Abstract
What we direct our attention to is strongly influenced by both bottom-up and top-down processes. Moreover, the control of attention is biased by prior learning, such that attention is automatically captured by stimuli previously associated with either reward or threat. It is unknown whether value-oriented and threat-oriented mechanisms of selective information processing function independently of one another, or whether they interact with each other in the selection process. Here, we introduced the threat of electric shock into the value-driven attentional capture paradigm to examine whether the experience of threat influences the attention capturing quality of previously reward-associated stimuli. The results showed that value-driven attentional capture was blunted by the experience of threat. This contrasts with previous reports of threat potentiating attentional capture by physically salient stimuli, which we replicate here. Our findings demonstrate that threat selectively interferes with value-based but not salience-based attentional priority, consistent with a competitive relationship between value-based and threat-based information processing.
Keywords: selective attention, attentional capture, reward learning, threat, anxiety
Introduction
Attention is a selective cognitive process that filters sensory input to manage the limited representational capacity of our perceptual system (Desimone & Duncan, 1995). Although attention can be voluntarily directed to objects (Corbetta, Kincade, Ollinger, McAvoy, & Shulman, 2000; Corbetta & Shulman, 2002; Duncan & Humphreys, 1989; Shulman et al., 1999; Wolfe, 1994) and spatial locations (Abrams, Barbot, & Carrasco, 2010; Gabay & Henik, 2010; Hayward & Ristic, 2013; Posner, 1980; Tipper & Kingstone, 2005) in accordance with behavioral goals, mechanisms of involuntary attentional capture reflexively orient attention under certain circumstances. The physical salience of stimuli (Kahneman, Treisman, & Gibbs, 1992; Pashler, 1988; Theeuwes, 1991, 1992, 2010) and the similarity between a stimulus and a searched-for target (Anderson & Folk, 2010; Becker, Dutt, Vromen, & Horstmann, 2017; Duncan & Humphreys, 1989; Folk, Remington, & Johnston, 1992; Wolfe, 1994) have been well-documented to influence attentional capture. More recently, learned associations between stimuli and reward have been shown to influence the orienting of attention (Anderson, Laurent, & Yantis, 2013; Della Libera & Chelazzi, 2006; Engelmann & Pessoa, 2007; Hickey, Chelazzi, & Theeuwes, 2010a, 2010b; Kiss, Driver, & Eimer, 2009; Navalpakkam, Koch, Rangel, & Perona, 2010). Previously reward-associated stimuli capture attention even when physically non-salient and task-irrelevant, suggesting that reward learning has a direct impact on the attention system, what has been referred to as value-driven attentional capture (Anderson, Laurent, & Yantis, 2011, 2014).
It has long been understood that fearful or threatening stimuli also automatically capture attention. Experimental paradigms have used fearful faces (Dimberg & Ohman, 1996; Eastwood, Smilek, & Merikle, 2001; Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010; Vuilleumier, 2005), threatening animals (e.g., snakes, spiders; Ohman, Flykt, & Esteves, 2001; Ohman & Mineka, 2003), negative-valence images (Derryberry & Reed, 2002; Most, Chun, Widders, & Zald, 2005; Quigley, Nelson, Carriere, Smilek, & Purdon, 2012), or threatening words (Mathews & Macleod, 1985, 1994) to capture attention. More recent evidence demonstrates that such automatic orienting translates to arbitrary stimuli previously paired with electric shock (Schmidt, Belopolsky, & Theeuwes, 2015b, 2015c; Wang, Yu, & Zhou, 2013) or aversive white noise (Chubala & Smith, 2009; Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2004) during a conditioning phase, suggesting that the influence of associative learning on automatic attention extends to learning from aversive outcomes.
The relationship between the mechanisms underlying value-driven attention and attention to aversively conditioned stimuli is not known. One possibility is that each of these two sources of attentional priority are represented in dedicated neural and cognitive systems for reward and threat, respectively, which independently bias attention. By this account, the experience of threat would not be expected to interfere with the influence of reward on attention. A second possibility is that these two sources of attentional priority compete with one another, such that the processing of threat information interferes with value-based guidance. By this account, value-driven attentional capture should be reduced under conditions of threat.
The role of anxiety and threat in attention and cognition has been a topic of broad research interest. Typical approaches include comparing the performance of individuals who differ in trait-level anxiety (e.g., Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007; Derryberry & Reed, 2002; Koster, Crombez, Van Damme, Verschuere, & De Houwer, 2005; Koster, Crombez, Verschuere, & De Houwer, 2006; Moser, Becker, & Moran, 2012) or assessing performance with and without concurrent threat (e.g., threat of shock: Eldar et al., 2010; Robinson, Vytal, Cornwell, & Grillon, 2013; Shackman, Maxwell, McMenamin, Greischar, & Davidson, 2011). Attentional biases towards threat-related stimuli are generally more pronounced in anxious individuals (Bar-Haim et al., 2007; Derryberry & Reed, 2002; Shechner & Bar-Haim, 2016; Shechner et al., 2017). Current threat has been shown to interfere with ongoing cognitive processes (Curtin, Patrick, Lang, Cacioppo, & Birbaume, 2001; Lang, 1995; Pessoa, Kastner, & Ungerleider, 2002; Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Wyczesany, Ligeza, & Grzybowski, 2015), although cases of unimpaired cognition have also been reported (Bechara, Damasio, & Damasio, 2000; Damasio, Tranel, & Damasio, 1991; Dias, Robbins, & Roberts, 1996; Esteves & Ohman, 1993; Maxwell & Davidson, 2004; Yamaguchi & Harwood, 2017). Most relevant to the present study, anxiety disorders have been linked to increased distractibility and impaired concentration (Eldar et al., 2010; Eysenck, Derakshan, Santos, & Calvo, 2007), including increased susceptibility to attentional capture by physically salient stimuli (Esterman et al., 2013). Furthermore, the signaling of threat via fearful facial expressions enhances detection of peripheral visual events (Phelps, Ling, & Carrasco, 2006; Susskind et al., 2008), and negative arousal biases perception towards stimuli with high priority (often operationalized as physically salient stimuli) at the expense of less-salient stimuli (Sutherland & Mather, 2012, 2015), consistent with the arousal-biased competition hypothesis (Mather & Sutherland, 2011).
In the present study, we examined how threat modulates the influence of reward history on the allocation of attention. If threat is processed independently of the mechanisms by which learned value biases attention, threatening conditions either should not influence the magnitude of attentional capture by previously reward-associated stimuli or attentional capture should be potentiated by threat. The latter possibility would be consistent with arousal-biased competition under the assumption that the valuable distractors are afforded high priority, which is typically the case without concurrent threat manipulations (e.g., Anderson et al., 2011, 2013, 2014; Anderson & Kim, in press; Anderson & Yantis, 2012), or with descreased cognitive control or otherwise increased distractibility in a threatened state. On the other hand, if systems for representing threat and value share a competitive relationship, the magnitude of value-driven attentional capture should instead be reduced under threatening conditions.
Experiment 1
To examine the modulatory influence of threat on value-driven attentional biases, we examined attentional capture by reward cues with and without the threat of shock. To quantify the effects of reward history on selective attention, we utilized the value-driven attentional capture paradigm in which a participant is rewarded for orienting towards a valuable stimulus during a training phase, and this valuable stimulus then serves as a task-irrelevant distractor during a subsequent test phase (Anderson et al., 2011). We combined our attentional capture paradigm with the translational threat of shock paradigm, which has been used to induce anxiety in within-subject behavioral designs (Davis, Walker, Miles, & Grillon, 2010; Schmitz & Grillon, 2012). There is emerging evidence that experimental induction of anxiety, particularly through threat of shock, evokes neural circuitry and patterns of behavior characteristic of pathological anxiety (Robinson, Bond, & Roiser, 2015; Robinson et al., 2014; Robinson, Letkiewicz, Overstreet, Ernst, & Grillon, 2011; Robinson et al., 2013). We measured attentional capture by previously reward-associated stimuli under conditions in which the threat of shock was and was not present. To test whether the effect of the threat of shock differed for participants with different proclivities towards anxiety and depression, we included a battery of questionnaires assessing relevant constructs (Beck, Steer, Ball, & Ranieri, 1996; Carver & White, 1994; Ferreira & Murray, 1983; Patton, Stanford, & Barratt, 1995). Our objective was to test between the competing accounts of threat and reward processing outlined above, and we did not have specific predictions concerning which outcome was more likely.
Methods
Participants
Thirty-eight participants (23 females), between the ages of 18 and 35 inclusive, were recruited from the Texas A&M University community. All participants were English-speaking, reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant. The sample size was informed by a power analysis in which the power to detect value-driven attentional capture and the power to detect threat-dependent modulations in attentional capture were considered. The effect size for attentional capture by a high-value distractor was estimated from Anderson and Kim (in press), on which the design of the task was based (dz = 0.55). The effect size for threat-dependent modulations in attentional capture was estimated at η2 = 0.09 from Sutherland and Mathur (2012). At α = 0.05, a sample size of at least 28 participants would provide β > 0.80 to detect each of the two effects.
Apparatus
A Dell OptiPlex 7040 (Dell, Round Rock, TX, USA) equipped with Matlab software (Mathworks, Natick, MA, USA) and Psychophysics Toolbox extensions (Brainard, 1997) was used to present the stimuli on a Dell P2717H monitor. The participants viewed the monitor from a distance of approximately 70 cm in a dimly lit room. Paired electrodes (EL500, BioPac Systems, Inc., Goleta, CA, USA) were attached to the left forearm of each participant, and electric shocks were delivered through an isolated linear stimulator under the constant current setting (STMISOLA, BioPac Systems), which was controlled by custom Matlab scripts. Eye-tracking was conducted using the EyeLink 1000 Plus system (SR Research Ltd., Ottawa, Ontario, Canada), and head position was maintained using a manufacturer-provided chin rest (SR Research Ltd.).
