Abstract
Glucocorticoids are potent anti-inflammatory and immunosuppressant medications and remain the mainstay of systemic lupus erythematosus (SLE) therapy. The potency of a specific glucocorticoid, i.e., the dose of glucocorticoid that is required to produce a specific effect, is dependent on its pharmacokinetic (PK) and pharmacodynamic (PD) properties. In this review, we summarize the PK/PD properties of commonly used glucocorticoids in an attempt to better delineate their role in the management of children with childhood-onset SLE (cSLE). We also address glucocorticoid side effects as these play a major role when deciding on the dose, frequency, and duration of use. A better understanding of the pharmacology of glucocorticoids appears useful to achieve improved outcomes in the management of cSLE.
Keywords: Glucocorticoids, pharmacokinetics, pharmacodynamics, childhood-onset systemic lupus erythematosus
Introduction
Since Philip Hench introduced glucocorticoids into clinical medicine in 1949 [1], these powerful anti-inflammatory medications are commonly prescribed in rheumatology. The pleiotropic effects of glucocorticoids are advantageous in the treatment of many autoimmune diseases. As such glucocorticoids have been used in the treatment of systemic lupus erythematosus (SLE) since the 1950s [2–5]. Indeed, either used alone or in combination with other immunosuppressive agents, glucocorticoids remain the cornerstone of chronically active SLE and are commonly used in the setting of SLE flares [6]. A deeper understanding of the pharmacology of specific glucocorticoids is needed along with the physician’s medical decision to guide the most appropriate treatment regimen for patients with childhood-onset SLE (cSLE). The purpose of this review is to provide a high-level summary of the pharmacology of glucocorticoids with a focus on their use in rheumatology in general, and cSLE in particular. We searched the PubMed/Medline database, using indexing (Medical subHeadings, MeSH) terms ‘Systemic Lupus Erythematosus, childhood-onset’, ‘glucocorticoids’, ‘pharmacology’, ‘pharmacokinetics’, ‘adverse effects’, and searched through references of the articles to establish a deeper understanding of glucocorticoids pharmacology and their application in cSLE.
Natural and synthetic glucocorticoids - pharmacology
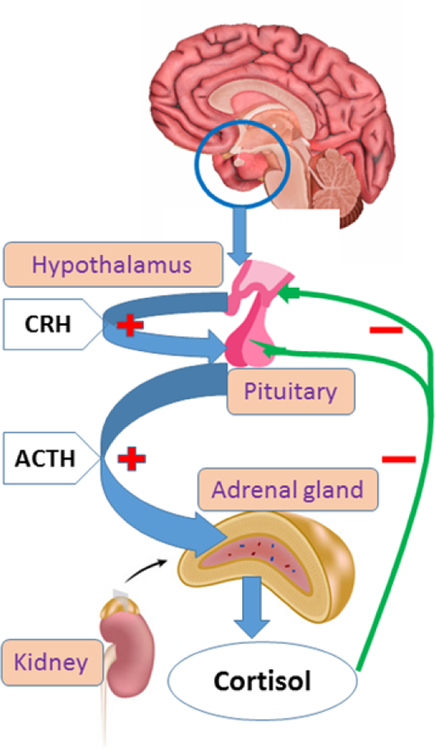
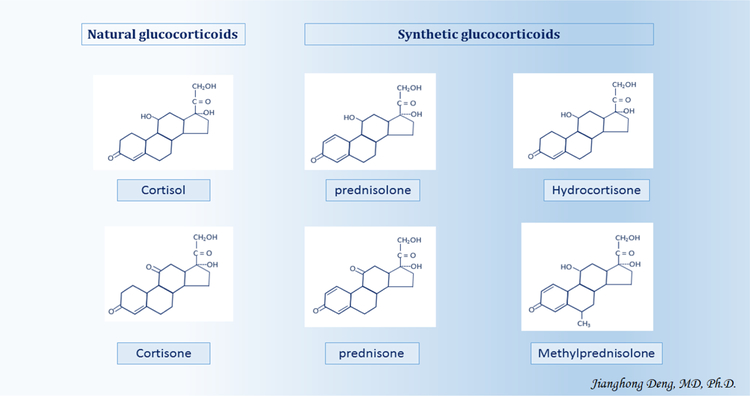
The term ‘glucocorticoids’ is a composite of glucose, cortex, and steroid. It was coined to point out the role of these compounds in the regulation of glucose metabolism, their synthesis in the adrenal cortex, and the steroidal chemical structure [7]. So-called natural or endogenous glucocorticoids, i.e., glucocorticoids naturally produced by the adrenal cortex, include cortisol - the biologically active hormonal compound and cortisone - a biologically inactive form of cortisol. Cortisol production and secretion are regulated by the hypothalamic-pituitary-adrenal (HPA) axis in response to the adrenocorticotropic hormone (ACTH) secretion by the pituitary gland [8] (Figure 1). Synthetic glucocorticoids used in the treatment of cSLE include prednisone, prednisolone, hydrocortisone, and methylprednisolone (6-α methylprednisolone); the latter is chemically identical to prednisolone with the exception of a carbon group at position 6 [9]. The structure of the synthetic glucocorticoid hydrocortisone is chemically identical to that of the endogenous glucocorticoid cortisol [10].
Figure 1. Hypothalamic-pituitary-adrenal axis.
Cortisol secretion is primarily regulates by the ACTH. CRH (Corticotrophin-releasing hormone) is principal hypothalamic factor that stimulate the pituitary secretion of ACTH. Cortisol is the primary negative regulator of hypothalamic-pituitary- adrenal (HPA) axis activity through negative feedback upon the pituitary and the hypothalamus. Thus, both ACTH and CRH creation are inhibited.
The enzyme 11-beta-hydroxysteroid-dehydrogenase (11β-HSD) converts biologically active forms of synthetic and endogenous glucocorticoids into their biologically inactive forms and vice versa [11]. As such 11β-HSD transforms the biologically active prednisolone (or dehydrocortisol) to its inactive form prednisone (or dehydrocortisone), and vice versa [12]. Healthy adults produce about 10 mg of cortisol daily [13]. Cortisol levels show a circadian pattern, with peak plasma concentrations reached in the morning between 6:00am and 9:00am while cortical concentrations are lowest at night between 8:00pm and 2:00am [13].
In general, synthetic glucocorticoids are more potent than natural glucocorticoids (Figure2), due to their pharmacokinetic (PK) and pharmacodynamic (PD) properties [14]. The potency of a specific glucocorticoid, i.e., the dose needed to produce a particular biologic effect, is determined by its PK and PD parameters. The glucocorticoid PK properties include the time course for its absorption, distribution, metabolism, and excretion from the body, while its PD characteristics pertain to biochemical and physiologic effects on cell types and organs [15].
Figure 2.
Chemical structures of natural and synthetic glucocorticoids
Glucocorticoid pharmacokinetics - absorption
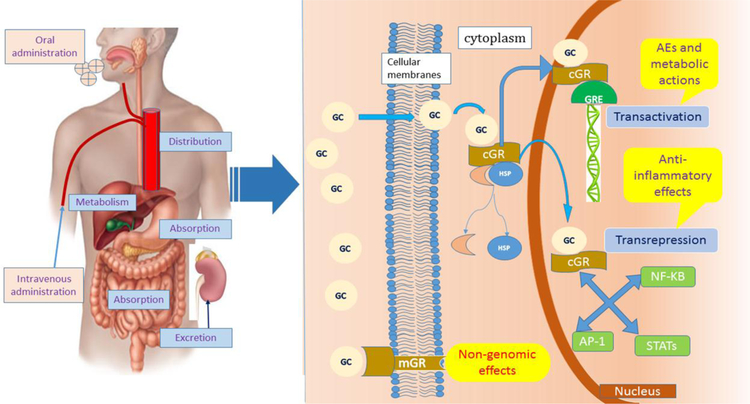
Oral administration is the most commonly used form of systemic glucocorticoid intake in the treatment of cSLE. Irrespective of concurrent food intake, the absorption of glucocorticoids is excellent, ranging from 60 to 100% [10]. The solubility of glucocorticoids in both lipid (fat) and water enhances absorption by the gastrointestinal tract, especially if the hydroxyl group of the synthetic glucocorticoids is acetylated, e.g. hydrocortisone. The addition of phosphate groups to synthetic glucocorticoids increases their aqueous solubility. Prednisolone sodium phosphate, the oral or ophthalmic preparation of commercially available prednisolone, is a classic example of a phosphate prodrug with high water solubility (>30 times greater than prednisolone without phosphate group) [16]. Intravenous administration bypasses the gastrointestinal absorption process and allows for a more rapid onset of action as compared to oral administration [17, 18].
