Abstract
Purpose of Review
We review recent developments on risk factors in schizophrenia.
Recent Findings
The way we think about schizophrenia today is profoundly different from the way this illness was seen in the twentieth century. We now know that the etiology of schizophrenia is multifactorial and reflects an interaction between genetic vulnerability and environmental contributors. Environmental risk factors such as pregnancy and birth complications, childhood trauma, migration, social isolation, urbanicity, and substance abuse, alone and in combination, acting at a number of levels over time, influence the individual’s likelihood to develop the disorder.
Summary
Environmental risk factors together with the identification of a polygenic risk score for schizophrenia, research on gene–environment interaction and environment–environment interaction have hugely increased our knowledge of the disorder.
Keywords: Schizophrenia, Psychosis, Risk factors, Environment, Gene–environment interaction, Childhood trauma, Cannabis
Introduction
Schizophrenia has a well-established genetic component, which can now be estimated using the polygenic risk score for schizophrenia [1, 2••]. In the ground-breaking meta-analysis of genome-wide association study (GWAS) of schizophrenia, 108 schizophrenia-associated loci were identified [2••]. The loci implicated include genes involved in dopamine synthesis, calcium channel regulation, immunity, and glutamate neuroreceptors. However, this and subsequent GWAS studies explain only a minority of the variance in the liability for schizophrenia in the general population. This reflects the fact that a significant proportion of the liability may be due to gene–environment interactions [3] or to epigenetic mechanisms reflecting the effect of environmental factors. Indeed, growing evidence shows that non-genetic risk factors not only contribute to the illness but also suggest ways in which we may find potential subgroups of subjects at higher risk and therefore influence clinical management.
Pregnancy and Birth Complications
Obstetric complications are well-documented as a risk factor for schizophrenia [4•,5–7]; increased susceptibility has been associated with emergency cesarean section, bleeding during pregnancy, preeclampsia [6], and low birth weight [8–10]. Use of forceps and low birth weight predict earlier age of onset of psychosis [11].
Some epidemiologic studies have also suggested that exposure to viruses and other infectious agents such as influenza, toxoplasmosis, and herpes simplex virus type 2, contracted during pregnancy [12, 13] and around the time of conception [14], are associated with a later risk of psychotic disorders. However, these results have not always been replicated. For example, a recent meta-analysis by Selten et al. has shown insufficient evidence to prove an association between second-trimester influenza exposure and psychotic outcome in the offspring [15••].
Four main pathogenic mechanisms have been suggested as involved: fetal malnutrition, prematurity, hypoxic-ischemic events, and maternal infections during pregnancy or delivery [4•, 16–20]. Moreover, elevated markers of inflammation, including maternal C-reactive protein [21] and interleukin-8 [22] have been found in mothers of patients with schizophrenia. Late winter or spring birth has often been reported as risk factor for schizophrenia [8, 23–25]. However, it has a very small effect [26]; whether it is secondary to maternal infection or nutritional deficiency remains unclear.
Advanced Parental Age
Increased paternal age, from age higher than 34 and upwards [27, 28], has been associated with schizophrenia [29–31]. An attractive theory suggests that age-associated increase in sporadic de novo mutations in male germ cells may play a role [32–34]. However, this was discounted by a study from Denmark that suggests that late marriage and reproduction may be due to personality attributes of fathers [35].
A less consistent pattern of findings has emerged regarding maternal age at birth and risk of schizophrenia in offspring. In one study, age younger than 19 and age older than 40 years [36] appeared to increase the risk. However, in another cohort study, the risk appeared decreased in offspring of mothers older than 30 years [37]. Lopez-Castroman et al. (2010) found a significant linear association increase only with advancing maternal age [38].
Trauma and Social Adversities
Trauma and social adversities in different forms, either during childhood or adulthood, have been extensively investigated as potential risk factors for schizophrenia. Varese and colleagues, in a meta-analysis of case-control, prospective, and cross-sectional cohort studies, reported strong evidence that childhood adversity (defined as sexual abuse, physical abuse, emotional/psychological abuse, neglect, parental death, and bullying) was associated with increased risk for psychosis in adulthood (overall OR = 2.78) [39•]. There is an association between permanent separation from, or death of, one or both parents and psychosis [40–43], victimization and bullying and psychosis [44–46]. A robust link between childhood trauma and schizophrenic symptoms has been found [47–49] with childhood trauma being associated with the most severe forms of positive symptomatology in adulthood, particularly hallucinations [49–51], and affective symptoms [52]. Life events more proximal to the onset of illness, defined as situations that bring about positive or negative changes in personal circumstances and/or involve an element of threat, have been investigated [53–55]. The most recent review and meta-analysis of the relationship between life events and psychosis has suggested around a threefold increased odds of life events in the period prior to psychosis onset, with the time period under consideration ranging between 3 months and 3.6 years [55].
Social Class and Isolation
Some reports link social inequality at birth with schizophrenia. Socioeconomic status (usually measured by paternal occupation) has been reported to be associated with an increased risk of psychosis [56–60]. However, while some findings are positive, there are a number of conflicting studies showing no association between psychosis and low social class at birth [41] or even a link with high social class [61, 62].
Markers of isolation/disadvantage, alone and cumulative, are also associated with psychosis [42, 43, 63]. First-episode psychosis patients are more likely to live alone; be single or unemployed; live in a rented accommodation, in overcrowded conditions; and receive an income below official poverty, not only at first contact with psychiatric services but up to 5 years prior to the onset of psychosis, with around a twofold increased odds [43]. The World Health Organization (WHO) studies have reported that despite the better access to biomedical treatment, higher rates of chronic disability and dependency in schizophrenia occur in high- than low-income countries and suggest that something essential to recovery is missing in the social fabric [64].
Migration
Meta-analytic reviews show that migrant groups are at increased risk of schizophrenia and other psychotic disorders [65, 66•]. These findings have been consistently replicated in a number of high-income countries: the UK [67], the Netherlands [68], Germany [69], Denmark [70], France [71], Italy [72], and to a lesser extent Canada [73] with some evidence that the risk of schizophrenia and other non-affective psychotic disorders is especially high among refugees compared with non-refugee migrants [74]. Interestingly, the risk appears to persist into the second and third generations [66•, 75].
The level of risk appears to vary by country of origin. A recent meta-analysis of schizophrenia incidence in the UK reported almost a five times greater risk of schizophrenia among people of black Caribbean origin compared with reference UK population (usually white) [76]. Numerous hypotheses have been tested. Higher incidence rates in the country of origin, selective migration, or misdiagnosis of mood disorders do not seem to explain the phenomena [77–81]. However, social adversity exposures at all stages of the migration process (before, during, and after) [82], low ethnic density [83], social isolation [84], discrimination [68], and lack of access to private accommodation and economic opportunities [85] have all been suggested as contributing, so has vitamin D deficiency [86, 87] but as yet little direct evidence for the latter has been found.
