Abstract
Attention is biased towards stimuli that have been associated with aversive outcomes in the past. This bias has previously been interpreted as reflecting automatic orienting toward threat signals. However, in many prior studies, either the threatening stimulus provided valuable predictive information, signaling the possibility of an otherwise unavoidable punishment and thereby allowing participants to brace themselves, or the aversive event could be avoided with fast and accurate task performance. Under these conditions, monitoring for threat could be viewed as an adaptive strategy. In the present study, fixating a color stimulus immediately resulted in a shock on some trials, providing a direct incentive not to look at the stimulus. Nevertheless, this contingency resulted in participants fixating the shock-associated stimulus more frequently than a neutral distractor matched for physical salience. Our findings demonstrate that threatening stimuli are automatically attended even when attending such stimuli is actually responsible for triggering the aversive event, providing compelling evidence for automaticity.
Keywords: selective attention, attentional capture, threat, aversive conditioning, associative learning
It is critically important to our survival that potential threats be rapidly detected and acted upon. Given the limited representational capacity of the human perceptual system (Desimone & Duncan, 1995), threat detection is often an attention-demanding process. To more effectively cope with this demand, it has been hypothesized that humans have evolved a bias to automatically direct attention to signals for potential threat (e.g., Mulckhuyse, 2018; Öhman & Mineka, 2011; Vuilleumier, 2005).
Consistent with this hypothesis, a variety of experiments have demonstrated attentional biases towards aversively conditioned stimuli. For example, stimuli previously associated with aversive electric shock (e.g., Schmidt et al., 2015a; Wang et al., 2013), white noise (e.g., Koster et al., 2004; Smith et al., 2006), monetary loss (e.g., Wentura et al., 2014), or negative social feedback (Anderson, 2017; Anderson & Kim, 2018) during a conditioning phase impair performance on visual tasks, consistent with distraction by aversively-conditioned stimuli. Furthermore, goal-directed eye movements are biased towards aversively conditioned stimuli, which are more frequently fixated when presented as task-irrelevant distractors compared to otherwise equivalent distractors without such association (Mulckhuyse et al., 2013; Mulckhuyse & Dalmaijer, 2016; Schmidt et al., 2015b).
Although each of these cases provides strong evidence for biased attention to aversively conditioned stimuli, the degree of automaticity involved in this bias is less clear. In many of these prior studies, the aversively conditioned cues provide useful information about whether otherwise unavoidable punishment can be anticipated, with attention to the aversively conditioned stimulus allowing the observer to prepare. Cues that are informative of outcomes are generally thought to be prioritized by attention (Gottlieb et al., 2013). Although there is no benefit to continuing to monitor for the aversively conditioned stimulus in a subsequent task in which distraction is assessed, there is also little motivation for participants to stop explicitly monitoring for potential threat, as threat monitoring has no direct cost associated with it in this context. A strong case for automaticity requires that attention to the stimulus of interest be explicitly counterproductive (Anderson, in press). Furthermore, to the degree that participants actively monitor for, and preferentially attend to, shock-predictive stimuli during conditioning, it could be this difference in selection history (Awh et al., 2012) rather than the punishment association per se that is responsible for the attentional biases towards the CS+ evident in extinction (e.g., Schmidt et al., 2015a; Wang et al., 2013).
Recently, Nissens and colleagues (2017) attempted to overcome these limitations by presenting participants with two color distractors, one of which signaled potential shock at the end of the trial (CS+). Importantly, shock was only delivered following CS+ trials on which participants were slow to fixate the target. Reorienting to the target after fixating the distractor takes time; therefore, fixating the distractor was counterproductive, increasing the probability of receiving a shock. In spite of this contingency, participants more frequently fixated the CS+ distractor compared to the neutral (CS-) distractor.
In the design of Nissens et al. (2017), however, the CS+ still provided useful information about the possibility of shock, which was inevitable on some trials given the individually-adjusted response thresholds that were used. Furthermore, as shock could be avoided on some trials with fast and accurate performance, the CS+ indicated to participants when they should exert the most effort in the task; this indication might have encouraged explicit threat monitoring, particularly given that the majority of CS+ trials did not result in shock. In this regard, the withholding of punishment on these trials may have negatively reinforced the rapid orienting of attention to the distractor followed by motivated re-orienting to the target.