Glucocorticoid pharmacokinetics - distribution
Endogenous and some synthetic glucocorticoids have a high affinity to transcortin, also known as corticosteroid binding globulin (CBG) [19]. Hydrocortisone and prednisolone have a higher affinity to transcortin than albumin, while albumin has a higher binding capacity than transcortin. Indeed, transcortin becomes fully saturated with plasma cortisol concentrations exceeding 200 ug/L, or equivalent doses of hydrocortisone or prednisolone of 20 mg, rendering the excess cortisol, hydrocortisone or prednisolone to be either bound to albumin or remain unbound [20]. In healthy individuals, more than 90% of circulating cortisol is bound tightly to transcortin [8], and about 5% of cortisol circulates in the unbound state in the plasma, while the remaining 5% is loosely bound to serum albumin or remains unbound [19].
The PK of prednisolone and prednisone are dose-dependent, given nonlinear protein binding. Therefore, with increasing concentrations from 200 μg/L to 800 μg/L protein binding of prednisolone decreases non-linearly from 95% to 60–70% [21]. Conversely, the PK of methylprednisolone is strictly linear, likely because it does not bind to transcortin but only binds to albumin [22].
Glucocorticoid pharmacokinetics - metabolism
The primary sites of cortisol metabolism in humans are the liver, kidneys and specific target tissues, including the lungs, adipose tissues, vascular beds, ovaries, and the central nervous system. Cytosolic and microsomal enzymes, such as cytochrome 450, 5α/5β reductase, 3α/3β oxidoreductase, and 11β-HSD, play an important role in the hepatic metabolism of cortisol [23]. There are two isoenzymes, 11β-HSD1 and 11β-HSD2 [11]: 11β-HSD1 is widely distributed in the glucocorticoid target tissues and acts as a reductase, catalyzing the conversion of hormonally inactive cortisone to hormonally active cortisol, and may contribute to the tissue hypersensitivity to glucocorticoids [24]; and 11β-HSD2 is only expressed in classic mineralocorticoid target tissues (kidney, colon, sweat glands and the placenta). Cushing disease results from absent or markedly decreased 11β-HSD2 activity [25].
As mentioned earlier cortisol and cortisone can be converted by the action of the enzyme 11β-HSD. Both cortisone and cortisol can be acted upon by 5α and 5β-reductases and 3α hydroxysteroid dehydrogenase, ultimately leading to the generation of tetrahydrocortisone (THE), 5β-tetrahydrocortisol (THF), and 5α-tetrahydrocortisol (allo-THF) [26].
Glucocorticoid pharmacokinetics - excretion
The inactive glucuronide and sulphate metabolites of glucocorticoids are eliminated via the kidneys. Only less than 1% of cortisol is excreted unchanged in the urine. The clearance of prednisolone is 210 mL/min per 1.73 m2, with an elimination half-life of approximately 3 hours [27, 28]. Clearance varies with the time of the day. Both prednisolone and methylprednisolone clearances are lower by 18 to 28% in the morning compared to the evening [28]. The above differences with glucocorticoid metabolism help explain the clinically noted variations in efficacy when the drug is given at different times of the cortisol diurnal rhythm and provides a rationale why morning intake of glucocorticoids is preferred.
Glucocorticoid pharmacodynamics- mechanisms of action and resistance
Only free or unbound glucocorticoids can interact with corticosteroid receptors and reach sites of action. Glucocorticoids exert their actions principally via intracellular receptors which belong to the nuclear receptor superfamily and regulate the transcription of target genes [29]. Two principal types of corticosteroid receptors have been identified, the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR), both belonging to the nuclear receptor superfamily [30]. The GR, also known as NR3C1 (nuclear receptor subfamily 3, group C, member 1) is bound to receptor-associated proteins, including heat-shock proteins. Binding of glucocorticoids to the GR results in dissociation of molecular chaperones from the GR. The MR has a higher affinity to glucocorticoids than the GR. Indeed, the Kd constant, which represents the concentration at which 50% of binding sites of receptors are occupied by drug, is 0.5–2 nM for the MR as compared to 10–20 nM for the GR. Thus, the MRs are responsible for mediating the effects of very low concentrations of glucocorticoids, while the GRs mediate the glucocorticoid biological effects once the MRs are saturated in the setting of free cortisol levels over 300 nM [29].
While the GRs are widely distributed throughout the body and present in almost all cell types, the MRs are principally localized to the distal renal tubules and cells/tissues concerned with Na+/K+ balance (e.g., sweat glands, parotid glands, and colon) [20, 31]. MRs are also located on neurons in specific brain regions, such as the limbic system, entorhinal cortex and, to a lesser extent, the hypothalamus. The ultimate process by which glucocorticoids would exert their anti-inflammatory effects would depend on their interaction with the respective GR or MR in a given tissue, i.e., the extent (capacity) of receptor binding and/or downstream physiological signaling mechanisms [32].
There are four recognized mechanisms that mediate the therapeutic effects of glucocorticoids: (1) the classic genomic mechanisms due to activation of cytosolic glucocorticoid receptors; (2) secondary non-genomic mechanisms provoked by the cytosolic glucocorticoid-GR complex (GC-GR complex); (3) non-genomic effects of membrane-bound GR; and (4) nonspecific, nongenomic effects caused by interactions with cellular membranes, including mitochondrial membranes [33–36].
According to the classic genomic theory of action, glucocorticoid molecules are highly lipophilic molecules that easily diffuse through the plasma membrane prior to binding to specific GRs which are located in the cell cytoplasm to form the GC-GR complex. This complex translocates into the nucleus, where it modulates the transcriptional activity of glucocorticoid responsive genes either by ‘transrepression’ or by ‘transactivation’ [35]. In ‘transrepression’, the GC-GR complex interferes with the activity of pro-inflammatory transcription factors such as activator protein 1 (AP-1), nuclear factor kB (NF-kB) and several signal transducers and activators of transcription (STATs), leading to down-regulation of pro-inflammatory protein synthesis. In ‘transactivation,’ the GC-GR complex binds to specific gene sequences, also called glucocorticoid-response elements (GREs), leading to the up-regulated synthesis of certain regulatory proteins via transcriptional activation [34]. The anti-inflammatory effects of glucocorticoids depend mainly on ‘transrepression’ with a few exceptions such as the upregulation of Annexin A1 (lipocortin 1) [37]. Conversely, adverse effects and metabolic actions of glucocorticoid therapy are mostly the result of ‘transactivation’ (Figure 3).
Figure 3. PK/PD of glucocorticoids in human body.
GC, glucocorticoids; GR, glucocorticoid receptor; mGR, membrane glucocorticoid receptor; cGR, cytosolic glucocorticoid receptor; GRE, glucocorticoid-response elements; AE, adverse effect; AP-1, activator protein 1; NF-κB nuclear factor κB; STATs, signal transducers and activators of transcription.
Anti-inflammatory effects of glucocorticoids are mainly based on classic genomic pathways of glucocorticoids. These take hours to days to lead to changes at the cell, tissue or organ level. The extent of GR saturation is directly dependent on GC dosage. At prednisolone equivalent dosages of >100 mg/day, the GR becomes 100% saturated, leading to the emergence of non-genomic mechanisms [38, 39]. Non-genomic effects of glucocorticoids do not require protein synthesis and occur rapidly within seconds or minutes. Therefore, non-genomic effects are thought to play an important role in mediating the therapeutic effects of intermediate to high doses of glucocorticoids, including high-dose pulse glucocorticoids [40]. Rapid immunosuppressive and anti-inflammatory effects of glucocorticoids are likely the result of non-genomic mechanisms. For instance, membrane-bound GRs, which mediate non-genomic glucocorticoid effects, are upregulated in patients with SLE and by inflammatory stimuli. Conversely, membrane-bound GRs are down regulated upon glucocorticoid exposure, suggesting a negative feedback loop [41]. Glucocorticoids increase the glomerular filtration rate, proximal tubular epithelial sodium transport, and free water clearance. Another effect of glucocorticoids is to counteract the increases in permeability and dilation of the capillary blood vessels with inflammation [20, 31]. Table 1 summarizes key PK/PD parameters of commonly used glucocorticoids.
Table 1.
Pharmacodynamic and pharmacokinetic parameters of glucocorticoids
| Glucocorticoids | Potencya | Plasma half-lifeb (minutes) |
Biological half-life (hours) |
Dose equivalence (mg) |
|---|---|---|---|---|
| Cortisone | 1 | 60 | 8–12 | 25 |
| Hydrocortisone | 1 | 60 | 8–12 | 20 |
| Prednisone | 4 | 180 | 12–36 | 5 |
| Prednisolone | 4 | 180 | 12–36 | 5 |
| Methylprednisolone | 5 | 180 | 12–36 | 4 |
Potency is a measurement of the strength of a drug that is required to produce a specific effect on the body. Potency=1/CE50. CE50 is the drug concentration that will achieve 50% of the maximal response.