Urbanicity
Growing up and/or living in an urban environment has frequently been associated with an increased risk of schizophrenia or psychosis in general [88–91]. A meta-analysis, including a total of 47,087 cases with psychosis, shows a pooled OR for psychosis in urban environment compared with the rural environment of 2.39 (95% CI 1.62–3.51) [92•]. Changing residence in childhood from rural to urban environment doubles the risk of developing schizophrenia [93, 94], and the more years a child spends in an urban area, the greater the risk becomes [95]. Many explanations have been proposed such as greater exposure to prenatal influenza [96], maternal obstetrical complications [97, 98], toxoplasma gondii infection [99], cannabis use [100], social deprivation, income inequality, and social fragmentation [101, 102] but none of them has been verified. Furthermore, while the largest multicentric study of first-episode psychosis patients to date (EU-GEI study) confirmed higher incidence in Northern European cities including London, Amsterdam, and Paris, the increased density effect is not so clear in Southern European settings [103•].
Interestingly, a recent study conducted in Denmark, has pointed out for the first time the protective role of living in, or near to, a green area, showing a dose-response association between the magnitude of greenspace during childhood and the risk of later development of schizophrenia [104•].
Cannabis and Other Substance Use
Substance use is highly prevalent in psychotic patients [105–107]. There is good evidence that psychostimulants (such as amphetamines and cocaine) can induce psychosis [108]. There also have been a few suggestions that alcohol misuse and psychosis might be associated [109, 110], and recently, a meta-analysis raised the question of whether tobacco use could be a risk factor for psychosis [111]. However, much greater evidence points to an important aetiological role for cannabis use. Prospective epidemiological studies consistently report an association between cannabis use and schizophrenia [112–114] with an estimated two- to threefold increased risk [114, 115]. A dose–response relationship between extent of use and risk of psychosis has been shown in a meta-analysis [116]. The association is stronger in those individuals who used cannabis earlier [113], and who used high potency tetrahydrocannabinol (THC) cannabis or/and more frequently [112, 117, 118]. Indeed, the EU-GEI study has found that if high-potency cannabis was no longer available, around 12% of first-episode psychosis cases across 11 Europe-wide sites could be prevented, rising to 30% in London and 50% in Amsterdam [119•]. The age at which cannabis use begins appears to correlate with the age at onset of psychosis [118, 120, 121] while persistent cannabis use after a first episode is associated with poorer prognosis [122, 123], higher relapse rates, longer hospitalizations, and severe positive symptoms [124].
Cognitive Impairments and Brain Structural Abnormalities
Although schizophrenia usually manifests in adolescence and early adult life, numerous reports suggest that many patients with schizophrenia have a history of delayed developmental milestones in the first year of life [125], lower IQ in childhood [126–128], hearing impairment [129], emotional problems, and interpersonal difficulties early in life [130, 131]. Those people who develop psychosis following heavy cannabis use show less evidence of such neurodevelopmental deviance. In particular, they have higher premorbid IQ and better social functioning in childhood than psychotic patients who do not use cannabis [132]. Perhaps being smarter and more sociable enables them to find cannabis dealers and obtain the money for the drug!
Gene–Environment Interaction
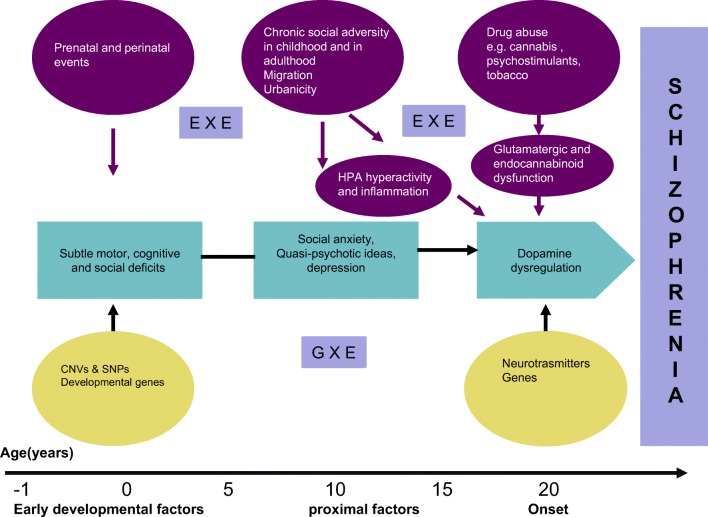
It is becoming increasingly clear that none of the risk factors discussed above, by itself, is either necessary or sufficient for the development of schizophrenia. Most of show a modest effect (twofold increase in risk) and none seem specific for schizophrenia. Different factors operating at various levels contribute to onset and progression of the disorder. The developmental cascade towards schizophrenia [133] should now include gene–environment interaction (GXE), and environment–environment interaction (EXE) (Fig. 1).
Fig. 1.
Developmental cascade towards schizophrenia. CNV, copy number variations; SNPs, single nucleotide polymorphisms; ExE, environment–environment interaction; GxE, gene–environment interaction
Interest has turned to the possibility of gene × environmental interactions. Preliminary reports suggested interactions between functional polymorphisms in the catechol-O-methyltransferase gene [134], or the DRD2 genotype (OMIM 126450) [135], and the AKT1 genotype (C/C rs2494732) [136, 137] and cannabis use, on risk of psychosis. However, all of these studies are relatively small and in need of replication. A few preliminary studies have examined interactions between the polygenic risk score for schizophrenia (e.g., Trotta et al. [138]; Ursini et al. [139]) but there are not sufficient studies yet to evaluate this adequately. The largest and most recently published study to date, analyzing the associations of polygenic risk score for schizophrenia and environmental exposures in 1699 patients and 1542 unrelated controls, shows an additive interaction between polygenic risk score and lifetime regular cannabis use and exposure to early life adversities (sexual abuse, emotional abuse, emotional neglect, and bullying), but not with the presence of other exposures such as hearing impairment, winter birth, physical abuse, or physical neglect [140••] confirming the need for future confirmatory studies.