In the present study, we provide a strong and direct test of the automaticity of attention to threatening stimuli. Participants performed a similar task to the one used by Nissens et al. (2017), although in our design shocks were delivered with 50% probability immediately upon fixating the CS+. Therefore, fixating the CS+ was directly and immediately punished, providing a strong incentive to curb the orienting behavior responsible for shock. The presence of an attentional bias towards the CS+ cue under these circumstances would provide compelling evidence for automaticity.
Methods
Participants
Twenty-eight participants (18–24 years of age, mean = 19.0y, 15 female) were recruited from the Texas A&M University community. Data were collected from two additional participants, who were replaced due to difficulty eye tracking. Participants were compensated with course credit. All reported normal or corrected-to-normal visual acuity and normal color vision, and all provided written informed consent. All procedures were approved by the Texas A&M University Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki. The sample size was informed by a power analysis. The effect size for the difference in performance between CS+ and CS- distractors was estimated at dz = 1.4, the effect size reported by Nissens et al. (2017). This analysis indicated power β > 0.9 with α = 0.05 (G*Power; http://www.gpower.hhu.de/).
Apparatus
A Dell OptiPlex equipped with MATLAB software and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Eye position was monitored using an EyeLink 1000 Plus desktop-mounted eye tracker (SR Research). Head position was maintained using an adjustable chin rest (SR Research). Paired electrodes (EL500, BioPac Systems, Inc.) were attached to the left forearm of each participant, and electric shocks were delivered through an isolated linear stimulator under the constant current setting (STMISOLA, BioPac Systems), which was controlled by custom Matlab scripts.
Stimuli
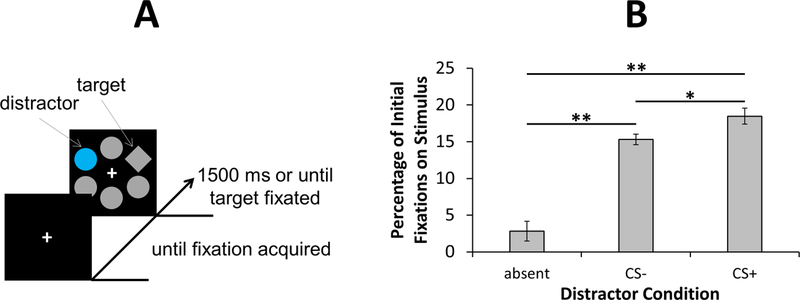
Each trial consisted of a fixation display, a search array (1500 ms or until a fixation on the target was registered), and a blank inter-trial-interval between 1400–1600 ms (Figure 1A). The fixation display remained on screen until eye position was registered within 2.4° of the center of the fixation cross for a continuous period of 500 ms. In the event that participants did not fixate the target within the timeout limit, the word “Miss” was centrally presented for 1000 ms immediately following the search array. The search array consisted of six shapes, each approximately 5.7° × 5.7° in visual angle, placed at equal intervals along an imaginary circle with a radius of 8.2°. On each trial, the target was either a single diamond in an array of circles or a single circle in an array of diamonds (shape singleton). On distractor-present trials, one of the non-targets was rendered in either red or blue while the rest were grey. On distractor-absent trials, all six shapes were grey. All shape stimuli were equiluminant.
Figure 1.
(A) Example trial. The task was to fixate the unique shape target. Fixations on one of the two color stimuli used as distractors immediately resulted in shock on 50% of associated trials (CS+), while the other color distractor was never paired with shock (CS-). (B) The percentage of initial fixations on a distractor for each distractor condition. Error bars reflect the within-subjects SEM.
Design
The target appeared in each of the six possible locations equally-often. The color singleton distractor was red on one third of trials, blue on one third of trials, and absent on one third of trials. For each color distractor, target and distractor position were fully crossed and counterbalanced. On half of all trials on which one color distractor was presented, the participant would receive an electric shock (2 ms pulse at the individually-calibrated intensity) immediately upon the eye tracker registering a fixation on the distractor (CS+). The CS+ color (red or blue) was counterbalanced across participants. Trials were presented in a random order.
