Abstract
Purpose:
To establish the incidence of ocular myasthenia gravis (OMG), as well as identify determinants of transformation to generalized MG (GMG), using a population-based record-linkage system.
Design:
Population-based, retrospective cohort study
Methods:
All adults (≥ 18 years) diagnosed with MG from January 1, 1990 through December 31, 2017 were identified using the Rochester Epidemiology Project. Sixty-five patients with MG were identified. Data was collected regarding symptom onset, diagnostic testing results, and conversion from OMG to GMG.
Results:
Median follow-up time was 91 months (range 17 to 333 months). The annual incidence of MG was 2.20/100,000 with a mean age at diagnosis of 59 years (SD=17) and 62% male sex. Thirty-three (51%) patients presented with OMG, providing an annual incidence of 1.13/100,000. Eighteen (55%) patients presenting with OMG converted to GMG at a median time of 13 months (range 2 to 180 months). 67% of OMG patients who were seropositive for acetylcholine receptor antibody (AchR Ab) converted to GMG at 5 years compared to 11 % of those who were seronegative (HR, 8.2, p=0.04). 77% of OMG patients with a positive single-fiber electromyography (sfEMG) at diagnosis converted with GMG at 5 years compared to 18% of patients that had a negative sfEMG (HR, 5.5, p=0.01).
Conclusions:
In our population-based study, 51% of patients with MG presented with isolated ocular involvement, with 55% of these patients converting to GMG at some point in the course of their disease. Positive sfEMG and AchR Ab seropositivity at the time of diagnosis increased the risk of conversion to GMG.
Keywords: myasthenia gravis, ocular, ocular myasthenia, incidence, epidemiology, Rochester Epidemiology Project, REP
Myasthenia Gravis (MG) is an uncommon disease in the adult population, with incidence reports ranging from 1.7 to 30 per million person-years.1–3 In up to 85% of patients, the initial presenting symptom is related to the extraocular muscles, eyelids, or both termed ocular MG (OMG).4 The reported transformation rate of OMG to generalized MG (GMG) has varies from 23.3% to 80%, depending on the setting in which the study has been performed.4–7 All of these studies were done at academic centers and therefore may have suffered from a tertiary referral bias that influenced the true incidence and risk of disease progression. A population-based study focusing on the incidence of OMG has yet to be reported. The goal of this study was to establish this incidence, as well as identify risk factors for transformation to GMG, using the Rochester Epidemiology Project (REP).
METHODS
Patient Data
This retrospective cohort study was conducted using the REP database, a multicenter medical records linkage system designed to capture data from all patient-physician encounters in Olmsted County which allows population-based evaluation of diseases.8 The medical records of all residents of Olmsted County, Minnesota 18 years or older with newly diagnosed MG from January 1, 1990 - December 31, 2017 were identified using the REP by searching for MG, myasthenic syndrome, or OMG diagnoses. This study was approved by the Mayo Clinic and Olmsted County institutional review boards. The medical records were individually reviewed to confirm the diagnosis of MG, determine residency of Olmsted County at time of diagnosis, and determine if patients presented initially with OMG or GMG. Patients who were referred to the institution but not residents of Olmsted County were excluded from consideration.
A diagnosis of OMG was defined by the presence of ptosis and/or diplopia with at least 1 of the following: (1) positive acetylcholine receptor antibody (AChR Ab) titer (AChR binding, blocking, or modulating), (2) significant jitter in single-fiber electromyography (sfEMG), or (3) unequivocal clinical response to edrophonium chloride (Tensilon test) or ice test. GMG was defined by any symptoms beyond the extraocular muscles or eyelid, including dysphagia, dysarthria, dyspnea, dysphonia, neck or extremity weakness with positive serological or physiological testing. Results of diagnostic tests including Tensilon, sfEMG, AChR Ab titer, ice test, and Cogan lid twitch, as well as other factors such as the presence/absence of thymoma, thyroid status, thyroid eye disease, and treatments utilized were documented. During the time of this study, Tensilon was available and therefore neostigmine (Prostigmin) was not used as a diagnostic test. Enhancement of ptosis with manual elevation of the contralateral eyelid and orbicularis strength were not routinely documented and therefore were not included in the study. Conversion from OMG to GMG was documented, including date and time from initial diagnosis.