Cumulative Effect of Environmental Risk Factors
A few studies have now started examining the additive effect of multiple environmental factors on risk of psychosis as aggregate index of total number of risk factors or weighted sum. Cougnard et al. reported an additive interaction between exposure to three risk factors—cannabis use, childhood trauma, and urbanicity—and baseline psychotic experiences in predicting persistent psychotic symptoms three years later in the general population [141]. Stepniak et al. found that individuals who had been exposed to 4 or more environmental risk factors had a significantly lower age of onset than those exposed to 3 factors [142]. As a predictor tool, Padmanabhan et al., in a pilot study, explored the association of cumulative environmental risk (including nine risk factors) with conversion to psychosis in a family high-risk population [143].
Neurochemical Mechanisms
Different systems: dopaminergic (DA), glutamatergic, neuroinflammation/immune, and more recently endocannabinoid (eCB), have all been investigated to understand the exact mechanism(s) by which some non-genetic risk factors can affect brain function. The predominant biological theory of schizophrenia highlights the role of the excess presynaptic synthesis of DA in the striatum in the onset of positive symptoms [144•, 145]. Consistent with this view, the diathesis–stress model suggests that the hypothalamus–pituitary–adrenal (HPA) axis may trigger a cascade of events resulting in neural circuit dysfunction, including alterations in DA signaling [146]. Epidemiological studies are consistent with a key role of stress/cortisol in the onset of psychosis. Higher levels of diurnal cortisol have been reported in patients compared with controls or patients on antipsychotic treatments for less than 2 weeks [147] and high baseline cortisol levels appear to facilitate transition to psychotic level symptoms in at-risk youths [148]. Mizrahi et al., investigating the DA release in response to a psychosocial stress challenge in psychosis-related disorders, found that the largest stress-induced changes in salivary cortisol was present in the schizophrenia group, followed by the clinical high-risk group, with an association between the percent change in the cortisol response and the stress-induced DA release in the associative striatum [149].
New data provide intriguing evidence of an association between migration [150], hearing impairment [151], childhood abuse [152], low parental care [153], and elevation in striatal dopamine synthesis. Acute administration of THC, the active ingredient of cannabis, has been reported to increase dopamine release [154]. However, paradoxically, chronic cannabis use [155] and also difficult premature birth [156] are associated with decreased striatal dopamine. Perhaps DA receptor sensitivity or dysregulation in response to stress may be one pathway through which the different exposures interact with genetic vulnerability to confer a higher risk of schizophrenia [144].
A compelling case is made for the role of glutamate/NMDA receptors in schizophrenia, originally suggested as a mechanism underlying the psychotogenic effects of PCP and ketamine [157]. There is now strong evidence in support of the hypothesis that hypofunction of NMDA receptors contributes to the symptoms of schizophrenia [158, 159]; some reports suggest that dopamine dysregulation in schizophrenia may be secondary to glutamatergic dysfunction in some cases at least [160, 161]. Furthermore, glutamatergic neurotransmission has been shown to mediate the effects of both acute and chronic stress [162].
Just as amphetamine-induced psychosis gave rise to the dopamine hypothesis and ketamine-induced psychosis to the glutamate hypotheses, so cannabis-induced psychosis has provoked interest in the endocannabinoid system [163]. Certainly, the endogenous cannabinoid system (eCB) is altered in schizophrenia. The CB1 receptor densities and anandamide levels have been reported abnormally in patients with schizophrenia [164, 165]; among other function, the eCB system appears to regulate the HPA axis [166–168]. It has been suggested that a dysregulation of this system (that could be induced for example by exogenous cannabis) can interact with neurotransmitter systems in such a way that an “endocannabinoid hypothesis” can be integrated into the neurobiological hypotheses of schizophrenia [164].
Another possible molecular mechanism underlying psychosis risk is neuroinflammation and abnormalities of the immune system [169, 170•]. A recent meta-analysis examining peripheral inflammatory markers shows that some markers such as interleukin 6 (IL-6), tumor necrosis factor α (TNFα), soluble IL-2 receptor (sIL-2R), and IL-1 receptor antagonist (IL-1RA) increase in acute episodes and tend to decrease after successful treatment [171]. The effects of childhood trauma on inflammation have been well-studied. Individuals exposed to childhood trauma have significantly elevated baseline peripheral inflammatory markers in adulthood [172, 173]. Another recently studied hypothesis implicates the immune system. Autoimmune diseases are reported to occur in 3.6% of patients with schizophrenia [174]. Mechanisms through which systemic immune activation affect risk of psychopathology include the effects of inflammation on concurrent brain function, the effects of early immune activation on brain development, the sensitization of immune brain cells to subsequent psychosocial stressors, and the cross-sensitization of the HPA axis response to subsequent psychosocial stressors [172]. It has been speculated that inflammation-mediated pathways may serve as a final common pathway for environmental risk factors such as early childhood adversity, adolescent cannabis use, and social exclusion [170]. However, hard evidence supporting this hypothesis remains elusive.
Conclusions
Epidemiological studies have consistently shown a pattern of association between environmental risk factors and later onset of psychosis, which is suggestive of a causal relationship. However, there are a number of reasons why the association between environmental risk factors and psychotic outcomes may be overestimated or underestimated such as bias (where incorrect estimates are due to measurements or sample selection), chance, confounding (third explanation for the association), and reverse causation (where psychosis increases risk of an environmental exposure), which should be taken in consideration when causality is inferred. Studying gene–environment interaction and gene–environment correlation (rGE) (genetic effects on environment exposure) may clarify the position [175, 176].
Future research should also explore potential protective factors in groups who have a lower risk of psychotic disorders. A new field of research includes big data and predictive models, where traditional paper notes have been replaced with electronic patient records. In line with this direction, there have been initial successful applications of machine learning algorithms to diagnose psychosis [177]. As with diagnostic tools for cardiovascular risk, schizophrenia will in the near future probably require a combination of diagnostic approaches, including measures of genetic risk, environmental risk factors, and imaging.
Acknowledgements
This article was made open access with the financial support of King’s College London.