Procedure
The experiment consisted of a 20-trial practice block with no shocks followed by 5 blocks of 108 trials each. Prior to the experiment task, the intensity of shock was calibrated to achieve a level that was “unpleasant, but not painful” (e.g., Schmidt et al., 2015a, 2017). Specifically, the intensity of a 2 ms shock was gradually increased from 8 mA until the participant first noted that the shock was painful, at which point the intensity was reduced by 1 mA and confirmed as “unpleasant, but not painful.” Eye position was calibrated prior to each block of trials using 9-point calibration and was manually drift corrected by the experimenter as necessary (the need for which was evident when acquiring initial fixation at the outset of each trial). Participants were instructed to fixate (“look directly at”) the unique shape, and were informed that sometimes they would receive a shock depending on where they looked. Participants were not informed of which color predicted shock, as such instruction to try to ignore a feature can ironically produce a bias to initially orient to that feature (Moher & Egeth, 2012).
Data Analysis
We recorded which of the six shape stimuli was initially fixated on each trial. Fixation of a stimulus was registered if eye position remained within a region extending 0.7° around the stimulus for a continuous period of at least 50 ms (100 ms on the target to trigger the termination of the stimulus array). Percentage of initial fixations on a distractor were taken over all trials within the respective condition. On distractor-absent trials, in order to quantify the probability of initially fixating a distractor for the sake of comparison, one of the non-targets was dummy-coded as the critical distractor on each trial using the same parameters that were used to define the position of the critical distractors on distractor-present trials (i.e., same counterbalance of position relative to the target position). Response time was measured from the onset of the display until a fixation on the target was registered; from the registered response time 100 ms was subtracted to yield the time at which eye position first entered into the region of the target.
Results
A fixation on the target was registered within the timeout limit on 98.1% of all trials. An analysis of variance (ANOVA) with distractor condition (absent, CS-, CS+) as a factor revealed a main effect of the manipulation, F(2,54) = 39.00, p < 0.001, η2p = 0.591 (Figure 1B). Replicating attentional capture by physically salient stimuli (Theeuwes, 1992), the CS+ and CS- distractors were both significantly more likely to be the first stimulus fixated compared to a non-target on distractor-absent trials, ts > 6.68, ps < 0.001, ds > 1.26. Importantly, participants were also significantly more likely to initially fixate a CS+ distractor compared to a CS- distractor, t(27) = 2.52, p = 0.018, d = 0.48. Unsurprisingly, given that reorienting attention from the distractor takes time, the same pattern of results was evident in response time (406, 447, and 457 ms, for the absent, CS-, and CS+ distractor conditions, respectively), F(2,54) = 58.51, p < 0.001, η2p = 0.684; CS+ vs. CS-: t(27) = 2.66, p = 0.013, d = 0.50.
Discussion
The present study provides clear and compelling evidence that signals for threat are preferentially attended automatically. Fixating the CS+ directly resulted in a shock on some trials (with 50% probability), which was delivered immediately upon fixating the stimulus. It was therefore explicitly counterproductive to fixate the CS+, which was made salient to participants from the immediacy of the feedback. The adaptive response in this context is to do everything possible to suppress eye movements to the CS+. In spite of this, participants were more likely to fixate the CS+ relative to a neutral CS-. Our results corroborate and extend the findings of Nissens et al., (2017), providing direct evidence for the automaticity of the attentional bias to threat.
Our findings also speak to the role of punishment in the control of eye movements. Punishment plays a general role in extinguishing behaviors that result in its delivery (e.g., Church, 1963). In the present study, this role for punishment in curbing behavior—in this case oculomotor behavior—was pitted against the influence of cue-based associative learning and its role in facilitating threat detection (e.g., Mulckhuyse, 2018; Schmidt et al., 2015a; Wang et al., 2013). Oculomotor capture was not high in our task (the CS+ was fixated on less than 20% of trials), such that participants had ample opportunity to learn that suppressing overt attention to the CS+ avoided shock, whereas fixating the CS+ but not the CS- reliably resulted in an immediate shock. That participants failed to adaptively adjust their behavior to this contingency is striking. In this sense, our findings suggest that the associative aspects of aversive conditioning influence the attention system more powerfully than does punishment learning, causing the punished behavior to be potentiated rather than extinguished when the two sources of learning compete against each other.
