Abstract
The purpose of this study is to describe lipid-lowering therapy (LLT) prescriptions and low-density lipoprotein cholesterol (LDL-C) monitoring in patients with diabetes mellitus (DM) with or without concomitant cardiovascular disease (CVD). Olmsted County, MN residents with a first-ever diagnosis of DM or CVD (ischemic stroke/transient ischemic attack, myocardial infarction, unstable angina pectoris, or revascularization procedure) between 2005 and 2012 were classified as having DM only, CVD only, or CVD + DM. All LLT prescriptions and LDL-C measurements were obtained for 2 years after diagnosis. A total of 4186, 2368, and 724 patients had DM, CVD, and CVD + DM, respectively. Rates of LDL-C measurement were 1.31, 1.66, and 1.88 per person-year and 14%, 32%, and 42% of LDL-C measurements were <70 mg/dL in those with DM, CVD, and CVD + DM. Within 3 months after diagnosis, 47%, 71%, and 78% of patients with DM, CVD, and CVD + DM were prescribed LLT. Most prescriptions were for moderate-intensity statins. Under one-fifth of patients with CVD and CVD + DM were prescribed high-intensity statins. Predictors of high-intensity statin prescriptions included male sex, having CVD or CVD + DM, increasing LDL-C, and LDL-C measured more recently (2012–2014 vs. before 2012). In conclusion, a large proportion of patients at high CVD risk are not adequately treated with LLT. Despite often being considered a risk-equivalent, patients with DM have substantially lower rates of LLT prescriptions and lesser controlled LDL-C than those with CVD or CVD + DM.
Keywords: statins, lipid-lowering therapy, atherosclerotic cardiovascular disease, diabetes
Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) is a well-established risk factor for the development of cardiovascular disease (CVD) and is highly prevalent.1 Although LDL-C reduction has improved in recent years with use of high-intensity statins, it has been estimated that up to 75% of high-risk statin-treated patients failed to achieve the guideline-recommended LDL-C thresholds.2–4 Furthermore, a substantial proportion of high risk patients (including those with CVD or diabetes mellitus [DM]) do not receive any lipid-lowering therapy (LLT).5–13 Despite this evidence, there are limited available data capturing longitudinal LLT prescribing patterns and LDL-C levels from all sources of healthcare, without restriction on insurance coverage or type of provider. Specifically, documentation of prescription patterns is lacking but is critical as it may more accurately reflect physician awareness of CVD risk. Thus, we aimed to describe LLT prescribing patterns, frequency of LDL-C monitoring, and predictors of high-intensity statin prescriptions over a 2-year period following a first diagnosis of CVD or DM in patients from a comprehensive linked medical records system in a community in southeastern Minnesota.
Methods
This study was conducted utilizing the Rochester Epidemiology Project (REP), a records-linkage system encompassing >6 million person-years of follow-up for >500,000 unique individuals residing in Olmsted County, Minnesota since 1966.14 Nearly all health care is captured because only a few providers (including Mayo Clinic and Olmsted Medical Center) deliver most health care to local residents and all medical record data from these providers are captured by the REP. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
This population-based cohort consisted of patients with incident (first-ever) diagnoses of CVD or DM from 1/1/2005 through 12/31/2012. The list of diagnostic codes and rules used to define the index events are included in Appendix Table 1. Patients with CVD had a diagnosis of ischemic stroke/transient ischemic attack (TIA), myocardial infarction (MI), unstable angina pectoris (UA), or a revascularization procedure. Patients with incident CVD were further classified into those with CVD only and those with CVD who had pre-existing DM (CVD + DM). Patients without CVD but who had an incident diagnosis of DM were classified as DM only.
Outpatient prescription data was obtained from Mayo Clinic and Olmsted Medical Center from 3 months prior through 2 years after initiating event. All outpatient prescriptions for LLT, including statin and non-statin therapy were obtained. For each statin prescription, the strength, dose, and frequency were used to calculate average daily potency in order to classify statin prescriptions into low-, moderate-, and high-intensity, as defined by the 2013 American College of ardiology/American Heart Association Practice Guideline (Appendix Table 2).15 Records were reviewed when data were missing or inconsistent to determine the correct daily potency.
