Abstract
Background
The Bladder Cancer Index (BCI) and Functional Assessment of Cancer Therapy-Bladder-Cystectomy (FACT-Bl-Cys) were developed to measure disease-specific health-related quality of life (HRQOL) in bladder cancer patients and patients treated with radical cystectomy, respectively. Both patient-reported outcome measures (PROMs) are frequently used in clinical practice, but are not yet validated according to the COSMIN criteria and not yet available in Dutch. Therefore, the aim of this study was to translate the BCI and FACT-Bl-Cys into Dutch and to evaluate their measurement properties according to the COSMIN criteria.
Methods
The BCI and FACT-Bl-Cys were translated into Dutch using a forward-backward method, and subsequently administered at baseline (pre-operatively) and 3 months post-operatively in bladder cancer patients who received a radical cystectomy. Validity (content and construct), reliability (internal consistency, test-retest reliability, and measurement error), floor and ceiling effects, and responsiveness were assessed according to the COSMIN criteria.
Results
Forward-backward translation encountered no particular linguistic problems. In total 260 patients completed the baseline measurement, while 182 patients completed the three-month measurement. Only a ceiling effect was identified for the BCI. Hypotheses testing for construct validity was satisfying, as 67% and 92% of the hypothesized correlations were confirmed. Structural validity was moderate for both measures, as confirmatory factor analyses showed limited fit. Reliability of both PROMs was good. The intraclass correlation coefficient (ICC) of the BCI domains ranged from 0.47 to 0.93, minimal value of Cronbach’s α was 0.70, smallest detectable change on group level (SDC group) ranged from 1.9 to 8.6. The ICC of the FACT-Bl-Cys domains ranged from 0.43 to 0.83, minimal value of Cronbach’s α was 0.77, SDC group was around 1. Only the FACT-Bl-Cys total score was found to be responsive to changes in generic quality of life.
Conclusions
The Dutch versions of the BCI and FACT-Bl-Cys were shown to be reliable and have good content validity. Structural validity was limited for both measures. Only the FACT-Bl-Cys total score was responsive to changes in generic HRQOL. Despite some limitations, both PROMs seem suitable for use in clinical practice and research.
Electronic supplementary material
The online version of this article (10.1186/s41687-019-0149-7) contains supplementary material, which is available to authorized users.
Keywords: Bladder cancer, Radical cystectomy, Patient-reported outcomes measures, Psychometrics, Validity, Reliability, Responsiveness
Introduction
Bladder cancer (BCa) ranks ninth in worldwide cancer incidence and is one of the most expensive malignancies to manage [1, 2]. The spectrum of BCa includes non-muscle-invasive, muscle-invasive, and metastatic disease, each with its own clinical behavior, prognosis, and treatment.
Due to the heterogeneous BCa population, the various treatments (e.g. transurethral surgery, chemotherapy, radical cystectomy), and the lack of long-term follow-up data, not much is known regarding the patient burden imposed by BCa [3–5]. Since BCa patients will usually undergo several treatments, measuring health-related quality of life (HRQOL) with valid instruments is important for clinicians and patients when making informed decisions about treatments, based on patients’ experiences [6, 7]. Increasingly more clinical trials [8] and comparative effectiveness studies [9] include patient-reported outcomes (PROs), in addition to objective outcomes.
In 2016, a systematic review was published in which Danna et al. [10] advised to use the Functional Assessment of Cancer Therapy-Bladder-Cystectomy (FACT-Bl-Cys, formerly known as the FACT-VCI) [11, 12] when studying HRQOL in patients undergoing radical cystectomy and to use the Bladder Cancer Index (BCI) [13, 14] when studying HRQOL in patients with nonmuscle-invasive or muscle-invasive BCa disease of any stage. Authors report that the FACT-Bl-Cys was the only available measure for muscle-invasive BCa patients and the BCI has unique “bother” scales, which quantifies the symptoms’ impact [10]. Both patient-reported outcomes measures (PROMs) are frequently used and have been translated into various languages (i.e. BCI is available in Spanish, French, Hungarian [15–17], and FACT-Bl-Cys is available in Korean and Swedish [18, 19]), but were not yet available in Dutch. In 2018, Mason et al. revealed in a systematic review that most PROMs related to BCa had limited information reported on their measurement properties [4]. The FACT-Bl-Cys and BCI are suggested to be most promising, but some measurement properties (e.g. content validity, measurement error, and responsiveness) are still unknown [4].
To assess whether both PROMs provide valuable information in clinical practice and research, it is warranted to study their measurement properties. The COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist was developed to assess the quality of validation studies and can be used to design and report on these studies [20–22]. The aim of this study was to translate the BCI and FACT-Bl-Cys into Dutch and to evaluate the validity, reliability, floor and ceiling effects, and responsiveness according to the COSMIN criteria.