Half-life is the time required for plasma serum concentration levels of an absorbed and distributed drug to decrease by one-half.
Patient & treatment factors that may impact the pharmacological properties of glucocorticoids
Besides dosing, the PK/PD properties of glucocorticoids are influenced, among others, by the patient sex, race, age, and concurrent medications used for SLE [14].
Indeed, white patients were found to have 50% higher methylprednisolone clearance than black patients in a sex and age matched study in renal transplant recipients [42]. Even after adjustment for body weight, free prednisolone oral clearance and apparent volume of distribution were higher in men compared with women, regardless of race [43]. The oral clearance was higher by 22% in white men compared to white women and by 40% in black men compared to black women [43]. The apparent volume of distribution was higher by 32% in white men compared to white women and by 38% in black men compared to black women [43]. Further, plasma clearance of unbound prednisolone decreases during adulthood [12]. Patients with hepatic or renal failure and renal transplant recipients show increased unbound prednisolone concentrations [44].
The PK of the glucocorticoids shows a circadian pattern. A specific glucocorticoid dose taken in the morning results in higher mean plasma concentrations than the very same dose taken in the evening [45]. Twice-daily administration of glucocorticoids has a higher total immunosuppressive effect but may also have adverse effects due to more significant suppression of the HPA axis [46].
Further, the female hormonal status influences the PK of glucocorticoids. During the luteal phase of the menstrual cycle, the methylprednisolone clearance is higher in women (0.45 versus 0.29 L/hr/kg) and the elimination half-life shorter (1.7 versus 2.6 hours) than in men [47]. A significantly smaller 50% inhibitory concentration (IC) value (0.1 versus 1.7 ng/ml) was seen in women as compared to men for suppression of cortisol secretion, indicating increased glucocorticoid sensitivity among women [47]. When considering increased clearance and increased glucocorticoid sensitivity together, it is generally presumed that there is a similar net response to methylprednisolone in men and women [47].
Multiple medications interact with the PK properties of glucocorticoids. The clearance of mycophenolate mofetil (MMF) is increased by glucocorticoids. This is because glucocorticoids induce uridine diphosphate glucuronosyl transferase, the enzyme involved in the MMF metabolism. Conversely, lowering or even discontinuing glucocorticoids will reduce the apparent plasma MMF clearance and thus enhance the bioavailability of mycophenolic acid, the pharmacologically active compound, after MMF intake [48]. Cyclophosphamide is converted by mixed-function oxidase enzymes (cytochrome P450 system) in the liver to its active metabolites. Glucocorticoids inhibit hepatic microsomal enzymes, resulting in a slower conversion of cyclophosphamide into its metabolites, consequently reducing its therapeutic and toxic effects [20, 31].
Individual responsiveness to therapy varies among patients with SLE, some patients requiring dose escalation to achieve adequate clinical response, suggesting the existence of glucocorticoid resistance. Several mechanisms for steroid resistance have been hypothesized. First, variability in the expression of GR has been linked to patterns of resistance [49]. A small study showed that increased expression of GR beta, a splice variant of GR, was related to high SLE activity as measured by the SLEDAI [50]. There are also specific genetic polymorphisms that help explain variability of GR expression and glucocorticoid responsiveness [51]. A second proposed mechanism underlying glucocorticoid resistance is the transport of intracellular steroids outside of the cell by membrane transporters. Patients with SLE with high disease activity who are considered resistant to glucocorticoids were found to have increased expression of the membrane associated transporter P-glycoprotein on lymphocytes [52]. The overproduction of nuclear factor kappa-B by activated plasmacytoid dendritic cells may be another source of glucocorticoid resistance in SLE [53].
Glucocorticoid use in SLE: past and present
Glucocorticoids are widely used for the management of SLE and have drastically improved the prognosis of this disease. The earliest report of a series of SLE patients receiving glucocorticoid treatment was published in 1952 [3, 4]. In this report, 18 patients were treated with high-dose of cortisone (200–300 mg/day) and tapering of cortisone occurred as SLE manifestations improved. During follow-up for 3 to 20 months, 12 patients survived, and 6 patients died of uncontrolled disease. These results were considered very promising, and authors even speculated that some patients might maintain remission indefinitely after corticosteroid treatment [4]. In 1967, a study at the Mayo clinic reported a definite trend toward prolonged survival among SLE patients who received high doses of prednisone (60–80 mg) compared to others not receiving prednisone or those receiving low-dose prednisone only (<40 mg/day) [54]. In 1977, Urman & Rothfield compared two SLE cohorts and reported that glucocorticoid therapy was associated with improved survival [55]. Two years later, investigators from the Massachusetts General Hospital showed that glucocorticoids were associated with improved survival among very ill (high-risk) patients [56]. Since the 1970’s very high doses of intravenous methylprednisolone (30 mg/kg/dose) are being used yielding rapid control of SLE associated inflammation, and leading to markedly sustained improvement of renal function among patients with lupus nephritis [57]. Taken together, the use of glucocorticoids, especially if combined with other immunosuppressants has led to a significant reduction of SLE related mortality, with 5-year and 10-year survival considerably increased from 74.8% to 94.8% and 63.2% to 91.4%, respectively [58].
The dose and duration of glucocorticoid therapy are essential considerations when treating SLE. Glucocorticoid regimens depend, among others, on the type of organ system involvement and the severity of symptoms [59]. The intensity of glucocorticoid therapy has been categorized into low intensity for prednisone equivalent doses <7.5 mg daily; medium intensity for doses ranging between 7.5 mg and 30 mg; high intensity for daily doses of 30 mg to 100 mg; and very high intensity for those exceeding 100 mg. The term ‘steroid pulse therapy’ is used when daily doses exceed 250 mg [7].
Glucocorticoids can be used for treating nearly every manifestation of SLE, although evidence guiding dose and duration of therapy remains limited. When systemic symptoms of SLE such as fever, fatigue, weight loss and lymphadenopathy occur in isolation, low to medium-dose glucocorticoids are often effective with the addition of hydroxychloroquine [39, 60]. Severe mucocutaneous manifestations often necessitate the use of medium to high dosages of glucocorticoids [39, 60]. When treating musculoskeletal manifestations up to 20 mg daily of prednisone are frequently used, and chronic low-dose glucocorticoids may occasionally be sufficient [60]. Myositis generally requires higher doses of glucocorticoids, especially in the initial treatment phase, with the addition of steroid sparing medications for severe or persistent symptoms [60]. More severe manifestations such as leukopenia, thrombocytopenia, antibody mediated hemolytic anemia, or aplastic anemia are typically treated with high-dose or pulse glucocorticoid therapy and immunosuppressive medications [60]. Pericarditis generally responds to moderate-dose steroid therapy while myocarditis is uncommon and requires high doses for effective control with concomitant aggressive immunosuppressants [60]. For severe pulmonary manifestations, such as pulmonary hemorrhage, pulse steroid is often administered along with cyclophosphamide whereas serositis is generally controlled with low to moderate dosing of glucocorticoids [60]. Shrinking lung disease, a rare complication, typically responds well to moderate to high-dose steroids, whereas pulmonary hypertension and interstitial lung disease are less likely to be steroid responsive [60]. Potentially severe gastrointestinal manifestations respond well to high-dose or pulse-dose glucocorticoids after other causes like infections are excluded [60]. Neuropsychiatric SLE (NPSLE) manifestations are potentially severe and oftentimes debilitating. Generally, glucocorticoid doses are dependent on the severity of symptoms but evidence-based dosing regimens are lacking [61]. Glucocorticoids are the first-line immunosuppressants indicated for severe or progressive NPSLE such as acute confusional state, myelitis, psychosis, and refractory seizures. Expert opinion supports the use of high-dose or pulse steroids in conjunction with aggressive immunosuppressive therapy for the treatment of NPSLE [61].
