatinine >2mg/dL; (3) more than moderate valvular disease; (4) COPD on oxygen therapy; (5) aortic valve replacement within 1 year; (6) pacemaker with <6 months of battery life and (7) patient life expectancy <6 months. The enrollment criteria are similar to the REVAMP study (NCT03210402) that is testing the safety and feasibility of a nocturnal HR elevation to 100bpm. The screening resulted in 49 eligible patients of whom 22 provided informed consent.
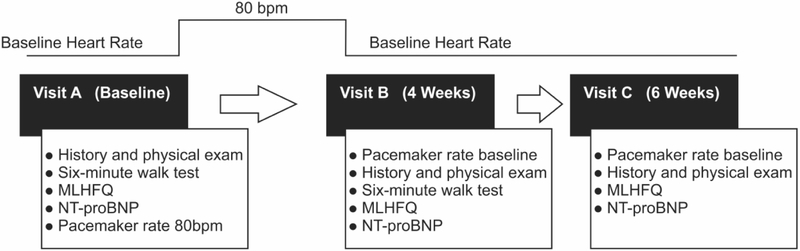
Enrolled patients had a baseline visit (A) after which the lower HR setting was increased to 80bpm. Following the cardiovascular exam at the beginning of the 4-week follow-up visit (B) the pacemaker setting was returned to the previous lower HR setting. This was followed by a repeat baseline assessment at 6 weeks (C) as shown in Figure 1. Each visit included a cardiovascular history, symptom assessment and physical exam to assess for signs or symptoms of heart failure and to solicit any subjective changes. A Minnesota Living with Heart Failure Questionnaire (MLHFQ), a NT-proBNP level, pacemaker interrogation and a 6MWT (A and B visit only) was also obtained. Study participants and investigators were blinded to prior results. Patient baseline characteristics and baseline ECGs were obtained from the electronic medical record. All echocardiograms were independently reviewed by a Level 3 certified echocardiographer using a volumetric (modified Simpson’s equation) EF assessment and guideline–based LV concentric remodeling and hypertrophy partition values.16
Figure 1. Study Design.
MLHFQ = Minnesota Living With Heart Failure Questionnaire
Of the 22 patients enrolled in the study, 20 successfully completed the A and B visit, and 18 patients completed all three visits. One patient who was enrolled and completed the study protocol was found to have an EF of 43% and was thus excluded from the analysis. One patient did not complete all 3 questionnaires and 2 patients were unable to perform the 6WMT.
Descriptive statistics of the cohort are presented as means and standard deviations for continuous variables, and as numbers and percentages for categorical variables. Paired t-tests were used to compare NT-proBNP, 6MWT distance and MLHFQ scores between the intervention and baseline visits. Linear regression models were used to investigate for interactions of the relative changes in NT-proBNP and baseline characteristics. Two-sample t-tests were used to compare subgroups followed by a statistical sensitivity analysis using nonparametric rank testing. A nonparametric Kruskal-Wallis test was used to confirm the significance of the interaction of more than 2 groups followed by a Dunn’s test. Formal tests utilized a 5% significance level. Parametric p values are reported.
RESULTS
The baseline patient characteristics of the enrolled study population are listed in Table 1. The patient population was older with a high prevalence of hypertension and atrial fibrillation. Two patients were observed to be in atrial fibrillation during the first visit, one of which had chronic atrial fibrillation. Pacing sites included the right atrial appendage, right ventricular apical septum and physiologic pacing sites (His or Bachmann’s bundle). The echocardiographic assessments confirmed the presence of concentric LV hypertrophy, left atrial dilatation and LV diastolic dysfunction.
Table 1.