Methods
Translation procedure
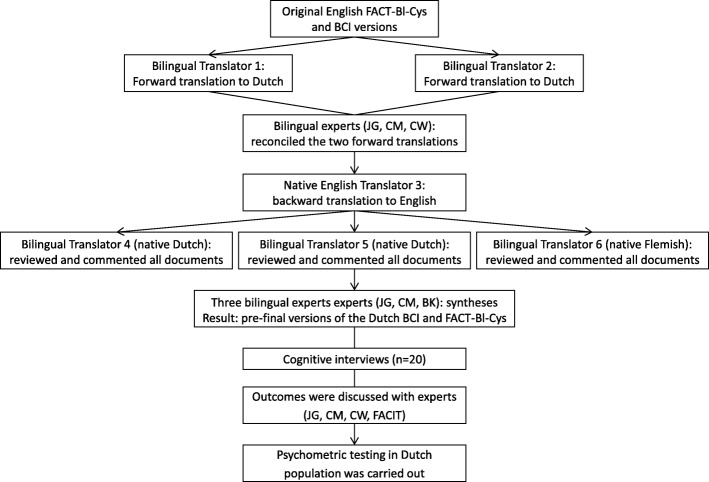
The BCI and FACT-Bl-Cys were translated into Dutch, using a forward–backward method according to published guidelines, involving six steps [23, 24]. A schematic overview of the translation procedure is presented in Fig. 1. First, two bilingual native Dutch translators independently translated the United States English versions of the BCI and FACT-Bl-Cys into Dutch (forward translation). Second, two independent native Dutch speakers (JG, CM) reconciled the two forward translations into one forward translation. Any uncertainties were discussed among JG, CM and a urologist (CW), and resolved by consensus. Third, one independent native English translator, who is fluent in Dutch, translated the forward translation back to English. The translator was uninformed of the concepts explored and blinded to the original instruments. Fourth, three independent bilingual experts (two native Dutch and one native Flemish speaker, all fluent in English) reviewed and commented on all documents (original version, forward and backward translations). The synthesis process was carefully documented and outcomes were evaluated by three Dutch speakers (a language coordinator, JG, CM). Conceptual rather than literal translation was leading, aiming to preserve the original meaning of each item. This resulted in pre-final versions of the Dutch BCI and FACT-Bl-Cys. Fifth, both pre-final versions were tested during a pilot study, in which cognitive interviews were carried out among 20 BCa patients who underwent a radical cystectomy. Patients completed both pre-final versions and were interviewed about the comprehension of items and the chosen response, according to Functional Assessment of Chronic Illness Therapy (FACIT) guideline and instructions [24]. Patients were randomly recruited from Radboud university medical center (Nijmegen, the Netherlands) and Rijnstate Hospital (Arnhem, the Netherlands). Finally, experts (JG, CM, CW, and FACIT representatives) discussed the outcomes and final versions were realized to enable the evaluation of their psychometrics.
Fig. 1.
Translation procedure
Study design and sample
As part of the RACE study (trial identifying number NTR5362, Dutch Trial Registry, www.trialregister.nl) patients were asked to complete four measures (i.e. BCI, FACT-Bl-Cys, EQ-5D-5 L, and EQ-VAS). Patients that completed the three-month follow-up period were included in this substudy. RACE is a comparative effectiveness study aiming to determine the (cost-)effectiveness of robot-assisted and open radical cystectomy [25]. Radical cystectomy is the standard treatment of non-metastatic, invasive BCa and is curative in the majority of patients with localized disease [1]. This surgery is associated with a high complication rate, varying between 49% and 68% [26]. Studies suggest that radical cystectomy affects urinary, sexual and bowel function, and body image which can lead to anxiety and depression [27–30].
All participants in this validation study fulfilled the inclusion criteria of the RACE study: they were 18 years or older, had an oncological indication for radical cystectomy, a histologically proven primary muscle invasive urothelial carcinoma or therapy resistant high-risk non muscle-invasive BCa (CIS, refractair pTa-1), a non-metastatic tumor (cT1a-cT4a, cN0M0), they were able to complete Dutch questionnaires, and provided written informed consent. Patients were excluded from RACE when they met one of the following criteria: previous major abdominal surgery (i.e. existing stomata, low anterior resection of the rectum or rectal amputation, status after open aortabifemoral graft, status after right hemicolectomy), pregnancy, morbid obesity (BMI ≥ 40 kg/m2), radical cystectomy performed in combination with a nephrectomy or a partial colon resection.
Measures used in this validation study were the translated Dutch versions of the BCI and FACT-Bl-Cys, and the validated Dutch versions of the EuroQol 5-Domain 5-Level (EQ-5D-5 L) [31] and the EuroQol Visual Analogue Scale (EQ-VAS). Participants could choose to complete the PROMs on paper or electronically. Participants completed the PROMs at baseline (T0, pre-operatively) and 3 months post-operatively (T3). Patients who completed the three-month measurement (T3) were asked to complete the PROMs again within 2 weeks (T3 retest).
Measures
Bladder Cancer index (BCI)
The BCI is developed to measure disease-specific HRQOL in patients with nonmuscle-invasive and muscle-invasive BCa disease of any stage. It consists of 36 items covering three domains: urinary (14 items), bowel (10 items), and sexual (12 items) [13, 14]. Each domain consists of two subdomains (function and bother). Item responses are based on 4- or 5-point Likert scales; domain and subdomain scores are standardized to a 0–100 point scale where higher scores indicate better HRQOL. No total BCI score is calculated. Function items focus on the frequency of the disease symptoms and bother items reflect the individual perception of these symptoms. According to BCI scoring instructions, the number of non-missing items needed to compute domain scores varied from 10 (urinary domain and sexual domain) to 4 (“urinary function”, “bowel function”, “sexual bother”), otherwise scores were set to missing.