Individual differences assessments
All participants completed electronic implementations of the State-Trait Anxiety Inventory (STAI-state, STAI-trait; Ferreira & Murray, 1983), Beck Depression Inventory (BDI-II; Beck et al., 1996), Behavioral Activation/Inhibition System Inventory (BAS/BIS; Carver & White, 1994), and Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) before completing the experimental task. After the experimental task, participants again completed the STAI-state inventory.
Stimuli
In the training phase, each trial consisted of a gaze-contingent fixation display, a stimulus array, and a feedback display (see Fig. 1A). The fixation display consisted of a box (3.3° x 2.5° visual angle) at the center of the screen. Each circle in the search array was 4.5° visual angle in diameter. Stimuli located on the left and right sides were 9.3° visual angle from the meridian. Vertically, stimuli were 3.3° visual angle and 7.4° visual angle above and below the horizontal equator. Targets were red and green, and the colors of the non-targets were drawn from the set {blue, cyan, pink, orange, yellow, white} without replacement (Anderson et al., 2011, 2014). If the target was fixated within the timeout limit, a feedback display was presented consisting of the amount of monetary reward earned on the current trial (+10¢ or +2¢), and the total reward accumulated across all trials. If the target was not fixated within the timeout limit, the word “Miss” would appear in the feedback in place of the trial earnings. Fixating a non-target did not trigger any outcome, and it was possible to fixate a non-target before fixating the target within the timeout limit and still receive the target-associated reward.
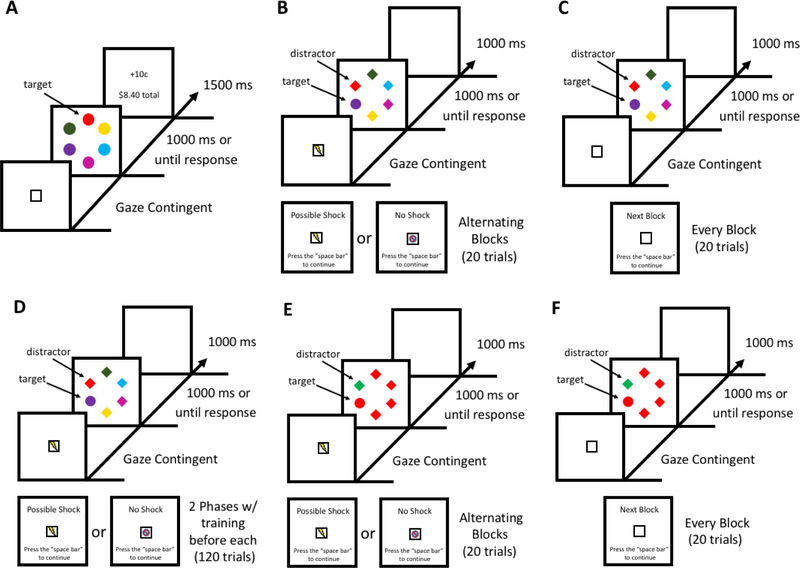
Fig. 1.
Sequence of trial events. (A) Training phase for Experiments 1–3. Each trial began after the participant fixated the white box located in the center of the screen for 500 ms. The target was defined by color (red or green, exactly one of which was present on each trial). Correct responses were followed by the delivery of monetary reward feedback. (B) Test phase of Experiment 1. Participants were informed whether it was or was not possible to receive an electric shock during the next block of 20 trials. Each trial began after the participant fixated the image corresponding to each block at the center of the screen for 500 ms. The target was defined as the unique shape, and no reward feedback was provided. (C) Test phase of Experiment 2, which mirrored Experiment 1 except that each block was the same and there was no reference to shock. (D) In Experiment 3, each of two 120-trial blocks of the test phase was preceded by a separate training phase. The stimuli were identical to Experiment 1. (E) In Experiment 4, there was no training phase and participants completed only the shape singleton search task with a color singleton as a distractor. The block design and procedure were identical to Experiment 1. (F) In Experiment 5, the same shape singleton search task as in Experiment 4 was completed without reference to shock.
In the test phase, before each block of trials, participants were presented with a display indicating whether shock was possible in that block. Each circle in the search array had a 4.5° visual angle diameter and diamonds were 4.1° x 3.7° visual angle. The target was defined as the unique shape. At the beginning of a possible shock block, the display would present the words “Possible Shock” along with a white box that contained an image of a lightning bolt. At the beginning of a no shock block, the display would present the words “No Shock” along with a white box that contained an image of a lightning bolt with a red hash over it. Each trial consisted of a gaze-contingent fixation display, a stimulus array, and a blank inter-trial-interval (see Fig. 1B). The fixation display included the identical image referenced at the start of each block, either a white box with a lightning bolt or red hash covering the bolt. The locations of the stimuli and the colors of the non-targets were identical to the training phase. If participants were unable to fixate the target within the timeout limit, the word “Miss” would appear during the inter-trial-interval. During possible shock blocks, a small number of trials were added in which electric shock was delivered in place of the stimulus array.
Design
Both the training phase and the test phase were split into two runs, with each run consisting of 120 trials (240 trials total in each phase). In the training phase, the target was equally-often red and green. Each target color appeared in each stimulus position equally-often within a run, and trials were presented in a random order. For each participant, one of the color targets (counterbalanced) would yield a monetary reward of 10¢ on 80% of trials and 2¢ on 20% of trials (high-value target); the other color target would yield 2¢ on 80% of trials and 10¢ on 20% of trials (low-value target). In the test phase, block order was counterbalanced across participants. On half of the trials, one of the non-target shapes was rendered in the color of the former high-value target during the training phase (referred to as the distractor). The other half of trials did not contain either of the prior target colors from training (distractor-absent trials); the low-value color did not appear during the test phase, in order to maximize the trials-per-cell in the factorial design. The target was equally-often a diamond among circles and a circle among diamonds, and was never red or green. Target and distractor position were fully crossed and counterbalanced, and trials were presented in a random order. In shock blocks, participants were shocked 2 times in 2 blocks, 3 times in 3 blocks, and 4 times in 1 block, with the assignment of number of shocks to blocks randomized. The pattern of shocks administered in the shock block across trials was pseudo-randomly determined with the constraint that shocks were never administered on consecutive trials nor on the last trial of a block. At the end of the experiment, participants were paid the total monetary reward obtained during the training phase.
Procedure
In the training phase, each trial began with the presentation of a white box that remained on-screen until the participant fixated on the box for 500 ms. The stimulus array would then be displayed for 1000 ms or until the target was fixated. Then the feedback display would appear for 1500 ms, indicating the monetary reward gained and the participant’s total earnings. Following the training phase, the participant was connected to the isolated linear stimulator and a shock calibration procedure was conducted for each participant to achieve a level that was “unpleasant, but not painful” (Murty, Labar, & Adcock, 2012; Schmidt, Belopolsky, & Theeuwes, 2015a, 2017). In the test phase, each trial began with the presentation of the block display, indicating whether the following block would contain a potential electric shock or no chance of an electric shock. The block began once the experimenter pressed the space bar. Each trial began with the presentation of the identical image referenced in the block display. Fixation on the image for 500 ms triggered the stimulus array, which again remained on screen until participants fixated the target or 1000 ms elapsed, and the inter-trial-interval lasted 1000 ms.
Head position was maintained throughout the experiment using an adjustable chin rest that included a bar upon which to rest the forehead (SR Research). Participants were provided a short break between each run of the task in which they were allowed to reposition their head to maintain comfort. Eye position was calibrated prior to each block of trials using 9-point calibration (Anderson & Yantis, 2012), and was manually drift corrected by the experimenter as necessary (the next trial could not begin until eye position was registered within 1.1° of the center of the fixation cross for 500 ms; see, e.g., (Nissens, Failing, & Theeuwes, 2017). During the presentation of the search array, the X and Y position of the eyes was continuously monitored in real time with respect to the six stimulus positions, such that fixations were coded on line (Le Pelley, Pearson, Griffiths, & Beesley, 2015).
Data Analysis
One participant withdrew from the experiment prior to completion and two participants were unable to be eye-tracked using our apparatus. Thus, 35 complete data sets were ultimately analyzed.
We measured which of the six shape stimuli was initially fixated on each trial, as well as whether the target was fixated before the timeout limit along with the time required to fixate the target (i.e., RT). Fixation of a stimulus was registered if eye position remained within a region extending 0.7° around the stimulus for a continuous period of at least 50 ms (100 ms on the target trigger the termination of the stimulus array; see, e.g., Le Pelley et al., 2015). Oculomotor capture was determined by comparing the probability of initially fixating the valuable distractor compared to the average of other non-target stimuli. RT was measured from the onset of the stimulus array until a valid target fixation was registered. RTs in fixating the target that exceeded three standard deviations of the mean for a given condition for a given participant were trimmed (Anderson & Yantis, 2012).