Statistical analysis
Descriptive statistics (mean, percentages, etc.) were used to summarize the data. Categorical variables were compared between groups using the Chi-square test for independence. Baseline characteristics were compared between patients with OMG that remained isolated to the eyes and those that became generalized over time in order to investigate any factors associated with secondary generalization of OMG.
Age- and sex-specific incidence rates were calculated using the number of incident cases of OMG and all MG as the numerators and population estimates for Olmsted County residents age ≥ 50 years based on decennial census counts as the denominator; linear interpolation was used to estimate population size for intercensal years. Overall rates were age- and sex-adjusted to the 2010 United States white population. Ninety-five percent confidence intervals (CI) were computed for incidence rates assuming that the incident cases follow a Poisson distribution. Potential differences in the incidence between males and females were investigated with Poisson regression models. The conversation rate of the presenting ocular patients was estimated using the Kaplan-Meier method. Potential risk factors for conversion were evaluated using Cox proportional hazards models. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
The median follow-up time was 91 months (range 17 to 333 months). Sixty-five patients (40 males and 25 females) were diagnosed with MG, which provided an overall age and sex adjusted incidence of 2.20 per 100,000 per year (95% CI 1.66-2.75).The mean age of diagnosis was 59 years (standard deviation (SD)=17).
Overall, 51 (78%) patients had ocular symptoms at initial presentation and 60 (92%) had ocular symptoms at some point in their disease. Thirty-three (51%) patients presented with OMG according to our criteria. Age and sex adjusted incidence of OMG was 1.13 per 100,000 per year (95% CI 0.74-1.52). Among these 33 patients, the mean age at diagnosis was 58.8 years (SD=16), which was similar to those presenting with GMG (59.3 years, SD=18) (Table 1). In patients presenting with OMG, 24 (73%) were males, whereas an equal number of males and females presented with GMG (Table 1).
Table 1.
Demographic information and clinical characteristics of patients with ocular and generalized myasthenia gravis from 1990-2017 in Olmstead County, MN
| Ocular | General | p-value | |
|---|---|---|---|
| Total, n/N(%) | 33/65 (51) | 32/65 (49) | |
| Age at Diagnosis, mean (SD) | 59 (16) | 59 (19) | 0.92 |
| Male, n(%) | 24 (73) | 16 (50) | 0.06 |
| Female, n(%) | 9 (27) | 16 (50) | |
| Race, n(%) | (N=33) | (N=32) | |
| White | 29 (88) | 25 (78) | 0.15 |
| Asian | 0 (0) | 1 (3) | |
| Black | 2 (6) | 0 (0) | |
| Unknown | 2 (6) | 6 | |
| Eye Findings On Initial Exam, n(%) | (N=33) | (N=32) | <0.001 |
| Only Diplopia | 9 (27) | 4 (13) | |
| Only Ptosis | 7 (21) | 3 (9) | |
| Both | 17 (52) | 11 (34) | |
| Neither | n/a | 14 (44) | |
| Eye Findings Ever in Course, n(%) | (N=33) | (N=32) | 0.03 |
| Only Diplopia | 3 (9) | 3 (9) | |
| Only Ptosis | 4 (12) | 8 (25) | |
| Both | 26 (79) | 16 (50) | |
| Neither | n/a | 5 (16) | |
| Seropositivity, n(%) | (N=33) | (N=32) | |
| Yes (AchR+Striated) | 27 (82) | 28 (88) | 0.53 |
| AchR only (AchR Seropositive) | 24 (73) | 28 (88) | 0.14 |
| Antibody Type, n(%) | (N=27) | (N=28) | |
| AchR Binding | 23 (85) | 27 (96) | 0.15 |
| AchR Modulating | 18 (67) | 24 (86) | 0.10 |
| AchR Blocking | 1 (4) | 8 (29) | 0.01 |
| Striated Muscle | 18 (67) | 15 (54) | 0.32 |
| Evidence on Single Fiber EMG, n(%) | (N=24) | (N=23) | |
| Positive | 13 (54) | 21 (91) | 0.004 |
| Negative | 11 (46) | 2 (9) | |
| Presence of Thymoma, n(%) | (N=33) | (N=32) | |
| Yes | 3 (9) | 4 (13) | 0.66 |
| No | 30 (91) | 28 (88) | |
| Thyroid Status, n(%) | (N=33) | (N=32) | |
| euthyroid | 25 (76) | 23 (72) | 0.26 |
| hypothyroid | 6 (18) | 9 (28) | |
| hyperthyroid | 2 (6) | 0 (0) | |
| hyperthyroid w/ thyroid eye disease | 1 (3) | 0 (0) | 0.32 |
AchR = acetylcholine receptor; Single Fiber EMG = single fiber electromyography
Seventeen of 33 (52%) OMG patients presented with both ptosis and diplopia, whereas 16 (48%) had one symptom (27% diplopia and 21% ptosis). Among the 16 patients who presented with one symptom, 9 developed the second symptom at some point in their course and 7 (21%) remained isolated to their initial symptom (9% diplopia and 12% ptosis).