Functional assessment of Cancer therapy-bladder-cystectomy (FACT-Bl-Cys)
The FACT-Bl-Cys is developed to measure condition-specific HRQOL in patients treated with radical cystectomy. It consists of the four general FACT-General (FACT-G) [32] domains (physical, social/family, emotional, and functional well-being) plus one additional Bl-Cys domain (17 items) covering urinary, sexual and bowel function, and body image [11, 12]. Thus, the FACT-Bl-Cys measure consists of five domains (physical, social/family, emotional, functional well-being, and Bl-Cys). The measure consists of 44 items and responses are based on 5-point Likert scales, with higher scores indicating better HRQOL. The total FACT-Bl-Cys score can range from 0 to 168. According to FACT-Bl-Cys scoring instructions, more than 50% of the items per domain need to be answered in order to calculate a domain score.
Statistical analyses
COSMIN recommendations were used as a guide for evaluating the measurement properties of the Dutch BCI and FACT-Bl-Cys [22]. Validity, reliability, floor and ceiling effects, and responsiveness were assessed as described below. Analyses were performed using IBM SPSS Statistics software (version 22.0, IBM Corp., Armonk, NY, USA). Additionally, R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria), including the packages mice (version 3.5.0) [33] and SemTools (version 0.5–1.928) [34] was used for multiple imputation and confirmatory factor analyses (CFA).
Because the BCI is developed for generic BCa patients, we determined its measurement properties at baseline (T0, pre-operatively), with the exception of test-retest and measurement error which were based on T3 and T3 retest. The test-retest and measurement error could not be assessed at T0, because there was no possibility to distribute and complete the PROMs again within two weeks since patients received a cystectomy on short term after T0. Because the FACT-Bl-Cys is developed for patients treated with radical cystectomy, we determined its measurement properties after the cystectomy had been performed, i.e. at T3 and T3 retest.
Floor and ceiling effects
Floor and ceiling effects are considered to be present if more than 15% of respondents achieved the lowest or highest possible score, respectively [21, 35]. We evaluated the presence of floor and ceiling effects of single scores in this study to assess interpretability. Interpretability [20] is the degree to which one can assign a qualitative meaning on a quantitative score or change in score. Additionally, floor and ceiling effects can also impact the responsiveness (if scores cannot further improve or deteriorate) and validity of an instrument (as patients with the lowest or highest possible score cannot be distinguished from each other).
Validity
Validity in this study was assessed by content validity and construct validity [20]. Content validity was evaluated from the perspective of researchers, patients, and urologists. First, the component of content validity known as face validity was determined by discussing with the research team whether the two measures gave the impression of adequately reflecting the construct to be measured. Second, content validity was evaluated during the above described cognitive interviews (n = 20). An interview guide was used for the interviews and respondents were invited to appraise the two measurement instruments on relevance, comprehensiveness, and comprehensibility [36]. Input from the interviews was summarized and notable similarities and differences in opinion among respondents were registered and closely evaluated in our research team.
Construct validity was evaluated by structural validity and hypotheses testing. First, structural validity was assessed by confirmatory factor analyses (CFA) to confirm the previously suggested dimensionality of both PROMs [37]. Regarding BCI, the original validation study [13] identified three primary domains (urinary, bowel and sexual) and one translation study [16] reported that data fitted to six subdomains (function and bother). Therefore, in this study BCI items were hypothesized to load on three or six factors. Regarding FACT-Bl-Cys, the original validation study [11] identified three factors in the Bl-Cys domain and one translation study [18] reported that the Bl-Cys domain fitted on three other factors. Therefore, the FACT-G (four domains: physical, social/family, emotional, functional well-being) was hypothesized to load on four factors and the individual Bl-Cys domain on two different three-factor structures. Which items are structured within which factor is presented in Table 4.
Table 4.