Results
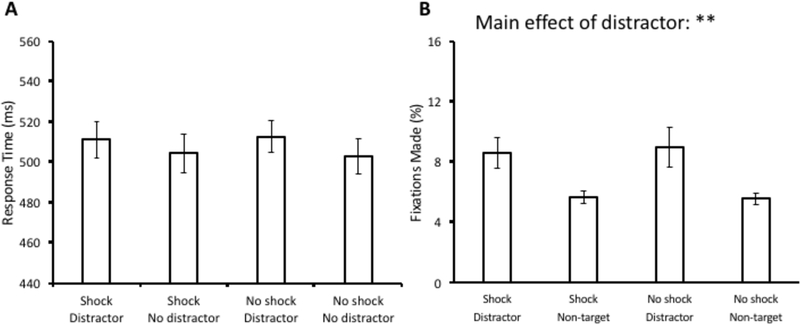
A 2 × 2 analysis of variance (ANOVA) with distractor condition (present vs. absent) and block (shock vs. no shock) as factors was conducted over mean RT. There was no main effect of distractor condition, F(1,34) = 3.01, p = 0.092, no main effect of block, F(1,34) < 0.01, p = 0.998, and also no interaction, F(1,34) = 0.16, p = 0.689 (see Fig. 2A). The same ANOVA conducted over oculomotor capture revealed a main effect of distractor condition, F(1,34) = 8.20, p = 0.007, η2 = 0.194, but there was no main effect of block, F(1,34) = 0.06, p = 0.802, nor an interaction F(1,34) = 0.15, p = 0.700 (see Fig. 2B). Post-hoc comparisons revealed that there were significantly more saccades to the distractor compared to a non-target within both the shock block, t(34) = 2.65, p = 0.012, d = 0.65, and the no-shock block, t(34) = 2.45, p = 0.02, d = 0.60.
Fig 2.
Response time (A) and fixation data (B) from the test phase of Experiment 1. Data are broken down by block (Shock vs. No shock) and distractor present vs. absent in (A) and fixations on the distractor vs. a non-target in (B). Error bars reflect the standard error of the mean. **p<0.01
Unsurprisingly given the lack of interaction effects between attentional capture and threat, no questionnaire measure was predictive of the difference in oculomotor capture between shock and no shock blocks (see Supplemental Table 1). State anxiety increased after the test phase, t(34) = 6.67, p < 0.001, d = 1.7, confirming the anxiety-provoking nature of the threat-of-shock manipulation.
Discussion
In Experiment 1, we found no effects of threat of shock on value-driven attentional capture. Eye movements were biased towards previously reward-associated stimuli, but the magnitude of this oculomotor bias did not differ between blocks with and without the threat of shock. State anxiety, as measured using the STAI, increased as a result of the test phase manipulation, suggesting that the threat of shock was effective in inducing a state of anxiety.
Threat can produce two distinct emotional states in an individual: fear or anxiety; these emotional states are behaviorally distinct, utilize separate brain networks, and show pharmacological differences (Blanchard, Yudko, Rodgers, & Blanchard, 1993; Davis et al., 2010; Grillon, Ameli, Woods, Merikangas, & Davis, 1991; Grillon et al., 2008). Fear is a response that occurs from predictable threat and is quick to dissipate, while anxiety results from the anticipation of an unpredictable threat and is longer-lasting. Anxiety is an adaptive mechanism that utilizes heightened vigilance and promotes rapid responses, particularly in unfamiliar and threatening conditions (Kalin & Shelton, 1989).
The threat of electric shock has been established as a well-controlled manipulation of state anxiety within-subjects (Clark et al., 2012; Corbetta & Shulman, 2002; Cornwell et al., 2007; Davis et al., 2010; Grillon et al., 2008; Robinson et al., 2015; Robinson et al., 2011; Robinson et al., 2013). However, null effects of this manipulation on behavior have been documented (Robinson et al., 2015), as in our experiment. One possibility is that the no-shock blocks in the present experiment were not sufficiently long for anxiety to dissipate, especially given the alternating nature of shock and no-shock blocks (where future epochs involving shock could be anticipated). Unlike in the manipulation of fear in which the removal of the fearful stimulus quickly returns a person to a baseline state (Davis et al., 2010; Grillon et al., 1991), a state of anxiety may be “bleeding over” into no-shock blocks in the present experiment, compromising the effectiveness of the block manipulation. A global state of threat could be influencing capture across the entire task, in similar measure across blocks.
Experiment 2
In Experiment 1, there was no difference in capture between “shock” and “no shock” blocks, which on the surface is consistent with the idea that brain systems for value and threat influence attention independently. However, it is unclear whether our manipulation of anxiety through threat varied substantively between blocks. Potentially, anxiety instilled through the threat of shock during the “shock” blocks is unable to be turned off in quick succession and resulted in a global state of threat over the entirety of the test phase of Experiment 1. Thus, in Experiment 2, we recruited a new group of participants to complete an otherwise identical task, but without any threat of electric shock. All mention of shock was removed from the task. Of interest was whether the magnitude of value-driven attentional capture would differ from the magnitude observed in Experiment 1 where participants were sometimes under threat of shock.
Methods
Participants
Thirty-two participants (18 females), between the ages of 18 and 35, were recruited from the Texas A&M University community. All participants reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and all study procedures were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant.
Apparatus and Stimuli
The apparatus and stimuli were identical to Experiment 1, with the exception that the isolated linear stimulator was not used and no shock-related images were presented.
Design and Procedure
The design and procedure were identical to Experiment 1, with the exception that the administration of electric shock and any references to electric shock, including in the instructions and images, were removed (see Fig. 1C).
Data Analysis
Data were analyzed in the same manner as Experiment 1. Two participants were unable to be eye-tracked using our apparatus. Thus, 30 complete data sets were analyzed.
Results
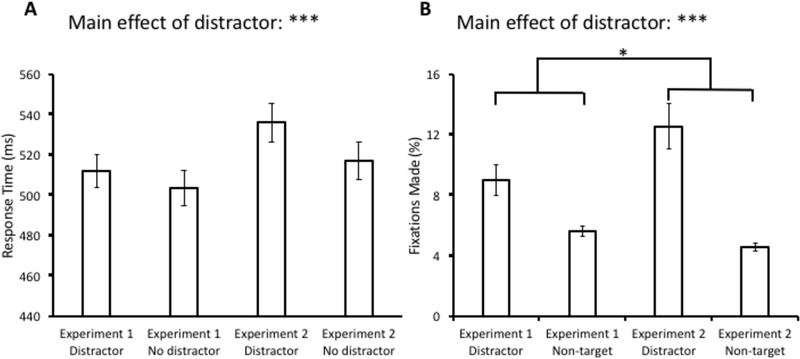
A 2 × 2 ANOVA with distractor condition (present vs. absent) and experiment (Experiment 1 vs. Experiment 2) as factors was conducted over mean RT. We collapsed performance across “shock” and “no shock” blocks for Experiment 1 because there were no differences found between them. This ANOVA revealed a main effect of distractor condition, F(1,63) = 12.06, p = 0.001, η2 = 0.161, but no effect of experiment, F(1,63) = 2.40, p = 0.127, or interaction, F(1,63) = 1.85, p = 0.179 (see Fig. 3A). The same ANOVA conducted over oculomotor capture revealed a main effect of distractor condition, F(1,63) = 34.60, p < 0.001, η2 = 0.354, and, critically, a significant interaction, F(1,63) = 5.96, p = 0.017, η2 = 0.086. The main effect of experiment was not significant, F(1,63) = 1.19, p = 0.175 (see Fig. 3B).
Fig 3.
Comparison of response time (A) and fixations (B) between the test phase of Experiment 1 (collapsed across shock and no-shock blocks) and Experiment 2. Data are broken down by experiment and distractor present vs. absent in (A) and fixations on the distractor vs. a non-target in (B). Error bars reflect the standard error of the mean. *p<0.05, ***p<0.001
Discussion
In Experiment 2, we conducted the identical task as Experiment 1 but with no reference to electric shock. This allowed us to compare the magnitude of value-driven attentional capture between conditions with (Experiment 1) and without (Experiment 2) a threat manipulation. As in Experiment 1, robust value-driven attentional capture was observed, this time in both RT and eye movements. In addition, oculomotor capture was significantly greater in magnitude in Experiment 2 (as evidenced by the experiment by distractor condition interaction), indicating that the threat of shock generally suppressed attentional capture by previously reward-associated stimuli.
Experiment 3
In Experiment 1, we were unable to produce a within-subject effect of shock using the alternating block design. As previously discussed, we hypothesized that participants were unable to reduce their anxiety levels back to baseline in the no-shock blocks given the timeframe of block switches. Experiment 2 showed that the threat of shock was indeed having a significant effect on oculomotor capture, consistent with the idea that participants in Experiment 1 were completing the test phase under a global state of anxiety. Given the novelty of this finding, which contrasts with the effects of threat on the processing of physically salient stimuli (e.g., Esterman et al., 2013; Mather & Sutherland, 2011; Sutherland & Mather, 2012, 2015), we wanted to replicate and extend the evidence for this relationship. Therefore, in Experiment 3, we examined whether within-subject effects of anxiety on value-driven attentional capture would be evident when threat of shock was confined to a distinct epoch of the task, providing a clear boundary between threatening and non-threatening contexts. Instead of alternating blocks after 20 trials, we modified the design to have participants complete two otherwise identical implantations of the test phase in which the delivery of shock was and was not possible.
Methods
Participants
Thirty-two participants (18 females), between the ages of 18 and 35, were recruited from the Texas A&M University community. All participants reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and all study procedures were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant.
Apparatus and Stimuli
The apparatus and stimuli were identical to Experiment 1.
Design and Procedure
The design and procedure were similar to Experiment 1. However, we changed the design to have two training and test phases (see Fig. 1D). Instead of an alternating block design of 20 trials, the test phase consisted of two blocks of 120 trials each, one with and one without the threat of shock (order counterbalanced between subjects). A training phase of 180 trials preceded each test phase. Such an alternating training-test design has been shown to have high test-retest reliability in measurements of value-driven attentional capture (Anderson & Kim, in press). In addition, each participant was only connected to the isolated linear stimulator before the test phase of the “shock” block and was immediately disconnected from the device after completion of the “shock” block. After disconnecting the stimulator from the participant, they completed the post-task STAI state inventory before proceeding (in addition to at the beginning of the experiment).