Compliance with Ethical Standards
Conflict of Interest
Simona A. Stilo declares no conflicts of interest. Robin M. Murray reports honoraria for lectures from Janssen, Sunovian, Lundbeck, and Otsuka.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Schizophrenia and Other Psychotic Disorders
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.International Schizophrenia C. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull. 2008;34(6):1066–1082. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 5.Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry. 2001;179:403–408. doi: 10.1192/bjp.179.5.403. [DOI] [PubMed] [Google Scholar]
- 6.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34(6):1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotlicka-Antczak M, Pawelczyk A, Rabe-Jablonska J, Smigielski J, Pawelczyk T. Obstetrical complications and Apgar score in subjects at risk of psychosis. Journal of psychiatric research. 2014;48(1):79–85. doi: 10.1016/j.jpsychires.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. BMJ. 1999;318(7181):421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abel KMWS, Susser ES, Dalman C, Pedersen MG, Mortensen PB, Webb RT. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67(9):923–930. doi: 10.1001/archgenpsychiatry.2010.100. [DOI] [PubMed] [Google Scholar]
- 10.Lahti M, Eriksson JG, Heinonen K, Kajantie E, Lahti J, Wahlbeck K, et al. Maternal grand multiparity and the risk of severe mental disorders in adult offspring. PloS one. 2014;9(12):e114679. doi: 10.1371/journal.pone.0114679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio-Abadal E, Ochoa S, Barajas A, Banos I, Dolz M, Sanchez B, et al. Birth weight and obstetric complications determine age at onset in first episode of psychosis. Journal of psychiatric research. 2015;65:108–114. doi: 10.1016/j.jpsychires.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58(11):1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 13.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 15.Selten JP, Termorshuizen F. The serological evidence for maternal influenza as risk factor for psychosis in offspring is insufficient: critical review and meta-analysis. Schizophr Res. 2017;183:2–9. doi: 10.1016/j.schres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155(3):355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 17.Geddes JR, Verdoux H, Takei N, Lawrie SM, Bovet P, Eagles JM, et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25(3):413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 18.Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26(2):351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 19.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157(5):801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 20.Byrne M, Agerbo E, Bennedsen B, Eaton WW, Mortensen PB. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res. 2007;97(1-3):51–59. doi: 10.1016/j.schres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomaki S, Leiviska J, Kellendonk C, et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171(9):960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161(5):889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 23.Torrey EF, Rawlings RR, Ennis JM, Merrill DD, Flores DS. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder and stillbirths. Schizophr Res. 1996;21(3):141–149. doi: 10.1016/0920-9964(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 24.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonality of births in schizophrenia and bipolar disorder: a review of the literature. Schizophr Res. 1997;28(1):1–38. doi: 10.1016/s0920-9964(97)00092-3. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340(8):603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 26.Carter JW, Schulsinger F, Parnas J, Cannon T, Mednick SA. A multivariate prediction model of schizophrenia. Schizophr Bull. 2002;28(4):649–682. doi: 10.1093/oxfordjournals.schbul.a006971. [DOI] [PubMed] [Google Scholar]
- 27.Hubert A, Szoke A, Leboyer M, Schurhoff F. Influence of paternal age in schizophrenia. Encephale. 2011;37(3):199–206. doi: 10.1016/j.encep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Buizer-Voskamp JE, Laan W, Staal WG, Hennekam EA, Aukes MF, Termorshuizen F, et al. Paternal age and psychiatric disorders: findings from a Dutch population registry. Schizophr Res. 2011;129(2-3):128–132. doi: 10.1016/j.schres.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AS, Schaefer CA, Wyatt RJ, Begg MD, Goetz R, Bresnahan MA, et al. Paternal age and risk of schizophrenia in adult offspring. Am J Psychiatry. 2002;159(9):1528–1533. doi: 10.1176/appi.ajp.159.9.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrin M, Harlap S, Kleinhaus K, Lichtenberg P, Manor O, Draiman B, et al. Older paternal age strongly increases the morbidity for schizophrenia in sisters of affected females. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(7):1329–1335. doi: 10.1002/ajmg.b.31116. [DOI] [PubMed] [Google Scholar]
- 31.Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, et al. Paternal age and schizophrenia: a population based cohort study. BMJ. 2004;329(7474):1070. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, et al. Paternal age and sporadic schizophrenia: evidence for de novo mutations. Am J Med Genet. 2002;114(3):299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crow JF. Development. There’s something curious about paternal-age effects. Science (New York, NY) 2003;301(5633):606–607. doi: 10.1126/science.1088552. [DOI] [PubMed] [Google Scholar]
- 34.Torrey EF, Buka S, Cannon TD, Goldstein JM, Seidman LJ, Liu T, et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res. 2009;114(1-3):1–5. doi: 10.1016/j.schres.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiatry. 2011;168(1):82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- 36.Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. 2012;69(6):E1–E8. doi: 10.1001/archgenpsychiatry.2011.1374. [DOI] [PubMed] [Google Scholar]
- 37.Haukka JK, Suvisaari J, Lonnqvist J. Family structure and risk factors for schizophrenia: case-sibling study. BMC psychiatry. 2004;4:41. doi: 10.1186/1471-244X-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez-Castroman J, Gomez DD, Belloso JJ, Fernandez-Navarro P, Perez-Rodriguez MM, Villamor IB, et al. Differences in maternal and paternal age between schizophrenia and other psychiatric disorders. Schizophr Res. 2010;116(2-3):184–190. doi: 10.1016/j.schres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38(4):661–671. doi: 10.1093/schbul/sbs050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 41.Morgan C, Kirkbride J, Leff J, Craig T, Hutchinson G, McKenzie K, et al. Parental separation, loss and psychosis in different ethnic groups: a case-control study. Psychol Med. 2007;37(4):495–503. doi: 10.1017/S0033291706009330. [DOI] [PubMed] [Google Scholar]