Overall, 35 of 48 (73%) of MG patients had abnormal (increased mean consecutive difference, i.e. jitter) sfEMG at presentation. Twenty-two of 24 (92%) patients presenting with GMG who were tested with sfEMG had an abnormal response compared with 13 of 24 (54%) patients presenting with OMG, p=0.003.
Overall, 52 of 65 (80%) of MG patients were AchR Ab seropositive (presence of AchR binding, blocking, or modulating Ab) at presentation. Twenty-eight of 32 (88%) of patients presenting with GMG were AchR Ab seropositive, compared with 24 of 33 (73%) OMG patients, p=0.14 (Table 1).
Eighteen of 33 (54.5%) patients presenting with OMG converted to GMG at a median time of 13 months. Fifty percent of those who generalized did so within 1 year, 72% within 2 years, and 94% within 5 years. AchR Ab seropositivity increased the risk of generalizing, with 67% of seropositive patients converting to GMG at 5 years compared to 11% of those who were seronegative (HR 8.2 (95% CI 1.1-61.6), p=0.04) (Figure 2; Table 2 and 3). SfEMG positivity was also associated with increased risk of conversion with 77% of those with a positive sfEMG converting to GMG at 5 years compared with 18% of patients who had a negative sfEMG (HR, 5.5 (95% CI 1.5-20.7), p=0.01) (Figure 3; Table 2 and 3).
Figure 2:
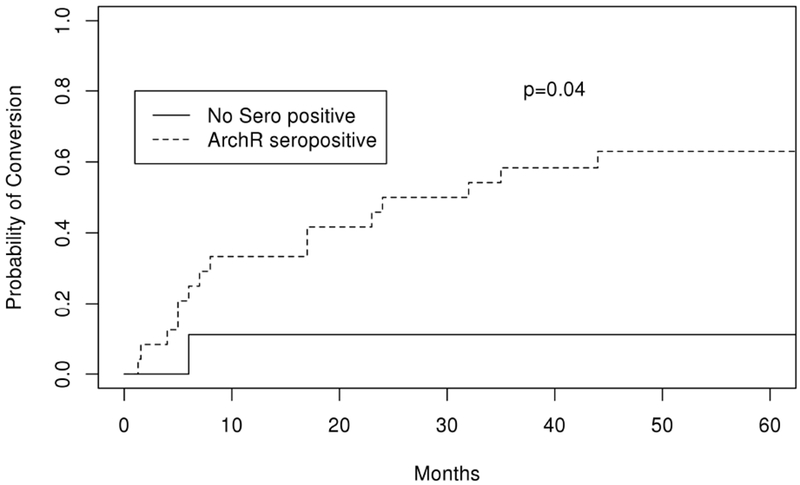
Kaplan Meier curve depicting probability of conversion over time (months) from ocular to generalized myasthenia gravis in patients who were acetylcholine receptor antibody seropositive compared to those who were not
Table 2.