Baseline Patient Characteristics (n = 20)
| Patient Data | Echocardiography | ||
|---|---|---|---|
| Age (years) | 79.0±8.4 | Left ventricular ejection fraction | 61±6% |
| Female | 11 (55%) | Septum (mm) | 13±1 |
| Height (cm) | 166±10 | Posterior wall (mm) | 12±1 |
| Weight (kg) | 86±16 | Left ventricular end diastolic diameter (mm) | 46±11 |
| Body mass index (kg/m2) | 31±6 | Left ventricular end-systolic diameter (mm) | 30±8 |
| Body surface area (BSA) (m2) | 2.0±0.2 | Relative wall thickness | 0.6±0.4 |
| Lower pacing rate (bpm) | 61.4±3.5 | Left ventricular mass (g) | 220±66 |
| Heart rate (bpm) | 70±12 | Left ventricular mass/BSA (g/m2) | 110±27 |
| Systolic blood pressure (mmHg) | 134±14 | Left ventricular end diastolic volume (LVEDV) (mL) | 77±23 |
| Diastolic blood pressure (mmHg) | 71±9 | Left ventricular end systolic volume (mL) | 30±13 |
| Baseline QRS interval (ms) | 134±50 | LVEDV/BSA (ml/m2) | 39±11 |
| Sinus node dysfunction | 14 (70%) | Left atrial volume (ml) | 69±21 |
| AV conduction disease | 6 (30%) | Left atrial volume/BSA (ml/m2) | 34±13 |
| Right atrial appendage paced | 8 (40%) | E-wave peak velocity (cm/sec) | 92±32 |
| His/Bachmann’s bundle paced | 5 (25%) | A-wave peak velocity (cm/sec) | 88±35 |
| Right ventricular apical septum paced | 7 (35%) | Ratio of mitral peak velocity of early filling (E) to early diastolic lateral mitral annular velocity (E/e'med) | 17±8 |
| Coronary artery disease | 8 (40%) | Ratio of mitral peak velocity of early filling (E) to early diastolic lateral mitral annular velocity (E/E'lat) | 13±6 |
| Hypertension | 17 (85%) | Pulmonary arterial systolic pressure (mmHg) | 36±9 |
| Diabetes mellitus | 6 (30%) | ||
| Atrial fibrillation | 13 (65%) | ||
| Stroke | 1 (5%) | ||
| Beta-blocker | 12 (60%) | ||
| ACE-inhibitor/Angiotensin Receptor Blocker | 12 (60%) | ||
| Diuretic | 7 (35%) | ||
| Anticoagulant | 8 (40%) | ||
| Calcium channel blocker | 5 (25%) |
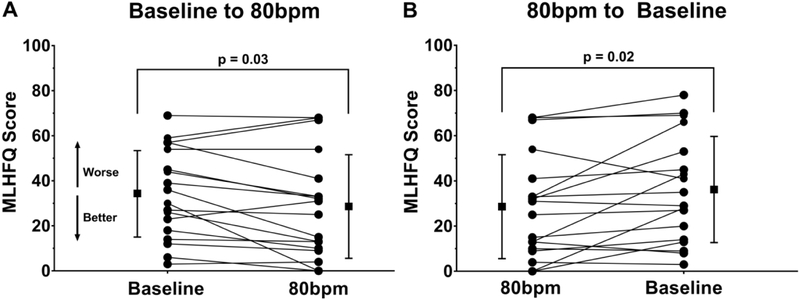
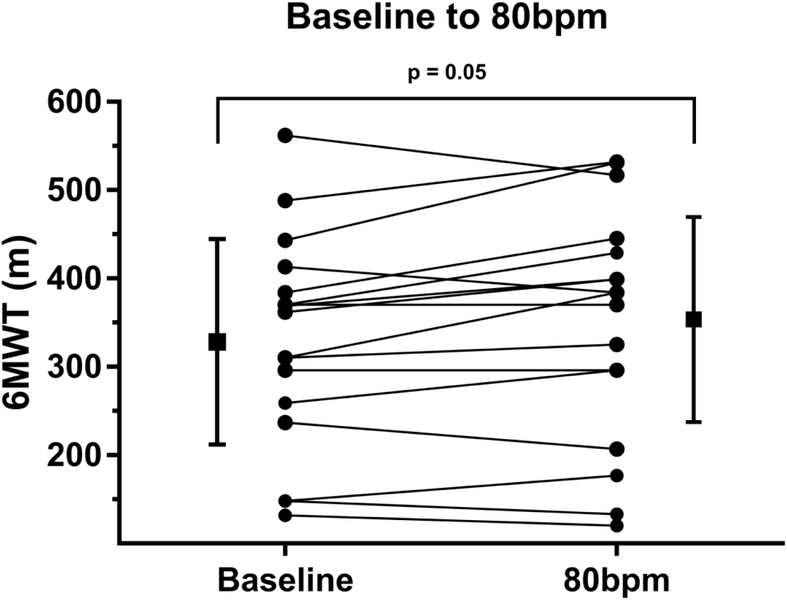