Use of glucocorticoids in SLE is best described for the treatment of lupus nephritis (LN). Pulse intravenous glucocorticoids (500–1,000 mg methylprednisolone daily for 3 doses) in combination with immunosuppressive therapy followed by daily oral glucocorticoids (0.5–1 mg/kg/day) have been recommended for induction therapy of adult patients with proliferative LN [62]. When cellular crescents are identified on renal biopsy, oral glucocorticoids exceeding 1 mg/kg/day are recommended. For adults with Class V LN and nephrotic range proteinuria, the suggested initial dose of prednisone is 0.5 mg/kg/day combined with MMF 2–3 gm total daily dose [62]. In recent years, the concept of “treat-to-target” therapy has been introduced with disease remission considered the ideal target when treating SLE patients in clinical trials and practice [63–65]. In treat-to-target therapy, glucocorticoid tapering is intended to reach disease remission. Particular concerns include the use of high doses of glucocorticoids, given the sizable risk of glucocorticoid related damage accrual. Glucocorticoid dosing is based on physician experience - or eminence - and remains non-standardized. There is no single evidence-based widely accepted recommendation for the reduction in glucocorticoid dose after successful control of the active disease manifestations, nor are there controlled studies to address glucocorticoid dosing in SLE [59, 66]. Nonetheless, it is generally accepted that for prolonged treatment, the glucocorticoid dosage should be kept to a minimum, and tapering should be attempted when remission or low disease activity states have been achieved [67].
Glucocorticoid use in childhood-onset SLE
Around 10–15% of all SLE patients experience disease onset during childhood, with cSLE patients experiencing more severe phenotypes, and more common kidney and neuropsychiatric involvement [59, 66]. Failure to achieve and maintain disease remission in children with LN reduces the overall 10-year survival by an estimated 15% [68]. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) recommend three distinct regimens for glucocorticoid dosing for the treatment of children with proliferative LN [69]. All three glucocorticoid regimens allow the use of three high-dose methylprednisolone pulses (30 mg/kg/dose up to 1,000 mg/dose) at the time of induction therapy initiation. The common goal for these three glucocorticoid regimens is to achieve a daily dose of oral glucocorticoid between 10 and 20 mg upon completion of the induction therapy after 24 weeks (or 6 months) [69]. In 2017, the SHARE (Single Hub and Access point for pediatric Rheumatology in Europe) initiative recommended that low-dose prednisone (<0.5 mg/kg/day) could be considered in Class I LN. First-line treatment of Class II LN consists of prednisone at a starting dose of 0.25–0.5 mg/kg/day, with a maximum of 30 mg/day followed by a tapering schedule over a total duration of 3–6 months. In case of severe disease, intravenous methylprednisolone pulses and high-dose prednisone (initially 1–2 mg/kg/day, gradually weaned) should be added to the treatment of LN [70, 71]. However, cSLE treatment approaches can vary among clinicians even within the same center [72], cSLE management remains dramatically variable, and additional efforts are needed to standardize treatment strategies.
Adverse effects of glucocorticoids
It is well known that glucocorticoids produce a broad spectrum of adverse effects at many organ levels (Table 2). Children may be even more susceptible to adverse events associated with glucocorticoid use than adults. Hirsutism, moon facies, buffalo hump, acne, striae and weight gain are well recognized side effects of glucocorticoids. Some side effects such as myopathy and hyperlipidemia occur with large doses of glucocorticoids. Skeletal growth inhibition, HPA-axis suppression, glucocorticoid induced osteonecrosis, cataract, acne, skin bruising, and weight gain have been reported to occur with chronic low-dose glucocorticoid use [73, 74]. Increased susceptibility to major infections is another notable adverse effect especially at higher glucocorticoid doses [75]. Psychological and behavioral disorders, including disturbances of mood, cognition, and psychosis, are also known steroid side effects and tend to occur early in the course of treatment and are dose dependent [76].
Table 2.
Major adverse effects of glucocorticoids
| Category | Adverse effect |
|---|---|
| General | Growth retardation |
| Weight gain | |
| Cushingoid features | Moon facies, facial plethora |
| Central obesity | |
| Buffalo hump | |
| Skin | Cutaneous atrophy |
| Increased skin fragility | |
| Hirsutism & Acne | |
| Striae | |
| Musculoskeletal | Myopathy |
| Osteoporosis | |
| Avascular necrosis of femur head | |
| Growth failure | |
| Metabolic and endocrine | Hyperglycemia/Diabetes |
| Lipolytic effects | |
| Perturbations of serum lipoproteins | |
| Suppression of HPA axis (adrenal insufficiency) | |
| Delayed puberty | |
| Gastrointestinal | Gastritis |
| Gastrointestinal bleed or ulcer | |
| Pancreatitis | |
| Cardiovascular system | Hypertension |
| Congestive heart failure | |
| Neuropsychiatric | Sleep disturbances, insomnia |
| Mood problems: Depression, anxiety, loss of emotional control | |
| Euphoria and hypomania | |
| Manic behavior | |
| Psychosis | |
| Ophthalmologic | Cataract formation |
| Glaucoma | |
| Immune system | Increased infections |
Previous reports from the Hopkins Lupus Cohort have demonstrated a reduced risk of organ damage associated with average doses of prednisone <6 mg/day [77]. It has been suggested that prednisone at <7.5 mg/day does not increase overall or glucocorticoid related damage in patients with SLE and that methylprednisolone pulses are not associated with new damage accrual in patients with SLE [78]. The relevance of low-dose glucocorticoid exposure on SLE damage is unclear given contradictory results from available studies [78, 79].
The strongest associations for glucocorticoid associated damage were observed with the development of cataracts, osteoporotic fractures, and cardiovascular damage. Glucocorticoids are proposed independent risk factors for cardiovascular diseases [80].
Data from the Toronto cohort showed that 49% of damage accrual in adults with SLE is likely to be attributable to glucocorticoid use [81]. In contrast to the damage in patients with adult-onset SLE, which is often steroid related (e.g. cataract, osteoporosis), damage in cSLE has been predominantly disease related, highlighting the severity of the disease and the relatively good tolerance of children under aggressive treatment [82]. In a study to identify risk factors for damage in cSLE, 66 patients with newly diagnosed cSLE were assessed retrospectively. It was found that ongoing disease activity leads to disease damage, and prolonged use of high-dose corticosteroids may further increase damage [83].
In a recent study of 2,265 patients from the Hopkins Cohort, a prednisone dose ≥7.5 mg/day was associated with an increased risk of developing cataract, osteoporotic fractures, and cardiovascular damage, but not renal damage [84]. After 15 years of follow-up, they found that every increase in the daily prednisone dose by 1 mg increases the risk of cataract by 3.8% and osteoporotic fractures by 4.2% [84]. Cataract and glaucoma can lead to visual impairment, resulting in significant disability and cost to the healthcare system [85, 86]. In particular, glucocorticoid exposure can lead to posterior subcapsular cataract. Similarly, steroid induced glaucoma, a type of open angle glaucoma occurs more frequently in glucocorticoid exposed patients [87]. Husher et al. have shown that the risk of glaucoma appears to increase for prednisone dosages over 7.5 mg per day [88] while posterior subcapsular cataract can develop even after chronic treatment with as little as 5 mg of prednisone per day [88]. Nonetheless, the relationship of dose and duration of glucocorticoid therapy and the development of ocular damage remains largely unknown. Although some observational studies found an association between glucocorticoid exposure and SLE damage, the amount of risk conveyed by chronic glucocorticoid exposure has not been well delineated. Therefore, it remains difficult to quantify the risk of ocular damage associated with the use of a certain glucocorticoid regimen in a way that can be used in discussions with patients about glucocorticoid treatment [89]. It has been recommended to screen patients on chronic glucocorticoid therapy for ocular toxicity at least annually, and more often if there are visual disturbances or a known eye pathology [90, 91].
The impact of glucocorticoids on the skeletal system, especially on the growing skeleton, deserves special attention. Glucocorticoids affect bone formation and slow longitudinal growth by reducing the proliferation of chondrocytes and inducing chondrocyte apoptosis [92]. Growth retardation and delayed puberty are common in children and growing adolescents with chronic glucocorticoid use. The suppression of linear growth in children is multifactorial. Chronic hypercortisolism caused by adrenal suppression results in reduced growth hormone secretion. The principal underlying mechanism seems to be an increase hypothalamic somatostatin tone [93]. Another proposed mechanism is glucocorticoid-induced resistance of target tissues to insulin-like growth factor-I (IGF-I) and other growth factors [94]. Glucocorticoids also inhibit intestinal calcium absorption and increase renal calcium excretion. Although there is generally a period of catch-up growth once glucocorticoid therapy is stopped, sustained glucocorticoid treatment during childhood is often associated with decreased adult stature. In a study by the Pediatric Rheumatology International Trials Organization (PRINTO), growth and puberty were evaluated in 1,015 children with cSLE. Growth failure and delayed puberty were observed in 15.3% and 11.3% of the children, respectively [95]. A study by Rygg et al. highlights the negative effects of glucocorticoids on height and pubertal development. Among 331 cSLE patients younger than 18 years of age, growth failure was seen in 14.7% of females and 24.5% of males. Delayed pubertal onset was seen in 15.3% and 24% of the females and males, respectively, while 36.1% of the females and 44% of the males had some degree of delayed pubertal development [96]. These detrimental effects were most pronounced in children whose cSLE commenced around the time of puberty, especially when treated with cumulative doses of prednisone exceeding 400 mg/kg body weight [96]. It has been suggested that delayed puberty should be an additional domain in a pediatric version of the Systemic Lupus International Collaborative Clinics/American College of Rheumatology Damage Index (SDI) [97].