Tobacco use, classified as ever/never, was based on patient-provided surveys. Body mass index was estimated as the median value of the 10 heights and 10 weights closest to and within 3 years of index. Select comorbidities were ascertained using the Centers for Medicare and Medicaid Chronic Conditions Data Warehouse algorithms.16 For all conditions we used the 5 years prior to the index date to determine the presence or absence of a comorbidity. Finally, all LDL-C measurements for the 1 year prior to 2 years after index were obtained.
Analyses were performed using SAS/STAT software, version 9.4. Patient characteristics across disease categories were compared using chi-squared tests for categorical variables and ANOVA for continuous variables. Characteristics of patients with 0 vs. ≥1 LDL measurements from index to 2 years after index were compared using chi-square and ANOVA tests. Rates of LDL-C measurements over follow-up were compared among the 3 groups using Poisson regression. Predictors of high-intensity statin prescriptions were assessed using Cox proportional hazards regression, using age as the time scale. This analysis was restricted to patients not on a high-intensity statin at index and who had a baseline LDL-C measurement (a measurement within 1 year prior to or 30 days after index [n=5670]). The proportional hazards assumption of the Cox model was tested by plotting scaled Schoenfeld residuals.
Results
Between 2005 and 2012, a total of 4186, 2368, and 724 patients were diagnosed with DM, CVD, and CVD + DM, respectively. Patients with CVD were older and had a higher prevalence of comorbidities compared to patients with DM; those with CVD + DM had the highest comorbidity burden (Table 1).
Table 1.
Characteristics of the cohort at baseline and summary information on all follow-up low-density lipoprotein cholesterol measurements, by index event.
| Variable | DM (N=4186) |
CVD (N=2368) |
CVD + DM (N=724) |
P-value |
|---|---|---|---|---|
| Age (years) | 56.6 ± 14.9 | 68.9 ± 15.2 | 70.2 ± 13.2 | <0.001 |
| Men | 2149 (51.3%) | 1324 (55.9%) | 392 (54.1%) | 0.002 |
| Ever tobacco use | 2404 (60.0%) | 1526 (65.4%) | 475 (66.8%) | <0.001 |
| Missing | 180 | 36 | 13 | |
| Body mass index (kg/m2) | ||||
| <25 | 402 (11.7%) | 655 (29.0%) | 116 (16.6%) | <0.001 |
| 25 to <30 | 806 (23.4%) | 898 (39.7%) | 213 (30.4%) | |
| ≥30 | 2241 (65.0%) | 708 (31.3%) | 372 (53.1%) | |
| Missing | 737 | 107 | 23 | |
| Hypertension | 2122 (50.7%) | 1435 (60.6%) | 638 (88.1%) | <0.001 |
| Heart failure | 177 (4.2%) | 320 (13.5%) | 197 (27.2%) | <0.001 |
| COPD | 354 (8.5%) | 325 (13.7%) | 123 (17.0%) | <0.001 |
| Chronic kidney disease | 270 (6.5%) | 297 (12.5%) | 239 (33.0%) | <0.001 |
| LLT prescriptions at indexa | ||||
| None | 2900 (69.3%) | 1684 (71.1%) | 314 (43.4%) | <0.001 |
| High-intensity statin | 156 (3.7%) | 80 (3.4%) | 64 (8.8%) | |
| Moderate-intensity statin | 761 (18.2%) | 435 (18.4%) | 256 (35.4%) | |
| Low-intensity statin | 211 (5.0%) | 124 (5.2%) | 61 (8.4%) | |
| Non-statin therapy only | 158 (3.8%) | 45 (1.9%) | 29 (4.0%) | |
| LDL-C summary information | ||||
| Patients who have full 2 years of | 3854 (92.1%) | 1919 (81.0%) | 566 (78.2%) | <0.001 |
| follow-up | ||||
| Patients with 0 LDL-C | 493 (11.8%) | 305 (12.9%) | 70 (9.7%) | 0.059 |
| measurements | ||||
| Rate (95% Cl) of LDL-C | 1.31 (1.28–1.33) | 1.66 (1.62–1.70) | 1.88 (1.81–1.96) | <0.001 |
| measurement per person-year | ||||
| Median (25th, 75th percentile) | 2.53 (2.04, 3.10) | 2.09 (1.66, 2.69) | 1.91 (1.53, 2.43) | <0.001 |
| LDL-C, mg/dL | ||||
| LDL-C ≥100 mg/dL | 5038 (48.3%) | 1943 (28.6%) | 454(19.6%) | <0.001 |
| LDL-C 70–99 mg/dL | 3898 (37.4%) | 2642 (38.9%) | 887 (38.3%) | |
| LDL-C <70 mg/dL | 1493 (14.3%) | 2203 (32.5%) | 975 (42.1%) |
Defined using prescription information from 1 day prior to index date.