Structural validity properties of the BCI and FACT-Bl-Cys
| CFI | RMSEA | SRMR | TLI | |
|---|---|---|---|---|
| T0 BCI | ||||
| Three-factor structure (Gilbert et al. [13]) | 0.69 | 0.09 | 0.09 | 0.67 |
| 1. Urinary domain (12 items) | ||||
| 2. Bowel domain (10 items) | ||||
| 3. Sexual domain (12 items) | ||||
| Six-factor structure (Hever et al. [16]) | 0.88 | 0.06 | 0.06 | 0.87 |
| 1. Urinary function (4 items) | ||||
| 2. Urinary bother (8 items) | ||||
| 3. Bowel function (4 items) | ||||
| 4. Bowel bother (6 items) | ||||
| 5. Sexual function (7 items) | ||||
| 6. Sexual bother (5 items) | ||||
| T3 FACT-Bl-Cys | ||||
| FACT-G in four-factor structure (27 items) | 0.88 | 0.05 | 0.09 | 0.86 |
| 1. PWB Physical well-being domain (7 items) | ||||
| 2. SWB Social/family well-being domain (7 items) | ||||
| 3. EWB Emotional well-being domain (6 items) | ||||
| 4. FWB Functional well-being domain (7 items) | ||||
| Bl-Cys domain in three-factor structure (Anderson et al. [11]) | 0.75 | 0.07 | 0.11 | 0.70 |
| 1. C2 Losing weight; C3 Bowel control; C4 Diarrhea; C6 Good appetite; ITU6 Embarrassed my condition; ITU1 Comfortable discussing with friends (6 items) | ||||
| 2. C7 Content with appearance; ITU3 Limit social interactions; ITU4 Limit physical activity; ITU5 Limit sexual activity (4 items) | ||||
| 3. Bl1 Trouble controlling urine; ITU7 Condition wakes me up at night; C9 Caring for condition difficult; VC1 Satisfied with urinary condition; ITU2 I am afraid to be far from a toilet (5 items) | ||||
| Bl-Cys domain in three-factor structure (Kim et al. [18]) | 0.85 | 0.06 | 0.09 | 0.81 |
| 1. C2 Losing weight; C4 Diarrhea; Bl1 Trouble controlling urine; ITU7 Condition wakes me up at night; ITU6 Embarrassed my condition; C9 Caring for condition difficult; ITU2 I am afraid to be far from a toilet (7 items) | ||||
| 2. C3 Bowel control; C6 Good appetite; C7 Content with appearance; ITU1 Comfortable discussing with friends; VC1 Satisfied with urinary condition (5 items) | ||||
| 3. ITU3 Limit social interactions; ITU4 Limit physical activity; ITU5 Limit sexual activity (3 items) | ||||
The CFA was performed using imputed data. CFI comparative fit index, RMSEA root mean square error of approximation; SRMR standardized root mean square residual, TLI Tucker-Lewis index, The criteria for unidimensionality include CFI ≥ 0.95, TLI ≥ 0.95, RMSEA≤0.06, and SRMR≤0.08
For a CFA, COSMIN advises to use a sample size of 7 patients per item, with a minimum number of 100 patients [38]. Since our sample size was too small according to the COSMIN criteria as cases with missing items are not analyzed in a CFA (i.e. listwise deletion), we performed multiple imputation by chained equations generating 25 independent imputation datasets. Data were imputed at item level using a predictive mean matching approach. CFA was performed on the imputed datasets. Model fit was evaluated based on the Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Root Means Square Error of Approximation (RMSEA), and Standardized Root Mean Square Residual (SRMR). The criteria for unidimensionality include CFI ≥ 0.95, TLI ≥ 0.95, RMSEA≤0.06, and SRMR≤0.08 [39].
Second, we tested various hypotheses by analyzing the association between the BCI domains and FACT-Bl-Cys domains, using Pearson’s correlation coefficients. Coefficients were considered low (< 0.30), moderate (0.30–0.69) or high (≥0.70). If ≥75% of the set number of hypotheses were confirmed, the construct validity was considered good [21]. Pre-specified hypotheses regarding BCI at T0 were that: a) correlations among different 3 domains (urinary, bowel, sexual) are low as they measure different constructs; b) correlations between the BCI domains and FACT-G, EQ-5D-5 L, EQ-VAS are moderate, due to differences between generic and disease-specific instruments. Pre-specified hypotheses regarding FACT-Bl-Cys at T3 were that: a) correlations among 5 different domains (physical, social/family, emotional, functional, Bl-Cys) are moderate based on original validation studies [11, 40]; b) correlation between the Bl-Cys domain and EQ-5D-5 L is moderate; c) correlation between FACT-Bl-Cys total score and the EQ-5D-5 L and EQ-VAS is moderate, due to differences between generic and disease-specific instruments. For the BCI and FACT-Bl-Cys there were 12 and 13 pre-specified hypotheses formulated, respectively.
Reliability
Reliability was assessed by analyzing internal consistency, test-retest reliability, and measurement error [20].
Internal consistency [20] of subscale items was measured using Cronbach’s α, to assess the degree of interrelatedness among the items of (sub) scales of the instrument. Values between 0.70 and 0.95 are considered adequate [21, 41].
Test-retest reliability [20] was assessed by calculating the intraclass correlation coefficient (ICC), using a two-way random effects model to compute absolute agreement (single measures) [21, 42]. Values ≥0.70 are considered to be reliable [21]. Patients who completed the three-month measure were assumed to be in a stable physical state and were asked to complete the PROMs again after 2 weeks.
Measurement error [20] was assessed by calculating the standard error of measurement (SEM) using the formula: √(σ2o + σ2residual) [22, 43]. Additionally, the smallest detectable change on group level (SDC group) was calculated from the SDC at individual level (SDCindividual = 1.96*√2*SEM), using the formula: SDCindividual/√n [44].