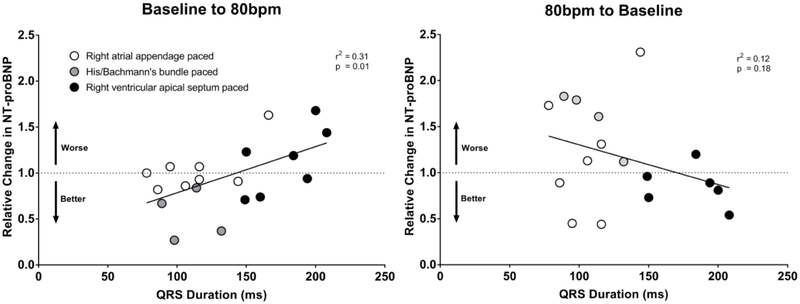
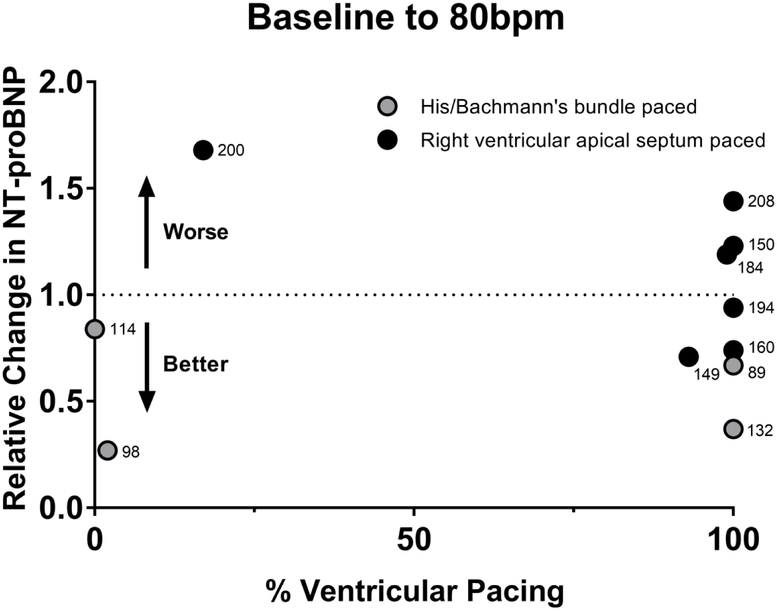
As shown in Figure 2 the MLHFQ composite score improved after being paced at 80bpm (baseline 34±19, 4wk 29±22, p=0.03). After returning the patients to the previous lower HR setting the MLHFQ scores worsened (6wk 36±23). The 6MWT improved after the patients had been paced at 80bpm for 4 weeks as compared to baseline (baseline 329±116m, 4wk 350±127m, p=0.05) as shown in Figure 3. Although the mean NT-proBNP was nominally lower after being paced at 80bpm and returned to previous higher levels after lowering the HR these differences were not significant as shown in Table 2. When the relative changes of NT-proBNP were evaluated for potentially interacting baseline variables such as age, gender, patient size, baseline QRS duration, lead position and ventricular pacing burden revealed a strong association between the relative NT-proBNP changes and QRS duration (r2=0.31, p<0.01), as shown in Figure 4. Stratification by QRS duration of <150ms was associated with a 21±26% reduction in NT-proBNP after being paced at 80bpm, as compared to patients with a QRS ≥150ms who had a 26±35% increase in NT-proBNP (p<0.01). Reversal to the lower HR had the opposite effect (p=0.03). Lead position had an important impact on the changes in NT-proBNP. Patients who were physiologically paced from the conduction system (Bachmann’s and/or His bundle) demonstrated a - 46±26% reduction in NT-proBNP at 80bpm as compared to a 4±26% and 13±26% increase with pacing from the right atrial appendage or the right ventricular apical septum (pinteraction=0.04). The impact of ventricular pacing burden is shown in Figure 5. Patients with a high ventricular pacing burden had beneficial effects on NT-proBNP levels if they were paced from the His bundle or had a shorter QRS duration. Non-obese patients (BMI<30) had significantly lower NT-proBNP levels with pacing at 80bpm when compared to obese patients (BMI≥30).
