Abstract
Purpose:
MDS with deletion of chromosome 7q/7 (−7/(del)7q MDS) is associated with worse outcomes and needs novel insights into pathogenesis. Reduced expression of signaling protein DOCK4 in −7/(del)7q MDS patients leads to a block in hematopoietic stem cell (HSC) differentiation. Identification of targetable signaling networks downstream of DOCK4 will provide means to restore hematopoietic differentiation in MDS.
Experimental design:
We utilized phospho-proteomics approaches to identify signaling proteins perturbed as a result of reduced expression of DOCK4 in human HSCs and tested their functional significance in primary model systems.
Results:
We demonstrate that reduced levels of DOCK4 lead to increased global tyrosine phosphorylation of proteins in primary human HSCs. LYN kinase and phosphatases INPP5D (SHIP1), and PTPN6 (SHP1) displayed greatest levels of tyrosine phosphorylation when DOCK4 expression levels were reduced using DOCK4-specific siRNA. Our data also found that increased phosphorylation of SHIP1 and SHP1 phosphatases were due to LYN kinase targeting these phosphatases as substrates. Increased migration and impediment of HSC differentiation were consequences of these signaling alterations. Pharmacological inhibition of SHP1 reversed these functional aberrations in HSCs expressing low DOCK4 levels. Additionally, differentiation block seen in DOCK4 haplo-insufficient (−7/(del)7q) MDS was rescued by inhibition of SHP1 phosphatase.
Conclusions:
LYN kinase and phosphatases SHP1 and SHIP1 are perturbed when DOCK4 expression levels are low. Inhibition of SHP1 promotes erythroid differentiation in healthy HSCs and in −7/(del)7q MDS samples with low DOCK4 expression. Inhibitors of LYN, SHP1 and SHIP1 also abrogated increased migratory properties in HSCs expressing reduced levels of DOCK4.
Introduction
Dedicator of cytokinesis 4 (DOCK4) is one of the members of the eleven DOCK family proteins, which are conserved across different mammalian species(1). It is a large protein of ~225 kilodaltons (KDa) with multiple signaling/protein-protein interaction domains(2). The gene for DOCK4 protein is located in the q arm of chromosome 7. Recent studies have highlighted the importance of normal levels of DOCK4 expression across multiple tissue types in maintaining cellular homeostasis(3–7). Mutations or reduced expressed of DOCK4 can lead to malignancies in prostate, breast, lung, brain and blood tissues as well as solid tumor metastasis(8–11). Its known functions include regulation of motility via Rac1 GTPases and actin cytoskeleton(12,13). However, very little is known with respect to the impact of reduced levels of DOCK4 expression within the stem cell compartment. Using healthy blood stem cells and blood stem cells expressing reduced levels of DOCK4, (as seen in the malignant blood disorder myelodysplastic syndromes), we identified downstream signaling networks regulated by DOCK4 and functional implications of reduced DOCK4 expression within the blood stem cell compartment.
Myelodysplastic syndromes are clonal stem cell disorders, where DOCK4 expression is reduced due to either deletion of chromosome 7q or mutations or promoter hypermethylation in DOCK4 gene(14). Patients with this disorder experience multi-lineage dysplasia and peripheral cytopenia(15). Our previous studies have shown that reduced levels of DOCK4 lead to dysplastic erythropoiesis and restoring DOCK4 expression in primary MDS erythroblasts improved erythroid differentiation(14,16). This work focused on the impact of DOCK4 aberrations on post-lineage committed erythroid progenitors and not in hematopoietic stem cells even though DOCK4 is highly expressed in early-stage stem cells. Moreover, downstream signaling networks regulated by DOCK4 in pre-lineage committed HSCs are not known.
In this report, we used a population of early-stage human primary hematopoietic stem cells (HSCs), as defined by expression of surface marker proteins CD34 and CD90 to identify downstream signaling networks regulated by DOCK4. Furthermore, we determined the functions of DOCK4 and the consequences of reduced DOCK4 expression in early hematopoietic stem cells. These studies revealed several phosphatases and kinases are regulated by DOCK4. We demonstrate that DOCK4 regulated tyrosine phosphorylation of a large number of signaling proteins resulting in significant increases in global phospho-tyrosine levels. Using mass spectrometry phosphoproteomic approaches, we precisely identified LYN (Tyrosine protein kinase-Lyn), PTPN6 (Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP1)) and INPP5D (Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1, also known as Src homology 2 domain containing inositol polyphosphate 5-phosphatase 1 (SHIP1)) as most significantly impacted (hyper tyrosine-phosphorylated) proteins. LYN kinase directly phosphorylated phosphatases SHP1 and SHIP1 at tyrosine sites 536 and 1021 respectively. Low DOCK4 levels led to increased stem cell migration, which was blunted in the presence of pharmacological inhibitors of LYN or SHP1 or SHIP1. In DOCK4 deficient MDS patient samples (−7/(del)7q), we observed increased numbers of CD34+/CD45+ cells in circulation. Lastly, we demonstrate that pharmacological inhibition of SHP1 in DOCK4 deficient HSCs from MDS patients can improve erythroid differentiation. Altogether, this study has identified a new signaling network that can be leveraged to potentially overcome the functional defects that arise due to reduced expression of DOCK4 in MDS.
Materials and Methods
Primary hematopoietic stem cell (HSC) isolation and culture
CD34+/CD90+ HSCs were purified from mobilized peripheral blood of healthy donors purchased from Key biologicals, Inc. (TN, USA) using a CliniMACS (Miltenyi Biotec, Inc. CA, USA) device(16–19). Purified cells were cultured in Stemspan SFEM II (Stemcell technologies Inc, BC, CN) supplemented with 50ng/ml thrombopoietin (TPO), 50ng/ml stem cell factor (SCF), 50ng/ml Fms-related tyrosine kinase-3 ligand (FLT3-L), 50ng/ml interleukin-3 (IL3), and 50ng/ml interleukin-6 (IL-6) until used for subsequent experiments. All the cytokines were purchased from R&D systems (MN, USA). Benzidine-hematoxylin staining of the cytospun HSCs were performed as previously described(20).
In the experiments involving cytokine deprivation and exposure, HSCs were washed twice to get rid of cytokines and cultured in Iscove’s modified Dulbecco’s medium (IMDM; Lonza, NJ, USA) containing 1% (v/v) BSA fraction V (Fisher Scientific, NH, USA) for 3 hours. Following this, the cells were exposed to cytokines for 15 minutes at a concentration of 250ng/ml. Specific cytokines and cytokine cocktail used in experiments are described in figure legends appropriately.
TF1 erythroleukemia cells were purchased from ATCC and cultured in Roswell Park Memorial Institute (RPMI; Gibco, USA) containing 10% (v/v) FBS (Life Technologies, USA), supplemented with 4ng/ml GM-CSF. HEK293 cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Gibco, USA) containing 10% (v/v) FBS.
All studies involving human subjects were conducted in accordance with U.S. Common Rule. Peripheral blood or bone marrow aspirates from each MDS patients were obtained after IRB approval and informed written consent. CD34+ HSPCs from MDS patients were purified as described previously using CD34+ selection kit purchased from Stemcell Technologies, Inc(16,21).
Nucleofection of CD34+ HSCs
Control (#D-001810–10, Dharmacon Inc. CO, USA) or DOCK4 siRNA (Dharmacon Inc. CO, USA) was nucleofected into CD34+ HSCs using CD34+ cells nucleofection kit (Lonza, NJ, USA) according to manufacturer’s protocol. Briefly, 2.5×10^6 cells were nucleofected with 300nM siRNA using the U-08 program in the Nucleofector II machine. Following nucleofection, cells were cultured in Stemspan SFEM II supplemented with 50ng/ml TPO, 50ng/ml SCF, 50ng/ml FLT3-L, 50ng/ml IL3, and 50ng/ml IL-6 until used for subsequent experiments. Similarly, in the experiments involving knockdown of LYN, SHIP1 or SHP1, respective smartpool siRNA (Dharmacon Inc. CO, USA) were used. In the experiments involving TF1 cells, control or DOCK4 siRNA was nucleofected using nucleofection kit T (Lonza, NJ, USA) according to manufacturer’s protocol.