- 42.Stilo SA, Di Forti M, Mondelli V, Falcone AM, Russo M, O’Connor J, et al. Social disadvantage: cause or consequence of impending psychosis? Schizophr Bull. 2013;39(6):1288–1295. doi: 10.1093/schbul/sbs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stilo SA, Gayer-Anderson C, Beards S, Hubbard K, Onyejiaka A, Keraite A, et al. Further evidence of a cumulative effect of social disadvantage on risk of psychosis. Psychol Med. 2017;47(5):913–924. doi: 10.1017/S0033291716002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. Am J Psychiatry. 2011;168(1):65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher HL, Schreier A, Zammit S, Maughan B, Munafo MR, Lewis G, et al. Pathways between childhood victimization and psychosis-like symptoms in the ALSPAC birth cohort. Schizophr Bull. 2013;39(5):1045–1055. doi: 10.1093/schbul/sbs088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trotta A, Di Forti M, Mondelli V, Dazzan P, Pariante C, David A, et al. Prevalence of bullying victimisation amongst first-episode psychosis patients and unaffected controls. Schizophr Res. 2013;150(1):169–175. doi: 10.1016/j.schres.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssen I, Krabbendam L, Bak M, Hanssen M, Vollebergh W, de Graaf R, et al. Childhood abuse as a risk factor for psychotic experiences. Acta Psychiatr Scand. 2004;109(1):38–45. doi: 10.1046/j.0001-690x.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 48.Morgan C, Fisher H. Environment and schizophrenia: environmental factors in schizophrenia: childhood trauma-a critical review. Schizophr Bull. 2007;33(1):3–10. doi: 10.1093/schbul/sbl053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Read J, van Os J, Morrison AP, Ross CA. Childhood trauma, psychosis and schizophrenia: a literature review with theoretical and clinical implications. Acta Psychiatr Scand. 2005;112(5):330–350. doi: 10.1111/j.1600-0447.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 50.Bentall RP, de Sousa P, Varese F, Wickham S, Sitko K, Haarmans M, et al. From adversity to psychosis: pathways and mechanisms from specific adversities to specific symptoms. Soc Psychiatry Psychiatr Epidemiol. 2014;49(7):1011–1022. doi: 10.1007/s00127-014-0914-0. [DOI] [PubMed] [Google Scholar]
- 51.Whitfield CL, Dube SR, Felitti VJ, Anda RF. Adverse childhood experiences and hallucinations. Child abuse & neglect. 2005;29(7):797–810. doi: 10.1016/j.chiabu.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Matheson SL, Shepherd AM, Pinchbeck RM, Laurens KR, Carr VJ. Childhood adversity in schizophrenia: a systematic meta-analysis. Psychol Med. 2013;43(2):225–238. doi: 10.1017/S0033291712000785. [DOI] [PubMed] [Google Scholar]
- 53.Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, et al. Life events and psychosis. Initial results from the Camberwell Collaborative Psychosis Study. Br J Psychiatry. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- 54.Raune D, Kuipers E, Bebbington P. Stressful and intrusive life events preceding first episode psychosis. Epidemiol Psichiatr Soc. 2009;18(3):221–228. [PubMed] [Google Scholar]
- 55.Beards S, Gayer-Anderson C, Borges S, Dewey ME, Fisher HL, Morgan C. Life events and psychosis: a review and meta-analysis. Schizophr Bull. 2013;39(4):740–747. doi: 10.1093/schbul/sbt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castle DJ, Scott K, Wessely S, Murray RM. Does social deprivation during gestation and early life predispose to later schizophrenia? Soc Psychiatry Psychiatr Epidemiol. 1993;28(1):1–4. doi: 10.1007/BF00797825. [DOI] [PubMed] [Google Scholar]
- 57.Harrison G, Gunnell D, Glazebrook C, Page K, Kwiecinski R. Association between schizophrenia and social inequality at birth: case-control study. Br J Psychiatry. 2001;179:346–350. doi: 10.1192/bjp.179.4.346. [DOI] [PubMed] [Google Scholar]
- 58.Wicks S, Hjern A, Gunnell D, Lewis G, Dalman C. Social adversity in childhood and the risk of developing psychosis: a national cohort study. Am J Psychiatry. 2005;162(9):1652–1657. doi: 10.1176/appi.ajp.162.9.1652. [DOI] [PubMed] [Google Scholar]
- 59.O’Donoghue B, Lyne JP, Fanning F, Kinsella A, Lane A, Turner N, et al. Social class mobility in first episode psychosis and the association with depression, hopelessness and suicidality. Schizophr Res. 2014;157(1-3):8–11. doi: 10.1016/j.schres.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Seidman LJ, Cherkerzian S, Goldstein JM, Agnew-Blais J, Tsuang MT, Buka SL. Neuropsychological performance and family history in children at age 7 who develop adult schizophrenia or bipolar psychosis in the New England Family Studies. Psychol Med. 2013;43(1):119–131. doi: 10.1017/S0033291712000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makikyro T, Isohanni M, Moring J, Oja H, Hakko H, Jones P, et al. Is a child’s risk of early onset schizophrenia increased in the highest social class? Schizophr Res. 1997;23(3):245–252. doi: 10.1016/s0920-9964(96)00119-3. [DOI] [PubMed] [Google Scholar]
- 62.Mulvany F, O’Callaghan E, Takei N, Byrne M, Fearon P, Larkin C. Effect of social class at birth on risk and presentation of schizophrenia: case-control study. BMJ. 2001;323(7326):1398–1401. doi: 10.1136/bmj.323.7326.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morgan C, Kirkbride J, Hutchinson G, Craig T, Morgan K, Dazzan P, et al. Cumulative social disadvantage, ethnicity and first-episode psychosis: a case-control study. Psychol Med. 2008;38(12):1701–1715. doi: 10.1017/S0033291708004534. [DOI] [PubMed] [Google Scholar]
- 64.Jablensky A, Sartorius N. What did the WHO studies really find? Schizophr Bull. 2008;34(2):253–255. doi: 10.1093/schbul/sbm151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 66.Bourque F, van der Ven E, Malla A. A meta-analysis of the risk for psychotic disorders among first- and second-generation immigrants. Psychol Med. 2011;41(5):897–910. doi: 10.1017/S0033291710001406. [DOI] [PubMed] [Google Scholar]
- 67.Harrison G, Glazebrook C, Brewin J, Cantwell R, Dalkin T, Fox R, et al. Increased incidence of psychotic disorders in migrants from the Caribbean to the United Kingdom. Psychol Med. 1997;27(4):799–806. doi: 10.1017/s0033291796004643. [DOI] [PubMed] [Google Scholar]
- 68.Veling W, Selten JP, Susser E, Laan W, Mackenbach JP, Hoek HW. Discrimination and the incidence of psychotic disorders among ethnic minorities in The Netherlands. International journal of epidemiology. 2007;36(4):761–768. doi: 10.1093/ije/dym085. [DOI] [PubMed] [Google Scholar]
- 69.Haasen C, Yagdiran O, Mass R, Krausz M. Schizophrenic disorders among Turkish migrants in Germany. A controlled clinical study. Psychopathology. 2001;34(4):203–208. doi: 10.1159/000049308. [DOI] [PubMed] [Google Scholar]
- 70.Cantor-Graae E, Pedersen CB. Risk of schizophrenia in second-generation immigrants: a Danish population-based cohort study. Psychol Med. 2007;37(4):485–494. doi: 10.1017/S0033291706009652. [DOI] [PubMed] [Google Scholar]