The 2000 National Institutes of Health Osteoporosis Prevention, Diagnosis, and Therapy Consensus Development Conference identified bone accrual during childhood as a critical determinant of lifelong skeletal health [80, 98]. Children with Cushing syndrome have delayed or arrested growth and achieve a final adult height, which is on average 7.5–8.0 cm below their predicted height [99, 100]. In children with rheumatic diseases exposed to moderate and high doses of glucocorticoids, the estimated mean height Z scores declined, and there was no catch-up growth during the 18-month study period [101]. The American College of Rheumatology guidelines for prevention or reversal of bone loss suggest that all patients taking glucocorticoids - at any dose with an anticipated duration of over 3 months - should maintain a total calcium intake of 1000 to 1200 mg/day and vitamin D intake of 600 to 800 international units/day [102]. The same has been suggested for the treatment of children with cSLE [103]. The American College of Rheumatology guidelines for prevention or reversal of bone loss emphasize starting bisphosphonate therapy for adults receiving long-term glucocorticoids (≥5 mg/day prednisone or equivalent) [104].
Prolonged courses of glucocorticoids increase risk of major infections, a leading cause of mortality in SLE. In a nested case-control lupus cohort, prednisone dose was found to be an independent predictor of serious infections. The median prednisone dose in patients with major infections was only 7.5 mg/day. It was also noted that each increase by 10 mg/day of prednisone multiplied by 11 the risk of suffering a serious infection [105]. A large cohort study of 15,597 rheumatoid arthritis patients from a Medicare beneficiary database reported that glucocorticoid use doubled the rate of serious bacterial infections compared with methotrexate use (RR 2.1, 95% CI 1.5–3.1). There was a dose-dependent risk for prednisone dosages greater than 5 mg/day (≤5 mg/day, RR [1.34; 6–9 mg/day, RR 1.53; 10–19 mg/day, RR 2.97; ≥20 mg/day, RR 5.48; P for trend <0.0001]) [106]. Fungal, bacterial and viral infections may also complicate glucocorticoid use [107–109]. A multicenter study in a large cSLE population identified prednisone as being an independent predictor for the development of herpes zoster infection [109].
Finally, glucocorticoids may produce many psychological and behavioral disorders. The available data suggest that the psychiatric symptoms during glucocorticoid therapy include mania, depression, lability, and psychosis. The most common adverse effects of short-term glucocorticoid treatment are euphoria and hypomania, whereas long-term therapy may induce depressive symptoms [110]. Such disturbances are dose related and may occur immediately after the initiation of treatment and even after discontinuation of treatment. The Boston Collaborative Drug Surveillance Program found that the incidence of psychiatric manifestations was 1.3% in patients receiving 40 mg/day prednisone equivalent or less and 18.4% in patients receiving doses >80 mg/day [111]. Headache, cognitive dysfunction, mood disturbances and seizures were reported to be the most common neuropsychiatric manifestations of cSLE [112]. In a study aiming to assess neuropsychiatric manifestations in cSLE, 41 (28%) of 146 patients developed neurological disease during a median follow-up period of 6 years (range 1–20 years). The mean age at the onset of symptoms was 10.2 ± 3 years. Headache was the most common feature (13%), cognitive disorders were diagnosed in 17 patients (11.6%), 8.9% had psychiatric disorders, and 9.5% developed seizures [113].
Quality indicators in patients on long term glucocorticoid treatment
Quality indicators are minimum standards of medical care. It is postulated that if medical care follows standards suggested by the quality indicators then disease prognosis will be improved [114, 115]. In a study by Hollander et al, emphasis was placed on the fact that quality indicators proposed for adults with SLE do not sufficiently capture issues important to the delivery of medical care in cSLE [103]. Aspects of care that need special consideration in cSLE include growth and development as well as transition of care. Some of the quality indicators can be considered especially relevant for the surveillance of children with cSLE exposed to chronic glucocorticoids. Table 3 summarizes quality indicators that can be considered relevant for glucocorticoid surveillance in cSLE.
Table 3.
Quality indicators for monitoring of glucocorticoid use in childhood-onset SLE1
|
|
Adapted from Hollander MC, Sage JM, Greenler AJ, et al. International consensus for provisions of quality-driven care in childhood-onset systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2013; 65: 1416–23.
Conclusion
Glucocorticoids are potent anti-inflammatory and immunosuppressant medications that rapidly and effectively suppress the immune system. The treatments of SLE and cSLE remain complex and continue to rely heavily on glucocorticoids in the absence of other potent anti-inflammatory drugs with comparable effectiveness. Glucocorticoids need to be vigorously monitored to ensure their use at the lowest level, given side effects especially with chronic exposure. A better understanding of the pharmacology of glucocorticoids may assist clinicians in adjusting glucocorticoid regimens. Despite their widespread use for the treatment of lupus, evidence-based guidelines on initiation, tapering, and cessation of glucocorticoids for lupus management remain elusive. Such guidelines would likely help to limit glucocorticoid associated damage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Burns CM, “The History of Cortisone Discovery and Development,” (in eng), Rheum Dis Clin North Am, vol. 42, no. 1, pp. 1–14, vii, February 2016. [DOI] [PubMed] [Google Scholar]
- [2].Bollet AJ, Segal S, and Bunim JJ, “Treatment of systemic lupus erythematosus with prednisone and prednisolone,” (in eng), J Am Med Assoc, vol. 159, no. 16, pp. 1501–7, December 17 1955. [DOI] [PubMed] [Google Scholar]
- [3].Du BE, Commons RR, Starr P, Stein CS Jr., and Morrison R, “Corticotropin and cortisone treatment for systemic lupus erythematosus,” (in eng), J Am Med Assoc, vol. 149, no. 11, pp. 995–1002, July 12 1952. [DOI] [PubMed] [Google Scholar]
- [4].Soffer LJ and Bader R, “Corticotropin and cortisone in acute disseminated lupus erythematosus; results of long-term use,” (in eng), J Am Med Assoc, vol. 149, no. 11, pp. 1002–8, July 12 1952. [DOI] [PubMed] [Google Scholar]
- [5].Dubois EL, “Prednisone and prednisolone in the treatment of systemic lupus erythematous,” (in eng), J Am Med Assoc, vol. 161, no. 5, pp. 427–33, June 2 1956. [DOI] [PubMed] [Google Scholar]
- [6].Ardoin SP and Schanberg LE, “The management of pediatric systemic lupus erythematosus,” (in eng), Nat Clin Pract Rheumatol, vol. 1, no. 2, pp. 82–92, December 2005. [DOI] [PubMed] [Google Scholar]
- [7].Buttgereit F et al. , “Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology,” (in eng), Ann Rheum Dis, vol. 61, no. 8, pp. 718–22, August 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yaffe SJ and Aranda JV, Neonatal and pediatric pharmacology: therapeutic principles in practice, 4th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health, 2010, pp. xxiii, 1042 p. [Google Scholar]
- [9].Ebling WF, Milsap RL, Szefler SJ, and Jusko WJ, “6 alpha-Methylprednisolone and 6 alpha-methylprednisone plasma protein binding in humans and rabbits,” (in eng), J Pharm Sci, vol. 75, no. 8, pp. 760–3, August 1986. [DOI] [PubMed] [Google Scholar]
- [10].Derendorf H, Mollmann H, Barth J, Mollmann C, Tunn S, and Krieg M, “Pharmacokinetics and oral bioavailability of hydrocortisone,” (in eng), J Clin Pharmacol, vol. 31, no. 5, pp. 473–6, May 1991. [DOI] [PubMed] [Google Scholar]