Data Analysis
Data were analyzed in the same manner as Experiment 1, with the exception that the order of blocks (shock block first vs. no-shock block first) was included as a factor in the ANOVAs. Data from two participants were excluded from analyses because their accuracy was lower than 70% and two participants were unable to be eye-tracked using our apparatus. Thus, 28 complete data sets were analyzed.
Results
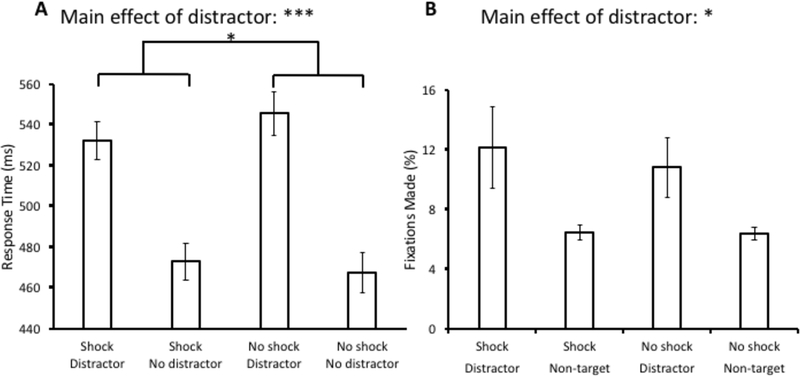
A 2 × 2 × 2 ANOVA with distractor condition (present vs. absent), block (shock vs. no shock), and order (shock block first vs. no-shock block first) as factors was conducted over mean RT. Unlike in Experiment 1, there was a significant main effect of distractor condition, F(1,26) = 108.77, p < 0.001, η2 = 0.807. There was no main effect of block, F(1,26) = 0.50, p = 0.485, or order, F(1,26) = 1.03, p = 0.321. Importantly, there was a significant interaction between distractor condition and block, F(1,26) = 4.35, p = 0.047, η2 = 0.143 (see Fig. 4A), with attentional capture being reduced under threat of shock. A significant interaction was observed between block and the order of blocks, F(1,26) = 11.20, p = 0.003, η2 = 0.301, reflecting the fact that participants were generally faster during the second block (regardless of whether that block involved shock of not). The order of blocks did not interact with distractor condition, F(1,26) = 0.29, p = 0.593, nor was the three-way interaction significant, F(1,26) = 1.90, p = 0.18.
Fig 4.
Response time (A) and fixation data (B) from the test phase of Experiment 3. Data are broken down by block (Shock vs. No shock) and distractor present vs. absent in (A) and fixations on the distractor vs. a non-target in (B). Error bars reflect the standard error of the mean. *p<0.05, ***p<0.001
The same ANOVA conducted over oculomotor capture revealed a main effect of distractor condition, F(1,26) = 6.89, p = 0.015, η2 = 0.21. No other main effects or interactions were significant, Fs < 1.7, ps > 0.2 (see Fig. 4B). As in Experiment 1, state anxiety increased after the test phase of the shock block, t(27) = 7.71, p < 0.001, d = 1.9, confirming the anxiety-provoking nature of the threat-of-shock manipulation.
Discussion
In Experiment 3, we modified the design of Experiment 1 to facilitate assessment of the threat of electric shock within-subjects, separately training and then testing participants with and without the use of the isolated linear stimulator. Here, we found robust value-driven attentional capture and a significant effect of the threat manipulation on the response time measure, replicating reduced distractibility in a threatening context. The results provide converging evidence for the modulatory role of threat in reducing the magnitude of value-driven attentional capture.
In the present experiment, the measure of value-driven attentional capture sensitive to the threat manipulation was RT, which differs from Experiments 1–2 in which threat modulated oculomotor selection. Each of these measures have been consistently implicated in distraction by reward cues (e.g., Anderson et al., 2011, 2013, 2014; Anderson & Kim, in press; Anderson & Yantis, 2012). In general, the RT cost associated with the distractor was also numerically much larger in Experiment 3 compared to the prior two experiments. The reason for this apparent discrepancy is unclear and reflects a limitation of the present study, although we do note that the implementation of the reward training, as well as the period over which attentional capture was measured both in relation to the threat manipulation and in relation to training, was quite different across experiments. Fewer trials of training preceded each epoch of the test phase in the present experiment, although the two total epochs of training resulted in more training trials overall. Given that each epoch of the test phase was only half as long as the test phase of Experiments 1–2, the test phase of Experiment 3 was likely less subject to extinction, which might explain the overall more robust attentional capture measured in this implementation. Any of these differences could have shifted the sensitivity of the paradigm to the effects of reward history on attention, although in each case some indication of attentional capture was significantly reduced under threat.
Experiment 4 & 5
Experiments 1–3 demonstrate reduced value-driven attentional capture under conditions of threat. This finding contrasts with previous demonstrations of increased attentional capture by physically salient stimuli in anxious individuals (Esterman et al., 2013; Moser et al., 2012) and more preferential processing of physically salient stimuli following induction of negative arousal (Mather & Sutherland, 2011; Sutherland & Mather, 2012, 2015). It is tempting to conclude that threat and anxiety influence value-driven and salience-driven attention differently, suppressing one while potentiating the other. However, it is unclear whether this is indeed the case, or whether a particular aspect of our experimental design led to fundamentally different results. To our knowledge, threat of shock has not been examined in the context of the additional singleton task (Theeuwes, 1992), which serves as the basis of our experimental paradigm.
Our goal here was to conceptually replicate findings supporting arousal-biased competition in the context of the processing of physically salient stimuli (Mather & Sutherland, 2011; Sutherland & Mather, 2012, 2015) and links between anxiety and increased attentional capture by physically salient stimuli (Esterman et al., 2013; Moser et al., 2012), but in the specific context of our visual search paradigm using a threat of shock manipulation. This would provide a more direct contrast to the findings of our prior experiments. Therefore, Experiments 4 and 5 paralleled Experiments 1 and 2, but using physically salient color singleton distractors (see Theeuwes, 1992, 2010) in the absence of prior reward training. We hypothesized that threat of shock would magnify rather than suppress attentional capture by physically salient distractors, consistent with prior findings using different experimental tasks and different manipulations of threat and anxiety (Esterman et al., 2013; Moser et al., 2012). To maintain consistency with the prior experiments, we retained the rapid-switching block structure of Experiment 4 and anticipated the need for Experiment 5 to provide a comparison condition with no threat of shock. We chose this between-subjects manipulation of threat of shock, rather than the within-subjects approach adopted in Experiment 3, given that the interaction with threat was more robust in Experiments 1–2 and oculomotor indicators of attentional capture, including value-driven attentional capture, tend to have higher reliability as a dependent measure (Anderson & Kim, in press).
Methods
Participants
Thirty-eight unique participants were recruited for both Experiment 4 and 5 (18 females and 20 females, respectively), between the ages of 18 and 35, from the Texas A&M University community. All participants reported normal or corrected-to-normal visual acuity and normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and all study procedures were conducted in accordance with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant.
Apparatus and Stimuli
The apparatus was identical to Experiment 1. The stimuli were identical to Experiment 1 except for the colors of the shapes. On distractor-absent trials, all of the shapes were a single color (red or green, counterbalanced across participants). On distractor-present trials, one of the non-target shapes was shown in the other color (red or green), which constituted the physically salient distractor (see Fig. 1E & 1F).
Design and Procedure
There were no training phases in Experiments 4 and 5. The design and procedure of Experiments 4 and 5 were identical to those corresponding to the test phase of Experiments 1 and 2, respectively.
Data Analysis
Data were analyzed in the same manner as Experiments 1 and 2. For Experiment 4, data from one participant was excluded because their accuracy was lower than 70% for the task, two participants withdrew from the study prior to completion, one participant was unable to be eye-tracked using our apparatus, and data from one participant was identified as an outlier and removed from further analysis (capture score exceeded 2.5 SD of the mean). For Experiment 5, four participants were unable to be eye-tracked using our apparatus and data from one participant was identified as an outlier and removed from further analysis (using the same 2.5 SD criterion). Thus, 33 complete data sets were analyzed for each experiment.
Results
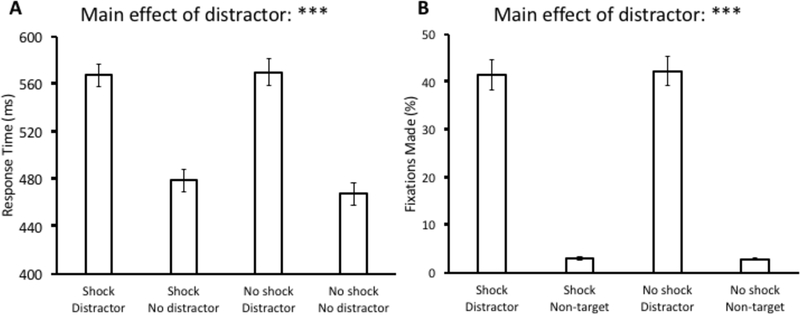
For Experiment 4, a 2 × 2 ANOVA with distractor condition (present vs. absent) and block (shock vs. no shock) as factors was conducted over mean RT. We found a main effect of distractor condition, F(1,32) = 231.47, p < 0.001, η2 = 0.879, but there was no main effect of block, F(1,32) = 1.38, p = 0.249, and also no interaction, F(1,32) = 1.85, p = 0.183 (see Fig. 5A). Post-hoc comparisons revealed that response times were significantly slower during distractor-present trials within both the shock block, t(32) = 11.80, p < 0.001, d = 1.71, and the no-shock block, t(32) = 13.75, p < 0.001, d = 1.85. The same ANOVA conducted over oculomotor capture revealed a main effect of distractor condition, F(1,32) = 153.86, p < 0.001, η2 = 0.828, but there was neither a main effect of block, F(1,32) = 0.06, p = 0.816, nor an interaction F(1,32) = 0.14, p = 0.708 (see Fig. 5B). Post-hoc comparisons revealed that there were significantly more saccades to the distractor compared to a non-target within both the shock block, t(32) = 11.71, p < 0.001, d = 2.93, and the no-shock block, t(32) = 12.09, p < 0.001, d = 3.1.
