Summary:
The MERIT study was a single-arm, phase 2 clinical trial of nivolumab for the 2nd or 3rd line treatment of patients with malignant pleural mesothelioma in Japan. MERIT confirmed that PD-1 inhibition has activity in mesothelioma and led to the regulatory approval of nivolumab for the treatment of mesothelioma in Japan.
In this issue of Clinical Cancer Research, Okada and colleagues report that nivolumab has promising efficacy and reasonable safety for the second- or third-line treatment of malignant pleural mesothelioma (1). The subsequent approval of nivolumab by the Ministry of Health, Labor and Welfare of Japan represents the first ever second-line approval for mesothelioma and a true milestone for this disease.
Mesothelioma is a devastating disease with only one treatment regimen approved by the United States (US) Food and Drug Administration (FDA): cisplatin and pemetrexed (2). Most patients diagnosed with this disease are not able to undergo surgical resection and recent SEER analyses suggest that many patients in the United States do not receive any treatment for this disease (3). Of the treated patients, most receive palliative chemotherapy with a platinum agent, pemetrexed and sometimes bevacizumab, based on the French MAPS trial (4). While bevacizumab was added to the NCCN Guidelines based on the MAPS trial, there is no FDA approval for this agent and there are no approvals for second-line systemic therapy in the United States and many other countries.
Historically, no therapeutic agent has demonstrated strong activity against mesothelioma in second or later lines of treatment. Immune checkpoint inhibitors that block CTLA-4, PD-1 or its ligand PD-L1 have been approved by the US FDA for the treatment of several solid tumors and hematologic malignancies as well as in the disease agnostic setting of microsatellite instability (MSI) high tumors. Many of these agents have recently been studied in mesothelioma with mixed results. Notably, despite an encouraging disease control rate with the CTLA-4 inhibitor tremelimumab in a non-randomized phase 2 clinical trial (5), there was no survival benefit over placebo with this agent in a phase 3 randomized trial (6). The signals with PD-1 inhibitors have been more encouraging. KEYNOTE-028 was a non-randomized, open-label, multi-cohort trial that tested the PD-1 inhibitor pembrolizumab in patients with tumors that expressed PD-L1 (≥1% tumor cell expression) and included a mesothelioma cohort (7). The response rate was 20% with a median response duration of 12 months (95%CI: 3.7 months to not reached) and a median overall survival of 18 months (95%CI: 9.4 months to not reached)(7). In Amsterdam, a single center non-randomized, single-arm trial of nivolumab regardless of PD-L1 expression demonstrated results similar to KEYNOTE-028 with a response rate of 24% (95% CI:11-42%) and a median response duration of 7 months. However, the median overall survival was shorter at 11.8 months (95% CI: 9.7-15.7 months)(8).
Immune checkpoint inhibitor combinations have also been tested after platinum-based therapy for patients with mesothelioma at multiple European centers. Remarkably, similar outcomes were seen in one study that tested tremelimumab with the PD-L1 inhibitor durvalumab (9), and two studies that tested the CTLA-4 inhibitor ipilimumab with nivolumab (10,11). Overall response rates of almost 30%, and median progression-free survival around 6 months were observed in all three studies. In the one study that included a treatment arm of nivolumab alone, the adverse event rates were numerically higher in the ipilimumab with nivolumab arm (11). Although outcomes with the combination of CTLA-4 and PD-(L)1 inhibitors have not been compared directly with single agent PD-(L)1 inhibitors, these combinations seem to result in minimal improvement in response rates and survival but increase toxicity.
Okada and colleagues report the results of the MERIT trial in this issue of Clinical Cancer Research for the second- or third-line treatment of malignant pleural mesothelioma (1). Thirty-four patients enrolled in this open-label, single-arm, phase 2 study for the 2nd or 3rd line treatment of mesothelioma at multiple centers in Japan. Overall, there was an objective response rate of 29%. Although there were only three patients with the typically refractory sarcomatoid histology who participated in this trial, two responded to nivolumab. The median duration of response was 11.1 months with median progression-free and overall survival of 6.1 months and 17.3 months, respectively. For comparison, the median overall survival in a separate trial with similar enrollment criteria was 7.7 months in patients treated with tremelimumab and 7.3 months in patients who received placebo. Among those whose tumors had ≥ 1% PD-L1 expression, the response rate was 40% and only 8% in those whose tumors did not have PD-L1 expression. Recently, the Ministry of Health, Labor and Welfare of Japan approved nivolumab for the second or later line treatment of mesothelioma based on the MERIT trial results.