- [11].Diederich S et al. , “11beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids,” (in eng), J Clin Endocrinol Metab, vol. 87, no. 12, pp. 5695–701, December 2002. [DOI] [PubMed] [Google Scholar]
- [12].Frey BM and Frey FJ, “Clinical pharmacokinetics of prednisone and prednisolone,” (in eng), Clin Pharmacokinet, vol. 19, no. 2, pp. 126–46, August 1990. [DOI] [PubMed] [Google Scholar]
- [13].Esteban NV et al. , “Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry,” (in eng), J Clin Endocrinol Metab, vol. 72, no. 1, pp. 39–45, January 1991. [DOI] [PubMed] [Google Scholar]
- [14].Czock D, Keller F, Rasche FM, and Haussler U, “Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids,” (in eng), Clin Pharmacokinet, vol. 44, no. 1, pp. 61–98, 2005. [DOI] [PubMed] [Google Scholar]
- [15].Johnston M, Davis K, and Gricar J, The pharmacy technician: foundations and practices. Upper Saddle River, NJ: Pearson, 2009, pp. xxiii, 788 p. [Google Scholar]
- [16].Huttunen KM, Raunio H, and Rautio J, “Prodrugs--from serendipity to rational design,” (in eng), Pharmacol Rev, vol. 63, no. 3, pp. 750–71, September 2011. [DOI] [PubMed] [Google Scholar]
- [17].Al-Habet SM and Rogers HJ, “Methylprednisolone pharmacokinetics after intravenous and oral administration,” (in eng), Br J Clin Pharmacol, vol. 27, no. 3, pp. 285–90, March 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Boumpas DT et al. , “Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis,” (in eng), Lancet, vol. 340, no. 8822, pp. 741–5, September 26 1992. [DOI] [PubMed] [Google Scholar]
- [19].Brien TG, “Human corticosteroid binding globulin,” (in eng), Clin Endocrinol (Oxf), vol. 14, no. 2, pp. 193–212, February 1981. [DOI] [PubMed] [Google Scholar]
- [20].Abraham DJ, Rotella DP, and Burger A, Burgers medicinal chemistry, drug discovery and development, 7th ed. Hoboken, N.J.: Wiley, 2010. [Google Scholar]
- [21].Wald JA, Law RM, Ludwig EA, Sloan RR, Middleton E Jr., and Jusko WJ, “Evaluation of dose-related pharmacokinetics and pharmacodynamics of prednisolone in man,” (in eng), J Pharmacokinet Biopharm, vol. 20, no. 6, pp. 567–89, December 1992. [DOI] [PubMed] [Google Scholar]
- [22].Rohatagi S et al. , “Pharmacokinetics of methylprednisolone and prednisolone after single and multiple oral administration,” (in eng), J Clin Pharmacol, vol. 37, no. 10, pp. 916–25, October 1997. [DOI] [PubMed] [Google Scholar]
- [23].Draper N and Stewart PM, “11beta-hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action,” (in eng), J Endocrinol, vol. 186, no. 2, pp. 251–71, August 2005. [DOI] [PubMed] [Google Scholar]
- [24].Diederich S et al. , “Metabolism of synthetic corticosteroids by 11 beta-hydroxysteroiddehydrogenases in man,” (in eng), Steroids, vol. 63, no. 5–6, pp. 271–7, May-Jun 1998. [DOI] [PubMed] [Google Scholar]
- [25].Seckl JR, “11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action,” (in eng), Curr Opin Pharmacol, vol. 4, no. 6, pp. 597–602, December 2004. [DOI] [PubMed] [Google Scholar]
- [26].Tomlinson JW and Stewart PM, “Cortisol metabolism and the role of 11beta-hydroxysteroid dehydrogenase,” (in eng), Best Pract Res Clin Endocrinol Metab, vol. 15, no. 1, pp. 61–78, March 2001. [DOI] [PubMed] [Google Scholar]
- [27].Hill MR, Szefler SJ, Ball BD, Bartoszek M, and Brenner AM, “Monitoring glucocorticoid therapy: a pharmacokinetic approach,” (in eng), Clin Pharmacol Ther, vol. 48, no. 4, pp. 390–8, October 1990. [DOI] [PubMed] [Google Scholar]
- [28].Xu J, Winkler J, Sabarinath SN, and Derendorf H, “Assessment of the impact of dosing timeon the pharmacokinetics/pharmacodynamics of prednisolone,” (in eng), Aaps j, vol. 10, no. 2, pp. 331–41, June 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Buckingham JC, “Glucocorticoids: exemplars of multi-tasking,” (in eng), Br J Pharmacol, vol. 147 Suppl 1, pp. S258–68, January 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Evans RM, “The steroid and thyroid hormone receptor superfamily,” (in eng), Science, vol. 240, no. 4854, pp. 889–95, May 13 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Avery MA and Chittiboyina AG, Anti-inflammatory glucocorticoids in Burgers Medicinal Chemistry, drug discovery and development. John Wiley& Sons Inc. [Google Scholar]
- [32].Jusko WJ, “Moving from basic toward systems pharmacodynamic models,” (in eng), J Pharm Sci, vol. 102, no. 9, pp. 2930–40, September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rhen T and Cidlowski JA, “Antiinflammatory action of glucocorticoids--new mechanisms for old drugs,” (in eng), N Engl J Med, vol. 353, no. 16, pp. 1711–23, October 20 2005. [DOI] [PubMed] [Google Scholar]
- [34].Spies CM, Strehl C, van der Goes MC, Bijlsma JW, and Buttgereit F, “Glucocorticoids,” (in eng), Best Pract Res Clin Rheumatol, vol. 25, no. 6, pp. 891–900, December 2011. [DOI] [PubMed] [Google Scholar]
- [35].Coutinho AE and Chapman KE, “The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights,” (in eng), Mol Cell Endocrinol, vol. 335, no. 1, pp. 2–13, March 15 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stahn C and Buttgereit F, “Genomic and nongenomic effects of glucocorticoids,” (in eng), Nat Clin Pract Rheumatol, vol. 4, no. 10, pp. 525–33, October 2008. [DOI] [PubMed] [Google Scholar]
- [37].Goodman LS, Brunton LL, Chabner B, and Knollmann B. r. C., Goodman & Gilmans pharmacological basis of therapeutics, 12th ed. New York: McGraw-Hill, 2011, p. 2084p. [Google Scholar]
- [38].Buttgereit F, Wehling M, and Burmester GR, “A new hypothesis of modular glucocorticoid actions: steroid treatment of rheumatic diseases revisited,” (in eng), Arthritis Rheum, vol. 41, no. 5, pp. 761–7, May 1998. [DOI] [PubMed] [Google Scholar]
- [39].Luijten RK, Fritsch-Stork RD, Bijlsma JW, and Derksen RH, “The use of glucocorticoids in systemic lupus erythematosus. After 60 years still more an art than science,” (in eng), Autoimmun Rev, vol. 12, no. 5, pp. 617–28, March 2013. [DOI] [PubMed] [Google Scholar]
- [40].Lipworth BJ, “Therapeutic implications of non-genomic glucocorticoid activity,” (in eng), Lancet, vol. 356, no. 9224, pp. 87–9, July 8 2000. [DOI] [PubMed] [Google Scholar]
- [41].Spies CM et al. , “Membrane glucocorticoid receptors are down regulated by glucocorticoids in patients with systemic lupus erythematosus and use a caveolin-1-independent expression pathway,” (in eng), Ann Rheum Dis, vol. 65, no. 9, pp. 1139–46, September 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tornatore KM, Reed KA, and Venuto RC, “Racial differences in the pharmacokinetics of methylprednisolone in black and white renal transplant recipients,” (in eng), Pharmacotherapy, vol. 13, no. 5, pp. 481–6, Sep-Oct 1993. [PubMed] [Google Scholar]
- [43].Magee MH, Blum RA, Lates CD, and Jusko WJ, “Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race,” (in eng), J Clin Pharmacol, vol. 41, no. 11, pp. 1180–94, November 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Honore PM et al. , “What do we know about steroids metabolism and PK/PD approach in AKI and CKD especially while on RRT--current status in 2014,” (in eng), Blood Purif, vol. 38, no. 2, pp. 154–7, 2014. [DOI] [PubMed] [Google Scholar]
- [45].Barth J, Damoiseaux M, Mollmann H, Brandis KH, Hochhaus G, and Derendorf H, “Pharmacokinetics and pharmacodynamics of prednisolone after intravenous and oral administration,” (in eng), Int J Clin Pharmacol Ther Toxicol, vol. 30, no. 9, pp. 317–24, September 1992. [PubMed] [Google Scholar]
- [46].Uhl A, Czock D, Boehm BO, Zellner D, Mertz A, and Keller F, “Pharmacokinetics and pharmacodynamics of methylprednisolone after one bolus dose compared with two dose fractions,” (in eng), J Clin Pharm Ther, vol. 27, no. 4, pp. 281–7, August 2002. [DOI] [PubMed] [Google Scholar]