Mass spectrometry phosphoproteomics
HSCs that were briefly cultured for three hours were used to knockdown DOCK4 and recultured for twenty four hours prior to lysing both DOCK4 knockdown (50%) and DOCK4 intact cells using phosphorylation lysis buffer (50 mM Hepes (pH 7.3), 150 mM sodium chloride, 1 mM EDTA, 1.5 mM magnesium chloride, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 200 μM sodium orthovanadate, 10% glycerol, 0.5% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride) as described previously(22). Protein concentration in the supernatants was determined by BCA assay. 450 μg of total protein for each of the two samples were reduced and alkylated prior to trypsin digestion. Phosphopeptides from the digests were enriched using TiO2 beads and fractionated them by high pH reverse phase into four fractions each(23). Each fraction was desalted prior to LC-MS analysis. Nano LC-MS/MS analyses were performed with a 75 μm × 10.5 cm PicoChip column packed with 3 μm Reprosil C18 beads with Dionex UltiMate 3000 Rapid Separation nanoLC coupled to a Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific Inc, San Jose, CA). A 150 μm × 3 cm trap packed with 3um beads was installed in-line. Peptides were separated in 120min gradient. Data was acquired in data-dependent MS/MS mode with a top-15 method. Dynamic exclusion was set to 20 s and charge 1+ ions were excluded. MS1 scans were collected from 300–2000 m/z with resolving power equal to 60,000. The MS1 automatic gain control (AGC) was set to 3×106. Precursors were isolated with a 2.0 m/z isolation width, and the HCD normalized collision energy was set to 30%. The MS2 AGC was set to 1×105 with the resolving power set at 30,000. Phosphopeptides in which the phosphorylation sites that can be assigned to a single amino acid with 75% probability or better in at least one sample were filtered. Robust z-scores were computed from the log2–fold changes to compare knocked down cells vs. control cells and defined a z-score of >= 1.5 as up-regulated and <= −1.5 as down-regulated.
In vitro kinase assays
Multiple doses of recombinant active LYN kinase (SignalChem, BC, CN) were incubated along with 250ng recombinant active SHIP1 (SignalChem, BC, CN) or recombinant SHP1 (SignalChem, BC, CN) in the presence of ATP at a final concentration of 100uM. The total volume of each in vitro reaction was 25μl using kinase dilution buffer I (SignalChem, BC, CN) and deionized water. The samples were incubated in a 30°C water bath for 15 minutes. The kinase reaction was stopped by adding 8.3μl 4X laemlii buffer (Bio-Rad, CA, USA) and boiling the samples for 5 minutes. The samples were then analyzed by immunoblotting. In the samples where inhibitor was added, the LYN/Src inhibitor, RK20449 (Selleck Inc.TX, USA) was added to a final concentration of 500nM.
LYN kinase activity assay (in vitro)
LYN kinase activities in the presence of flag-tagged DOCK4 or recombinant DOCK4 C-terminus were measured using the universal tyrosine kinase activity assay kit (Takara, USA) according to the manufacturer’s guidelines. In experiments involving flag-tagged DOCK4, we ectopically expressed Flag-tagged full length DOCK4 in HEK293 cells and immunoprecipitated flag-tagged DOCK4 using anti-Flag coated protein G dynabeads (ThermoFisher, USA) according to the manufacturer’s protocol. Following this, increasing amounts of immunoprecipitated flag-tagged DOCK4 as indicated was incubated with 20ng of active recombinant LYN kinase or active recombinant JAK2 kinase. Similarly, in experiments involving recombinant DOCK4 C-terminus, increasing concentration of DOCK4 C-terminus (Proteintech, USA) as indicated was incubated with 20ng of active recombinant LYN kinase or active recombinant JAK2 kinase.
In vitro binding assay
Flag-tagged recombinant DOCK4 C-terminus was generated by cloning DOCK4 C-terminus using primers DOCK4 C-terminus forward primer - 5’-GGTGCCATGGGCCACCATCACCACCATCATCACCACCATCACCCTTTGTTGTCTGACAAACACAC and DOCK4 C-terminus reverse primer - 5’- CACCCTCGAGTCACTTGTCGTCATCGTCTTTGTAGTCTAACTGAGAGACCTTGCGG into pET15b plasmid purchased from Novagen. Following cloning, we produced recombinant flag-tagged DOCK4-C-terminus according to the manufacturer’s protocol. 100ng recombinant LYN and 400ng of flag-tagged recombinant DOCK4 C-terminus was incubated overnight along with anti-IgG or anti-Flag coated magnetic beads in a rotating shaker at 4°C. Immunoprecipitation followed by immunoblot analysis was performed as described earlier.
Transfection and Immunoprecipitation
Flag-tagged full length DOCK4 plasmid(8) (gift from Dr. Linda Van Aelst, CSHL) was co-transfected along with GFP-tagged LYN plasmid(24) (gift from Dr. Anna Huttenlocher, University of Wisconsin, USA) or GFP-tagged SHIP1 plasmid(25) (gift from Dr. Aaron Marshall, University of Manitoba, CN) or GFP-tagged SHP1 plasmid(26) (gift from Dr. Stephen Shaw, National Cancer Institute) using Lipofectamine 2000 reagent (ThermoScientific, USA) according to manufacturer’s protocol. 24 hours later cells were harvested, lysed and protein concentration was calculated using Lowry-Bradford assay. Protein G dynabeads (ThermoScientific, USA) were coated with indicated antibodies and immunoprecipitation was carried out according to the manufacturer’s protocol. GFP-trap beads (Chromotek Inc, NY, USA) were used to immunoprecipitate GFP-tagged proteins. The eluted samples after immunoprecipitation were analyzed by immunoblotting as described earlier.
Database for Annotation Visualization and Integrated Discovery (DAVID) analysis
Proteins that were hyper-tyrosine phosphorylated greater that 1.5 fold and tyrosine phosphorylated proteins identified only in DOCK4 knockdown samples were subjected to functional annotation analysis available in the DAVID website (http://david.abcc.ncifcrf.gov/) Gene ontology option GOTERM_BP_ALL was selected and a functional annotation chart generated.
Immunofluorescence
Control and DOCK4 knockdown HSCs were immobilized on Alcian blue-coated coverslips and stained for actin with Phalloidin (ThermoFisher, USA) as previously described (16). Images were captured using Leica STED-SP5 confocal microscope at 63X magnification. Fiji Image J software was used to quantify the promigratory cells using the circularity feature. At least 100 cells from 4 random fields were analyzed.
Transwell migration assays
HSC migration assays were performed using transwells. Briefly, 150,000 HSCs were suspended in 100μl of starvation media (IMDM containing 1% (vol/vol) BSA fract V) and was added to the upper chamber of the 5-μm-pore transwell insert (24-well plate format transwell, Corning, NY, USA). 0.5ml of starvation media with various concentrations (0–100ng/ml) of chemokine stromal derived factor-1 alpha (SDF-1α) was added to the bottom chamber. The transwell plates were incubated for 4 hours in a 37°C 5% CO2. The transwell inserts were carefully removed and the migrated cells in the bottom chamber were resuspended. Samples were obtained from the bottom chamber and stained with acridine orange and propidium iodide nuclear dyes (AO-PI dye, Nexcelom, MA, USA). The stained samples were enumerated using Nexcelom auto 2000 cell counter (Nexcelom, MA, USA). All the migration experiments were performed in triplicates. In the experiments involving inhibitor treatments (LYN/Src inh. – RK20449 (Selleck Inc. TX, USA), SHIP1 inh. – 3AC (EMD Millipore Inc. MA, USA) and SHP1 inh. – TPI-1 (Cayman Inc. MI, USA)), the cells were exposed to inhibitors for 1 hour in IMDM containing 1% BSA media prior to adding to the top chamber of the transwell. Migration assays with TF1 cells were performed as described previously(27).
Methylcellulose colony assays and hemoglobin ELISA
1500 HSCs from healthy or MDS patients were cultured in methylcellulose (Stemcell technologies Inc. #H4434) supplemented with 2 Units/ml erythropoietin for 14–16 days in the presence/absence of SHP1 inhibitor, 4μM TPI-1 (Cayman Inc.). DMSO was used as a vehicle control. After 14–16 days, the colonies were enumerated and imaged. Images of the colonies were captured using Olympus microscope at 10X magnification. Following colony enumeration, cells were collected from the methylcellulose plates and hemoglobin levels were quantitated using hemoglobin ELISA kit (Abcam #ab157707 CA, USA) according to the manufacturer’s guidelines.