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; LDL-C, low-density lipoprotein cholesterol; LLT, lipid lowering therapy; SD, standard deviation.
A total of 493 (11.8%), 305 (12.9%), and 70 (9.7%) patients with DM, CVD, and CVD + DM, respectively, had no LDL-C measurements within the two years after index (Table 1). Patients with no LDL-C measurements were older (65.6 vs. 61.5 years), more likely to be female (53.8% vs. 46.0%), and more likely to have heart failure (21.1% vs. 8.0%), chronic obstructive pulmonary disease (19.5% vs. 9.9%), and chronic kidney disease (19.8% vs. 9.9%) compared to patients who had at least 1 LDL-C measurement (all p-values <0.001).
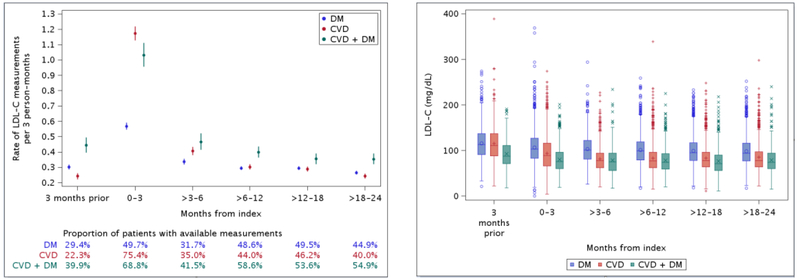
Patients with DM had the most infrequent rates of measurement of LDL-C, at 1.31 per person-year, with the highest rates found in those with CVD + DM (1.88 per person-year; Table 1). Among patients with DM, 14.3% of all LDL-C measurements over follow-up were <70 mg/dL; nearly half (48.3%) were ≥100 mg/dL. Among patients with CVD and CVD + DM, respectively, 32.5% and 42.1% were <70 mg/dL whereas 28.6% and 19.6% were ≥100 mg/dL. The proportion of patients with available LDL-C measurements across time periods over follow-up ranged from 22.3% to 75.4% (Figure 1). For all groups, the rate of LDL-C measurement decreased substantially after the 0–3 months post-index. Rates were generally lower for patients with DM compared to patients with CVD + DM. Furthermore, the mean and median values of LDL-C were highest across all periods of follow-up for patients with DM (Figure 1).
Figure 1.
Overall rates (95% confidence interval) of low-density lipoprotein cholesterol measurements before and after index event in patients with diabetes mellitus, cardiovascular disease, and cardiovascular disease with concomitant diabetes mellitus (panel A). Distribution of low-density lipoprotein cholesterol measurements before and after index event in patients with diabetes mellitus, cardiovascular disease, and cardiovascular disease with concomitant diabetes mellitus (panel B). CVD, cardiovascular disease; DM, diabetes mellitus.