Fig 5.
Response time (A) and fixation data (B) from the test phase of Experiment 4. Data are broken down by block (Shock vs. No shock) and distractor present vs. absent in (A) and fixations on the distractor vs. a non-target in (B). Error bars reflect the standard error of the mean. ***p<0.001
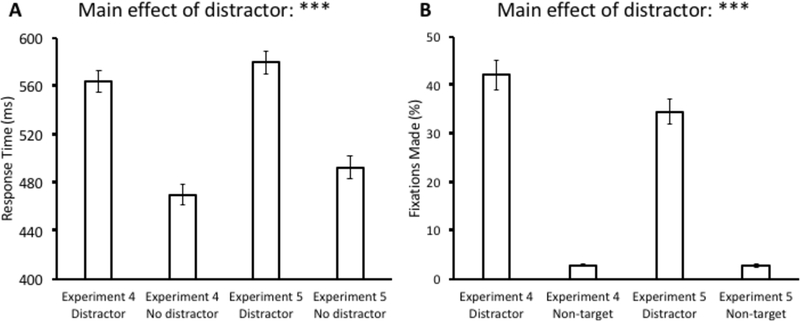
For Experiment 5, a 2 × 2 ANOVA with distractor condition (present vs. absent) and experiment (Experiment 4 vs. Experiment 5) as factors was conducted over mean RT. As in Experiment 2, we collapsed performance across “shock” and “no shock” blocks for Experiment 4 because there were no differences found between them. This ANOVA revealed a main effect of distractor condition, F(1,64) = 446.75, p < 0.001, η2 = 0.875, but there was neither a main effect of experiment, F(1,64) = 2.65, p = 0.108, nor an interaction, F(1,64) = 0.62, p = 0.433 (see Fig. 6A). The same ANOVA conducted over oculomotor capture revealed a main effect of distractor condition, F(1,64) = 284.26, p < 0.001, η2 = 0.816. In addition, we found a marginal effect of experiment, F(1,64) = 3.73, p = 0.058, η2 = 0.055, and a marginal interaction, F(1,64) = 3.22, p = 0.078, η2 = 0.048. Importantly, this trend was in the opposite direction compared to our experiments of value-driven attentional capture (see Fig. 6B), with capture being greater in magnitude during the experiment with threat of shock.
Fig 6.
Comparison of response time (A) and fixations (B) between the test phase of Experiment 4 (collapsed across shock and no-shock blocks) and Experiment 5. Data are broken down by experiment and distractor present vs. absent in (A) and fixations on the distractor vs. a non-target in (B). Error bars reflect the standard error of the mean. ***p<0.001
To statistically assess whether threat differentially modulates value-driven and salience-driven oculomotor capture, we conducted a follow-up 2 × 2 × 2 ANOVA with distractor condition (present vs. absent), presence of shock (shock vs. no shock), and type of distractor (valuable vs. physically salient) as factors, and probed the three-way interaction. The three-way interaction was indeed significant, F(1,127) = 6.79, p = 0.010, η2 = 0.051, confirming a significant difference in how threat modulates value-driven and salience-driven attentional capture.
Discussion
In Experiments 4 and 5, we examined how the threat of electric shock modulates attentional capture to physically-salient stimuli in the additional singleton paradigm. We emulated the design and procedure of our experiments examining value-driven attentional capture (Experiments 1 and 2), but removed the training phase and replaced previously reward-associated distractors with physically salient color singleton distractors. We found a robust effect of the distractor for both RT and eye movements in Experiment 4, but again found no difference between blocks with and without the threat of shock in a rapid switching design. In anticipation of the anxiety “bleed-over” between blocks, we conducted Experiment 5 without the threat of shock to serve as a comparison condition (as in Experiment 2). A robust effect of the distractor was again observed in Experiment 5. Contrary to Experiments 1 and 2, however, the influence of shock on attentional capture trended in the opposite direction, with capture being greater in magnitude under threat of shock, consistent with arousal-biased competition (Mather & Sutherland, 2011; Sutherland & Mather, 2012, 2015). A significant three-way interaction across experiments confirmed that the impact of threat differently affects value-driven and salience-driven attentional capture; while the threat of shock suppressed attentional capture by previously reward-associated stimuli, it tended to increase attentional capture to the physical salience of objects.
General Discussion
In the present study, we used the value-driven attentional capture paradigm to assess the influence of threat-induced anxiety on attentional capture by reward-associated stimuli. We used the threat of electric shock to manipulate anxiety, as in previous studies (Clark et al., 2012; Cornwell et al., 2007; Davis et al., 2010; Grillon et al., 2008; Robinson et al., 2015; Robinson et al., 2011; Robinson et al., 2013). Experiments 1 and 2 demonstrated attenuated value-driven attentional capture when previously reward-associated stimuli are encountered in a threatening situation, and this basic pattern was replicated in Experiment 3. Changes in self-reported state anxiety confirmed the effectiveness of our threat of shock manipulation. On the other hand, in Experiments 4 and 5, threat of shock showed a trend towards increasing susceptibility to attentional capture by physically salient stimuli, consistent with prior reports (Esterman et al., 2013; Moser et al., 2012). Our findings reveal a striking dissociation in which the threat of electric shock suppresses oculomotor capture by reward cues, while increasing oculomotor capture by physically salient stimuli.
The threat of shock paradigm has reliably induced anxiety in both human and animal studies (e.g., Davis et al., 2010; Grillon et al., 2008). However, null effects of the threat of shock paradigm have also been reported in two different decision-making tasks utilizing a similar fast-alternating block design (Robinson et al., 2015; compare to Experiments 1 and 4 of the present study). Robinson et al. (2015) speculated that the threat of shock manipulation may not have been significant enough to elicit behavioral change; however, in the present study, between-experiment measures of oculomotor capture and a within-subject manipulation involving a longer epoch of no threat produced reliable effects of threat of shock. Therefore, we hypothesize that the null effect of threat within-subjects in Experiments 1 and 4 was due to the slow-dissipating nature of anxiety (Kalin & Shelton, 1989), which bled over into the no-shock blocks and produced a global state of anxiety. In utilizing the threat of shock paradigm, a fast-alternating block design may be suboptimal, and either a between-subjects manipulation or a manipulation involving an extended epoch with and without the threat of shock may be more robust.
Previous studies observing the effects of anxiety on attentional capture by physically salient stimuli have tended to find evidence for increased attentional capture (Esterman et al., 2013; Moser et al., 2012). These studies supported the theory that anxiety produces a vigilant state within an individual and results in heightened responsiveness to external events under threatening conditions (Armony & Dolan, 2002; Kalin & Shelton, 1989; Mogg & Bradley, 1999; Pourtois, Grandjean, Sander, & Vuilleumier, 2004). The vigilance hypothesis of anxiety has also been supported in the context of attention to threatening facial expressions (Hahn & Gronlund, 2007; Sussman, Jin, & Mohanty, 2016; Sussman, Szekely, Hajcak, & Mohanty, 2016; Williams, McGlone, Abbott, & Mattingley, 2005). Similarly, the arousal-biased competition hypothesis suggests that negative arousal enhances high-priority visual signals at the expense of less-salient signals, biasing perceptual processing more strongly in favor of physically salient stimuli (Mather & Sutherland, 2011). A state of heightened vigilance has also been shown to reduce errors in Go/No-Go tasks (Grillon et al., 2017), indicating that anxiety may be effective and beneficial in facilitating rapid and accurate information processing.
Previously reward-associated stimuli preferentially draw attention (e.g., Anderson et al., 2011), which is thought to in part reflect stronger signals evoked by previously reward-associated stimuli in the visual cortex (Anderson, 2016a, 2017; Anderson et al., 2014; Hickey & Peelen, 2015; Hickey & Peelen, 2017). To the degree that such value-biased visual signals are processed in a similar fashion to differences in feature contrast, or to the degree that anxiety invokes a general tendency to monitor for unexpected visual events at the expense of goal-directed attention, more robust attentional capture by reward cues would be expected under conditions of threat. However, our results reveal the exact opposite pattern. We show that the processing of negative emotional information such as threat interacts with the ability of learned value to guide attention, aligning with the dual competition framework (Pessoa, 2009). This model proposes that task-irrelevant threat information competes for central processing resources with cognition, potentially impairing cognitive processes. Our findings are consistent with the idea that negative valence states interfere with value-based attentional guidance, competing for limited processing resources.