The findings of the present study fit into a broader literature examining the role of associative learning in the control of attention (Le Pelley et al., 2016). In particular, stimuli previously associated with reward also capture attention, in a manner that is hypothesized to be similarly automatic (see Anderson, 2016, for a review). The similarities and differences between these two influences on the attention system, particularly with respect to the underlying neural systems involved (Anderson, 2019), are largely unexplored and reflect a promising direction for future research.
Acknowledgements
This research was supported by a start-up package from Texas A&M University to BAA and grants from the Brain & Behavior Research Foundation [NARSAD 26008] and NIH [R01-DA046410] to BAA. The authors report no conflicts of interest.
References
- Anderson BA (2016). The attention habit: How reward learning shapes attentional selection. Annals of the New York Academy of Sciences, 1369, 24–39. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2017). Counterintuitive effects of negative social feedback on attention. Cognition & Emotion, 31, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2019). Neurobiology of value-driven attention. Current Opinion in Psychology, 29, 27–33. [DOI] [PubMed] [Google Scholar]
- Anderson BA (in press). Controlled information processing, automaticity, and the burden of proof. Psychonomic Bulletin & Review. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (2018). Relating attentional biases for stimuli associated with social reward and punishment to autistic traits. Collabra: Psychology, 4(1), 10. doi: 10.1525/collabra.119 [DOI] [Google Scholar]
- Awh E Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox, Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Church RM (1963). The varied effects of punishment on behavior. Psychological Review, 70, 369–402. [DOI] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Oudeyer P-Y, Lopes M, & Baranes A (2013). Information seeking, curiosity, and attention: Computational and neural mechanisms. Trends in Cognitive Sciences, 17, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, & De Houwer J (2004). Does imminent threat capture and hold attention? Emotion, 4, 312–317. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME, Mitchell CJ, Beesley M, George DN, & Wills AJ (2016). Attention and associative learning in humans: An integrative review. Psychological Bulletin, 142, 1111–1140. [DOI] [PubMed] [Google Scholar]
- Moher J, & Egeth HE (2012). The ignoring paradox: Cueing distractor features leads first to selection, then to inhibition of to-be-ignored items. Attention, Perception, and Psychophysics, 74, 1590–1605. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M (2018). The influence of emotional stimuli on the oculomotor system: A review of the literature. Cognitive, Affective, & Behavioral Neuroscience, 18(3), 411–425. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, Crombez G, & Van der Stigchel S (2013). Conditioned fear modulates visual selection. Emotion, 13(3), 529–536. [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, & Dalmaijer ES (2016). Distracted by danger: Temporal and spatial dynamics of visual selection in the presence of threat. Cognitive, Affective, & Behavioral Neuroscience, 16(2), 315–324. [DOI] [PubMed] [Google Scholar]
- Nissens T, Failing M, & Theeuwes J (2017). People look at the object they fear: oculomotor capture by stimuli that signal threat. Cognition & Emotion, 31, 1707–1714. [DOI] [PubMed] [Google Scholar]
- Öhman A, & Mineka S (2001). Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review, 108, 483–522. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015a). Attentional capture by signals of threat. Cognition and Emotion, 29, 687–694. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015b). Potential threat attracts attention and interferes with voluntary saccades. Emotion, 15, 329–338. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2017). The time course of attentional bias to cues of threat and safety. Cognition & Emotion, 31, 845–857. [DOI] [PubMed] [Google Scholar]
- Smith SD, Most SB, Newsome LA, & Zald DH (2006). An “emotional blink” of attention elicited by aversively conditioned stimuli. Emotion, 6, 523–527. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual selectivity for color and form. Perception and Psychophysics, 51, 599–606. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9, 585–594. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu H, & Zhou X (2013). Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision, 13(3:5), 1–13. [DOI] [PubMed] [Google Scholar]
- Wentura D, Muller P, & Rothermund K (2014). Attentional capture by evaluative stimuli: gain- and loss-connoting colors boost the additional-singleton effect. Psychonomic Bulletin and Review, 21, 701–707. [DOI] [PubMed] [Google Scholar]