- 71.Tortelli A, Morgan C, Szoke A, Nascimento A, Skurnik N, de Caussade EM, et al. Different rates of first admissions for psychosis in migrant groups in Paris. Soc Psychiatry Psychiatr Epidemiol. 2014;49(7):1103–1109. doi: 10.1007/s00127-013-0795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarricone I, Boydell J, Kokona A, Triolo F, Gamberini L, Sutti E, et al. Risk of psychosis and internal migration: results from the Bologna First Episode Psychosis study. Schizophr Res. 2016;173(1-2):90–93. doi: 10.1016/j.schres.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 73.Anderson KK, Cheng J, Susser E, McKenzie KJ, Kurdyak P. Incidence of psychotic disorders among first-generation immigrants and refugees in Ontario. CMAJ. 2015;187(9):E279–EE86. doi: 10.1503/cmaj.141420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hollander AC, Dal H, Lewis G, Magnusson C, Kirkbride JB, Dalman C. Refugee migration and risk of schizophrenia and other non-affective psychoses: cohort study of 1.3 million people in Sweden. BMJ. 2016;352:i1030. doi: 10.1136/bmj.i1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amad A, Guardia D, Salleron J, Thomas P, Roelandt JL, Vaiva G. Increased prevalence of psychotic disorders among third-generation migrants: results from the French Mental Health in General Population survey. Schizophr Res. 2013;147(1):193–195. doi: 10.1016/j.schres.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Tortelli A, Errazuriz A, Croudace T, Morgan C, Murray RM, Jones PB, et al. Schizophrenia and other psychotic disorders in Caribbean-born migrants and their descendants in England: systematic review and meta-analysis of incidence rates, 1950-2013. Soc Psychiatry Psychiatr Epidemiol. 2015;50(7):1039–1055. doi: 10.1007/s00127-015-1021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahy GE, Mallett R, Leff J, Bhugra D. First-contact incidence rate of schizophrenia on Barbados. Br J Psychiatry. 1999;175:28–33. doi: 10.1192/bjp.175.1.28. [DOI] [PubMed] [Google Scholar]
- 78.Selten JP, Hoek HW. Does misdiagnosis explain the schizophrenia epidemic among immigrants from developing countries to Western Europe? Soc Psychiatry Psychiatr Epidemiol. 2008;43(12):937–939. doi: 10.1007/s00127-008-0390-5. [DOI] [PubMed] [Google Scholar]
- 79.van der Ven E, Dalman C, Wicks S, Allebeck P, Magnusson C, van Os J, et al. Testing Odegaard’s selective migration hypothesis: a longitudinal cohort study of risk factors for non-affective psychotic disorders among prospective emigrants. Psychol Med. 2015;45(4):727–734. doi: 10.1017/S0033291714001780. [DOI] [PubMed] [Google Scholar]
- 80.Kirkbride JB, Barker D, Cowden F, Stamps R, Yang M, Jones PB, et al. Psychoses, ethnicity and socio-economic status. Br J Psychiatry. 2008;193(1):18–24. doi: 10.1192/bjp.bp.107.041566. [DOI] [PubMed] [Google Scholar]
- 81.Kirkbride JB, Hameed Y, Ioannidis K, Ankireddypalli G, Crane CM, Nasir M, et al. Ethnic minority status, age-at-immigration and psychosis risk in rural environments: evidence from the SEPEA study. Schizophr Bull. 2017;43(6):1251–1261. doi: 10.1093/schbul/sbx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophr Bull. 2010;36(4):655–664. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boydell J, van Os J, McKenzie K, Allardyce J, Goel R, McCreadie RG, et al. Incidence of schizophrenia in ethnic minorities in London: ecological study into interactions with environment. BMJ. 2001;323(7325):1336–1338. doi: 10.1136/bmj.323.7325.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veling W, Susser E, van Os J, Mackenbach JP, Selten JP, Hoek HW. Ethnic density of neighborhoods and incidence of psychotic disorders among immigrants. Am J Psychiatry. 2008;165(1):66–73. doi: 10.1176/appi.ajp.2007.07030423. [DOI] [PubMed] [Google Scholar]
- 85.Porter M, Haslam N. Predisplacement and postdisplacement factors associated with mental health of refugees and internally displaced persons: a meta-analysis. JAMA. 2005;294(5):602–612. doi: 10.1001/jama.294.5.602. [DOI] [PubMed] [Google Scholar]
- 86.McGrath JJ, Welham JL. Season of birth and schizophrenia: a systematic review and meta-analysis of data from the Southern Hemisphere. Schizophr Res. 1999;35(3):237–242. doi: 10.1016/s0920-9964(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 87.Dealberto MJ. Why are immigrants at increased risk for psychosis? Vitamin D insufficiency, epigenetic mechanisms, or both? Medical hypotheses. 2007;68(2):259–267. doi: 10.1016/j.mehy.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 88.Marcelis M, Navarro-Mateu F, Murray R, Selten JP, Van Os J. Urbanization and psychosis: a study of 1942-1978 birth cohorts in The Netherlands. Psychol Med. 1998;28(4):871–879. doi: 10.1017/s0033291798006898. [DOI] [PubMed] [Google Scholar]
- 89.Krabbendam L, van Os J. Schizophrenia and urbanicity: a major environmental influence-conditional on genetic risk. Schizophr Bull. 2005;31(4):795–799. doi: 10.1093/schbul/sbi060. [DOI] [PubMed] [Google Scholar]
- 90.van Os J, Hanssen M, Bijl RV, Vollebergh W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry. 2001;58(7):663–668. doi: 10.1001/archpsyc.58.7.663. [DOI] [PubMed] [Google Scholar]
- 91.van Os J, Pedersen CB, Mortensen PB. Confirmation of synergy between urbanicity and familial liability in the causation of psychosis. Am J Psychiatry. 2004;161(12):2312–2314. doi: 10.1176/appi.ajp.161.12.2312. [DOI] [PubMed] [Google Scholar]
- 92.Vassos E, Pedersen CB, Murray RM, Collier DA, Lewis CM. Meta-analysis of the association of urbanicity with schizophrenia. Schizophr Bull. 2012;38(6):1118–1123. doi: 10.1093/schbul/sbs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marcelis M, Takei N, van Os J. Urbanization and risk for schizophrenia: does the effect operate before or around the time of illness onset? Psychol Med. 1999;29(5):1197–1203. doi: 10.1017/s0033291799008983. [DOI] [PubMed] [Google Scholar]
- 94.Pedersen CB, Mortensen PB. Are the cause(s) responsible for urban-rural differences in schizophrenia risk rooted in families or in individuals? Am J Epidemiol. 2006;163(11):971–978. doi: 10.1093/aje/kwj169. [DOI] [PubMed] [Google Scholar]
- 95.March D, Hatch SL, Morgan C, Kirkbride JB, Bresnahan M, Fearon P, et al. Psychosis and place. Epidemiologic reviews. 2008;30:84–100. doi: 10.1093/epirev/mxn006. [DOI] [PubMed] [Google Scholar]
- 96.Westergaard T, Mortensen PB, Pedersen CB, Wohlfahrt J, Melbye M. Exposure to prenatal and childhood infections and the risk of schizophrenia: suggestions from a study of sibship characteristics and influenza prevalence. Arch Gen Psychiatry. 1999;56(11):993–998. doi: 10.1001/archpsyc.56.11.993. [DOI] [PubMed] [Google Scholar]
- 97.Eaton WW, Mortensen PB, Frydenberg M. Obstetric factors, urbanization and psychosis. Schizophr Res. 2000;43(2-3):117–123. doi: 10.1016/s0920-9964(99)00152-8. [DOI] [PubMed] [Google Scholar]
- 98.Harrison G, Fouskakis D, Rasmussen F, Tynelius P, Sipos A, Gunnell D. Association between psychotic disorder and urban place of birth is not mediated by obstetric complications or childhood socio-economic position: a cohort study. Psychol Med. 2003;33(4):723–731. doi: 10.1017/s0033291703007591. [DOI] [PubMed] [Google Scholar]