Clearly, PD-(L)1 inhibitors have single agent activity that is possibly enhanced with CTLA-4 inhibition and PD-L1 expression enriches for responses to PD-(L)1 inhibitors even though responses may be seen in the absence of detected expression. Despite these advances, many issues remain to be solved. Even though PD-L1 expression enriches for responses, the dynamics and heterogeneity of its expression encourage the development of more robust predictive biomarkers. In this regard, yes-associated protein expression is correlated with PD-L1 expression and may have immunologic significance (12). Also, despite the relatively low tumor mutation burden in mesothelioma, chromosomal rearrangements are common in this cancer and may have neoantigenic potential (13).
Unfortunately, we do not have randomized data demonstrating a survival benefit with PD-(L)1 inhibitors but a trial is underway in the United Kingdom (). Even though mesothelioma has modest incidence, we must consider whether this justifies using the results of single arm phase 2 clinical trials for regulatory approval. While approval helps more patients access these novel agents, premature adoption of therapy may potentially harm patients and may stymie clinical trial development and execution. One way to augment the information from single arm phase 2 clinical trials would be to use contemporaneous synthetic control groups matched by prognostic factors and line of therapy as well as to develop surrogate markers for survival and predictive markers for response (Figure). This paradigm has the potential to expedite drug development in clinically meaningful ways requiring the least number of trial participants. Recently the appropriations bill included funding for the Centers for Disease Control to develop a pilot Mesothelioma Patient Registry. Such a registry could be a powerful resource for generating data for synthetic control arms and will hopefully enable us to learn from each and every patient with this disease, treated on or off protocol.
Figure 1:
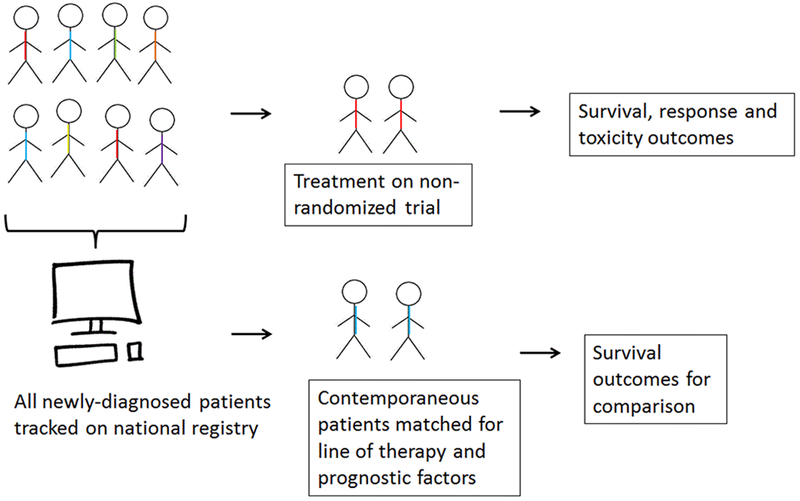
Synthetic control groups derived from national mesothelioma registry. Single-arm clinical trial results for rare tumors like mesothelioma could be augmented by contemporaneous synthetic control groups matched for prognostic factors and derived from the national mesothelioma registry that is in development.
The next wave of clinical trials with immune checkpoint inhibitors is already underway and in a few years we will have insight into (1) whether combined PD-1 and CTLA-4 inhibition improves survival over first line chemotherapy, (2) whether combined chemotherapy and PD-L1 inhibition improves survival over chemotherapy alone, (3) whether PD-(L)1 inhibitors are synergistic with other agents in second or later lines of therapy (4) how neo-adjuvant chemo-immunotherapy affects surgical outcomes, and (5) the role of other immune checkpoints in mesothelioma.