Statistical analysis
The error bars are computed as mean ± SEM. Student’s t tests were performed to determine the statistical significance between the samples. In experiments involving dose responses, one-way ANOVA test was performed to determine statistical significance. Experiments were from three biological replicates, and values with P < 0.05 were considered statistically significant.
Results
Reduction of DOCK4 increases global tyrosine phosphorylation in HSCs
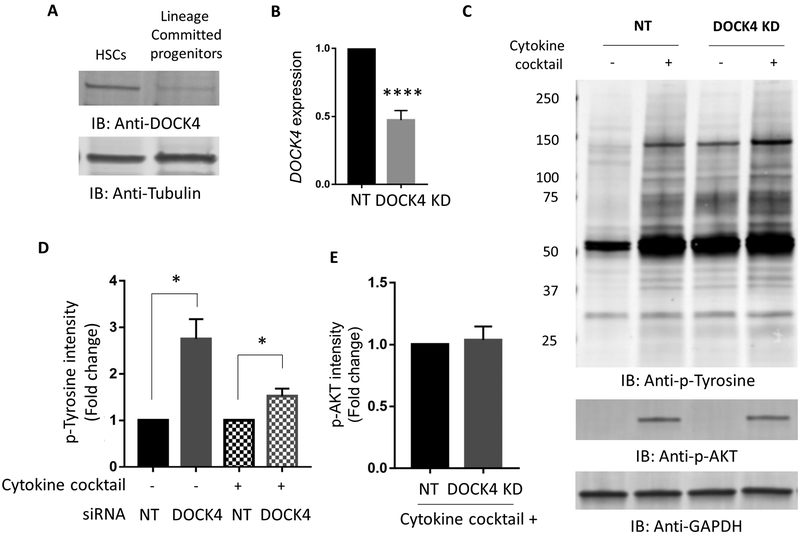
We first examined steady state expression levels of DOCK4 in HSCs and in lineage committed progenitors, which showed a relatively high level of expression in HSCs but low levels in committed progenitors (Figure 1A). We confirmed that cells used in our studies were highly enriched for the hematopoietic stem cell phenotype by determining the CD34+/CD90+ expression and cell morphology (supplemental Figure 1A–C), which showed 84% cells were double positive and greater that 99% were positive for CD34 early stem/progenitor cell marker. We then knocked down DOCK4 in these cells by siRNA, which enabled us to reduce the levels by fifty percent consistently in multiple primary samples as determined by quantitative PCR (qPCR) (Figure 1B). We then determined HSC response to cytokines in cells that are expressing DOCK4 at 50% of their normal levels, by exposing them to a cocktail of stem cell cytokines (TPO, SCF, Flt3L, IL3 and IL6)(28) after a short deprivation of cytokines. We also exposed cells expressing DOCK4 at their normal levels to the same cocktail before harvesting cells and performing immunoblotting against an anti-phospho tyrosine antibody. These experiments revealed increased tyrosine phosphorylation of a large number of proteins in DOCK4 knockdown samples compared to the ones that had normal levels of DOCK4 expression (Figure 1C–D). This increase was observed regardless of cytokine stimulation suggesting DOCK4 levels alone were sufficient to elicit global phosphorylation response. Levels of protein under each condition were equivalent as reflected by no difference in AKT phosphorylation or GAPDH in DOCK4 knockdown cells and non-targeting control cells (Figure 1C, 1E). Collectively, these data demonstrated that reduced levels of DOCK4 increased global tyrosine phosphorylation and suggested that DOCK4 functions as a signaling intermediate downstream of several cytokine receptors in HSCs.
Figure 1: Expression of DOCK4 and increased global phosphorylation of proteins in human primary hematopoietic stem cells with reduced DOCK4 levels.
A) Expression of DOCK4 in CD34+/CD90+ HSCs and in lineage committed progenitors. B) qPCR analysis for DOCK4 expression following knockdown of DOCK4 by fifty percent in primary hematopoietic stem cells. The data are 24 hours post-nucleofection. Data are represented as mean ±SEM from six biological replicates (****P < 0.0005; Student’s t test). C) Immunoblot analysis 24 hrs following DOCK4 knockdown in HSCs with or without exposure to a five-cytokine cocktail (Thrombopoietin (TPO), stem cell factor (SCF), FLT3 ligand, Interleukin-3 (IL-3) and Interleukin-6 (IL-6)) for 15 mins as described in methods. An anti-pan phosphotyrosine antibody was used to detect phosphorylated proteins. The same membrane was stripped and re-probed with an anti-phospho-AKT antibody in order to show activity of signaling in response to cytokine stimulation as a control. GAPDH was used as a loading control. D) Quantitation of fold change in phospho-tyrosine levels in panel C. Comparisons were made between cells expressing DOCK4 at normal levels and cells expressing DOCK4 at 50% levels with and without cytokine exposure. Data are represented as mean±SEM from four biological replicates (*P < 0.05; Student’s t test). E) Quantitation of the levels of phospho-AKT in HSCs from control and DOCK4 knockdown samples. Data are represented as mean ±SEM from four biological replicates. NT – Non-targeting control.
DOCK4 regulates the phosphorylation of kinases and phosphatases
In order to identify the proteins that were impacted in their phosphorylation due to reduced DOCK4 expression, we performed phosphoproteomic analysis by mass spectrometry. These experiments uncovered a large number of phospho-peptides belonging to mostly kinases and phosphatases that were hyperphosphorylated in cells that expressed low levels of DOCK4. Among them, SHP1, SHIP1 and LYN exhibited greatest increase in tyrosine phosphorylation (Table 1). We then focused on LYN kinase, and phosphatases SHP1 and SHIP1 for further study since these enzymes have been extensively studied during blood cell development (29). We used commercially available phospho-specific antibodies against each of the three proteins to confirm our mass spectrometry results, which showed LYN, SHIP1 and SHP1 proteins were phosphorylated at tyrosine sites 397, 1021 and 536 respectively (Figure 2A–D). These results were also confirmed in TF-1 erythroleukemia cells, which are at early phase (CD34+) of blood cell development (supplemental Figure 2A–F).
Table 1:
Differentially tyrosine phosphorylated proteins in cells with reduced DOCK4 levels
| Protein names | Gene names | Phospho Site | Fold Change (Z-score) |
|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 6 | PTPN6 (SHP1) | 536 | 5.43 |
| Phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 1 | INPP5D (SHIP1) | 865 | 4.36 |
| Neural Wiskott-Aldrich syndrome protein | WASL | 256 | 2.77 |
| Tyrosine-protein kinase Lyn;Tyrosine-protein kinase HCK | LYN;HCK | 397;411 | 2.45 |
| Signal transducer and activator of transcription 3 | STAT3 | 705 | 1.76 |
| Glycogen synthase kinase-3 beta;Glycogen synthase kinase-3 alpha | GSK3B;GSK3A | 216;279 | 1.58 |
| SHC-transforming protein 1 | SHC1 | 427 | −2.76 |
| CWF19-like protein 2 | CWF19L2 | 201 | −2.8 |
| Glucose 1,6-bisphosphate synthase | PGM2L1 | 383 | −4.2 |
Figure 2: Reduced levels of DOCK4 leads to increased phosphorylation of LYN kinase and phosphatases SHIP1 and SHP1 and their interactions with DOCK4.