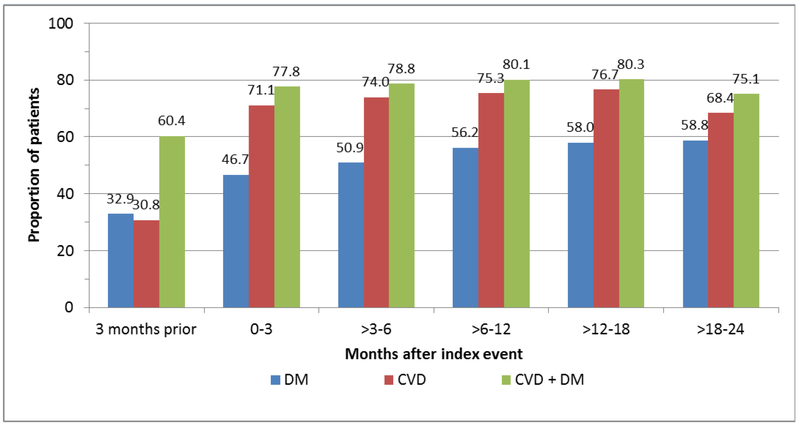
Patients with DM were prescribed LLT at the lowest rates (Figure 2). Within the first 3 months after index, 47% of patients with DM were prescribed LLT, which increased modestly over time but remained under 60% over the 2 years of follow-up. Patients with CVD + DM had higher rates of LLT during the 3 months prior to index compared with patients with CVD only; however, the patterns for these 2 groups of patients were generally similar after index. Within the first 3 months after index, 71% and 78% of patients with CVD and CVD + DM, respectively, were prescribed LLT and the proportions remained relatively stable thereafter.
Figure 2.
Proportion of patients on any lipid-lowering therapy before and after index event in patients with diabetes mellitus, cardiovascular disease, and cardiovascular disease with concomitant diabetes mellitus. CVD, cardiovascular disease; DM, diabetes mellitus.
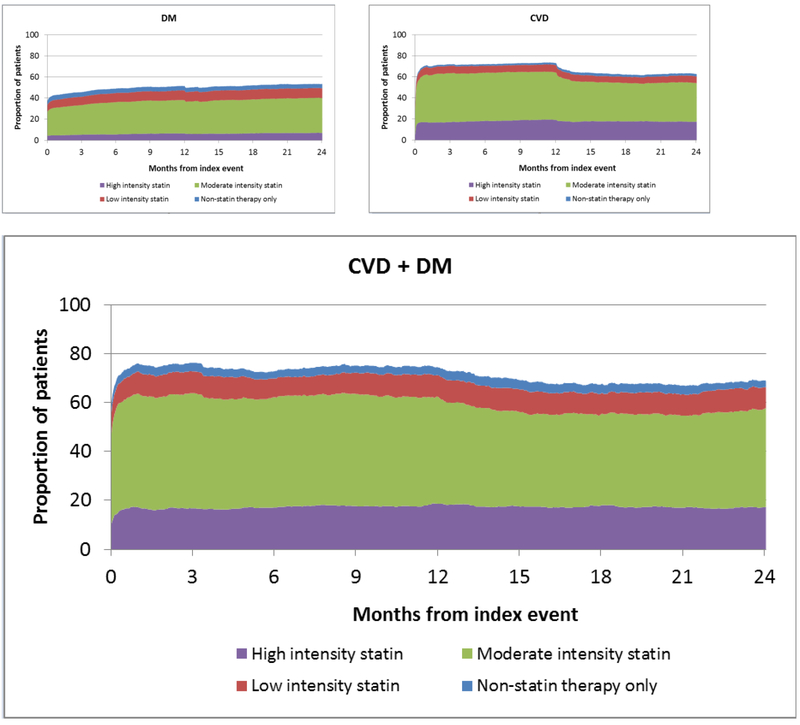
Daily rates of LLT by type and intensity of therapy are displayed in Figure 3. An increase in prescriptions for LLT was observed immediately after index and the majority of prescriptions were for moderate-intensity statins. Approximately 6% of people with DM were prescribed high-intensity statins, and <20% of patients with CVD and CVD + DM were prescribed high-intensity statins. At 1 year after index, a drop in prescription rates was observed, which may indicate a lack of renewal of prescriptions for some patients.