Figure 2. Change in Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores after lower pacing rate was increased at 80bpm.
Panel A depicts improvement in MLHFQ scores at 80bpm as compared to baseline, followed by the subsequent worsening of MLHFQ scores when the lower pacing rate was returned to baseline (B).
Figure 3.
Change in six-minute walk test (6MWT) distance after lower pacing rate was increased at 80bpm as compared to baseline.
Table 2.
Comparison of NT-proBNP levels (pg/mL)
| QRS Duration | Pacing Site | BMI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 19) | p value | < 150ms (n = 12) | ≥ 150ms (n = 7) | p value | RA* (n = 8) | Physiologic† (n = 4) | RV‡ (n = 7) | p value | < 30 (n = 10) | ≥ 30 (n = 9) | P value | |
| Visit A (Baseline) | 915±920 | 1029±1114 | 719±437 | 0.5 | 484±449 | 1363±1033 | 1152±1150 | 0.21 | 1159±1164 | 644±469 | 0.23 | |
| Visit B (80bpm) | 815±731 | 729±791 | 963±643 | 0.51 | 495±439 | 781±775 | 1201±875 | 0.18 | 843±851 | 785±620 | 0.87 | |
| A-B Absolute Change in NT-proBNP | −100±452 | 0.35 | −300±410 | 243±294 | <0.01 | 12±143 | −582±410 | 49±554 | 0.04 | −316±466 | 141±301 | 0.02 |
| Visit C# (Baseline) | 911±922 | 868±1037 | 1015±648 | 0.78 | 366±181 | 1352±1465 | 1254±824 | 0.12 | 1082±1116 | 668±528 | 0.38 | |
| B-C Absolute Change in NT-proBN P | 53±493 | 0.66 | 139±546 | −151±284 | 0.28 | −74±345 | 572±692 | −143±254 | 0.04 | 239±495 | −211±377 | 0.04 |
| A-C Absolute Change in NT-proBN P | −78±475 | 0.51 | −161±512 | 123±331 | 0.27 | −110±379 | −11±576 | −84±587 | 0.95 | −78±531 | −77±422 | 0.99 |
Values are mean ± SD
RA = Right atrial appendage pacing
physiologic = His/Bachmann’s bundle pacing
RV = Right ventricular apical septum pacing
As one patient did not complete visit C, all analyses involving data from visit C included n = 18 patients
Figure 4.
Relationship of relative change in NT-proBNP and baseline QRS duration after lower pacing rate was increased to 80bpm.
Figure 5. Relative change in NT-proBNP after lower pacing rate was increased to 80bpm as compared to baseline among ventricular-paced patients.
The annotated numbers represent the baseline QRS intervals in milliseconds.
Pacing at 80bpm was not associated with any obvious adverse effects or arrhythmias and upon study completion five patients (25%) requested to have their lower rate setting permanently increased citing the following reasons: “felt more lively”; “more active with less napping”; “more perky”; “more pep” and “less winded”. Among these patients, the mean age was 76±9 years, BMI 29±4 and 60% were female. The mean QRS duration was 124±43ms and 80% were paced from the right atrial appendage or from the conduction system.
DISCUSSION
In this prospective exploratory study, we found that patients with preclinical and overt HFpEF and pacemakers experienced a reduction in heart failure symptoms and demonstrated an improved functional capacity after increasing the lower HR setting to 80bpm. In patients with shorter QRS durations this was associated with lower NT-proBNP levels. The benefits from a higher HR reversed after lowering the HR to previous baseline levels. These findings suggest that higher HRs may provide a meaningful benefit to patients with diastolic dysfunction and HFpEF.