Responsiveness
Responsiveness should be determined in a population that shows actual change [20]. First, we assessed the change scores of both measures between T0 and T3. Second, we evaluated the occurrence of complications (defined as grade 1 to 5 according to the Clavien-Dindo classification) in relation to the change scores of both measures between T0 and T3. Third, we assessed the correlation and effect size of change score of both measures with the change score of the EQ-VAS between T0 and T3. We used the EQ-VAS score as this might cover a broader underlying concept of overall health than the EQ-5D-5 L, which seems applicable for this population considering the heterogeneous and multidimensional character of BCa disease. If there is an actual change in HRQOL, we assumed that the EQ-VAS score shows this change. We constructed hypotheses about the change scores between T0 and T3 of the BCI and FACT-Bl-Cys, in comparison to the change scores of the EQ-VAS. We expected that patients with an improved or decreased EQ-VAS score (i.e. improvement or deterioration of ≥10 points), the BCI and FACT-Bl-Cys values also increased or declined, respectively. Previous studies in other patient populations reported a minimal important change (MIC) varying from 6.9 to 8.9 [45–48]. Based on these MIC values we assumed that an EQ-VAS change of 10 points might indicate actual change. Pre-specified hypotheses regarding the correlations and effect sizes between changes were that in patients with an improved or declined EQ-VAS score (i.e. plus or minus ≥10 points), we expected a) to find moderate correlations of ≥0.30 between change in EQ-VAS and the change in BCI and FACT-Bl-Cys values; b) to find a larger effect size, in comparison to patients that did not show a change in EQ-VAS (i.e. plus or minus ≤5 points). We analyzed the effect size between the changes by calculating Cohens d, using the formula: (T3mean-T0mean)/T0SD.
Results
Translation procedure
Conceptual rather than literal translation was leading to preserve the original meaning of each item. There was agreement between the forward and backward translations, and no cultural adaptions regarding translation were made. Based on the cognitive interviews, two items regarding the interpretation and meaning were discussed with the authors (JG, CM, CW) and FACIT representatives. First, we added one additional phrase “for men only” (in Dutch: “alleen voor mannen”) to the beginning of FACT-Bl-Cys item Bl5 “I am able to maintain an erection”, as this item is specifically aimed at men. According to FACIT scoring instructions Bl5 was not included in the scoring algorithm of the scale. Therefore, adapting this item could not influence the total score. Second, the BCI items where patients are asked about their “urine loss” could be interpreted according to their own specific situation. In case of a neobladder, stoma, or own bladder questions about “urine loss” could be answered as problems with catheterizing incontinence, emptying the stoma bag or leakage from the stoma bag, or urination, respectively.
Patient population
In total 260 patients completed the baseline measure (T0), followed by 182 patients who completed the three-month measure post-operatively (T3). The T3 evaluation was completed at a mean of 106 days (SD:14 days). Sixty-two patients participated in the test–retest measurement (T3 retest). The T3 retest was completed at a mean of 14 days (SD:6 days) after T3. In Table 1 the characteristics of the study population are shown. Of the 260 patients who completed T0, the mean age was 67 years (SD:10 years), 207 (80%) were male, 177 (68%) were married, and 149 (57%) were retired. These characteristics were not notably different at T3. We did not find any major change scores between T0 and T3 for both measures, as presented in Table 2.
Table 1.
Patient characteristics of the test and re-test groups
| Baseline (T0, pre-operatively) | 3 months (T3, post-operatively) | Test-retest (T3 retest)a | |
|---|---|---|---|
| Population size (n) | 260 | 182 | 62 |
| Age (years) | |||
| Mean ± SD | 67 ± 10 | 67 ± 9 | 67 ± 8 |
| Median (min-max) | 68 (35–88) | 68 (38–82) | 67 (40–82) |
| Gender, n (%) | |||
| Women | 53 (20%) | 37 (20%) | 14 (23%) |
| Men | 207 (80%) | 145 (80%) | 48 (77%) |
| Type of surgery, n (%) | |||
| RARC | 142 (55%) | 99 (54%) | 32 (52%) |
| ORC | 112 (43%) | 78 (43%) | 30 (48%) |
| LRC | 6 (2%) | 5 (3%) | 0 (0%) |
| Diversion type, n (%) | |||
| Own bladder | 233 (90%) | 0 (0%) | 0 (0%) |
| Ileal conduit | 11 (4%) | 114 (63%) | 45 (72%) |
| Neobladder | 1 (0%) | 33 (18%) | 11 (18%) |
| Other | 3 (1%) | 31 (17%) | 6 (10%) |
| Missing | 12 (5%) | 4 (2%) | 0 (0%) |
| Preoperative chemotherapy (yes), n (%) | 38 (15%) | 28 (15%) | 8 (13%) |
| Education, n (%) | |||
| Elementary school or less | 22 (8%) | 17 (9%) | 3 (5%) |
| Secondary education | 94 (36%) | 67 (37%) | 20 (32%) |
| Postsecondary education | 116 (45%) | 87 (48%) | 36 (58%) |
| Missing | 28 (11%) | 11 (6%) | 3 (5%) |
| Living status, n (%) | |||
| Married | 177 (68%) | 136 (75%) | 51 (82%) |
| Other | 83 (32%) | 46 (25%) | 11 (18%) |
| Employment status, n (%) | |||
| Pension | 149 (57%) | 108 (59%) | 36 (58%) |
| Salaried employment | 61 (24%) | 44 (25%) | 19 (31%) |
| Other | 23 (9%) | 19 (10%) | 4 (6%) |
| Missing | 27 (10%) | 11 (6%) | 3 (5%) |
aTwo weeks after T3. RARC robot-assisted radical cystectomy, ORC open radical cystectomy, LRC laparoscopic-assisted radical cystectomy
Table 2.