The nature of this hypothesized competition in the processing of emotionally valent information is unclear. Broadly, the processing of salient features of objects have been organized into an oculomotor control network, starting from neuronal activation in early visual areas V1 (Knierim & Vanessen, 1992) and V4 (Burrows & Moore, 2009) to later cortical areas such as the parietal cortex (Balan & Gottlieb, 2006), and the frontal eye field (Bichot & Schall, 1999; Moore, Armstrong, & Fallah, 2003; Thompson & Bichot, 2005), in addition to the superior colliculus (Fecteau, Bell, & Munoz, 2004). Likewise, value-driven attentional capture also recruits the early visual cortex, ventral visual cortex, and the posterior parietal cortex (Anderson, 2017; Anderson et al., 2014; Hickey & Peelen, 2015; Hickey & Peelen, 2017; Hopf et al., 2015; Serences, 2008). However, additional regions have been linked to value-driven attentional capture specifically, both in the basal ganglia (e.g., caudate tail; Anderson, 2016a, 2017; Anderson, Kuwabara, et al., 2016; Anderson et al., 2014; Kim & Hikosaka, 2013; Yamamoto, Kim, & Hikosaka, 2013) and in the limbic system (e.g., amygdala; Ousdal et al., 2014; Peck, Lau, & Salzman, 2013; Peck & Salzman, 2014). The processing of threat also recruits the amygdala (e.g., Cisler & Koster, 2010; Ohman, 2002, 2005). One possibility is that the reduced influence of reward associations on the control of attention under threat is a result of the competition between the processing of threat and value-dependent information processing within the limbic system and/or basal ganglia. Further consistent with this hypothesis, attention to emotional targets in a visual search task has been shown to activate both areas of the spatial attention network and the limbic system, including the amygdala (Mohanty, Egner, Monti, & Mesulam, 2009). In addition, studies of non-human primates have identified projections from the basolateral amygdala to the caudate tail (Griggs et al., 2017), suggesting that amygdala-dependent processing and other regions involved in value-driven attention are interconnected.
An alternative possibility, not mutually exclusive with the prior, is that threat biases attention towards a more stimulus-driven mode of information processing in which salient external events more effectively drive selection. Learned value associations reflect internally-generated bias signals, which may be generally suppressed when under threat. Interactions between different valence-dependent processing mechanisms in the control of attention are largely unexplored, and the present study suggests that this is an area of inquiry ripe for future investigation.
Previously reward-associated stimuli have been consistently shown to compete effectively with a more physically salient target for attention under conditions without an explicit threat manipulation (e.g., Anderson et al., 2011, 2013, 2014; Anderson & Kim, in press; Anderson & Yantis, 2012), suggesting that valuable stimuli have high attentional priority (Anderson, 2016a). In this sense, our findings suggest a limitation to the arousal-biased competition model of information processing (Mather & Sutherland, 2011; Sutherland & Mather, 2012, 2015). It seems not to be the case than any high-priority information is biased under states of negative arousal, as manipulated here via threat of shock. Rather, as outlined above, the kind of priority enhanced by threat and/or negative arousal may be restricted to stimulus-driven representations or might not translate to positively valenced representations.
In addition to supporting competition in the processing of reward and threat, our findings have other important theoretical implications. First, it is clear that value-driven attentional priority cannot be reduced to a change in the perceived salience of a stimulus at the sensory level. If this were the case, threat would be expected to influence attention to valuable stimuli and physically salient stimuli in the same manner, which is clearly inconsistent with our results. It seems more likely that distinctly valence-dependent representations contribute, at least in part, to the control of value-driven attention. In addition, our findings suggest that susceptibility to distraction is not a uniformly state-dependent phenomenon. Although the threat of shock creates an anxiety-induced state, this state of anxiety has a fundamentally different effect on the orienting of attention depending on the eliciting stimulus. That is, anxiety does not have general effect on distractibility that can be reduced to heightened vigilance, but rather, its effect appears to be contingent upon the nature of the distracting information. Future research might seek to investigate the influence of threat on other factors involved in the control of attentional control, such as selection history (Awh, Belopolsky, & Theeuwes, 2012) and goal-contingent attentional capture (Folk et al., 1992).
The present study focused on the influence of threat on value-driven attention. The extent to which the competitive relationship observed in the present study is particular to value-driven attention, or the extent to which it reflects a broader principle of valenced-dependent competition, is unclear. It is possible that the processing of negatively valenced information competes with the processing of positively valenced information more broadly, which would predict the same pattern of results for attention to aversively conditioned stimuli with and without a positive arousal manipulation. Another interesting question not addressed by the present study concerns the influence of threat on attention to aversively conditioned stimuli. Valence-dependent competition might predict enhanced attentional capture in this situation. Future research should explore these possibilities. Relatedly, it is unclear whether the observed pattern of results is particular to the influence of associative reward learning on attention, or whether attention to arguably more “hard-wired” positively valenced stimuli such as erotica (Most, Smith, Cooter, Levy, & Zald, 2005) would be similarly subject to threat-dependent suppression. Future research might also explore the influence of trial-by-trial modulations in threat and/or negative arousal on the capture of attention, potentially using pupil dilation or electrodermal activity (EDA) as an on-line indicator.
The findings of the present study also have potential implications for our understanding of addiction. An important component of addiction is attentional bias (see Anderson, 2016b; Field & Cox, 2008; for reviews). Drug cues capture the attention of drug-dependent patients (e.g., Hogarth, Dickinson, & Duka, 2003; Lubman, Peters, Mogg, Bradley, & Deakin, 2000; Mogg, Bradley, Field, & De Houwer, 2003), and attentional biases towards drug cues are related to craving (Field, Mogg, & Bradley, 2004, 2005; Field, Mogg, Mann, Bennett, & Bradley, 2013; Franken, Kroon, Wiers, & Jansen, 2000) and relapse (Carpenter, Schreiber, Church, & McDowell, 2006; Cox, Hogan, Kristian, & Race, 2002; Marissen et al., 2006; Powell, Dawkins, West, Powell, & Pickering, 2010; Waters et al., 2003). Furthermore, drug dependence is also associated with more pronounced attentional biases for stimuli previously associated with non-drug reward (Albertella et al., 2017; Anderson, Faulkner, Rilee, Yantis, & Marvel, 2013; Anderson, Kronemer, Rilee, Sacktor, & Marvel, 2016), suggesting that susceptibility to automatic reward-related influences on attention may play a role in the addiction process (see Anderson, 2016b). At the same time, it is well known that stress can facilitate relapse (e.g., Sinha, 2007), and yet the present study demonstrates that stress actually suppresses attention to reward cues at least in a college-age sample not screened for substance abuse or dependence. One possibility suggested by our data is that value-driven attention is unrelated to the influence of stress on relapse. It is also possible that stress and anxiety have a different impact on the attention system in drug-dependent individuals, or that stress and anxiety suppress attention to cues for positive reinforcers (such as money) while facilitating attention to cues for negative reinforcers (which can include drug cues associated with relief from symptoms of withdrawal). In either case, our findings suggest a more complex relationship between reward-related attentional bias and processes relevant to addiction, and more research is needed to explore these and other interesting possibilities.
Supplementary Material
Acknowledgements
This research was supported by a start-up package from Texas A&M University to BAA and grants from the Brain & Behavior Research Foundation [NARSAD 26008] and NIH [R01-DA046410] to BAA. The authors report no conflicts of interest.
References
- Abrams J, Barbot A, & Carrasco M (2010). Voluntary attention increases perceived spatial frequency. Atten Percept Psychophys, 72(6), 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertella L, Copeland J, Pearson D, Watson P, Wiers RW, & Le Pelley ME (2017). Selective attention moderates the relationship between attentional capture by signals of nondrug reward and illicit drug use. Drug and Alcohol Dependence, 175, 99–105. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2016a). The attention habit: how reward learning shapes attentional selection. Year in Cognitive Neuroscience, 1369, 24–39. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2016b). What is abnormal about addiction-related attentional biases? Drug and Alcohol Dependence, 167, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA (2017). Reward processing in the value-driven attention network: reward signals tracking cue identity and location. Social Cognitive and Affective Neuroscience, 12(3), 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Faulkner ML, Rilee JJ, Yantis S, & Marvel CL (2013). Attentional Bias for Nondrug Reward Is Magnified in Addiction. Experimental and Clinical Psychopharmacology, 21(6), 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Folk CL (2010). Variations in the magnitude of attentional capture: testing a two-process model. Atten Percept Psychophys, 72(2), 342–352. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Kim H (in press). Test-retest reliability of value-driven attentional capture. Behavior Research Methods. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Kronemer SI, Rilee JJ, Sacktor N, & Marvel CL (2016). Reward, attention, and HIV-related risk in HIV plus individuals. Neurobiology of Disease, 92, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kuwabara H, Wong DF, Gean EG, Rahmim A, Brasic JR, … Yantis S (2016). The Role of Dopamine in Value-Based Attentional Orienting. Current Biology, 26(4), 550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011). Value-driven attentional capture. Proc Natl Acad Sci U S A, 108(25), 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2013). Reward predictions bias attentional selection. Frontiers in Human Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2014). Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Research, 1587, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Yantis S (2012). Value-driven attentional and oculomotor capture during goal-directed, unconstrained viewing. Attention Perception & Psychophysics, 74(8), 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, & Dolan RJ (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia, 40(7), 817–826. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in Cognitive Sciences, 16(8), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan PF, & Gottlieb J (2006). Integration of exogenous input into a dynamic salience map revealed by perturbing attention. Journal of Neuroscience, 26(36), 9239–9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, & van IJzendoorn MH (2007). Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin, 133(1), 1–24. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, & Damasio AR (2000). Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex, 10(3), 295–307. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, & Ranieri WF (1996). Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment, 67(3), 588–597. [DOI] [PubMed] [Google Scholar]