- 99.Torrey EF, Yolken RH. The urban risk and migration risk factors for schizophrenia: are cats the answer? Schizophr Res. 2014;159(2-3):299–302. doi: 10.1016/j.schres.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 100.Lewis G, David A, Andreasson S, Allebeck P. Schizophrenia and city life. Lancet. 1992;340(8812):137–140. doi: 10.1016/0140-6736(92)93213-7. [DOI] [PubMed] [Google Scholar]
- 101.Zammit S, Lewis G, Rasbash J, Dalman C, Gustafsson JE, Allebeck P. Individuals, schools, and neighborhood: a multilevel longitudinal study of variation in incidence of psychotic disorders. Arch Gen Psychiatry. 2010;67(9):914–922. doi: 10.1001/archgenpsychiatry.2010.101. [DOI] [PubMed] [Google Scholar]
- 102.Kirkbride JB, Jones PB, Ullrich S, Coid JW. Social deprivation, inequality, and the neighborhood-level incidence of psychotic syndromes in East London. Schizophr Bull. 2014;40(1):169–180. doi: 10.1093/schbul/sbs151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jongsma HE, Gayer-Anderson C, Lasalvia A, Quattrone D, Mule A, Szoke A, et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry. 2018;75(1):36–46. doi: 10.1001/jamapsychiatry.2017.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engemann K, Pedersen CB, Arge L, Tsirogiannis C, Mortensen PB, Svenning JC. Childhood exposure to green space-a novel risk-decreasing mechanism for schizophrenia? Schizophr Res. 2018;199:142–148. doi: 10.1016/j.schres.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 105.Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Patterns and predictors of substance use disorders and daily tobacco use in first-episode psychosis. Aust N Z J Psychiatry. 2005;39(10):892–898. doi: 10.1080/j.1440-1614.2005.01699.x. [DOI] [PubMed] [Google Scholar]
- 106.Barnett JH, Werners U, Secher SM, Hill KE, Brazil R, Masson K, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190:515–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 107.Mazzoncini R, Donoghue K, Hart J, Morgan C, Doody GA, Dazzan P, et al. Illicit substance use and its correlates in first episode psychosis. Acta Psychiatr Scand. 2010;121(5):351–358. doi: 10.1111/j.1600-0447.2009.01483.x. [DOI] [PubMed] [Google Scholar]
- 108.Sara GE, Large MM, Matheson SL, Burgess PM, Malhi GS, Whiteford HA, et al. Stimulant use disorders in people with psychosis: a meta-analysis of rate and factors affecting variation. Aust N Z J Psychiatry. 2015;49(2):106–117. doi: 10.1177/0004867414561526. [DOI] [PubMed] [Google Scholar]
- 109.Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Prevalence of alcohol use disorders in schizophrenia-a systematic review and meta-analysis. Acta Psychiatr Scand. 2009;120(2):85–96. doi: 10.1111/j.1600-0447.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 110.Abdel-Baki A, Ouellet-Plamondon C, Salvat E, Grar K, Potvin S. Symptomatic and functional outcomes of substance use disorder persistence 2 years after admission to a first-episode psychosis program. Psychiatry Res. 2017;247:113–119. doi: 10.1016/j.psychres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 111.Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. 2015;2(8):718–725. doi: 10.1016/S2215-0366(15)00152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. 2002;325(7374):1199. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156(4):319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- 115.Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2(3):233–238. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- 116.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42(5):1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195(6):488–491. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509–1517. doi: 10.1093/schbul/sbt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Di Forti M, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. 2019;6(5):427–436. doi: 10.1016/S2215-0366(19)30048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Large Matthew, Sharma Swapnil, Compton Michael T., Slade Tim, Nielssen Olav. Cannabis Use and Earlier Onset of Psychosis. Archives of General Psychiatry. 2011;68(6):555. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 121.Galvez-Buccollini JA, Proal AC, Tomaselli V, Trachtenberg M, Coconcea C, Chun J, et al. Association between age at onset of psychosis and age at onset of cannabis use in non-affective psychosis. Schizophr Res. 2012;139(1-3):157–160. doi: 10.1016/j.schres.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alvarez-Jimenez M, Priede A, Hetrick SE, Bendall S, Killackey E, Parker AG, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1-3):116–128. doi: 10.1016/j.schres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 123.Seddon JL, Birchwood M, Copello A, Everard L, Jones PB, Fowler D, et al. Cannabis use is associated with increased psychotic symptoms and poorer psychosocial functioning in first-episode psychosis: a report from the UK National EDEN study. Schizophr Bull. 2016;42(3):619–625. doi: 10.1093/schbul/sbv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, et al. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3(3):215–225. doi: 10.1016/S2215-0366(15)00363-6. [DOI] [PubMed] [Google Scholar]
- 125.Sorensen HJ, Mortensen EL, Schiffman J, Reinisch JM, Maeda J, Mednick SA. Early developmental milestones and risk of schizophrenia: a 45-year follow-up of the Copenhagen Perinatal Cohort. Schizophr Res. 2010;118(1-3):41–47. doi: 10.1016/j.schres.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 127.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Agnew-Blais J, Seidman LJ, Fitzmaurice GM, Smoller JW, Goldstein JM, Buka SL. The interplay of childhood behavior problems and IQ in the development of later schizophrenia and affective psychoses. Schizophr Res. 2017;184:45–51. doi: 10.1016/j.schres.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van der Werf M, Thewissen V, Dominguez MD, Lieb R, Wittchen H, van Os J. Adolescent development of psychosis as an outcome of hearing impairment: a 10-year longitudinal study. Psychol Med. 2011;41(3):477–485. doi: 10.1017/S0033291710000978. [DOI] [PubMed] [Google Scholar]