Change in scores between T0 (baseline) and T3 (90 days post-operative) of the measures
| Domains | No. of items per domain | T0 Valid population (n)a | T0 Mean (SD) | T0 Median (min-max) | T3 Valid population (n) a | T3 Mean (SD) | T3 Median (min-max) | T0-T3 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Valid population (n) | Mean change (SD) | Min-max | SE | ||||||||
| BCI | |||||||||||
| Urinary domain | 12 | 223 | 81.6 (18.2) | 86.4 (7–100) | 158 | 82.9 (17.6) | 89.6 (25–100) | 145 | 1.7 (21.5) | −55; 60 | 1.8 |
| Function | 4 | 242 | 84.8 (25.1) | 100.0 (0–100) | 144 | 74.8 (33.2) | 100.0 (0–100) | 137 | −11.0 (39.6) | −100; 100 | 3.4 |
| Bother | 8 | 212 | 79.1 (20.7) | 84.4 (11–100) | 159 | 86.6 (13.0) | 90.6 (47–100) | 136 | 7.6 (20.4) | −41; 75 | 1.7 |
| Bowel domain | 10 | 239 | 86.6 (14.4) | 91.7 (34–100) | 170 | 81.4 (16.6) | 86.7 (18–100) | 160 | −5.4 (18.9) | −79; 58 | 1.5 |
| Function | 4 | 234 | 86.3 (15.5) | 91.8 (15–100) | 163 | 83.8 (17.1) | 87.5 (6–100) | 152 | −2.5 (20.3) | −79; 65 | 1.7 |
| Bother | 6 | 239 | 86.7 (17.0) | 95.8 (25–100) | 196 | 80.0 (18.5) | 83.3 (21–100) | 159 | −6.5 (21.3) | −79; 63 | 1.7 |
| Sexual domain | 12 | 157 | 49.1 (19.6) | 51.4 (9–85) | 64 | 37.1 (17.7) | 37.4 (3–75) | 52 | −15.6 (19.8) | − 62; 18 | 2.8 |
| Function | 7 | 148 | 33.0 (19.3) | 31.3 (0–82) | 52 | 22.7 (16.6) | 25.0 (0–61) | 42 | −14.2 (18.6) | −52; 18 | 2.9 |
| Bother | 5 | 200 | 67.2 (30.7) | 75.0 (0–100) | 130 | 52.0 (31.0) | 40.0 (0–100) | 116 | −13.5 (37.8) | −100; 100 | 3.5 |
| FACT-Bl-Cys | |||||||||||
| FACT-PWB | 7 | 254 | 23.5 (4.6) | 25.0 (7–28) | 180 | 24.4 (3.6) | 25.0 (8–28) | 177 | 0.5 (4.5) | −16; 18 | 0.34 |
| FACT-SWB | 7 | 255 | 22.0 (4.4) | 22.2 (0–28) | 177 | 21.3 (4.5) | 22.0 (0–28) | 175 | −1.0 (4.6) | −24; 22 | 0.35 |
| FACT-EWB | 6 | 253 | 17.8 (4.8) | 19.0 (0–24) | 173 | 20.4 (3.5) | 21.0 (5–24) | 167 | 2.8 (4.1) | −8; 23 | 0.32 |
| FACT-FWB | 7 | 257 | 17.7 (5.8) | 18.0 (0–28) | 175 | 19.0 (4.9) | 20.0 (6–28) | 172 | 1.1 (5.0) | −14; 20 | 0.38 |
| Bl-Cys domain | 15 | 257 | 40.7 (10.0) | 41.8 (9–60) | 175 | 43.9 (8.0) | 45.0 (11–58) | 172 | 2.5 (10.3) | −34; 28 | 0.79 |
| FACT-Bl-Cys total score | 42 | 247 | 121.8 (22.3) | 124.0 (38–168) | 169 | 129.5 (18.0) | 132.0 (47–163) | 161 | 5.7 (19.0) | −55; 71 | 1.5 |
| EQ-5D | |||||||||||
| EQ-5D-5 L | 5 | 245 | 0.80 (0.1) | 0.85 (0–1) | 176 | 0.84 (0.13) | 0.88 (0–1) | 167 | 0.03 (0.17) | −1; 1 | 0.1 |
| EQ-VAS | 1 | 260 | 72.0 (20.5) | 75.0 (0–100) | 229 | 74.9 (19. 5) | 80.0 (0–100) | 229 | −13.5 (36.9) | −96; 81 | 2.4 |
aThe number of patients that completed enough items to calculate a total score, PWB Physical well-being domain, SWB Social/family well-being domain, EWB Emotional well-being domain, FWB Functional well-being domain, −FACT-Bl-Cys total score is only calculated when all items have been completed
Floor and ceiling effects
Seven out of nine BCI domains showed a ceiling effect (meaning few problems), while no floor effects were observed (see Table 3). Most missing values were reported in the BCI “sexual function” domain at T0; for 112 of 260 patients (43%) we could not calculate a domain score.
Table 3.