- [47].Lew KH et al. , “Gender-based effects on methylprednisolone pharmacokinetics and pharmacodynamics,” (in eng), Clin Pharmacol Ther, vol. 54, no. 4, pp. 402–14, October 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cattaneo D, Perico N, Gaspari F, Gotti E, and Remuzzi G, “Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation,” (in eng), Kidney Int, vol. 62, no. 3, pp. 1060–7, September 2002. [DOI] [PubMed] [Google Scholar]
- [49].Du J et al. , “Flow cytometry analysis of glucocorticoid receptor expression and binding in steroid-sensitive and steroid-resistant patients with systemic lupus erythematosus,” (in eng), Arthritis Res Ther, vol. 11, no. 4, p. R108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Piotrowski P et al. , “Glucocorticoid receptor beta splice variant expression in patients with high and low activity of systemic lupus erythematosus,” (in eng), Folia Histochem Cytobiol, vol. 45, no. 4, pp. 339–42, 2007. [PubMed] [Google Scholar]
- [51].Zou YF et al. , “Association study of glucocorticoid receptor genetic polymorphisms with efficacy of glucocorticoids in systemic lupus erythematosus: a prospective cohort study,” (in eng), Autoimmunity, vol. 46, no. 8, pp. 531–6, December 2013. [DOI] [PubMed] [Google Scholar]
- [52].Tsujimura S, Saito K, Nakayamada S, Nakano K, and Tanaka Y, “Clinical relevance of the expression of P-glycoprotein on peripheral blood lymphocytes to steroid resistance in patients with systemic lupus erythematosus,” (in eng), Arthritis Rheum, vol. 52, no. 6, pp. 1676–83, June 2005. [DOI] [PubMed] [Google Scholar]
- [53].Guiducci C et al. , “TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus,” (in eng), Nature, vol. 465, no. 7300, pp. 937–41, June 17 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hagge WW, Burke EC, and Stickler GB, “Treatment of systemic lupus erythematosus complicated by nephritis in children,” (in eng), Pediatrics, vol. 40, no. 5, pp. 822–7, November 1967. [PubMed] [Google Scholar]
- [55].Urman JD and Rothfield NF, “Corticosteroid treatment in systemic lupus erythematosus. Survival studies,” (in eng), Jama, vol. 238, no. 21, pp. 2272–6, November 21 1977. [PubMed] [Google Scholar]
- [56].Albert DA, Hadler NM, and Ropes MW, “Does corticosteroid therapy affect the survival of patients with systemic lupus erythematosus?,” (in eng), Arthritis Rheum, vol. 22, no. 9, pp. 945–53, September 1979. [DOI] [PubMed] [Google Scholar]
- [57].Chatham WW and Kimberly RP, “Treatment of lupus with corticosteroids,” (in eng), Lupus, vol. 10, no. 3, pp. 140–7, 2001. [DOI] [PubMed] [Google Scholar]
- [58].Mak A, Cheung MW, Chiew HJ, Liu Y, and Ho RC, “Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and meta-regression of observational studies from the 1950s to 2000s,” (in eng), Semin Arthritis Rheum, vol. 41, no. 6, pp. 830–9, June 2012. [DOI] [PubMed] [Google Scholar]
- [59].Lahita RG, Systemic lupus erythematosus, 5th ed. Amsterdam; New York: Elsevier Academic Press, 2011, pp. xix, 1134 p. [Google Scholar]
- [60].Kasturi S and Sammaritano LR, “Corticosteroids in Lupus,” (in eng), Rheum Dis Clin North Am, vol. 42, no. 1, pp. 47-62, viii, February 2016. [DOI] [PubMed] [Google Scholar]
- [61].Kamen DL and Zollars ES, “Corticosteroids in Lupus Nephritis and Central Nervous System Lupus,” (in eng), Rheum Dis Clin North Am, vol. 42, no. 1, pp. 63–73, viii, February 2016. [DOI] [PubMed] [Google Scholar]
- [62].Hahn BH et al. , “American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis,” (in eng), Arthritis Care Res (Hoboken), vol. 64, no. 6, pp. 797–808, June 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].van Vollenhoven R et al. , “A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS),” (in eng), Ann Rheum Dis, vol. 76, no. 3, pp. 554–561, March 2017. [DOI] [PubMed] [Google Scholar]
- [64].Tani C, Vagelli R, Stagnaro C, Carli L, and Mosca M, “Remission and low disease activity in systemic lupus erythematosus: an achievable goal even with fewer steroids? Real-life data from a monocentric cohort,” (in eng), Lupus Sci Med, vol. 5, no. 1, p. e000234, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].van Vollenhoven RF et al. , “Treat-to-target in systemic lupus erythematosus: recommendations from an international task force,” (in eng), Ann Rheum Dis, vol. 73, no. 6, pp. 958–67, June 2014. [DOI] [PubMed] [Google Scholar]
- [66].Olga Dvorkina EMG, Corticosteroid and Nonsteroidal Anti-Inflammatory Drug use in Systemic Lupus Erythematosus, in Systemic Lupus Erythematosus( 5th edition). 2011. [Google Scholar]
- [67].Hoes JN et al. , “EULAR evidence-based recommendations on the management of systemic glucocorticoid therapy in rheumatic diseases,” (in eng), Ann Rheum Dis, vol. 66, no. 12, pp. 1560–7, December 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Marks SD, Sebire NJ, Pilkington C, and Tullus K, “Clinicopathological correlations of paediatric lupus nephritis,” (in eng), Pediatr Nephrol, vol. 22, no. 1, pp. 77–83, January 2007. [DOI] [PubMed] [Google Scholar]
- [69].Mina R et al. , “Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus,” Arthritis Care Res (Hoboken), vol. 64, no. 3, pp. 375–83, March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Groot N et al. , “European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative,” (in eng), Ann Rheum Dis, vol. 76, no. 11, pp. 1788–1796, November 2017. [DOI] [PubMed] [Google Scholar]
- [71].Groot N et al. , “European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative,” (in eng), Ann Rheum Dis, vol. 76, no. 12, pp. 1965–1973, December 2017. [DOI] [PubMed] [Google Scholar]
- [72].Brunner HI, Klein-Gitelman MS, Ying J, Tucker LB, and Silverman ED, “Corticosteroid use in childhood-onset systemic lupus erythematosus-practice patterns at four pediatric rheumatology centers,” (in eng), Clin Exp Rheumatol, vol. 27, no. 1, pp. 155–62, Jan-Feb 2009. [PubMed] [Google Scholar]
- [73].Curtis JR et al. , “Population-based assessment of adverse events associated with long-term glucocorticoid use,” (in eng), Arthritis Rheum, vol. 55, no. 3, pp. 420–6, June 15 2006. [DOI] [PubMed] [Google Scholar]
- [74].McDonough AK, Curtis JR, and Saag KG, “The epidemiology of glucocorticoid-associated adverse events,” (in eng), Curr Opin Rheumatol, vol. 20, no. 2, pp. 131–7, March 2008. [DOI] [PubMed] [Google Scholar]
- [75].Apostolopoulos D and Morand EF, “It hasnt gone away: the problem of glucocorticoid use in lupus remains,” (in eng), Rheumatology (Oxford), vol. 56, no. suppl_1, pp. i114–i122, April 1 2017. [DOI] [PubMed] [Google Scholar]
- [76].Brown ES and Chandler PA, “Mood and Cognitive Changes During Systemic Corticosteroid Therapy,” (in eng), Prim Care Companion J Clin Psychiatry, vol. 3, no. 1, pp. 17–21, February 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Thamer M, Hernan MA, Zhang Y, Cotter D, and Petri M, “Prednisone, lupus activity, and permanent organ damage,” (in eng), J Rheumatol, vol. 36, no. 3, pp. 560–4, March 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I, Medina JA, Moran MA, and Ruiz-Irastorza G, “Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus,” (in eng), Rheumatology (Oxford), vol. 53, no. 8, pp. 1470–6, August 2014. [DOI] [PubMed] [Google Scholar]
- [79].Bruce IN et al. , “Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort,” (in eng), Ann Rheum Dis, vol. 74, no. 9, pp. 1706–13, September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].“Osteoporosis prevention, diagnosis, and therapy,” (in eng), Jama, vol. 285, no. 6, pp. 785–95, February 14 2001. [DOI] [PubMed] [Google Scholar]
- [81].Gladman DD, Urowitz MB, Rahman P, Ibanez D, and Tam LS, “Accrual of organ damage over time in patients with systemic lupus erythematosus,” (in eng), J Rheumatol, vol. 30, no. 9, pp. 1955–9, September 2003. [PubMed] [Google Scholar]