Demographic information and clinical characteristics of patients with ocular myasthenia gravis converting to generalized myasthenia gravis from 1990-2017 in Olmstead County, MN.
| All | Remained Ocular | Become Generalized | |
|---|---|---|---|
| Total, n/N(%) | 33 (100) | 15 (45) | 18 (55) |
| Age at Diagnosis, age (SD) | 59 (16) | 53 (14.2) | 64 (16.8) |
| Male, n(%) | 24 (73) | 10 (67) | 14 (78) |
| Female, n(%) | 9 (27) | 5 (33) | 4 (22) |
| Eye Findings On Initial Exam, n(%) | (N=33) | (N=15) | (N=18) |
| Only Diplopia | 9 (27) | 3 (20) | 6 (33) |
| Only Ptosis | 7 (21) | 4 (27) | 3 (17) |
| Both | 17 (52) | 8 (53) | 9 (50) |
| Eye Findings Ever in Course, n(%) | (N=33) | (N=15) | (N=18) |
| Only Diplopia | 3 (9) | 1 (7) | 2 (11) |
| Only Ptosis | 4 (12) | 2 (13) | 2 (11) |
| Both | 26 (79) | 12 (80) | 14 (78) |
| Motility Deficits, n(%) | (N=33) | (N=15) | (N=18) |
| Abduction | 10 (30) | 3 (20) | 7 (39) |
| Adduction | 9 (27) | 3 (20) | 6 (33) |
| Depression | 8 (24) | 4 (27) | 4 (22) |
| Elevation | 5 (15) | 2 (13) | 3 (17) |
| No Motility Deficit (full) | 14 (42) | 7 (47) | 7 (39) |
| Alignment in primary gaze, n(%) | (N=33) | (N=15) | (N=18) |
| Hypertropia | 23 (70) | 9 (60) | 14 (78) |
| Exotropia | 8 (24) | 3 (20) | 5 (28) |
| Esotropia | 7 (21) | 2 (13) | 5 (28) |
| Ortho | 7 (21) | 4 (27) | 3 (17) |
| Seropositivity, n(%) | (N=33) | (N=15) | (N=18) |
| Yes (AchR+Striated) | 27 (82) | 10 (67) | 17 (94) |
| AchR only (AchR Seropositive) | 24 (73) | 7 (47) | 17 (94) |
| No (Seronegative) | 6 (18) | 5 (33) | 1 (6) |
| Antibody Type, n(%) | (N=27) | (N=15) | (N=18) |
| AchR Binding | 23 (85) | 7 (47) | 16 (89) |
| AchR Modulating | 18 (67) | 3 (20) | 15 (83) |
| AchR Blocking | 1 (4) | 0 (0) | 1 (6) |
| Striated Muscle | 18 (67) | 5 (33) | 13 (72) |
| Evidence on Single Fiber EMG, n(%) | (N=24) | (N=11) | (N=13) |
| Positive | 13 (54) | 3 (27) | 10 (77) |
| Negative | 11 (46) | 8 (73) | 3 (23) |
| Ice Test, n(%) | (N=8) | (N=4) | (N=4) |
| Positive | 7 (88) | 3 (75) | 4 (100) |
| Negative | 1 (13) | 1 (25) | 0 (0) |
| Tensilon Test, n(%) | (N=23) | (N=12) | (N=11) |
| Positive | 19 (83) | 9 (75) | 10 (91) |
| Negative | 4 (17) | 3 (25) | 1 (9) |
| Fatiguability of Ptosis, n(%) | (N=33) | (N=15) | (N=18) |
| Yes | 29 (88) | 12 (80) | 17 (94) |
| No | 4 (12) | 3 (20) | 1 (6) |
| Cogan Lid Twitch, n(%) | (N=5) | (N=0) | (N=5) |
| Positive | 3 (60) | 0 (0) | 3 (60) |
| Negative | 2 (40) | 0 (0) | 2 (40) |
| Presence of Thymoma, n(%) | (N=33) | (N=15) | (N=18) |
| Yes | 3 (9) | 0 (0) | 3 (17) |
| No | 30 (91) | 15 (100) | 15 (83) |
| Thyroid Status, n(%) | (N=33) | (N=15) | (N=18) |
| euthyroid | 25 (76) | 11 (73) | 14 (78) |
| hypothyroid | 6 (18) | 2 (13) | 4 (22) |
| hyperthyroid | 2 (6) | 2 (13) | 0 (0) |
| hyperthyroid w/ thyroid eye disease | 1 (3) | 1 (7) | 0 (0) |
AchR = acetylcholine receptor; Single Fiber EMG = single fiber electromyography
Table 3.