A) Immunoblot analysis 24 hours following DOCK4 knockdown in HSCs with or without exposure to a five-cytokine cocktail (TPO, SCF, FLT3 ligand, IL-3 and IL-6) for 15 mins as described in methods. An anti-phospho LYN (Y397) antibody, an anti-phospho SHIP1 (Y1021) antibody or an anti-phospho SHP1 (Y536) antibody was used for detection. Same membranes were stripped and re-probed with anti-LYN, SHIP1, SHP1 or anti-GAPDH antibody as protein loading controls. B, C and D) Quantitation of the levels of phospho-LYN (Y397), phospho-SHIP1 (Y1021) and phospho-SHP1 (Y536) in panel A. Data are represented as mean ±SEM from four biological replicates (*P < 0.05; Student’s t test.). NT– Non-targeting control. E) An in vitro kinase assay was performed using recombinant SHIP1 and increasing concentrations of active LYN kinase under cell-free conditions for ascertaining whether SHIP1 is a direct target for phosphorylation by LYN. JAK2 kinase and LYN/Src inhibitor were used as controls. In vitro kinase reaction products were resolved on SDS-PAGE gel and immunoblot analysis performed using an anti-phospho SHIP1 (Y1021) antibody. The same immunoblots were also probed with anti-SHIP1 antibody as protein loading control. Representative data from three independent experiments are shown. F) An in vitro kinase experiment as described in panel E was set up to test whether SHP1 is a direct substrate of LYN. In vitro kinase reaction products were resolved on a SDS-PAGE gel and immunoblot analysis performed using an anti-phospho SHP1 (Y536) antibody. The same immunoblots were also probed with anti-SHP1 antibody as loading control. Representative data from three independent experiments are shown. G) Cultured HSCs were exposed to increasing doses of LYN/Src inhibitor, RK20449 in the presence or absence of stem cell cytokines. Samples were resolved and immunoblotted for phospho-LYN (Y397), phospho-SHIP1 (Y1021) and phospho-SHP1 (Y536). The same blot was probed with LYN, SHIP1 or SHP1 antibodies as protein loading controls. Representative data from three biological replicates are shown. H, I, J) Determination of DOCK4 interaction with LYN, SHIP1 and SHP1. Flag-tagged DOCK4 together with either H) GFP-tagged LYN or I) GFP-tagged SHIP1 or J) GFP-tagged SHP1 was transfected into HEK293 cells and protein lysates were used in reciprocal immunoprecipitation assays. Immunoprecipitations were performed with an anti-Flag antibody followed by immunoblot analysis using either anti-LYN antibody, anti-SHIP1 antibody or anti-SHP1 antibodies. As controls immunoprecipitations were also performed using GFP-trap beads followed by an anti-Flag antibody. Anti-DOCK4 or GFP antibodies were also used as additional controls. K) Flag-tagged DOCK4 was transfected into HEK293 cells and immunoprecipitated with anti-Flag antibody followed by immunoblot analysis using anti-Flag and anti-DOCK4 antibodies. Samples were prepared using different amounts of anti-flag immunoprecipitated beads. L) LYN kinase activity assay was performed using recombinant LYN kinase and increasing amounts of flag-DOCK4 to ascertaining whether DOCK4 controls LYN kinase activity. JAK2 kinase and LYN/Src inhibitor were used as controls. Data are mean ±SEM from three independent experiments. (****P < 0.00005; One way ANOVA)
SHIP1 and SHP1 are substrates for LYN kinase
Next, we wanted to determine whether LYN kinase was directly responsible for phosphorylating the two phosphatases, SHIP1 and SHP1 as a result of LYN Kinase activation due to reduced expression of DOCK4 (supplemental Figure 2G). In order to determine whether SHIP1 and SHP1 are direct targets of LYN kinase, we designed cell-free in vitro kinase assays, where either recombinant full-length SHIP1 protein or recombinant full-length SHP1 protein was incubated with active form of recombinant LYN kinase in a biochemical assay in the presence of a kinase buffer. A parallel assay using the same substrates but recombinant JAK2 as the kinase enzyme was also performed as a control. The reaction products were then analyzed by immunoblotting using an anti-phospho SHIP1 (Y1021) antibody or an anti- phospho SHP1 (Y536) antibody. The results of these experiments showed that LYN kinase phosphorylated SHIP1 at tyrosine 1021 and SHP1 at tyrosine 536 in a dose dependent manner whereas JAK2 kinase did not show such phosphorylation of SHIP1 or SHP1 (Figure 2E–F, supplemental Figure 2H–I). Furthermore, the presence of the LYN/Src inhibitor, abrogated phosphorylation of both SHIP1 and SHP1, re-enforces the specificity of the in vitro kinase assays (Figure 2E–F, supplemental Figure 2H–I). In order to determine whether SHIP1 and SHP1 are substrates of LYN kinase in HSCs, we inhibited LYN using the LYN/Src inhibitor in cultured HSCs. Immunoblotting analysis showed that SHIP1 (Y1021) and SHP1 (Y536) phosphorylation decreased in a dose dependent manner when LYN was inhibited (Figure 2G, supplemental Figure 2J–L). Taken together these results demonstrated that reduced expression of DOCK4 initiate a sequential phosphorylation events impacting the signaling cascade involving LYN kinase, SHIP1 and SHP1.
DOCK4 interacts with LYN kinase and SHIP1 but not SHP1
Next, we wanted to ascertain whether DOCK4 directly interacts with LYN kinase as well as SHIP1 and SHP1 phosphatases in addition to whether DOCK4 regulates LYN kinase activity. In order to test this, we ectopically expressed Flag-tagged full length DOCK4 and GFP-tagged full length LYN in HEK293 cells and performed reciprocal co-immunoprecipitation experiments coupled with immunoblot analysis. Immunoprecipitation of Flag-tagged DOCK4 followed by using anti-GFP/LYN antibody for detection as well as immunoprecipitation with GFP-trap beads and immunoblot analysis with anti-FLAG/DOCK4 antibody showed that DOCK4 directly interacted with LYN (Figure 2H). Similarly, we ectopically expressed Flag-tagged full length DOCK4 and GFP-tagged full length SHIP1 in HEK293 cells and performed reciprocal co-immunoprecipitation experiments coupled with immunoblot analysis. Immunoprecipitation of Flag-tagged DOCK4 followed by using anti-GFP/SHIP1 antibody for detection as well as immunoprecipitation with GFP-trap beads and immunoblot analysis with anti-FLAG/DOCK4 antibody showed that DOCK4 directly interacted with SHIP1 (Figure 2I). However, when similar experiments were performed using GFP-tagged full-length SHP1, we did not detect a direct interaction between DOCK4 and SHP1 suggesting that changes in phosphorylation seen in SHP1 is indirect and most likely only through LYN kinase (Figure 2J).
We then determined whether LYN kinase activity is regulated by DOCK4 levels. In order to accomplish this, we set up an in vitro kinase assay where varying amounts recombinant DOCK4 protein was incubated with recombinant active form of LYN kinase and measured LYN kinase activity using a commercially available ELISA. These experiments showed that increasing amounts of DOCK4 protein decreased LYN kinase activity (Figure 2K, L and supplemental Figure 2M, N). However, when recombinant JAK2 was used as a control no modulation of JAK2 activity was observed when increasing amounts of DOCK4 was used in the assay (Figure 2L and supplemental Figure 2M).
Decreased DOCK4 expression leads to increased HSC migration
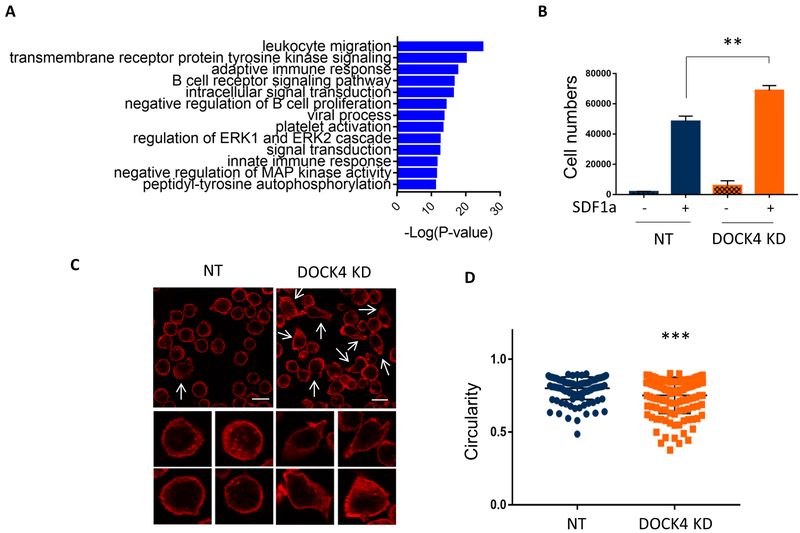
To identify the functional implications of increased tyrosine signaling in DOCK4 deficient HSCs, we performed in silico Database for Annotation Visualization and Integrated Discovery (DAVID) analysis using the list of proteins that were identified by mass spectrometry to be highly phosphorylated (Table 1). This analysis revealed cell migration as one of the highly enriched biological pathways (Figure 3A). We tested this prediction experimentally by carrying out transwell migration assays using HSCs expressing normal levels and reduced levels of DOCK4, which showed increased rates of migration of cells expressing reduced levels of DOCK4 (Figure 3B). Similar results were also observed in TF1 cells following DOCK4 knockdown (supplemental Figure 3A). Since F-actin in the cytoskeleton play a key function in cell migration, we examined for changes in the F-actin network in HSCs expressing reduced levels of DOCK4 and compared them to HSCs expressing normal levels of F-actin. These experiments revealed significantly increased numbers of cells displaying pro-migratory features such as cell spreading and bundled F-actin in the leading edges of the cells (Figure 3C). In follow up experiments we quantified the extent of cell spreading in DOCK4 knocked down (50% knockdown) cells and cells expressing normal levels of DOCK4 by computing circularity values using the built-in circularity feature available in the Fiji software. This quantitation revealed that cell spreading was significantly increased when DOCK4 levels were reduced in HSCs as indicated by the decrease in circularity values (Figure 3D). Similar pro-migratory features were also observed in TF1 cells when DOCK4 levels were reduced (supplemental Figure 3B).