Proposing to use higher HRs as a treatment for heart failure may, at first glance, appear ill-advised, as it breaks with longstanding clinical paradigms. Typical concerns are that high HRs have been associated with adverse outcomes.17 Moreover, pharmacological HR lowering with beta-blockers and ivabradine provide unequivocal benefits to patients with HFrEF.1,3,17 Under the assumption that they may provide some benefit beta-blockers are also commonly used in HFpEF.18,19 However, contemporary trials that examined clinical outcomes on HR lowering medications in related patient populations with a normal EF have unexpectedly revealed adverse outcomes.4-8 This resulted in a downgrade of beta-blockers as a preferred treatment in hypertension. Additionally, there is also an emerging recognition that pharmacological HR lowering is detrimental in patients with coronary artery disease with a normal EF as this was found to be associated with an excess in heart failure and atrial fibrillation. 7,8,20,21
Contrary to a commonly held belief that higher HRs result in higher filling pressures, it has been repeatedly demonstrated that atrial pacing acutely reduces filling pressures in resting patients with a normal EF, including those with heart failure.11,12 Higher HRs also accelerate relaxation, through an increased activity of the calcium pump of the sarcoplasmatic reticulum.22 This lusiotropic effect of HR is preserved in isolated contracting myocardium from patients with HFpEF despite an intrinsic prolongation of relaxation.23 Lower HRs on the other hand result in a slowed relaxation and prolonged LV filling that results in higher filling pressures, wall stress and NT-proBNP levels as evident in historic beta-blocker studies.10,24
Another conceptual benefit of an HR elevation is that it provides a trigger for ventricular remodeling which can only be accomplished through reorganization of the extracellular matrix. Experimental models of prolonged tachycardia demonstrated that this process effectively reduces myocardial fibrosis, a finding that was recently confirmed in a human biopsy study.13,15,25 These mechanisms may combine to improve left ventricular compliance.15 Prior to the current study, we performed a safety and feasibility evaluation in patients with diastolic dysfunction and established that an automated nocturnal HR elevation to 100bpm was well tolerated and safe.26
Without evidence-based guidance, the lower HR setting of pacemakers is typically left at 60bpm. If the discussed mechanisms are indeed at play, raising the lower HR setting of a pacemaker could provide a long-term hemodynamic benefit that would be expected to result in symptom alleviation with a potential for improvements in functional capacity. Particular attention was given to the analysis of NT-proBNP levels as these have been demonstrated to predict long term outcomes in HFpEF.27 The pacing-induced changes in NT-proBNP levels were most apparent when stratified by baseline QRS intervals. In patients with shorter QRS durations the higher HR led to a reduction in NT-proBNP. This effect was most apparent in patients with pacing leads in either the Bachmann’s bundle and/or the His bundle. By contrast, NT-proBNP levels increased in patients with a wider QRS and a high ventricular pacing burden from the right ventricular apical septum, presumably due to pacing-induced ventricular dyssynchrony. This may indicate that patients with a wider QRS are at risk for adverse long-term effects if paced at higher HRs. Our findings are in line with a recent study demonstrating that His-bundle pacing may reduce heart failure hospitalizations, mortality and other outcomes as compared to standard right ventricular pacing.28 Our data also suggests that the atrial pacing site may be important as well. Bachmann’s bundle pacing generates a P wave that has an axis and duration nearly identical to sinus P waves and was found to decrease the incidence of atrial fibrillation in patients with sinus node dysfunction when compared with standard right atrial appendage pacing.29 Conceivably, this could be due to a more physiological intra and interatrial conduction that minimizes atrial dyssynchrony.
We did not expect that several patients requested to return to a higher HR setting after completion of the study. After consultation with the patient’s primary cardiologist we either followed through with the patient request or recommended that the patients first try to discontinue HR lowering medications.