Figure 3.
Daily rates of statin use in patients with diabetes mellitus (panel A), cardiovascular disease (panel B), and cardiovascular disease with concomitant diabetes mellitus (panel C). CVD, cardiovascular disease; DM, diabetes mellitus.
The daily denominators were adjusted accordingly to account for deaths (n=167, 408, and 152 patients in the diabetes mellitus, cardiovascular disease, and cardiovascular disease + diabetes mellitus groups, respectively) and losses to follow-up (n=165, 41, and 6 patients in the diabetes mellitus, cardiovascular disease, and cardiovascular disease + diabetes mellitus groups, respectively).
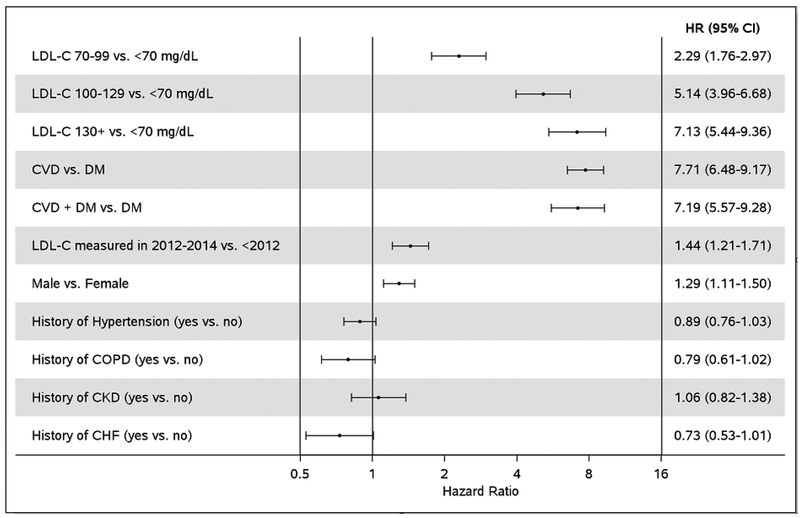
Among the subset of patients with available baseline LDL-C who were not on high-intensity statins at index (n=5670), predictors of receiving a high-intensity statin prescription post-index are presented in Figure 4. We observed a graded association with increasing LDL-C level, with a 7.1-fold higher probability of high-intensity statin prescription among those with LDL-C ≥130 mg/dL compared to LDL-C <70 mg/dL. Men and patients with LDL-C measured in more recent years (2012–2014 vs. prior to 2012) were also more likely to be prescribed high-intensity statins. Patients with CVD alone or CVD + DM were 7 times more likely to be prescribed high-intensity statins than those with DM. The proportional hazards assumption was not met for patients in the CVD and CVD + DM groups aged <55 years. The estimates were higher for patients <55 years than for patients ≥55 years (HR (95% CI) 11.33 (8.50–15.12) vs. 6.02 (4.86–7.47) for CVD and 10.32 (6.29–16.30) vs. 5.72 (4.24–7.71) for CVD + DM). Finally, we tested whether the association of LDL-C with high-intensity statin prescriptions differed by disease category. We found no difference in the effect of LDL-C with high-intensity statin prescriptions between patients with DM alone, CVD, and CVD + DM (p-interaction=0.11).
Figure 4.
Predictors of high-intensity statin prescriptions among patients with an available baseline low-density lipoprotein cholesterol measurement (defined as a measure within 1 year prior to 30 days after index) and who were not on a high-intensity statin prior to the baseline low-density lipoprotein cholesterol measurement (n=5670). Hazard ratios are adjusted for all other variables in the figure. Low-density lipoprotein cholesterol and year of low-density lipoprotein cholesterol measurement were modeled as time-dependent variables.
CHF, chronic heart failure; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol.