Reliability properties of the BCI (T0, baseline) and FACT-Bl-Cys (T3, 90 days post-operative)
| Domains | Population size (n) | Valid population (n)a | No. of items per domain | Minimal no. of missing items (%)b | Maximal no. of missing items (%)c | Mean (SD) | Median (min-max) | Floor effect | Ceiling effect | Internal consistency (Cronbach’s α) |
|---|---|---|---|---|---|---|---|---|---|---|
| T0 BCI | ||||||||||
| Urinary domain | 260 | 223 | 12 | 0 (66%) | 12 (3%) | 82 (18) | 86 (7–100) | 0 (0%) | 41 (16%) | 0.83 |
| Function | 260 | 241 | 4 | 0 (93%) | 4 (4%) | 85 (25) | 100 (0–100) | 5 (2%) | 144 (55%) | 0.86 |
| Bother | 260 | 212 | 8 | 0 (67%) | 8 (4%) | 79 (21) | 84 (11–100) | 0 (0%) | 48 (19%) | 0.82 |
| Bowel domain | 260 | 239 | 10 | 0 (88%) | 10 (4%) | 87 (15) | 92 (34–100) | 0 (0%) | 40 (15%) | 0.83 |
| Function | 260 | 234 | 4 | 0 (90%) | 4 (4%) | 86 (15) | 92 (15–100) | 0 (0%) | 92 (35%) | 0.70 |
| Bother | 260 | 239 | 6 | 0 (90%) | 6 (4%) | 87 (17) | 96 (25–100) | 0 (0%) | 73 (35%) | 0.77 |
| Sexual domain | 260 | 157 | 12 | 0 (42%) | 12 (12%) | 49 (20) | 51 (9–85) | 0 (0%) | 0 (0%) | 0.91 |
| Function | 260 | 148 | 7 | 0 (45%) | 7 (12%) | 33 (19) | 31 (0–82) | 5 (2%) | 0 (0%) | 0.90 |
| Bother | 260 | 200 | 5 | 0 (69%) | 5 (15%) | 67 (31) | 75 (0–100) | 5 (2%) | 61 (24%) | 0.85 |
| T3 FACT-Bl-Cys | ||||||||||
| FACT-PWB | 182 | 180 | 7 | 0 (91%) | 6 (1%) | 24 (4) | 25 (0–28) | 0 (0%) | 25 (14%) | 0.80 |
| FACT-SWB | 182 | 177 | 7 | 0 (63%) | 7 (2%) | 21 (4) | 22 (0–28) | 2 (1%) | 11 (6%) | 0.81 |
| FACT-EWB | 182 | 173 | 6 | 0 (92%) | 6 (4%) | 20 (3) | 21 (5–24) | 0 (0%) | 1 (1%) | 0.77 |
| FACT-FWB | 182 | 175 | 7 | 0 (90%) | 7 (4%) | 19 (5) | 20 (6–28) | 1 (1%) | 1 (1%) | 0.85 |
| Bl-Cys domain | 182 | 175 | 15 | 0 (63%) | 15 (4%) | 44 (8) | 45 (11–58) | 0 (0%) | 0 (0%) | 0.77 |
| FACT-Bl-Cys total− | 182 | 169 | 42 | – | – | 129 (18) | 132 (74–163) | 0 (0%) | 0 (0%) | 0.90 |
aThe number of patients that completed enough items to calculate a total score, bMinimal number of missings per domain (i.e. 67% misses 0 items), cMaximal number of missings per domain (i.e. 3% misses 12 items), PWB Physical well-being domain, SWB Social/family well-being domain, EWB Emotional well-being domain, FWB Functional well-being domain, −FACT-Bl-Cys total score is only calculated when all items have been completed
Regarding the FACT-Bl-Cys, no floor or ceiling effects were detected (see Table 3). The FACT-EWB domain at T3 was missing for 9 out of 182 patients (5%). For 13 out of 182 patients (7%) the FACT-Bl-Cys total score could not be calculated since they did not complete all items.
Validity
Face validity was considered to be good for both measures, as they appeared to address all relevant aspects of HRQOL in Dutch BCa adults treated with radical cystectomy.
Regarding content validity, interviewed patients expressed an overall positive opinion regarding relevance and comprehensiveness of the measures and their evaluative purposes. Nonetheless, one item of FACT-Bl-Cys (Bl1 “I have trouble controlling my urine”) raised concerns by some respondents, as patients with different bladder diversions might interpret this differently (e.g. stoma, neobladder of bladder replacement). We discussed these findings with FACIT representatives, and decided that patients could respond to this item in any way they feel appropriate.
The CFA was performed on the imputed datasets and the model fit was evaluated based on Comparative Fit Index (CFI), Tucker-Lewis index (TLI), Root Means Square Error of Approximation (RMSEA), and Standardized Root Mean Square Residual (SRMR). Considering structural validity for BCI, the CFA resulted in a moderate fit (see Table 4). The BCI in six factors showed a better fit (CFI:0.88, TLI:0.87, RMSEA:0.06, SRMR:0.07) in comparison to three factors (CFI:0.69, TLI:0.67, RMSEA:0.09, SRMR:0.09). Considering structural validity for FACT-Bl-Cys, the CFA also resulted in a moderate fit (see Table 4). For FACT-G in four factors, the CFI was 0.88, the TLI was 0.86, the RMSEA was 0.05, and the SRMR was 0.09. The Bl-Cys domain in three factors as proposed by Kim et al. [18] showed better fit (CFI:0.85, TLI:0.81, RMSEA:0.06, SRMR:0.09). None of the hypothesized factor structures for both PROMs fulfilled the CFI or TLI thresholds of ≥0.95.