Efforts were made to reduce bias with regards to the collection of the qualitative and subjective data. For example, study participants and investigators were blinded to prior MLHFQ results. Nonetheless, effective blinding of study participants and investigators is difficult, if not impossible to accomplish when the HR is changed. However, the changes in NT-proBNP levels are objective and the reported variations of natriuretic peptide levels in stable heart failure patients are relatively low.30
In summary, a 4 week increase in the lower pacing rate to 80bpm in patients with diastolic dysfunction appears to improve symptoms, quality of life and functional capacity, without any obvious adverse effects. Improvements in NT-proBNP levels were demonstrated in patients with a QRS <150ms and physiologic pacing from the conduction system. Prospective studies in larger populations will be necessary to confirm our findings and investigate if the short-term benefits of a higher HR translate into long-term improvements in outcomes in patients with diastolic dysfunction and HFpEF.
ACKNOWLEDGEMENTS
We would like to thank Dr. Trina Brand and Paul Ziegler from Medtronic for their expert advice and Marilynn Roth for her help with the patient visits. Biostatistical support was provided by Dr. Peter Callas from the UVM biostatistics unit. We thank Dr. Margaret Infeld and Dr. Daniel Silverman for their constructive review of the manuscript.
Sources of Funding
This study was funded by a Medtronic grant. In addition, M. Meyer is supported by a National Institutes of Health Grant (R01 HL-122744).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The University of Vermont has licensed patents and applications on the use of pacemakers to prevent and treat HFpEF.
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics - 2017 Update: A Report From the American Heart Association. Circulation. 2017; 135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG, Böhm M, Andersson B, Kjekshus J, Packer M, Rigby AS, Rosano G, Wedel H, Hjalmarson A, Wikstrand J, Kotecha D. Beta-blockers in Heart Failure Collaborative Group. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018; 39(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe-Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. LIFE Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002; 359(9311):1004–10. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Parkar S, Grossman E, Messerli FH. A meta-analysis of 94,492 patients with hypertension treated with beta-blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol. 2007; 100(8):1254–62. [DOI] [PubMed] [Google Scholar]
- 6.Bangalore S, Sawhney S, Messerli FH. Relation of beta-blocker-induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008; 52(18):1482–9. [DOI] [PubMed] [Google Scholar]
- 7.Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, DiNicolantonio JJ, Devereaux PJ, Alexander KP, Wetterslev J, Messerli FH. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014; 127(10):939–53. [DOI] [PubMed] [Google Scholar]
- 8.Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R. SIGNIFY Investigators. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014; 371(12):1091–9. [DOI] [PubMed] [Google Scholar]
- 9.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. CAFE Investigators; Anglo-Scandinavian Cardiac Outcomes Trial Investigators; CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006; 113(9):1213–25. [DOI] [PubMed] [Google Scholar]
- 10.Meyer M, Rambod M, LeWinter M. Pharmacological heart rate lowering in patients with a preserved ejection fraction-review of a failing concept. Heart Fail Rev. 2018; 23(4):499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermann D, Kasner M, Steendijk P, Spillmann F, Riad A, Weitmann K, Hoffmann W, Poller W, Pauschinger M, Schultheiss HP, Tschöpe C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008; 117(16):2051–60. [DOI] [PubMed] [Google Scholar]
- 12.Wachter R, Schmidt-Schweda S, Westermann D, Post H, Edelmann F, Kasner M, Lüers C, Steendijk P, Hasenfuss G, Tschöpe C, Pieske B. Blunted frequency-dependent upregulation of cardiac output is related to impaired relaxation in diastolic heart failure. Eur Heart J. 2009; 30(24):3027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997. March 15;29(4):709–15. [DOI] [PubMed] [Google Scholar]