Discussion
In this community-based study focused on clinician prescribing patterns, patients with DM were the least likely to receive a prescription for LLT, despite the fact that many clinicians consider DM to be a risk-equivalent. The majority of prescriptions were for moderate-intensity statins. Fewer than 20% of patients with CVD or CVD + DM were prescribed high-intensity statins, and the proportion was much lower (6%) among those with DM alone. High-intensity statins were more likely to be prescribed in males, those with CVD or CVD + DM, those with increasing levels of LDL-C, and those having more recent LDL-C measurements. We found that patients with DM alone, as compared to those with CVD and CVD + DM, had the lowest rates of LDL-C measurement and lowest proportion of LDL-C values <70 mg/dL.
In our population, <50% of patients with DM received a prescription for LLT in the 3 months after diagnosis, and while the proportion treated increased with follow-up, <60% were prescribed LLT 2 years after diagnosis. Prior studies similarly report that patients with DM are undertreated. In the National Health and Nutritional Examination Survey, although use of statins among patients aged 40–75 years with DM increased from 26.2% in 1999–2002 to 49.5% in 2011–2014, the rates of high-intensity statins remained constant.17 Data from the 2010 Medical Expenditure Panel Survey (MEPS) indicated that 52.0% of patients ≥40 years of age with DM self-reported use of statins.6 Based on the MEPS data, a staggering 9 million individuals in the US with DM aged ≥40 years are not using statins.6 In addition, approximately half of US adults with DM have a LDL-C of ≥100 mg/dL,7 which was confirmed by our findings. Reasons for LLT underutilization may include provider opinions on the benefits of statins given the small risk of increasing hemoglobin A1c and fasting glucose.18 Yet, the underutilization of LLT is particularly concerning given the evidence that statins in patients with DM reduce the risk of cardiovascular events by 22–37%.19–21 The newest cholesterol guidelines call out the importance of regular monitoring of LDL-C levels, but also the importance of shared decision making.22 This could potentially improve the utilization of statins in this high-risk patient group.
Although LLT prescription rates were higher compared to DM, we nonetheless observed a substantial treatment gap with nearly one-quarter of patients with CVD not receiving a LLT prescription. In this population, prescriptions for LLT were higher than estimates from the 2010 MEPS (58.2%)6 and the Optum Insight database from 2012 (67.9%),3 but lower than the proportion of acute MI patients discharged on statins from hospitals in the Worcester, MA area,8 from Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status study,23 and the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry–Get With The Guidelines and Catheterization PCI databases,24 which ranged between 85–91%. Among a 5% random sample of Medicare beneficiaries, only 58.8% of patients with coronary heart disease filled a statin prescription in the 4th quarter of 2011, of which 14.2% were for high-intensity statins.5 The observed under-prescribing of LLT in this study is concerning given that patients had their LDLC monitored between 1–2 times per year and nearly 30% of LDL-C measurements in patients with CVD and 20% of LDL-C measurements in patients with CVD + DM were ≥100 mg/dL.
Moreover, the extent of the problem of undertreatment with LLT is compounded by poor adherence and persistence.25 In a meta-analysis of >3 million statin users aged ≥65 years, 82.6% of patients using statins for secondary prevention were persistent at 1-year, but only 62.3% were adherent.26 Data from MarketScan and Medicare found that both persistence and adherence to statin therapy in the year following treatment initiation were markedly lower in patients with DM compared to MI.27 Thus, the proportion of patients with clear indications for LLT who are actually taking it is sorely inadequate, and those with DM are less likely to be adherent than patients with CVD. This is particularly concerning given the high medication burden of newly-diagnosed DM patients (mean of 6.6 therapeutic classes of drugs),28 which could heighten issues with non-adherence. Furthermore, it is unknown whether physician prescribing of LLT or patient adherence will be affected by use of newer anti-diabetic agents, including sodium-glucose co-transporter 2 inhibitors and glucagon-like peptide 1 analogues, some of which have shown a CVD benefit, including reduced cardiovascular mortality and reduced heart failure admissions.29 Thus, additional research is warranted to describe whether trends in LLT prescribing and adherence differ by anti-diabetic treatment strategy.