Risk factors and conversion rate of Ocular to Generalized Myasthenia Gravis
| Risk Factor | Hazard Ratio (95% Confidence Interval) | p-value |
|---|---|---|
| Immunosuppression | 0.43 (0.10-1.88) | 0.26 |
| AchR Seropositivity | 8.18(1.09-61.61) | 0.04 |
| AchR Binding Antibody | 4.47 (1.03-19.50) | 0.05 |
| AchR Blocking Antibody | 2.67 (0.34-20.90) | 0.35 |
| AchR Modulating Antibody | 5.96 (1.72-20.74) | 0.005 |
| Striational Ab Seropositivity | 2.83 (1.00-7.97) | 0.05 |
| Presence of Thymoma | 2.48 (0.70-8.71) | 0.16 |
| Pathologic Thyroid status | 0.85 (0.28-2.62) | 0.78 |
| sfEMG Pathologic Response | 5.57 (1.50-20.70) | 0.01 |
| Positive clinical results | ||
| Tensilon Test | 3.29 (0.41-26.42) | 0.26 |
| Cogan Lid Twitch | 1.80 (0.18-17.92) | 0.62 |
AchR = acetylcholine receptor; sfEMG = single fiber electromyography; Ab = antibody
Figure 3:
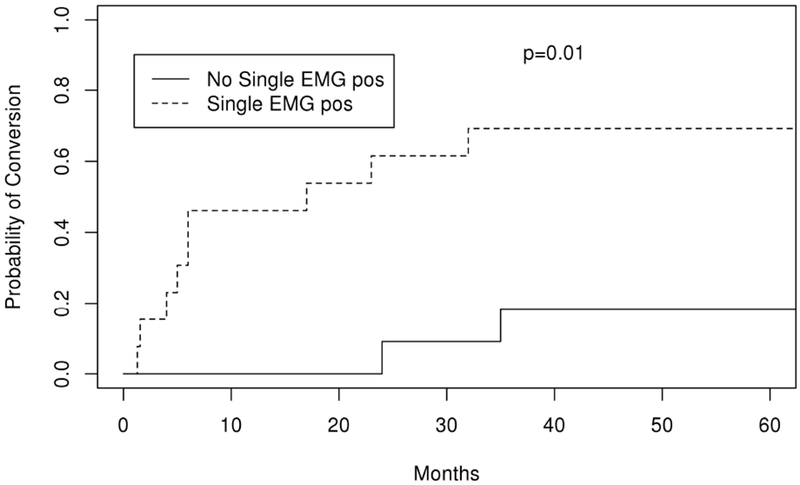
Kaplan Meier curve depicting probability of conversion over time (months) from ocular to generalized myasthenia gravis in patients who had an abnormal single fiber electromyography test compared to those who were normal
No other risk factor analyzed, including presence of thymoma (HR 2.48 (95% CI 0.7 – 8.71), p=0.16), tensilon test positivity (HR 3.29 (95% CI 0.41 – 26.42), p=0.26), or immunosuppressive treatment significantly influenced conversion to GMG (HR 0.43 (95% CI 0.1 – 1.88), p=0.24) (Table 2 and 3). Of patients who were negative for both AchR seropositivity and sfEMG abnormality, none converted to GMG; however, there were only four patients in this category.