Figure 3: Reduced levels of DOCK4 leads to increased cell migration and cell morphology in HSCs.
A) Differentially phosphorylated proteins displayed in table 1 were used for in silico gene ontology pathway analysis to predict functional pathways associated with identified phosphoproteins. Predicted functional pathways are displayed graphically. B) Transwell cell migration assays performed to compare differences in cell mobility/migration in HSCs expressing normal and reduced levels of DOCK4. Data are represented as mean ±SEM from four biological replicates (**P < 0.005; Student’s t test.). C) Changes in HSC morphology following knockdown of DOCK4 was determined by staining for cytoskeletal F-actin and analyzed by immunofluorescence microscopy. Cells which depicted spread morphology with bundled actin at the leading edges are shown in white arrows (Scale bar, 15μM). Individual cells at high magnification showing pronounced cell spreading and bundled F-actin when DOCK4 levels were reduced. D) Cell shape/circularity was measured in HSCs expressing normal and reduced levels of DOCK4 using circularity parameter in Fiji image analysis software. A minimum of 100 cells from four random fields were quantified (***P < 0.0005; Student’s t test.). NT – Non-targeting control.
Inhibition of LYN, SHIP1 or SHP1 protein levels or their activity decreases HSC migration
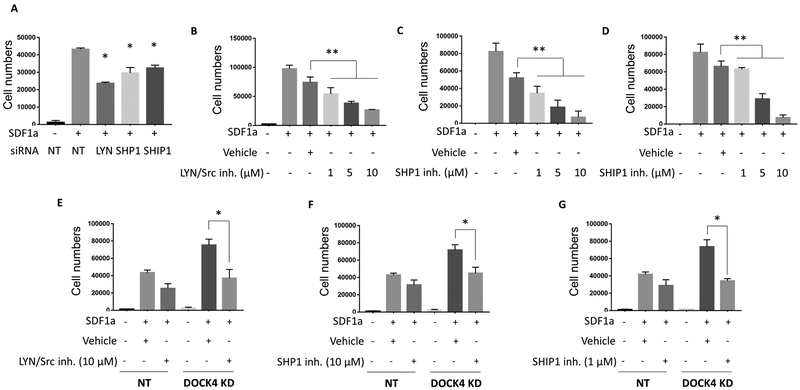
Given that LYN, SHIP1 and SHP1 are downstream of DOCK4, we next determined whether the LYN kinase and its two down-stream targets SHIP1 and SHP1 were involved in regulating HSC migration. We reduced the expression levels of LYN, SHIP1 or SHP1 in HSCs by 50% or greater by knocking down these proteins using specific siRNAs (supplemental Figure 4A–C). Using these cells, we performed in vitro transwell migration assays and compared their migration to cells that expressed LYN, SHIP1 and SHP1 at normal levels. The results of these experiments revealed that reduced expression of LYN or SHIP1 or SHP1 led to a significant decrease in the migration of HSCs compared to the controls (Figure 4A). We extended these studies and performed a series of experiments where we exposed HSCs to increasing concentrations of inhibitors of LYN/Src kinase, SHIP1 and SHP1 and evaluated their migration response. These studies demonstrated a dose dependent decrease in HSC migration (Figure 4B–D). Taken together increased migration of HSCs observed in cells expressing reduced levels of DOCK4 seemed to be as a result of each of the three signaling molecules identified in this study.
Figure 4: Inhibition of LYN or SHP1 or SHIP1 activities reverse increased migration exhibited by DOCK4 deficient HSCs.
A) Transwell migration assays performed following knockdown of LYN, SHP1 or SHIP1 by using specific siRNAs in HSCs. Data are represented as mean ±SEM from three technical replicates (*P < 0.05; Student’s t test). Data are representative of three biological replicates. B, C and D) Transwell migration assays performed on HSCs expressing normal levels of DOCK4 exposed to increasing concentrations of LYN/Src inhibitor (RK20449), SHP1 inhibitor (TPI-1) and SHIP1 inhibitor (3AC) respectively. Data are represented as mean ±SEM from three technical replicates (**P < 0.005; One-way ANOVA). E) Transwell migration assays performed on HSCs expressing reduced levels of DOCK4 in the presence or absence of LYN/Src inhibitor (RK20449) F) SHP1 inhibitor (TPI-1) and G) SHIP1 inhibitor (3AC). Data are represented as mean ±SEM from three technical replicates (*P < 0.05; Student’s t test). Data are representative of three biological replicates. NT – Non-targeting control.
Inhibitors of LYN, SHIP1 and SHP1 restore normal HSC migratory properties in DOCK4 deficient cells
Next, we interrogated whether inhibition of LYN kinase, SHIP1 or SHP1 can reverse the increased migration observed in HSCs expressing reduced levels of DOCK4. We performed in vitro transwell migration assays using HSCs expressing normal and reduced levels of DOCK4 in the presence and absence of pharmacological inhibitors of LYN/Src kinase, SHIP1 or SHP1. As expected, in the absence of LYN/Src inhibitor, DOCK4 deficient HSCs exhibited significant increase in transwell migration when compared to the controls (Figure 4E). However, in the presence of LYN/Src inhibitor, the increased migration exhibited by the DOCK4 knocked down cells was significantly blunted and returned to migration levels exhibited by DOCK4 intact HSCs (Figure 4E). In a similar manner, increased migration exhibited by DOCK4 deficient HSCs was also significantly reduced in the presence of pharmacological inhibitors of SHIP1 and SHP1 (Figure 4F–G). Taken together, these studies provide evidence that aberrant migration resulted by reduced DOCK4 levels can be restored to normal levels by pharmacologically targeting its downstream targets LYN or SHIP1 or SHP1.