- [82].Watson L et al. , “Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort,” (in eng), Arthritis Rheum, vol. 64, no. 7, pp. 2356–65, July 2012. [DOI] [PubMed] [Google Scholar]
- [83].Brunner HI, Silverman ED, To T, Bombardier C, and Feldman BM, “Risk factors for damage in childhood-onset systemic lupus erythematosus: cumulative disease activity and medication use predict disease damage,” (in eng), Arthritis Rheum, vol. 46, no. 2, pp. 436–44, February 2002. [DOI] [PubMed] [Google Scholar]
- [84].Al Sawah S et al. , “Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort,” (in eng), Lupus Sci Med, vol. 2, no. 1, p. e000066, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Black RL, Oglesby RB, Von Sallmann L, and Bunim JJ, “Posterior subcapsular cataracts induced by corticosteroids in patients with rheumatoid arthritis,” (in eng), Jama, vol. 174, pp. 166–71, September 10 1960. [DOI] [PubMed] [Google Scholar]
- [86].Stern JJ, “Acute glaucoma during cortisone therapy,” (in eng), Am J Ophthalmol, vol. 36, no. 3, pp. 389–90, March 1953. [DOI] [PubMed] [Google Scholar]
- [87].Taylor HR et al. , “Vision loss in Australia,” (in eng), Med J Aust, vol. 182, no. 11, pp. 565–8, June 6 2005. [DOI] [PubMed] [Google Scholar]
- [88].Huscher D et al. , “Dose-related patterns of glucocorticoid-induced side effects,” (in eng), Ann Rheum Dis, vol. 68, no. 7, pp. 1119–24, July 2009. [DOI] [PubMed] [Google Scholar]
- [89].Black RJ, Hill CL, Lester S, and Dixon WG, “The Association between Systemic Glucocorticoid Use and the Risk of Cataract and Glaucoma in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis,” (in eng), PLoS One, vol. 11, no. 11, p. e0166468, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mosca M et al. , “European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies,” (in eng), Ann Rheum Dis, vol. 69, no. 7, pp. 1269–74, July 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mosca M et al. , “Development of quality indicators to evaluate the monitoring of SLE patients in routine clinical practice,” (in eng), Autoimmun Rev, vol. 10, no. 7, pp. 383–8, May 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chrysis D, Zaman F, Chagin AS, Takigawa M, and Savendahl L, “Dexamethasone induces apoptosis in proliferative chondrocytes through activation of caspases and suppression of the Akt-phosphatidylinositol 3-kinase signaling pathway,” (in eng), Endocrinology, vol. 146, no. 3, pp. 1391–7, March 2005. [DOI] [PubMed] [Google Scholar]
- [93].Giustina A and Wehrenberg WB, “The role of glucocorticoids in the regulation of Growth Hormone secretion: mechanisms and clinical significance,” (in eng), Trends Endocrinol Metab, vol. 3, no. 8, pp. 306–11, October 1992. [DOI] [PubMed] [Google Scholar]
- [94].Magiakou MA, Mastorakos G, Gomez MT, Rose SR, and Chrousos GP, “Suppressed spontaneous and stimulated growth hormone secretion in patients with Cushings disease before and after surgical cure,” (in eng), J Clin Endocrinol Metab, vol. 78, no. 1, pp. 131–7, January 1994. [DOI] [PubMed] [Google Scholar]
- [95].Gutierrez-Suarez R et al. , “A proposal for a pediatric version of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index based on the analysis of 1,015 patients with juvenile-onset systemic lupus erythematosus,” (in eng), Arthritis Rheum, vol. 54, no. 9, pp. 2989–96, September 2006. [DOI] [PubMed] [Google Scholar]
- [96].Rygg M et al. , “A longitudinal PRINTO study on growth and puberty in juvenile systemic lupus erythematosus,” (in eng), Ann Rheum Dis, vol. 71, no. 4, pp. 511–7, April 2012. [DOI] [PubMed] [Google Scholar]
- [97].Hiraki LT, Hamilton J, and Silverman ED, “Measuring permanent damage in pediatric systemic lupus erythematosus,” (in eng), Lupus, vol. 16, no. 8, pp. 657–62, 2007. [DOI] [PubMed] [Google Scholar]
- [98].“Recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis: 2001 update. American College of Rheumatology Ad Hoc Committee on Glucocorticoid-Induced Osteoporosis,” (in eng), Arthritis Rheum, vol. 44, no. 7, pp. 1496–503, July 2001. [DOI] [PubMed] [Google Scholar]
- [99].Magiakou MA, Mastorakos G, and Chrousos GP, “Final stature in patients with endogenous Cushings syndrome,” (in eng), J Clin Endocrinol Metab, vol. 79, no. 4, pp. 1082–5, October 1994. [DOI] [PubMed] [Google Scholar]
- [100].Charmandari E, Kino T, Souvatzoglou E, and Chrousos GP, “Pediatric stress: hormonal mediators and human development,” (in eng), Horm Res, vol. 59, no. 4, pp. 161–79, 2003. [DOI] [PubMed] [Google Scholar]
- [101].Shiff NJ et al. , “Glucocorticoid-related changes in body mass index among children and adolescents with rheumatic diseases,” (in eng), Arthritis Care Res (Hoboken), vol. 65, no. 1, pp. 113–21, January 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Buckley L et al. , “2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis,” (in eng), Arthritis Rheumatol, vol. 69, no. 8, pp. 1521–1537, August 2017. [DOI] [PubMed] [Google Scholar]
- [103].Hollander MC et al. , “International consensus for provisions of quality-driven care in childhood-onset systemic lupus erythematosus,” (in eng), Arthritis Care Res (Hoboken), vol. 65, no. 9, pp. 1416–23, September 2013. [DOI] [PubMed] [Google Scholar]
- [104].Grossman JM et al. , “American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis,” (in eng), Arthritis Care Res (Hoboken), vol. 62, no. 11, pp. 1515–26, November 2010. [DOI] [PubMed] [Google Scholar]
- [105].Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A, Egurbide MV, and Aguirre C, “Predictors of major infections in systemic lupus erythematosus,” (in eng), Arthritis Res Ther, vol. 11, no. 4, p. R109, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schneeweiss S et al. , “Anti-tumor necrosis factor alpha therapy and the risk of serious bacterial infections in elderly patients with rheumatoid arthritis,” (in eng), Arthritis Rheum, vol. 56, no. 6, pp. 1754–64, June 2007. [DOI] [PubMed] [Google Scholar]
- [107].Tanaka H et al. , “Disseminated candidiasis following prednisolone therapy in systemic lupus erythematosus,” (in eng), Pediatr Int, vol. 44, no. 6, pp. 702–4, December 2002. [DOI] [PubMed] [Google Scholar]
- [108].Pillay VK, Wilson DM, Ing TS, and Kark RM, “Fungus infection in steroid-treated systemic lupus erythematosus,” (in eng), Jama, vol. 205, no. 5, pp. 261–5, July 29 1968. [PubMed] [Google Scholar]
- [109].Ferreira JC et al. , “Herpes zoster infection in childhood-onset systemic lupus erythematosus patients: a large multicenter study,” (in eng), Lupus, vol. 25, no. 7, pp. 754–9, June 2016. [DOI] [PubMed] [Google Scholar]
- [110].Warrington TP and Bostwick JM, “Psychiatric adverse effects of corticosteroids,” (in eng), Mayo Clin Proc, vol. 81, no. 10, pp. 1361–7, October 2006. [DOI] [PubMed] [Google Scholar]
- [111].“Drug-induced convulsions. Report from Boston Collaborative Drug Surveillance Program,” (in eng), Lancet, vol. 2, no. 7779, pp. 677–9, September 30 1972. [PubMed] [Google Scholar]
- [112].Fernandes H and Brito I, “Juvenile Systemic Lupus Erythematosus: neuropsychiatric manifestations,” (in eng), Acta Reumatol Port, vol. 37, no. 2, pp. 117–25, Apr-Jun 2012. [PubMed] [Google Scholar]
- [113].Khajezadeh MA, Zamani G, Moazzami B, Nagahi Z, Mousavi-Torshizi M, and Ziaee V, “Neuropsychiatric Involvement in Juvenile-Onset Systemic Lupus Erythematosus,” (in eng), Neurol Res Int, vol. 2018, p. 2548142, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rubin HR, Pronovost P, and Diette GB, “The advantages and disadvantages of process-based measures of health care quality,” (in eng), Int J Qual Health Care, vol. 13, no. 6, pp. 469–74, December 2001. [DOI] [PubMed] [Google Scholar]
- [115].Passo MH and Taylor J, “Quality improvement in pediatric rheumatology: what do we need to do?,” (in eng), Curr Opin Rheumatol, vol. 20, no. 5, pp. 625–30, September 2008. [DOI] [PubMed] [Google Scholar]