A major strength of this study is the REP records-linkage system which provides for nearly complete coverage of patients’ medical history. Second, the longitudinal nature of the data allowed the examination of prescription patterns before, immediately after, and in the 2-year period following a CVD or DM event which is not commonly described in the existing literature and provides valuable insights into real-world treatment patterns. Finally, we documented the extent of under-prescribing of LLT which more accurately reflects awareness of CVD risk and benefits vs. harms of LLT treatment compared to studies using pharmacy claims data. A limitation of the REP is that while the demographic and ethnic characteristics of Olmsted County are representative of the Midwest region of the US,30 minority racial and ethnic groups are underrepresented. In addition, pharmacy claims data are not available in the REP, and thus, we could not calculate adherence to the prescribed therapy.
In this community-based study using high quality and largely complete linked medical records, we found substantial under-prescribing of LLT in patients who had higher risk conditions of CVD and/or DM. Of concern, despite the substantial cardiovascular risk of those with DM, these patients were found to have fewer measurements of LDL-C, lower LLT prescription rates, and less well-controlled LDL-C compared to patients with CVD or CVD + DM. Additional research and educational efforts are needed to better understand why a treatment with proven benefits remains underutilized in patients known to be at high risk for future CVD events, and how to best combine medications to treat the multiple risk factors which often exist.
Supplementary Material
Acknowledgments
We thank Ellen E. Koepsell, RN, Elizabeth Hedgeman, PhD, MPH, and Deborah S. Strain for their assistance with data collection and manuscript preparation.
Funding Sources: This work was supported by grants from Amgen, Inc. (A biopharmaceutical company) Thousand Oaks, CA, USA and the National Institute on Aging (R01 AG034676), Bethesda, MD, USA. The funding sources did not have a role in any of the writing or preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: During the course of this research, Keri L. Monda and Ted Okerson were employees and stockholders of Amgen, Inc. Sarah S. Cohen received research grants from Amgen, Inc. Alanna M. Chamberlain is a Co-Investigator of the Rochester Epidemiology Project (R01 AG034676). All other authors report no conflict of interest.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karalis DG, Subramanya RD, Hessen SE, Liu L, Victor MF. Achieving optimal lipid goals in patients with coronary artery disease. Am J Cardiol 2011;107:886–890. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez F, Olufade T, Heithoff K, Friedman HS, Navaratnam P, Foody JM. Frequency of high-risk patients not receiving high-potency statin (from a large managed care database). Am J Cardiol 2015;115:190–195. [DOI] [PubMed] [Google Scholar]
- 4.Toth PP, Foody JM, Tomassini JE, Sajjan SG, Ramey DR, Neff DR, Tershakovec AM, Hu XH, Tunceli K. Therapeutic practice patterns related to statin potency and ezetimibe/simvastatin combination therapies in lowering LDL-C in patients with high-risk cardiovascular disease. J Clin Lipidol 2014;8:107–116. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V, Deng L, Rosenson RS, Taylor B, Glasser SP, Kent ST, Farkouh ME, Muntner P. Trends in the use of nonstatin lipid-lowering therapy among patients with coronary heart disease: a retrospective cohort study in the Medicare population 2007 to 2011. J Am Coll Cardiol 2015;66:1864–1872. [DOI] [PubMed] [Google Scholar]