- Becker SI, Dutt N, Vromen JMG, & Horstmann G (2017). The capture of attention and gaze in the search for emotional photographic faces. Visual Cognition, 25(1–3), 241–261. [Google Scholar]
- Bichot NP, & Schall JD (1999). Saccade target selection in macaque during feature and conjunction visual search. Visual Neuroscience, 16(1), 81–89. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Rodgers RJ, & Blanchard DC (1993). Defense System Psychopharmacology - an Ethological Approach to the Pharmacology of Fear and Anxiety. Behavioural Brain Research, 58(1–2), 155–165. [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997). The Psychophysics Toolbox. Spat Vis, 10(4), 433–436. [PubMed] [Google Scholar]
- Burrows BE, & Moore T (2009). Influence and Limitations of Popout in the Selection of Salient Visual Stimuli by Area V4 Neurons. Journal of Neuroscience, 29(48), 15169–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, & McDowell D (2006). Drug Stroop performance: Relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addictive Behaviors, 31(1), 174–181. [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral-Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment - the Bis Bas Scales. Journal of Personality and Social Psychology, 67(2), 319–333. [Google Scholar]
- Chubala C, & Smith S (2009). An emotional blink of attention elicited by anticipation of an aversive event. Canadian Journal of Experimental Psychology-Revue Canadienne De Psychologie Experimentale, 63(4), 339–339. [Google Scholar]
- Cisler JM, & Koster EHW (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30(2), 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Li RR, Wright CM, Rome F, Fairchild G, Dunn BD, & Aitken MRF (2012). Risk-avoidant decision making increased by threat of electric shock. Psychophysiology, 49(10), 1436–1443. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, & Shulman GL (2000). Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience, 3(3), 292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Baas JMP, Johnson L, Holroyd T, Carver FW, Lissek S, & Grillon C (2007). Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage, 37(1), 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Hogan LM, Kristian MR, & Race JH (2002). Alcohol attentional bias as a predictor of alcohol abusers’ treatment outcome. Drug and Alcohol Dependence, 68(3), 237–243. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Patrick CJ, Lang AR, Cacioppo JT, & Birbaume N (2001). Alcohol affects emotion through cognition. Psychol Sci, 12(6), 527–531. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, & Damasio HC (1991). Somatic Markers and the Guidance of Behavior - Theory and Preliminary Testing. Frontal Lobe Function and Dysfunction, 217–229. [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Libera C, & Chelazzi L (2006). Visual selective attention and the effects of monetary rewards. Psychological Science, 17(3), 222–227. [DOI] [PubMed] [Google Scholar]
- Derryberry D, & Reed MA (2002). Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology, 111(2), 225–236. [DOI] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural mechanisms of selective visual attention. Annu Rev Neurosci, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1996). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380(6569), 69–72. [DOI] [PubMed] [Google Scholar]
- Dimberg U, & Ohman A (1996). Behold the wrath: Psychophysiological responses to facial stimuli. Motivation and Emotion, 20(2), 149–182. [Google Scholar]
- Duncan J, & Humphreys GW (1989). Visual-Search and Stimulus Similarity. Psychological Review, 96(3), 433–458. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, & Merikle PM (2001). Differential attentional guidance by unattended faces expressing positive and negative emotion. Perception & Psychophysics, 63(6), 1004–1013. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, & Bar-Haim Y (2010). Enhanced neural reactivity and selective attention to threat in anxiety. Biol Psychol, 85(2), 252–257. [DOI] [PubMed] [Google Scholar]
- Engelmann JB, & Pessoa L (2007). Motivation sharpens exogenous spatial attention. Emotion, 7(3), 668–674. [DOI] [PubMed] [Google Scholar]
- Esterman M, DeGutis J, Mercado R, Rosenblatt A, Vasterling JJ, Milberg W, & McGlinchey R (2013). Stress-Related Psychological Symptoms Are Associated with Increased Attentional Capture by Visually Salient Distractors. Journal of the International Neuropsychological Society, 19(7), 835–840. [DOI] [PubMed] [Google Scholar]
- Esteves F, & Ohman A (1993). Masking the Face - Recognition of Emotional Facial Expressions as a Function of the Parameters of Backward-Masking. Scandinavian Journal of Psychology, 34(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: attentional control theory. Emotion, 7(2), 336–353. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, & Munoz DP (2004). Neural correlates of the automatic and goal-driven biases in orienting spatial attention. Journal of Neurophysiology, 92(3), 1728–1737. [DOI] [PubMed] [Google Scholar]
- Ferreira R, & Murray J (1983). Spielberger State-Trait Anxiety Inventory - Measuring Anxiety with and without an Audience during Performance on a Stabilometer. Perceptual and Motor Skills, 57(1), 15–18. [DOI] [PubMed] [Google Scholar]
- Field M, & Cox WM (2008). Attentional bias in addictive behaviors: A review of its development, causes, and consequences. Drug and Alcohol Dependence, 97(1–2), 1–20. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, & Bradley BP (2004). Cognitive bias and drug craving in recreational cannabis users. Drug and Alcohol Dependence, 74(1), 105–111. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, & Bradley BP (2005). Craving and cognitive biases for alcohol cues in social drinkers. Alcohol and Alcoholism, 40(6), 504–510. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Mann B, Bennett GA, & Bradley BP (2013). Attentional Biases in Abstinent Alcoholics and Their Association With Craving. Psychology of Addictive Behaviors, 27(1), 71–80. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, & Johnston JC (1992). Involuntary Covert Orienting Is Contingent on Attentional Control Settings. Journal of Experimental Psychology-Human Perception and Performance, 18(4), 1030–1044. [PubMed] [Google Scholar]
- Franken IHA, Kroon LY, Wiers RW, & Jansen A (2000). Selective cognitive processing of drug cues in heroin dependence. Journal of Psychopharmacology, 14(4), 395–400. [DOI] [PubMed] [Google Scholar]
- Gabay S, & Henik A (2010). Temporal expectancy modulates inhibition of return in a discrimination task (vol 17, pg 47, 2010). Psychonomic Bulletin & Review, 17(2), 273–273. [DOI] [PubMed] [Google Scholar]
- Griggs WS, Kim HF, Ghazizadeh A, Costello MG, Wall KM, & Hikosaka O (2017). Flexible and Stable Value Coding Areas in Caudate Head and Tail Receive Anatomically Distinct Cortical and Subcortical Inputs. Frontiers in Neuroanatomy, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, & Davis M (1991). Fear-Potentiated Startle in Humans - Effects of Anticipatory Anxiety on the Acoustic Blink Reflex. Psychophysiology, 28(5), 588–595. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, & Pine DS (2008). Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry, 165(7), 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Robinson OJ, Krimsky M, O’Connell K, Alvarez G, & Ernst M (2017). Anxiety-Mediated Facilitation of Behavioral Inhibition: Threat Processing and Defensive Reactivity During a Go/No-Go Task. Emotion, 17(2), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, & Gronlund SD (2007). Top-down guidance in visual search for facial expressions. Psychonomic Bulletin & Review, 14(1), 159–165. [DOI] [PubMed] [Google Scholar]
- Hayward DA, & Ristic J (2013). Measuring attention using the Posner cuing paradigm: the role of across and within trial target probabilities. Frontiers in Human Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, & Theeuwes J (2010a). Reward Changes Salience in Human Vision via the Anterior Cingulate. Journal of Neuroscience, 30(33), 11096–11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Chelazzi L, & Theeuwes J (2010b). Reward Guides Vision when It’s Your Thing: Trait Reward-Seeking in Reward-Mediated Visual Priming. Plos One, 5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, & Peelen MV (2015). Neural Mechanisms of Incentive Salience in Naturalistic Human Vision. Neuron, 85(3), 512–518. [DOI] [PubMed] [Google Scholar]
- Hickey C, & Peelen XV (2017). Reward Selectively Modulates the Lingering Neural Representation of Recently Attended Objects in Natural Scenes. Journal of Neuroscience, 37(31), 7297–7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, & Duka T (2003). Discriminative stimuli that control instrumental tobacco-seeking by human smokers also command selective attention. Psychopharmacology, 168(4), 435–445. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Schoenfeld MA, Buschschulte A, Rautzenberg A, Krebs RM, & Boehler CN (2015). The modulatory impact of reward and attention on global feature selection in human visual cortex. Visual Cognition, 23(1–2), 229–248. [Google Scholar]
- Kahneman D, Treisman A, & Gibbs BJ (1992). The Reviewing of Object Files - Object-Specific Integration of Information. Cognitive Psychology, 24(2), 175–219. [DOI] [PubMed] [Google Scholar]
- Kalin NH, & Shelton SE (1989). Defensive Behaviors in Infant Rhesus-Monkeys - Environmental Cues and Neurochemical Regulation. Science, 243(4899), 1718–1721. [DOI] [PubMed] [Google Scholar]
- Kim HF, & Hikosaka O (2013). Distinct Basal Ganglia Circuits Controlling Behaviors Guided by Flexible and Stable Values. Neuron, 79(5), 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Driver J, & Eimer M (2009). Reward Priority of Visual Target Singletons Modulates Event-Related Potential Signatures of Attentional Selection. Psychological Science, 20(2), 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, & Vanessen DC (1992). Neuronal Responses to Static Texture Patterns in Area-V1 of the Alert Macaque Monkey. Journal of Neurophysiology, 67(4), 961–980. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, & De Houwer J (2004). Does imminent threat capture and hold attention? Emotion, 4(3), 312–317. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Crombez G, Van Damme S, Verschuere B, & De Houwer J (2005). Signals for threat modulate attentional capture and holding: Fear-conditioning and extinction during the exogenous cueing task. Cognition & Emotion, 19(5), 771–780. [Google Scholar]
- Koster EHW, Crombez G, Verschuere B, & De Houwer J (2006). Attention to threat in anxiety-prone individuals: Mechanisms underlying attentional bias. Cognitive Therapy and Research, 30(5), 635–643. [Google Scholar]
- Lang PJ (1995). The emotion probe. Studies of motivation and attention. Am Psychol, 50(5), 372–385. [DOI] [PubMed] [Google Scholar]