We are encouraged by the ongoing pharmaceutical and academic interest in finding effective, safe therapies for our patients and this approval based on the MERIT trial is likely the first of many to come. Immune checkpoint inhibitors, PD-(L)1 inhibitors in particular, are changing the natural history of this disease but this treatment approach requires more investigation and refinement for optimal use. The first ever second line approval for mesothelioma is revolutionary; however, we anticipate that the role of immunotherapy will continue to evolve and these agents will become a new standard of care in many disease settings and may eventually be incorporated into frontline, systemic treatment regimens for mesothelioma.
Acknowledgments:
A. Mansfield was supported by NIH award P30CA015083.
Conflicts of interest:
Aaron S. Mansfield reports receiving commercial research grants from Novartis and Verily (paid to Dr. Mansfield’s institution) and has served on advisory boards for AbbVie, Genentech, and Bristol-Myers Squibb (honoraria paid to Dr. Mansfield’s institution). In the last 3 years, Marjorie G. Zauderer has received consulting fees from Epizyme, Sellas Life Sciences, and Aldeyra Therapeutics. Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, MedImmune, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium, and Curis for research conducted by Dr. Zauderer. Dr. Zauderer serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation. Memorial Sloan Kettering has an institutional agreement with IBM for Watson for Oncology and receives royalties from IBM. Dr. Zauderer is an employee of Memorial Sloan Kettering.
Abbreviations list:
- CTLA-4
Cytotoxic T-Lymphocyte Associated Protein 4
- MSI
microsatellite instability
- NCCN
National Comprehensive Cancer Network
- PD-1
Programmed Cell Death 1 (aka CD279)
- PD-L1
Programmed Cell Death 1 Ligand 1 (aka CD274, B7-H1)
- SEER
Surveillance, Epidemiology, and End Results
- US FDA
United States Food and Drug Administration
References:
- 1.Okada M, Kijima T, Aoe K, Kato T, Fujimoto N, Nakagawa K, et al. Clinical efficacy and safety of nivolumab: results of a multicenter, open-label, single-arm, Japanese phase 2 study in malignant pleural mesothelioma (MERIT). Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-19-0103. [DOI] [PubMed] [Google Scholar]
- 2.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21(14):2636–44 doi 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 3.Zauderer MG, Tsao AS, Fennell DA, Wong WB, Pattipaka T, Bretscher MT, et al. Patterns of comorbidity, treatment, resource utilization, and referral in malignant pleural mesothelioma patients in the US. J Clin Oncol 2017;35. [Google Scholar]
- 4.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387(10026):1405–14 doi 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]
- 5.Calabro L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14(11):1104–11 doi 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 6.Maio M, Scherpereel A, Calabro L, Aerts J, Cedres Perez S, Bearz A, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18(9):1261–73 doi 10.1016/S1470-2045(17)30446-1. [DOI] [PubMed] [Google Scholar]
- 7.Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18(5):623–30 doi 10.1016/S1470-2045(17)30169-9. [DOI] [PubMed] [Google Scholar]
- 8.Quispel-Janssen J, van der Noort V, de Vries JF, Zimmerman M, Lalezari F, Thunnissen E, et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13(10):1569–76 doi 10.1016/j.jtho.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Calabro L, Morra A, Giannarelli D, Amato G, D’Incecco A, Covre A, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med 2018;6(6):451–60 doi 10.1016/S2213-2600(18)30151-6. [DOI] [PubMed] [Google Scholar]
- 10.Disselhorst MJ, Quispel-Janssen J, Lalezari F, Monkhorst K, de Vries JF, van der Noort V, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med 2019;7(3):260–70 doi 10.1016/S2213-2600(18)30420-X. [DOI] [PubMed] [Google Scholar]
- 11.Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Do P, Bylicki O, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20(2):239–53 doi 10.1016/S1470-2045(18)30765-4. [DOI] [PubMed] [Google Scholar]
- 12.Hsu PC, Miao J, Wang YC, Zhang WQ, Yang YL, Wang CW, et al. Inhibition of yes-associated protein down-regulates PD-L1 (CD274) expression in human malignant pleural mesothelioma. J Cell Mol Med 2018;22(6):3139–48 doi 10.1111/jcmm.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansfield AS, Peikert T, Smadbeck JB, Udell JBM, Garcia-Rivera E, Elsbernd L, et al. Neoantigenic Potential of Complex Chromosomal Rearrangements in Mesothelioma. J Thorac Oncol 2019;14(2):276–87 doi 10.1016/j.jtho.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