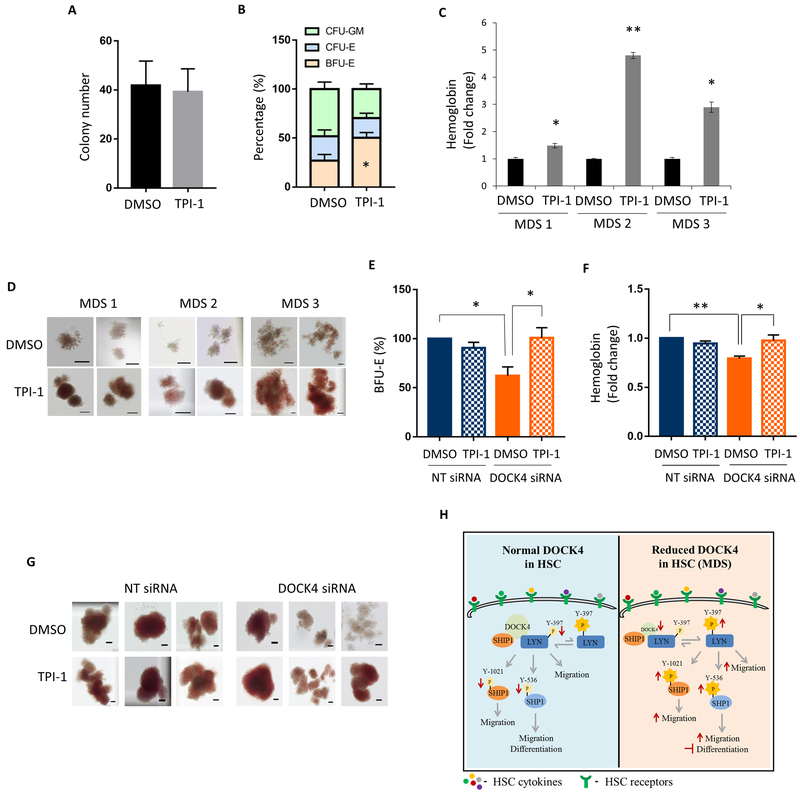
Inhibition of SHP1 promotes erythroid differentiation in −7/(del)7q MDS samples
Since anemia is central to morbidity and mortality of MDS patients, we investigated whether one or more of the inhibitors of down-stream effectors of DOCK4 are capable of improving erythroid differentiation with minimal toxicity to HSCs (supplemental Figure S5A). We setup hematopoietic colony assays using HSCs from MDS patients in the presence and absence of inhibitors of LYN/SRC kinase (RK20449), SHIP1 (3AC) and SHP1 (TPI-1) under conditions to promote erythroid colony formations. The results of these experiments revealed that LYN/SRC kinase inhibitors suppressed formation of erythroid colonies, whereas the SHIP1 inhibitor, 3AC, showed no change in colony numbers in −7/(del)7q MDS patient HSCs (data not shown). However, MDS patient samples that were exposed to the SHP1 inhibitor exhibited a 50% increase in erythroid colonies as well as up to five-fold increase in hemoglobin content without suppressing overall colony numbers (Figure 5A–D). In agreement with these data morphology of the erythroid colonies was larger and brighter red in intensity compared to HSCs from −7/(del)7q MDS patients that were not exposed to the inhibitor. In addition, enumeration of differential colonies showed a shift from myeloid to erythroid under the culture conditions that was used in these experiments. To test whether pharmacological inhibition of SHP1 under conditions where DOCK4 expression was at 50% will result in improved erythroid differentiation, we performed methyl cellulose colony assays using HSCs that have been treated with DOCK4 siRNAs to reduce DOCK4 expression to haploinsufficient levels in the presence or absence of SHP1 inhibitor. These experiments revealed that exposure of cells expressing reduced levels of DOCK4 to SHP1 inhibitor significantly increased the erythroid colonies, whereas cells expressing normal levels of DOCK4 showed no increase in colony numbers after exposure to the same inhibitor in comparison with the vehicle treated controls (Figure 5E). In addition, SHP1 inhibitor increased the hemoglobin levels and size of the erythroid colonies in the experimental arm of the study compared to the control arm (Figure 5F, G). SHP1 inhibition did not have any impact on differentiation of HSCs expressing normal levels of DOCK4 (Figure 5E, F, G and supplemental Figure S5B–E).
Figure 5: Inhibition of SHP1 activity promotes erythroid differentiation in DOCK4 deficient MDS.
CD34+ stem/progenitor cells were purified from −7/(del)7q MDS patients bone marrow mononucleated cells and methylcellulose colony assays were setup in the presence or absence of SHP1 inhibitor, TPI-1 (4μM). After 14–16 days of culture under differentiation conditions, A) Total number of colonies enumerated from each arm of the experiment. Data are mean ±SEM from seven different −7/(del)7q MDS patients. B) Scoring for early erythroid (BFU-E), late erythroid (CFU-E) and myeloid (CFU-GM) (*P < 0.05; Student’s t test.). Data are mean ±SEM from seven different −7/(del)7q MDS patients. C) ELISA performed to determine beta hemoglobin expression. Data are represented as mean ±SEM from three technical replicates (*P < 0.05; **P < 0.005; Student’s t test.). D) Photomicrographs depicting colony morphology and the extent of hemoglobin after 14–16 days in methylcellulose (Scale bar, 100μM). 24 hrs following DOCK4 knockdown in HSCs, methylcellulose colony assays were setup in the presence or absence of SHP1 inhibitor, TPI-1 (4μM). After 14–16 days of culture under erythroid differentiation conditions, E) Percentage of erythroid colonies enumerated from each arm of the experiment. Data are mean ±SEM from four biological replicates. (*P < 0.05; Student’s t test.). F) ELISA performed to determine hemoglobin expression. Data are representative of five biological replicates (*P < 0.05; ** P < 0.005; Student’s t test.). G) Photomicrographs depicting colony morphology and the extent of hemoglobin after 14–16 days in methylcellulose (Scale bar, 100μM). H) Schematic of the DOCK4 signaling pathway in HSCs.
Increased HSPC mobilization into the peripheral circulation in −7/(del)7q MDS patients
Since increased migration of HSCs within the bone marrow can lead to increased HSPC mobilization, we examined peripheral blood samples from patients that are haplo-insufficient for DOCK4 (−7/(del)7q) expression. Flow cytometry analysis was performed to determine the percentages of CD34+ sub-population within the CD45+ population using peripheral blood samples from healthy individuals, non −7q MDS patients and −7/(del)7q MDS patients. We found that compared to non −7q MDS samples, percent CD34+ cells in −7/(del)7q MDS samples were approximately 8.8 fold higher (P=0.05 supplemental Figure 6A–B).
Discussion
In this study, we identify key signaling pathways regulated by the adaptor protein DOCK4 (Figure 5E). As a classical adaptor protein DOCK4 lacks catalytic activity but provide multiple docking sites for other signaling elements and regulate their catalytic activities or stabilities via protein-protein interaction(30,31). In our previous work we demonstrated that in differentiating primary human erythroblasts DOCK4 activates one of its downstream targets, RAC1 GTPase, which in turn promotes formation of the actin skeletal network required for terminal differentiation(16). Now we show in hematopoietic stem cells that reduced levels of DOCK4 results in global increase in tyrosine phosphorylation both with and without exposure to hematopoietic cytokines. Among the increased phosphorylated proteins LYN kinase, SHP1 and SHIP1 were prominent. Although activation of phosphatases, SHP1 and SHIP1 leads to de-phosphorylation of their targets, overall reduced expression of DOCK4 resulted in phosphorylation of a large number of proteins due to activation of kinases. As result we observed a net gain in global phosphorylation when DOCK4 levels were low. Based on our results the mechanism of action of DOCK4 seems to act as a negative regulator of protein phosphorylation and such LYN kinase, SHP1 and SHIP1 which are examples of its target proteins.
Increased cell migration and morphological changes we observed when DOCK4 levels were low are consistent with functions that have been ascribed to LYN kinase based on previously published work (32–36). Previous studies have also shown that increased HSC migration was consistent with increase in HSC mobilization (37,38), which we also observed in −7/(del)7q MDS patient blood samples. Our current work seemed to suggest that increased HSC mobilization is specifically associated with −7/(del)7q MDS since MDS samples with other chromosomal abnormalities did not exhibit increased HSC mobilization. Furthermore, our current findings showing increased phosphorylation/activation of SHIP1 and SHP1 is also consistent with previous findings showing both these phosphatases are substrates for LYN kinase(39,40). In fact, Lyn deficient mice exhibit similar phenotypic characteristics to mice lacking Ship1 and Shp1(29). Based on these data we show DOCK4, LYN kinase, SHIP1 and SHP1 are all part of the same signaling cascade and because deficiency of DOCK4 in HSCs impacts all three enzymes one could target this pathway to reverse functional deficiency observed in these HSCs.
In fact when we evaluated for terminal differentiation of MDS patient derived CD34+ cells under culture conditions that was permissive for erythroid differentiation, cells that were exposed to the SHP1 inhibitor (TPI-1) showed pro-differentiation characteristics (increased BFU-Es and hemoglobinization). These results were in agreement with previous studies using healthy cells, which had demonstrated that SHP1 phosphatase is a negative regulator of erythroid differentiation(41–43). Therefore, by blocking SHP1 activity one can potentially reverse anemia in −7/(del)7q MDS patients. Recent work by Taolin Yi and colleagues(44) have demonstrated that TPI-1 and its analogues are non-toxic when administered to mice and is effective in reducing the melanoma tumor burden in mice(44). In another study it was shown that mice lacking Shp1 do not respond to TGF-beta(45). Since previous work by us and others have shown that in MDS TGF-beta signaling is overactive and inhibiting this pathway can restore hematopoiesis in MDS(46–48), our current work identifying SHP1 inhibition leads to terminal erythroid differentiation provides a unique opportunity to develop inhibitors of SHP1 that might be effective in treating MDS patients.
Kinase inhibitors such as midostaurin are effective in high-risk Flt3 mutated MDS/AML patients, whereas pro-erythroid differentiation agents such as luspatercept and erythropoietin are effective in low-risk MDS patients. Our findings in this study highlight the potential use of SHP1 inhibitor as a pro-erythroid differentiation agent in intermediate/high risk MDS patients with chromosome 7 deletions. Future investigation of SHP1 inhibitor as single agent and in combination with luspatercept or 5-azacytidine/decitabine as a treatment strategy in −7/del(7q) MDS is warranted. Taken together, the current study has uncovered a signaling network regulated by DOCK4 that can be targeted to reverse the aberrant phenotypes arising due to reduced expression of DOCK4.