- 130.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 131.Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry. 2002;59(5):449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 132.Ferraro L, Russo M, O’Connor J, Wiffen BD, Falcone MA, Sideli L, et al. Cannabis users have higher premorbid IQ than other patients with first onset psychosis. Schizophr Res. 2013;150(1):129–135. doi: 10.1016/j.schres.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 133.Stilo SA, Murray RM. The epidemiology of schizophrenia: replacing dogma with knowledge. Dialogues Clin Neurosci. 2010;12(3):305–315. doi: 10.31887/DCNS.2010.12.3/sstilo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57(10):1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 135.Colizzi M, Iyegbe C, Powell J, Ursini G, Porcelli A, Bonvino A, et al. Interaction between functional genetic variation of DRD2 and cannabis use on risk of psychosis. Schizophr Bull. 2015;41(5):1171–1182. doi: 10.1093/schbul/sbv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Winkel R, van Beveren NJ, Simons C, Genetic R. Outcome of Psychosis I. AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology. 2011;36(12):2529–2537. doi: 10.1038/npp.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry. 2012;72(10):811–816. doi: 10.1016/j.biopsych.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 138.Trotta A, Iyegbe C, Di Forti M, Sham PC, Campbell DD, Cherny SS, et al. Interplay between schizophrenia polygenic risk score and childhood adversity in first-presentation psychotic disorder: a pilot study. PloS one. 2016;11(9):e0163319. doi: 10.1371/journal.pone.0163319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ursini G, Punzi G, Chen Q, Marenco S, Robinson JF, Porcelli A, et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat Med. 2018;24(6):792–801. doi: 10.1038/s41591-018-0021-y. [DOI] [PubMed] [Google Scholar]
- 140.Guloksuz S, Pries LK, Delespaul P, Kenis G, Luykx JJ, Lin BD, et al. Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry. 2019;18(2):173–182. doi: 10.1002/wps.20629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cougnard A, Marcelis M, Myin-Germeys I, De Graaf R, Vollebergh W, Krabbendam L, et al. Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness-persistence model. Psychol Med. 2007;37(4):513–527. doi: 10.1017/S0033291706009731. [DOI] [PubMed] [Google Scholar]
- 142.Stepniak B, Papiol S, Hammer C, Ramin A, Everts S, Hennig L, et al. Accumulated environmental risk determining age at schizophrenia onset: a deep phenotyping-based study. Lancet Psychiatry. 2014;1(6):444–453. doi: 10.1016/S2215-0366(14)70379-7. [DOI] [PubMed] [Google Scholar]
- 143.Padmanabhan JL, Shah JL, Tandon N, Keshavan MS. The “polyenviromic risk score”: aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophr Res. 2017;181:17–22. doi: 10.1016/j.schres.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383(9929):1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 146.Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological review. 1997;104(4):667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 147.Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D’Albenzio A, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116(2-3):234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410–417. doi: 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71(6):561–567. doi: 10.1016/j.biopsych.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 150.Egerton A, Howes OD, Houle S, McKenzie K, Valmaggia LR, Bagby MR, et al. Elevated striatal dopamine function in immigrants and their children: a risk mechanism for psychosis. Schizophr Bull. 2017;43(2):293–301. doi: 10.1093/schbul/sbw181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gevonden M, Booij J, van den Brink W, Heijtel D, van Os J, Selten JP. Increased release of dopamine in the striata of young adults with hearing impairment and its relevance for the social defeat hypothesis of schizophrenia. JAMA Psychiatry. 2014;71(12):1364–1372. doi: 10.1001/jamapsychiatry.2014.1325. [DOI] [PubMed] [Google Scholar]
- 152.Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology (Berl) 2014;231(12):2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- 155.Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Delta(9)-tetrahydrocannabinol on the dopamine system. Nature. 2016;539(7629):369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Froudist-Walsh S, Bloomfield MA, Veronese M, Kroll J, Karolis VR, Jauhar S, et al. The effect of perinatal brain injury on dopaminergic function and hippocampal volume in adult life. Elife. 2017;6. [DOI] [PMC free article] [PubMed]
- 157.Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79(2):565–575. doi: 10.1111/j.1476-5381.1983.tb11031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26(4-6):365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- 159.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29(2):97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(22):8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McGuire P, Howes OD, Stone J, Fusar-Poli P. Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci. 2008;29(2):91–98. doi: 10.1016/j.tips.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 162.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews Neuroscience. 2011;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Murray RM, Paparelli A, Morrison PD, Marconi A, Di Forti M. What can we learn about schizophrenia from studying the human model, drug-induced psychosis? Am J Med Genet B Neuropsychiatr Genet. 2013;162B(7):661–670. doi: 10.1002/ajmg.b.32177. [DOI] [PubMed] [Google Scholar]
- 164.Fernandez-Espejo E, Viveros MP, Nunez L, Ellenbroek BA, Rodriguez de Fonseca F. Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology (Berl). 2009;206(4):531–549. doi: 10.1007/s00213-009-1612-6. [DOI] [PubMed] [Google Scholar]
- 165.Desfosses J, Stip E, Bentaleb LA, Lipp O, Chiasson JP, Furtos A, et al. Plasma endocannabinoid alterations in individuals with substance use disorder are dependent on the “Mirror Effect” of schizophrenia. Front Psychiatry. 2012;3:85. doi: 10.3389/fpsyt.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30(3):508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- 167.Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54(1):108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 169.Carter CS, Bullmore ET, Harrison P. Is there a flame in the brain in psychosis? Biol Psychiatry. 2014;75(4):258–259. doi: 10.1016/j.biopsych.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 170.Radhakrishnan R, Kaser M, Guloksuz S. The link between the immune system, environment, and psychosis. Schizophr Bull. 2017;43(4):693–697. doi: 10.1093/schbul/sbx057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21(12):1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Danese A, S JL Psychoneuroimmunology of early-life stress: the hidden wounds of childhood trauma? Neuropsychopharmacology. 2017;42(1):99–114. doi: 10.1038/npp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry. 2016;21(5):642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. Am J Psychiatry. 2014;171(2):218–226. doi: 10.1176/appi.ajp.2013.13010086. [DOI] [PubMed] [Google Scholar]
- 175.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neiderhiser JM, Reiss D, Pedersen NL, Lichtenstein P, Spotts EL, Hansson K, et al. Genetic and environmental influences on mothering of adolescents: a comparison of two samples. Dev Psychol. 2004;40(3):335–351. doi: 10.1037/0012-1649.40.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alghamdi W, Stamate D, Stahl D, Zamyatin A, Murray R, Di Forti M. A new machine learning framework for understanding the link between cannabis use and first-episode psychosis. Stud Health Technol Inform. 2018;248:9–16. [PubMed] [Google Scholar]