- Le Pelley ME, Pearson D, Griffiths O, & Beesley T (2015). When Goals Conflict With Values: Counterproductive Attentional and Oculomotor Capture by Reward-Related Stimuli. Journal of Experimental Psychology-General, 144(1), 158–171. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Peters LA, Mogg K, Bradley BP, & Deakin JFW (2000). Attentional bias for drug cues in opiate dependence. Psychological Medicine, 30(1), 169–175. [DOI] [PubMed] [Google Scholar]
- Marissen MAE, Franken IHA, Waters AJ, Blanken P, van den Brink W, & Hendriks VM (2006). Attentional bias predicts heroin relapse following treatment. Addiction, 101(9), 1306–1312. [DOI] [PubMed] [Google Scholar]
- Mather M, & Sutherland MR (2011). Arousal-Biased Competition in Perception and Memory. Perspectives on Psychological Science, 6(2), 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, & Macleod C (1985). Selective Processing of Threat Cues in Anxiety-States. Behaviour Research and Therapy, 23(5), 563–569. [DOI] [PubMed] [Google Scholar]
- Mathews A, & Macleod C (1994). Cognitive Approaches to Emotion and Emotional Disorders. Annual Review of Psychology, 45, 25–50. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, & Davidson RJ (2004). Unequally masked: Indexing differences in the perceptual salience of “unseen” facial expressions. Cognition & Emotion, 18(8), 1009–1026. [Google Scholar]
- Mogg K, & Bradley BP (1999). Orienting of attention to threatening facial expressions presented under conditions of restricted awareness. Cognition & Emotion, 13(6), 713–740. [Google Scholar]
- Mogg K, Bradley BP, Field M, & De Houwer J (2003). Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction, 98(6), 825–836. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Egner T, Monti JM, & Mesulam MM (2009). Search for a Threatening Target Triggers Limbic Guidance of Spatial Attention. Journal of Neuroscience, 29(34), 10563–10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, & Fallah M (2003). Visuomotor origins of covert spatial attention. Neuron, 40(4), 671–683. [DOI] [PubMed] [Google Scholar]
- Moser JS, Becker MW, & Moran TP (2012). Enhanced Attentional Capture in Trait Anxiety. Emotion, 12(2), 213–216. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, & Zald DH (2005). Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin & Review, 12(4), 654–661. [DOI] [PubMed] [Google Scholar]
- Most SB, Smith SD, Cooter AB, Levy BN, & Zald DH (2005). The naked truth: Positive, arousing distractors impair rapid target perception. Cognition & Emotion, 21(5), 964–981. [Google Scholar]
- Murty VP, Labar KS, & Adcock RA (2012). Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. J Neurosci, 32(26), 8969–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalpakkam V, Koch C, Rangel A, & Perona P (2010). Optimal reward harvesting in complex perceptual environments. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 5232–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissens T, Failing M, & Theeuwes J (2017). People look at the object they fear: oculomotor capture by stimuli that signal threat. Cognition & Emotion, 31(8), 1707–1714. [DOI] [PubMed] [Google Scholar]
- Ohman A (2002). Automaticity and the amygdala: Nonconscious responses to emotional faces. Current Directions in Psychological Science, 11(2), 62–66. [Google Scholar]
- Ohman A (2005). The rote of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology, 30(10), 953–958. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, & Esteves F (2001). Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology-General, 130(3), 466–478. [DOI] [PubMed] [Google Scholar]
- Ohman A, & Mineka S (2003). The malicious serpent: Snakes as a prototypical stimulus for an evolved module of fear. Current Directions in Psychological Science, 12(1), 5–9. [Google Scholar]
- Ousdal OT, Specht K, Server A, Andreassen OA, Dolan RJ, & Jensen J (2014). The human amygdala encodes value and space during decision making. Neuroimage, 101, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H (1988). Familiarity and Visual Change Detection. Perception & Psychophysics, 44(4), 369–378. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, & Barratt ES (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51(6), 768–774. [DOI] [PubMed] [Google Scholar]
- Peck CJ, Lau B, & Salzman CD (2013). The primate amygdala combines information about space and value. Nature Neuroscience, 16(3), 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CJ, & Salzman CD (2014). Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment. Elife, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2009). How do emotion and motivation direct executive control? Trends in Cognitive Sciences, 13(4), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, & Ungerleider LG (2002). Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res, 15(1), 31–45. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, & Ungerleider LG (2002). Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A, 99(17), 11458–11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, & Carrasco M (2006). Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science, 17(4), 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI (1980). Orienting of attention. Q J Exp Psychol, 32(1), 3–25. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, & Vuilleumier P (2004). Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex, 14(6), 619–633. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, & Pickering A (2010). Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology, 212(4), 537–549. [DOI] [PubMed] [Google Scholar]
- Quigley L, Nelson AL, Carriere J, Smilek D, & Purdon C (2012). The effects of trait and state anxiety on attention to emotional images: an eye-tracking study. Cogn Emot, 26(8), 1390–1411. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Bond RL, & Roiser JP (2015). The impact of threat of shock on the framing effect and temporal discounting: executive functions unperturbed by acute stress? Frontiers in Psychology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, & Grillon C (2014). The dorsal medial prefrontal (anterior cingulate) cortex-amygdala aversive amplification circuit in unmedicated generalised and social anxiety disorders: an observational study. Lancet Psychiatry, 1(4), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Letkiewicz AM, Overstreet C, Ernst M, & Grillon C (2011). The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn Affect Behav Neurosci, 11(2), 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Vytal K, Cornwell BR, & Grillon C (2013). The impact of anxiety upon cognition: perspectives from human threat of shock studies. Frontiers in Human Neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015a). Attentional capture by signals of threat. Cogn Emot, 29(4), 687–694. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015b). Attentional capture by signals of threat. Cognition & Emotion, 29(4), 687–694. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015c). Potential Threat Attracts Attention and Interferes With Voluntary Saccades. Emotion, 15(3), 329–338. [DOI] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2017). The time course of attentional bias to cues of threat and safety. Cogn Emot, 31(5), 845–857. [DOI] [PubMed] [Google Scholar]
- Schmitz A, & Grillon C (2012). Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nature Protocols, 7(3), 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT (2008). Value-Based Modulations in Human Visual Cortex. Neuron, 60(6), 1169–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Maxwell JS, McMenamin BW, Greischar LL, & Davidson RJ (2011). Stress Potentiates Early and Attenuates Late Stages of Visual Processing. Journal of Neuroscience, 31(3), 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, & Bar-Haim Y (2016). Threat Monitoring and Attention-Bias Modification in Anxiety and Stress-Related Disorders. Current Directions in Psychological Science, 25(6), 431–437. [Google Scholar]
- Shechner T, Jarcho JM, Wong S, Leibenluft E, Pine DS, & Nelson EE (2017). Threats, rewards, and attention deployment in anxious youth and adults: An eye tracking study. Biological Psychology, 122, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, & Corbetta M (1999). Areas involved in encoding and applying directional expectations to moving objects. Journal of Neuroscience, 19(21), 9480–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2007). The role of stress in addiction relapse. Curr Psychiatry Rep, 9(5), 388–395. [DOI] [PubMed] [Google Scholar]
- Susskind JM, Lee DH, Cusi A, Feiman R, Grabski W, & Anderson AK (2008). Expressing fear enhances sensory acquisition. Nature Neuroscience, 11(7), 843–850. [DOI] [PubMed] [Google Scholar]
- Sussman TJ, Jin JW, & Mohanty A (2016). Top-down and bottom-up factors in threat-related perception and attention in anxiety. Biological Psychology, 121, 160–172. [DOI] [PubMed] [Google Scholar]
- Sussman TJ, Szekely A, Hajcak G, & Mohanty A (2016). It’s All in the Anticipation: How Perception of Threat Is Enhanced in Anxiety. Emotion, 16(3), 320–327. [DOI] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M (2012). Negative Arousal Amplifies the Effects of Saliency in Short-Term Memory. Emotion, 12(6), 1367–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, & Mather M (2015). Negative Arousal Increases the Effects of Stimulus Salience in Older Adults. Experimental Aging Research, 41(3), 259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J (1991). Categorization and Identification of Simultaneous Targets. Acta Psychologica, 76(1), 73–86. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual Selectivity for Color and Form. Perception & Psychophysics, 51(6), 599–606. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychol (Amst), 135(2), 77–99. [DOI] [PubMed] [Google Scholar]
- Thompson KG, & Bichot NP (2005). A visual salience map in the primate frontal eye field. Development, Dynamics and Pathology of Neuronal Networks: From Molecules to Functional Circuits, 147, 251–262. [DOI] [PubMed] [Google Scholar]
- Tipper C, & Kingstone A (2005). Is inhibition of return a reflexive effect? Cognition, 97(3), B55–B62. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–594. [DOI] [PubMed] [Google Scholar]
- Wang LH, Yu HB, & Zhou XL (2013). Interaction between value and perceptual salience in value-driven attentional capture. Journal of Vision, 13(3). [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, & Balabanis MH (2003). Attentional bias predicts outcome in smoking cessation. Health Psychology, 22(4), 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, & Mattingley JB (2005). Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage, 24(2), 417–425. [DOI] [PubMed] [Google Scholar]
- Wolfe JM (1994). Guided Search 2.0 - a Revised Model of Visual-Search. Psychonomic Bulletin & Review, 1(2), 202–238. [DOI] [PubMed] [Google Scholar]
- Wyczesany M, Ligeza TS, & Grzybowski SJ (2015). Effective connectivity during visual processing is affected by emotional state. Brain Imaging Behav, 9(4), 717–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, & Harwood SL (2017). Threat captures attention but does not affect learning of contextual regularities. Cogn Emot, 31(3), 564–571. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kim HF, & Hikosaka O (2013). Reward Value-Contingent Changes of Visual Responses in the Primate Caudate Tail Associated with a Visuomotor Skill. Journal of Neuroscience, 33(27), 11227–11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.