- 14.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008; 358(13):1370–80. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 15.Klein FJ, Bell S, Runte KE, Lobel R, Ashikaga T, Lerman LO, LeWinter MM, Meyer M. Heart rate-induced modifications of concentric left ventricular hypertrophy: exploration of a novel therapeutic concept. Am J Physiol Heart Circ Physiol. 2016; 311(4):H1031–H1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28(1):1–39. [DOI] [PubMed] [Google Scholar]
- 17.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, Steg PG, Tardif JC, Tavazzi L, Tendera M. Heart Rate Working Group. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007; 50(9):823–30. [DOI] [PubMed] [Google Scholar]
- 18.Lam PH, Dooley DJ, Deedwania P, Singh SN, Bhatt DL, Morgan CJ, Butler J, Mohammed SF, Wu WC, Panjrath G, Zile MR, White M, Arundel C, Love TE, Blackman MR, Allman RM, Aronow WS, Anker SD, Fonarow GC, Ahmed A. Heart Rate and Outcomes in Hospitalized Patients With Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2017; 70(15):1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverría Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A, Goncalvesova E, Janssens S, Katova T, Køber L, Lelonek M, Linssen G, Lund LH, O'Meara E, Merkely B, Milicic D, Oh BH, Perrone SV, Ranjith N, Saito Y, Saraiva JF, Shah S, Seferovic PM, Senni M, Sibulo AS Jr, Sim D, Sweitzer NK, Taurio J, Vinereanu D, Vrtovec B, Widimský J Jr, Yilmaz MB, Zhou J, Zweiker R, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, Van Veldhuisen DJ, Zannad F, Zile MR, McMurray JJV. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 20.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, Mackenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311(5):507–20. [DOI] [PubMed] [Google Scholar]
- 21.Nambiar L, Meyer M. β-Blockers in myocardial infarction and coronary artery disease with a preserved ejection fraction: recommendations, mechanisms, and concerns. Coron Artery Dis. 2018; 29(3):262–270. [DOI] [PubMed] [Google Scholar]
- 22.Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000; 278(1):H249–55. [DOI] [PubMed] [Google Scholar]
- 23.Runte KE, Bell SP, Selby DE, Häußler TN, Ashikaga T, LeWinter MM, Palmer BM, Meyer M. Relaxation and the Role of Calcium in Isolated Contracting Myocardium From Patients With Hypertensive Heart Disease and Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2017; 10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luchner A, Burnett JC Jr, Jougasaki M, Hense HW, Riegger GA, Schunkert H. Augmentation of the cardiac natriuretic peptides by beta-receptor antagonism: evidence from a population-based study. J Am Coll Cardiol. 1998; 32(7):1839–44. [DOI] [PubMed] [Google Scholar]
- 25.Mueller KAL, Heinzmann D, Klingel K, Fallier-Becker P, Kandolf R, Kilias A, Walker-Allgaier B, Borst O, Kumbrink J, Kirchner T, Langer H, Geisler T, Schreieck J, Gramlich M, Gawaz M, Seizer P. Histopathological and Immunological Characteristics of Tachycardia-Induced Cardiomyopathy. J Am Coll Cardiol. 2017; 69(17):2160–2172. [DOI] [PubMed] [Google Scholar]
- 26.Yeshwant SC, Zile MR, Lewis MR, Lewinter M, Meyer M. Safety and Feasibility of a Nocturnal Heart Rate Elevation-Exploration of a Novel Treatment Concept. J Card Fail. 2018; S1071-9164(18)30275-6. [DOI] [PubMed] [Google Scholar]
- 27.Savarese G, Hage C, Orsini N, Dahlström U, Perrone-Filardi P, Rosano GM, Lund LH. Reductions in N-Terminal Pro-Brain Natriuretic Peptide Levels Are Associated With Lower Mortality and Heart Failure Hospitalization Rates in Patients With Heart Failure With Mid-Range and Preserved Ejection Fraction. Circ Heart Fail. 2016; 9(11). [DOI] [PubMed] [Google Scholar]
- 28.Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, Oren JW, Dandamudi G, Vijayaraman P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J Am Coll Cardiol. 2018; 71(20):2319–2330. [DOI] [PubMed] [Google Scholar]
- 29.Bailin SJ, Adler S, Giudici M. Prevention of chronic atrial fibrillation by pacing in the region of bachmann's bundle: Results of a multicenter randomized trial. J Cardiovascular Electrophysiology. 2001; 12(8):912–917. [DOI] [PubMed] [Google Scholar]
- 30.Schou M, Gustafsson F, Nielsen PH, Madsen LH, Kjaer A, Hildebrandt PR. Unexplained week-to-week variation in BNP and NT-proBNP is low in chronic heart failure patients during steady state. Eur J Heart Fail. 2007; 9(1):68–74. [DOI] [PubMed] [Google Scholar]