DISCUSSION
Our study, which represents the first population-based epidemiologic analysis of OMG, found an overall incidence of MG of 2.2 per 100,000 per year and an incidence of OMG of 1.13 per 100,000 per year. Previous MG epidemiologic studies have reported a large range of incidence from 0.17 to 7 per 100,000 per year.1,9 Prior to this study, there had not been a population-based study focusing on OMG; however population-based studies of MG have reported the percentage of populations presenting initially with solely ocular findings. In Cambridgeshire, England, of the 100 identified cases of MG, close to half (52%) of patients had ocular limited disease at presentation.10 This percentage is similar to that in our cohort, in which 51% of MG patients presented with ocular MG.
With regard to demographics, our population had a male prevalence of 61.5%, which was driven by a higher male prevalence (73%) among patients with OMG, wheras there was an equal number of males and females presenting with GMG. A slight male predominance in OMG has also been reported by others, but not all studies.4,5,11–13 Age at diagnosis did not differ between OMG and GMG (both 59 years of age), which was similar to prior non-population based studies.12–14
Overall, 78% of MG patients in our population had ocular symptoms at initial presentation, with 92% developing ocular manifestations at some point in their disease. Many others have reported similar findings, including Bever6 et al reporting ocular symptoms in 84% at onset and Grob4 et al reporting 85%.11 In our cohort, 27% experienced only diplopia, 21% only ptosis, and 52% had both symptoms at initial presentation. Nagia et al reported similar findings, with 34% experiencing only diplopia, 10% only ptosis, and 56% both symptoms.12
Fifty-five percent of our cohort presenting with OMG converted to GMG. Prior studies have reported a wide range of conversion rates generally falling into a low and high range. Initial studies reported overall conversion rates of 50% to 64%.4,6,11,15 More recent studies have described lower rates ranging from 21% to 31%, though many of these studies included a focus on the effect of immunosuppressive treatment.5,12,14,16,17 One potential cause of a lower reported conversion rate in certain studies may be a shorter follow-up time. For example, Hong et al reported a rate of 23.3% when following patients over a mean time of 11.8 months. At this same time point, the rate of conversion in our cohort was similar at 27% (Figure 1), but increased to 52% at 5 years. At final follow-up, a total of 23% of our population remained OMG. This coincides with other population-based prevalence studies that report OMG accounting for upwards of 20% of MG patients.9,18–21
Figure 1:
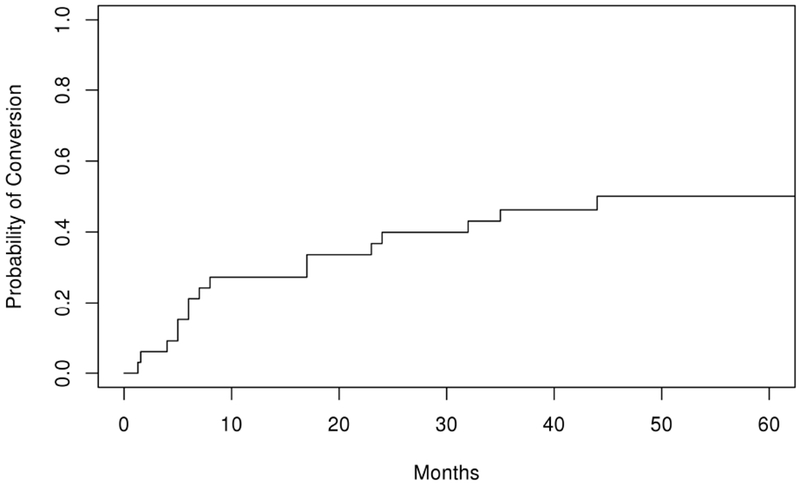
Kaplan Meier curve depicting probability of conversion from ocular to generalized myasthenia gravis over time (months)
Historically, it has been thought that nearly 80% of patients that generalize do so within the first year, and up to 90% within 3 years.4,6,11 Among the 55% OMG patients who generalized in our cohort, 50% generalized within 1 year, 72% within 2 years, and 94% within 5 years, which is in line with recent studies suggesting that generalization can occur later in the course of the disease. Nagia et al reported a median time of conversion of 20 months and found that 69.7% of the OMG patients who generalized did so within 2 years, but the remaining 30.3% converted after 2 years.12 Sommer et al and Antonini et al found that 50% converted to GMG within 2 years and 60–75% within 4 years.14,22
We found an abnormal sfEMG and AchR Ab positivity at presentation increased the risk of transforming to GMG. Previous studies have also found that an increased risk of conversion with AchR Ab positivity and a decreased risk of conversion with normal sfEMG testing.5,13,23 Because sfEMG and AchR antibody represent tests with the highest sensitivity and specificity, respectively, for diagnosing OMG,24 it is of interest that these tests also correlated with conversion to GMG. Our population showed no trend toward increased conversion with thymoma or thyroid derangements, despite these factors being shown to be significant or near significant by others.5,12 Other investigators also have reported that immunosuppression may decrease conversion from OMG to GMG.13,14,25 Although our study showed there was a trend toward decreased conversion with greater than 6 months of immunosuppression, the results were not significant possibly due to the small sample size.