- 6.Johansen ME, Green LA, Sen A, Kircher S, Richardson CR. Cardiovascular risk and statin use in the United States. Ann Fam Med 2014;12:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuznik A, Mardekian J. Trends in utilization of lipid- and blood pressure-lowering agents and goal attainment among the U.S. diabetic population, 1999–2008. Cardiovasc Diabetol 2011;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makam RC, Erskine N, McManus DD, Lessard D, Gore JM, Yarzebski J, Goldberg RJ. Decade-long trends (2001 to 2011) in the use of evidence-based medical therapies at the time of hospital discharge for patients surviving acute myocardial infarction. Am J Cardiol 2016;118:1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pauff BR, Jiroutek MR, Holland MA, Sutton BS. Statin prescribing patterns: an analysis of data from patients with diabetes in the National Hospital Ambulatory Medical Care Survey Outpatient Department and National Ambulatory Medical Care Survey databases, 2005–2010. Clin Ther 2015;37:1329–1339. [DOI] [PubMed] [Google Scholar]
- 10.Pokharel Y, Akeroyd JM, Ramsey DJ, Hira RS, Nambi V, Shah T, Woodard LD, Winchester DE, Ballantyne CM, Petersen LA, Virani SS. Statin use and its facility-level variation in patients with diabetes: insight from the Veterans Affairs national database. Clin Cardiol 2016;39:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Punekar RS, Fox KM, Richhariya A, Fisher MD, Cziraky M, Gandra SR, Toth PP. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol 2015;38:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah NS, Huffman MD, Ning H, Lloyd-Jones DM. Trends in vascular risk factor treatment and control in US stroke survivors: the National Health and Nutrition Examination Surveys (1999–2010). Circ Cardiovasc Qual Outcomes 2013;6:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma A, Visintainer P, Elarabi M, Wartak S, Rothberg MB. Overtreatment and undertreatment of hyperlipidemia in the outpatient setting. South Med J 2012;105:329–333. [DOI] [PubMed] [Google Scholar]
- 14.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Chronic Conditions Data Warehouse (CCW), CCW Condition Algorithms. https://www.ccwdata.org/web/guest/condition-categories accessed 6/25/19.
- 17.Gu A, Kamat S, Argulian E. Trends and disparities in statin use and low-density lipoprotein cholesterol levels among US patients with diabetes, 1999–2014. Diabetes Res Clin Pract 2018. [DOI] [PubMed] [Google Scholar]
- 18.Maki KC, Ridker PM, Brown WV, Grundy SM, Sattar N, The Diabetes Subpanel of the National Lipid Association Expert Panel. An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol 2014;8:S17–29. [DOI] [PubMed] [Google Scholar]
- 19.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 20.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 21.Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial--lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28:1151–1157. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation 2018:CIR0000000000000625. [Google Scholar]
- 23.Arnold SV, Kosiborod M, Tang F, Zhao Z, Maddox TM, McCollam PL, Birt J, Spertus JA. Patterns of statin initiation, intensification, and maximization among patients hospitalized with an acute myocardial infarction. Circulation 2014;129:1303–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, Chen AY, Klein LW, Masoudi FA, McKay C, Hewitt K, Brindis RG, Peterson ED, Rumsfeld JS. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol 2010;56:254–263. [DOI] [PubMed] [Google Scholar]
- 25.Bates TR, Connaughton VM, Watts GF. Non-adherence to statin therapy: a major challenge for preventive cardiology. Expert Opin Pharmacother 2009;10:2973–2985. [DOI] [PubMed] [Google Scholar]
- 26.Ofori-Asenso R, Jakhu A, Zomer E, Curtis AJ, Korhonen MJ, Nelson M, Gambhir M, Tonkin A, Liew D, Zoungas S. Adherence and persistence among statin users aged 65 years and over: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2018;73:813–819. [DOI] [PubMed] [Google Scholar]
- 27.Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE, Muntner P. Adherence to statin therapy among US adults between 2007 and 2014. J Am Heart Assoc 2019;8:e010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmittdiel JA, Raebel MA, Dyer W, Xu S, Goodrich GK, Schroeder EB, Segal JB, O’Connor P, Nichols GA, Lawrence JM, Kirchner HL, Karter AJ, Lafata JE, Butler MG, Steiner JF. Prescription medication burden in patients with newly diagnosed diabetes: a SUrveillance, PREvention, and ManagEment of Diabetes Mellitus (SUPREME-DM) study. J Am Pharm Assoc (2003) 2014;54:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S73–S85. [DOI] [PubMed] [Google Scholar]
- 30.St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.