Supplementary Material
Translational relevance.
Better understanding of mechanisms underlying ineffective hematopoiesis in myelodysplastic syndromes (MDS) is critically needed to develop novel therapeutic strategies. Reduced levels of the adaptor protein DOCK4 is frequently observed in −7/(del)7q MDS. Restoring DOCK4 expression in −7/(del)7q MDS overcomes differentiation block and improves erythroid differentiation. Here, we demonstrate avenues for restoring the DOCK4 functions by targeting signaling elements downstream of DOCK4 in human HSCs. Using HSCs from MDS patients expressing reduced levels (haploinsufficient) of DOCK4 due to chromosome 7 deletions, we demonstrate that inhibitors of one of the three identified regulators of DOCK4 is capable of relieving the differentiation block along the erythroid lineage. In addition, inhibitors of all 3 regulators restored aberrant stem cell migration properties observed in HSCs harboring aberrant DOCK4 expression.
Acknowledgements
This work was supported, in part, by National Institute of Health (NIH) R01 HL16336 (to A.V. and A.W.), Leukemia and Lymphoma Society (LLS) translational research program (to A.V. and A.W.) and National Cancer Institute (NCI) F99/K00 CA223044 predoctoral to postdoctoral fellow transition award (to S.S.). We thank Dr. Linda Van Aelst (Cold Spring Harbor Lab, US) for generously providing the flag-tagged DOCK4 construct and Dr. Aaron Marshall (University of Manitoba, CA) for generously providing the GFP-tagged SHIP1 construct. Proteomics services were performed by the Northwestern Proteomics Core Facility, generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center and the National Resource for Translational and Developmental Proteomics supported by P41 GM108569.
Footnotes
Disclosure of Potential Conflicts of interest
No relevant conflict of interests exists for this study. AV has received research funding from GSK, Incyte, Medpacto, and Eli Lilly. He is a scientific advisor for Stelexis, Novartis, Acceleron, and Celgene. He also holds equity stake in Stelexis.
References
- 1.Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DCR, et al. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell [Internet]. 2003. [cited 2014 Jan 10];112:673–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12628187 [DOI] [PubMed] [Google Scholar]
- 2.Upadhyay G, Goessling W, North TE, Xavier R, Zon LI, Yajnik V. Molecular association between beta-catenin degradation complex and Rac guanine exchange factor DOCK4 is essential for Wnt/beta-catenin signaling. Oncogene [Internet]. 2008. [cited 2014 Jan 10];27:5845–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18641688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Y, Peng Y, Wan J, Tang G, Chen Y, Tang J, et al. The Atypical Guanine Nucleotide Exchange Factor Dock4 Regulates Neurite Differentiation through Modulation of Rac1 GTPase and Actin Dynamics. J Biol Chem [Internet]. 2013. [cited 2018 Nov 27];288:20034–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23720743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan D, Li F, Hall ML, Sage C, Hu W-H, Giallourakis C, et al. An Isoform of GTPase Regulator DOCK4 Localizes to the Stereocilia in the Inner Ear and Binds to Harmonin (USH1C). J Mol Biol [Internet]. 2006. [cited 2018 Nov 27];357:755–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16464467 [DOI] [PubMed] [Google Scholar]
- 5.Kang H, Davis-Dusenbery BN, Nguyen PH, Lal A, Lieberman J, Van Aelst L, et al. Bone Morphogenetic Protein 4 Promotes Vascular Smooth Muscle Contractility by Activating MicroRNA-21 (miR-21), which Down-regulates Expression of Family of Dedicator of Cytokinesis (DOCK) Proteins. J Biol Chem [Internet]. 2012. [cited 2018 Nov 27];287:3976–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22158624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eguchi K, Yoshioka Y, Yoshida H, Morishita K, Miyata S, Hiai H, et al. The Drosophila DOCK family protein sponge is involved in differentiation of R7 photoreceptor cells. Exp Cell Res [Internet]. 2013. [cited 2018 Nov 27];319:2179–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23747680 [DOI] [PubMed] [Google Scholar]
- 7.Okamoto O, Carvalho ACS, Marti LC, Vêncio RZ, Moreira-Filho CA. Common molecular pathways involved in human CD133+/CD34+ progenitor cell expansion and cancer. Cancer Cell Int [Internet]. 2007. [cited 2018 Nov 27];7:11 Available from: http://www.ncbi.nlm.nih.gov/pubmed/17559657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J-R, Tai Y, Jin Y, Hammell MC, Wilkinson JE, Roe J-S, et al. TGF-β/Smad signaling through DOCK4 facilitates lung adenocarcinoma metastasis. Genes Dev [Internet]. 2015. [cited 2018 Nov 27];29:250–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25644601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham S, Scarcia M, Bagshaw RD, McMahon K, Grant G, Harvey T, et al. A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat Commun [Internet]. 2015. [cited 2018 Nov 27];6:7286 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26129894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debruyne DN, Turchi L, Burel-Vandenbos F, Fareh M, Almairac F, Virolle V, et al. DOCK4 promotes loss of proliferation in glioblastoma progenitor cells through nuclear beta-catenin accumulation and subsequent miR-302–367 cluster expression. Oncogene [Internet]. 2018. [cited 2018 Nov 27];37:241–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28925399 [DOI] [PubMed] [Google Scholar]
- 11.Kjeldsen E, Veigaard C. DOCK4 deletion at 7q31.1 in a de novo acute myeloid leukemia with a normal karyotype. Cell Oncol [Internet]. 2013. [cited 2018 Nov 27];36:395–403. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23979775 [DOI] [PubMed] [Google Scholar]
- 12.Hiramoto K, Negishi M, Katoh H. Dock4 is regulated by RhoG and promotes Rac-dependent cell migration. Exp Cell Res [Internet]. 2006. [cited 2018 Nov 27];312:4205–16. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17027967 [DOI] [PubMed] [Google Scholar]
- 13.Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, et al. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol [Internet]. 2010. [cited 2018 Nov 27];190:461–77. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20679435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Opalinska J, Sohal D, Yu Y, Mo Y, Bhagat T, et al. Aberrant epigenetic and genetic marks are seen in myelodysplastic leukocytes and reveal Dock4 as a candidate pathogenic gene on chromosome 7q. J Biol Chem [Internet]. 2011. [cited 2014 Jan 10];286:25211–23. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3137092&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med [Internet] 1999. [cited 2014 Jan 10];340:1649–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10341278 [DOI] [PubMed] [Google Scholar]
- 16.Sundaravel S, Duggan R, Bhagat T, Ebenezer DL, Liu H, Yu Y, et al. Reduced DOCK4 expression leads to erythroid dysplasia in myelodysplastic syndromes. Proc Natl Acad Sci U S A [Internet]. 2015. [cited 2016 Feb 24];112:E6359–68. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4655581&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Mo Y, Ebenezer D, Bhattacharyya S, Liu H, Sundaravel S, et al. High Resolution Methylome Analysis Reveals Widespread Functional Hypomethylation during Adult Human Erythropoiesis. J Biol Chem [Internet]. American Society for Biochemistry and Molecular Biology; 2013. [cited 2016 Aug 10];288:8805–14. Available from: http://www.jbc.org/cgi/doi/10.1074/jbc.M112.423756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madzo J, Liu H, Rodriguez A, Vasanthakumar A, Sundaravel S, Caces DBD, et al. Hydroxymethylation at Gene Regulatory Regions Directs Stem/Early Progenitor Cell Commitment during Erythropoiesis. Cell Rep [Internet] 2014. [cited 2014 Jan 21];6:231–44. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24373966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong JJ, Gu X, Nie J, Sundaravel S, Liu H, Kuo W-L, et al. Cytokine-Regulated Phosphorylation and Activation of TET2 by JAK2 in Hematopoiesis. Cancer Discov [Internet]. American Association for Cancer Research; 2019. [cited 2019 Jun 4];9:778–95. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30944118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickrema A, Krantz SB, Winkelmann JC, Bondurant MC. Differentiation and erythropoietin receptor gene expression in human erythroid progenitor cells. Blood [Internet] 1992. [cited 2014 Jan 10];80:1940–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1391953 [PubMed] [Google Scholar]