There were several limitations to our study, including sample size, and a racially homogenous (white) cohort from a single geographic area. Due to its retrospective nature, standardized evaluation and testing was not performed on every patient. For example, the presence or absence of a Cogan lid twitch was documented in a small percentage of patients and therefore its sensitivity for OMG could not be evaluated. In addition, the small sample size could result in missing potential factors that could influence generalization, such as immunosuppression.
In conclusion, this study represents the first population-based evaluation of OMG, which found an overall incidence of 1.13 cases per 100,000 per year. Fifty-one percent of patients with MG presented with isolated ocular involvement, with 55% of these patients converting to GMG. Twenty-eight percent of patients transformed to GMG after 2 years into their disease course, challenging the traditional thinking that converting to GMG rarely occurs after 2 years. Abnormal sfEMG and AchR Ab seropositivity at the time of diagnosis increased the risk of conversion of OMG to GMG.
The first population-based evaluation of ocular myasthenia gravis (OMG)
Overall incidence of OMG is 1.13 cases per 100,000 per year
Conversion rate from ocular to generalized myasthenia gravis of 55%
28% percent transformed to GMG after 2 years, challenging traditional thinking
Abnormal sfEMG and AchR Ab seropositivity increased the risk of conversion
ACKNOWLEDGEMENTS:
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors report no relevant conflict of interest in submitting this article for publication.
The currently submitted manuscript represents original research that is not under consideration for publication elsewhere. The corresponding abstract will be presented at the 2019 North American Neuro-Ophthalmology Society meeting in Las Vegas, NV. We performed this research with approval from the Mayo Clinic and Olmsted County Medical Center Institutional Review Boards (IRB 17-010518 and 059-OMC-17, respectively).
DISCLOSURES:
Dr. Bhatti discloses work at Celgene as a consultant. The remaining authors report no financial disclosures.
REFERENCES
- 1.Carr AS, Cardwell CR, McCarron PO, McConville J. A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC neurology. 2010;10(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGrogan A, Sneddon S, De Vries CS. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology. 2010;34(3):171–183. [DOI] [PubMed] [Google Scholar]
- 3.Casetta I, Groppo E, De Gennaro R, et al. Myasthenia gravis: a changing pattern of incidence. Journal of neurology. 2010;257(12):2015–2019. [DOI] [PubMed] [Google Scholar]
- 4.Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle & nerve. 2008;37(2):141–149. [DOI] [PubMed] [Google Scholar]
- 5.Hong Y-H, Kwon S-B, Kim B-J, et al. Prognosis of ocular myasthenia in Korea: a retrospective multicenter analysis of 202 patients. Journal of the neurological sciences. 2008;273(1):10–14. [DOI] [PubMed] [Google Scholar]
- 6.Bever CT, Aquino AV, Penn AS, Lovelace RE, Rowland LP. Prognosis of ocular myasthenia. Annals of neurology. 1983;14(5):516–519. [DOI] [PubMed] [Google Scholar]
- 7.Schlezinger N, Fairfax W. Evaluation of ocular signs and symptoms in myasthenia gravis. AMA Archives of Ophthalmology. 1959;62(6):985–990. [DOI] [PubMed] [Google Scholar]