- 21.Gilles L, Arslan AD, Marinaccio C, Wen QJ, Arya P, McNulty M, et al. Downregulation of GATA1 drives impaired hematopoiesis in primary myelofibrosis. J Clin Invest [Internet]. American Society for Clinical Investigation; 2017. [cited 2019 Feb 19];127:1316–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28240607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, et al. Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol Chem [Internet]. American Society for Biochemistry and Molecular Biology; 1999. [cited 2018 Nov 28];274:24469–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10455108 [DOI] [PubMed] [Google Scholar]
- 23.Yue X-S, Hummon AB. Combination of Multistep IMAC Enrichment with High-pH Reverse Phase Separation for In-Depth Phosphoproteomic Profiling. J Proteome Res [Internet]. 2013. [cited 2019 May 8];12:4176–86. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23927012 [DOI] [PubMed] [Google Scholar]
- 24.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature [Internet]. 2011. [cited 2018 Nov 28];480:109–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22101434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauls SD, Ray A, Hou S, Vaughan AT, Cragg MS, Marshall AJ. FcγRIIB-Independent Mechanisms Controlling Membrane Localization of the Inhibitory Phosphatase SHIP in Human B Cells. J Immunol [Internet]. 2016. [cited 2018 Nov 28];197:1587–96. Available from: www.jimmunol.org/cgi/doi/10.4049/jimmunol.1600105 [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Kruhlak MJ, Hao J-J, Shaw S. Rapid T cell receptor-mediated SHP-1 S591 phosphorylation regulates SHP-1 cellular localization and phosphatase activity. J Leukoc Biol [Internet]. 2007. [cited 2019 Mar 19];82:742–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17575265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prebet T, Lhoumeau A-C, Arnoulet C, Aulas A, Marchetto S, phane Audebert S, et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. 2010. [cited 2018 Nov 28]; Available from: www.bloodjournal.org [DOI] [PubMed] [Google Scholar]
- 28.Knapp DJHF Hammond CA, Aghaeepour N, Miller PH, Pellacani D, Beer PA, et al. Distinct signaling programs control human hematopoietic stem cell survival and proliferation. 2017. [cited 2019 Feb 17]; Available from: www.bloodjournal.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harder KW, Quilici C, Naik E, Inglese M, Kountouri N, Turner A, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood [Internet]. American Society of Hematology; 2004. [cited 2019 Mar 13];104:3901–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15339845 [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi M, Harada K, Negishi M, Katoh H. Dock4 forms a complex with SH3YL1 and regulates cancer cell migration. Cell Signal [Internet]. 2014. [cited 2018 Nov 27];26:1082–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24508479 [DOI] [PubMed] [Google Scholar]
- 31.Ueda S, Negishi M, Katoh H. Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol Biol Cell [Internet]. 2013. [cited 2019 Mar 13];24:1602–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23536706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Laughlin-Bunner B, Radosevic N, Taylor ML, Shivakrupa, DeBerry C, Metcalfe DD, et al. Lyn is required for normal stem cell factor-induced proliferation and chemotaxis of primary hematopoietic cells. Blood [Internet]. 2001. [cited 2019 Mar 13];98:343–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11435302 [DOI] [PubMed] [Google Scholar]
- 33.Orschell CM, Borneo J, Munugalavadla V, Ma P, Sims E, Ramdas B, et al. Deficiency of Src family kinases compromises the repopulating ability of hematopoietic stem cells. Exp Hematol [Internet]. Elsevier; 2008. [cited 2019 Feb 25];36:655–66. Available from: https://www.sciencedirect.com/science/article/pii/S0301472X08000167?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Röselová P, Obr A, Holoubek A, Grebeňová D, Kuželová K. Adhesion structures in leukemia cells and their regulation by Src family kinases. Cell Adh Migr [Internet]. 2018. [cited 2019 Mar 13];12:286–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28678601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler SE, Morariu EM, Bednash JS, Otte CG, Seethala RR, Chiosea SI, et al. Lyn Kinase Mediates Cell Motility and Tumor Growth in EGFRvIII-Expressing Head and Neck Cancer. Clin Cancer Res [Internet]. American Association for Cancer Research; 2012. [cited 2019 Mar 18];18:2850–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22490227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakata Y, Tomkowicz B, Gewirtz AM, Ptasznik A. Integrin inhibition through Lyn-dependent cross talk from CXCR4 chemokine receptors in normal human CD34 marrow cells. 2006. [cited 2019 Mar 16]; Available from: www.bloodjournal.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gur-Cohen S, Itkin T, Chakrabarty S, Graf C, Kollet O, Ludin A, et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med [Internet]. Nature Publishing Group; 2015. [cited 2019 Feb 18];21:1307–17. Available from: http://www.nature.com/articles/nm.3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borneo J, Munugalavadla V, Sims EC, Vemula S, Orschell CM, Yoder M, et al. Src family kinase–mediated negative regulation of hematopoietic stem cell mobilization involves both intrinsic and microenvironmental factors. Exp Hematol [Internet]. Elsevier; 2007. [cited 2019 Feb 25];35:1026–37. Available from: https://www.sciencedirect.com/science/article/pii/S0301472X07002020?via%3Dihub#fig3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5’-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor-induced Akt activity. J Biol Chem [Internet]. American Society for Biochemistry and Molecular Biology; 2003. [cited 2018 Nov 27];278:38628–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12882960 [DOI] [PubMed] [Google Scholar]
- 40.Mkaddem S Ben, Murua A, Flament H, Titeca-Beauport D, Bounaix C, Danelli L, et al. Lyn and Fyn function as molecular switches that control immunoreceptors to direct homeostasis or inflammation. Nat Commun [Internet]. Nature Publishing Group; 2017. [cited 2018 Nov 27];8:246 Available from: http://www.nature.com/articles/s41467-017-00294-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bittorf T, Seiler J, Zhang Z, Jaster R, Brock J. SHP1 Protein Tyrosine Phosphatase Negatively Modulates Erythroid Differentiation and Suppression of Apoptosis in J2E Erythroleukemic Cells. Biol Chem [Internet]. Walter de Gruyter; 1999. [cited 2019 Mar 17];380:1201–9. Available from: https://www.degruyter.com/view/j/bchm.1999.380.issue-10/bc.1999.152/bc.1999.152.xml [DOI] [PubMed] [Google Scholar]
- 42.Wickrema A, Chen F, Namin F, Yi T, Ahmad S, Uddin S, et al. Defective expression of the SHP-1 phosphatase in polycythemia vera. Exp Hematol [Internet]. 1999. [cited 2019 Mar 17];27:1124–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10390187 [DOI] [PubMed] [Google Scholar]
- 43.Sharlow ER, Pacifici R, Crouse J, Batac J, Todokoro K, Wojchowski DM. Hematopoietic cell phosphatase negatively regulates erythropoietin-induced hemoglobinization in erythroleukemic SKT6 cells. Blood [Internet]. 1997. [cited 2019 Mar 13];90:2175–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9310468 [PubMed] [Google Scholar]
- 44.Kundu S, Fan K, Cao M, Lindner DJ, Zhao ZJ, Borden E, et al. Novel SHP-1 Inhibitors Tyrosine Phosphatase Inhibitor-1 and Analogs with Preclinical Anti-Tumor Activities as Tolerated Oral Agents. J Immunol [Internet]. 2010. [cited 2019 Feb 19];184:6529–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20421638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang L, Han X, Wang J, Wang C, Sun X, Xie J, et al. SHP-1 regulates hematopoietic stem cell quiescence by coordinating TGF-β signaling. J Exp Med [Internet]. Rockefeller University Press; 2018. [cited 2019 Mar 20];215:1337–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29669741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou L, Nguyen AN, Sohal D, Ying Ma J, Pahanish P, Gundabolu K, et al. Inhibition of the TGF- receptor I kinase promotes hematopoiesis in MDS. Blood [Internet]. 2008. [cited 2019 Mar 20];112:3434–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18474728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, et al. Reduced SMAD7 Leads to Overactivation of TGF- Signaling in MDS that Can Be Reversed by a Specific Inhibitor of TGF- Receptor I Kinase. Cancer Res [Internet]. 2011. [cited 2019 Mar 20];71:955–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21189329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shastri A, Will B, Steidl U, Verma A. Stem and progenitor cell alterations in myelodysplastic syndromes. Blood [Internet]. 2017. [cited 2019 Mar 20];129:1586–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28159737 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.