- 8.Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic Proceedings. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald B, Cockerell O, Sander J, Shorvon S. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123(4):665–676. [DOI] [PubMed] [Google Scholar]
- 10.Robertson N, Deans J, Compston D. Myasthenia gravis: a population based epidemiological study in Cambridgeshire, England. Journal of Neurology, Neurosurgery & Psychiatry. 1998;65(4):492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommer N, Melms A, Weller M, Dichgans J. Ocular myasthenia gravis. Documenta ophthalmologica. 1993;84(4):309–333. [DOI] [PubMed] [Google Scholar]
- 12.Nagia L, Lemos J, Abusamra K, Cornblath WT, Eggenberger ER. Prognosis of ocular myasthenia gravis. Ophthalmology. 2015;122(7):1517–1521. [DOI] [PubMed] [Google Scholar]
- 13.Kupersmith MJ, Latkany R, Homel P. Development of generalized disease at 2 years in patients with ocular myasthenia gravis. Archives of Neurology. 2003;60(2):243–248. [DOI] [PubMed] [Google Scholar]
- 14.Sommer N, Sigg B, Melms A, et al. Ocular myasthenia gravis: response to long-term immunosuppressive treatment. Journal of Neurology, Neurosurgery & Psychiatry. 1997;62(2):156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evoli A, Tonali P, Bartoccioni E, Monaco ML. Ocular myasthenia: diagnostic and therapeutic problems. Acta neurologica scandinavica. 1988;77(l):31–35. [DOI] [PubMed] [Google Scholar]
- 16.Allen JA, Scala S, Jones HR. Ocular myasthenia gravis in a senior population: diagnosis, therapy, and prognosis. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2010;41(3):379–384. [DOI] [PubMed] [Google Scholar]
- 17.Zach H, Cetin H, Hilger E, et al. The effect of early prednisolone treatment on the generalization rate in ocular myasthenia gravis. European journal of neurology. 2013;20(4):708–713. [DOI] [PubMed] [Google Scholar]
- 18.Somnier FE, Keiding N, Paulson OB. Epidemiology of myasthenia gravis in Denmark a longitudinal and comprehensive population survey. Archives of neurology. 1991;48(7):733–739. [DOI] [PubMed] [Google Scholar]
- 19.Phillips LH, Torner JC. Epidemiologic evidence for a changing natural history of myasthenia gravis. Neurology. 1996;47(5):1233–1238. [DOI] [PubMed] [Google Scholar]
- 20.Phillips LH, Torner JC, Anderson MS, Cox GM. The epidemiology of myasthenia gravis in central and western Virginia. Neurology. 1992;42(10):1888–1888. [DOI] [PubMed] [Google Scholar]
- 21.Christensen P, Jensen T, Tsiropoulos I, et al. Incidence and prevalence of myasthenia gravis in western Denmark 1975 to 1989. Neurology. 1993;43(9):1779–1779. [DOI] [PubMed] [Google Scholar]
- 22.Antonini G, Morino S, Gragnani F, Fiorelli M. Myasthenia gravis in the elderly: a hospital based study. Acta neurologica scandinavica. 1996;93(4):260–262. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg DH, Rizzo JF III, Hayes MT, Kneeland MD, Kelly JJ Jr. Ocular myasthenia gravis: Predictive value of single-fiber electromyography. Muscle & nerve. 1999;22(9):1222–1227. [DOI] [PubMed] [Google Scholar]
- 24.Padua L, Stalberg E, LoMonaco M, Evoli A, Batocchi A, Tonali P. SFEMG in ocular myasthenia gravis diagnosis. Clinical neurophysiology. 2000;111(7):1203–1207. [DOI] [PubMed] [Google Scholar]
- 25.Monsul NT, Patwa HS, Knorr AM, Lesser RL, Goldstein JM. The effect of prednisone on the progression from ocular to generalized myasthenia gravis. Journal of the neurological sciences. 2004;217(2):131–133. [DOI] [PubMed] [Google Scholar]