Considering hypotheses testing for BCI, 67% of prior hypotheses (8 of 12) were confirmed (see Table 5). Correlations among the three BCI domains were expected to be low, however we found a moderate correlation between the urinary domain and the bowel domain (r = 0.35). Although we expected moderate correlations when comparing the urinary domain with FACT-G, the sexual domain with EQ-5D-5 L, and the sexual domain with EQ-VAS, we instead found low correlations of r = 0.29, r = 0.24, r = 0.28, respectively. For FACT-Bl-Cys, 92% of all a priori hypotheses (12 of 13) were confirmed (see Table 5). As hypothesized for FACT-Bl-Cys, we found moderate correlations when comparing the Bl-Cys domain with EQ-5D-5 L (r = 0.60), FACT-Bl-Cys with EQ-5D-5 L (r = 0.70), and FACT-Bl-Cys with EQ-VAS (r = 0.58). All correlations between the five domains were moderate as expected, with the exception of one low correlation was observed (EWB-SWB r = 0.29).
Table 5.
Construct validity properties of the BCI and FACT-Bl-Cys
| Correlation expected | Hypotheses tested | Correlation coefficient (r) | Confirmed? | |
|---|---|---|---|---|
| T0 BCI | ||||
| Low (< 0.30) | Correlations between 3 domains are low | Urinary – Bowel | 0.35 | No |
| Urinary – Sexual | 0.15 | Yes | ||
| Bowel – Sexual | 0.25 | Yes | ||
| Moderate (0.30–0.69) | Correlations between 3 domains and FACT-G are moderate | FACT-G – Urinary | 0.29 | No |
| FACT-G – Bowel | 0.39 | Yes | ||
| FACT-G – Sexual | 0.36 | Yes | ||
| Correlations between 3 domains and EQ-5D-5 L are moderate | EQ-5D-5 L – Urinary | 0.34 | Yes | |
| EQ-5D-5 L – Bowel | 0.38 | Yes | ||
| EQ-5D-5 L – Sexual | 0.24 | No | ||
| Correlations between 3 domains and EQ-VAS are moderate | VAS – Urinary | 0.37 | Yes | |
| VAS – Bowel | 0.32 | Yes | ||
| VAS – Sexual | 0.28 | No | ||
| T3 FACT-Bl-Cys | ||||
| Moderate (0.30–0.69) | Correlations between 5 domains are moderate | PWB – SWB | 0.30 | Yes |
| PWB – EWB | 0.51 | Yes | ||
| PWB – FWB | 0.65 | Yes | ||
| PWB – Bl-Cys | 0.63 | Yes | ||
| SWB – EWB | 0.29 | No | ||
| SWB – FWB | 0.41 | Yes | ||
| SWB – Bl-Cys | 0.35 | Yes | ||
| EWB – FWB | 0.59 | Yes | ||
| EWB – Bl-Cys | 0.50 | Yes | ||
| FWB – Bl-Cys | 0.64 | Yes | ||
| Correlation between Bl-Cys domain and EQ-5D-5 L is moderate | Bl-Cys domain – EQ-5D-5 L | 0.60 | Yes | |
| Correlation between total FACT-Bl-Cys and EQ-5D-5 L / EQ-VAS are moderate | FACT-Bl-Cys total score – EQ-5D-5 L | 0.70 | Yes | |
| FACT-Bl-Cys total score – VAS | 0.58 | Yes | ||
Reliability
Internal consistency (Cronbach’s α), test–retest reliability (ICC), and measurement error (SEM, SDC group, SDC individual) are presented in Tables 3 and 6.
Table 6.
Test-retest properties of the BCI and FACT-Bl-Cys (T3 and T3 retest)
| Domains | Valid population (n) | No. of items per domain | T3 Mean (SD) | T3 Median (min-max) | T3 retest Mean (SD) | T3 retest Median (min-max) | T3-T3 retest | T3 retest:a ICCagreement (95% CI) | Measurement error:b SEMagreement | Measurement error:c SDCindividual | Measurement error:d SDCgroup | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean change (SD) | 95% CI | SE | |||||||||||
| BCI | |||||||||||||
| Urinary domain | 53 | 12 | 84 (17) | 91 (32–100) | 86 (15) | 92 (31–100) | −2 (7) | −25; 15 | 0.9 | 0.90 (0.83; 0.91) | 4.98 | 13.82 | 1.90 |
| Function | 43 | 4 | 76 (33) | 100 (0–100) | 83 (28) | 100 (0–100) | −7 (16) | −57; 8 | 2.4 | 0.85 (0.71; 0.92) | 12.16 | 33.72 | 5.14 |
| Bother | 52 | 8 | 88 (12) | 91 (50–100) | 88 (14) | 92 (47–100) | 0 (9) | −17; 36 | 1.2 | 0.77 (0.63; 0.86) | 6.13 | 16.99 | 2.36 |
| Bowel domain | 61 | 10 | 84 (14) | 88 (37–100) | 86 (14) | 90 (47–100) | −2 (10) | −26; 26 | 1.3 | 0.76 (0.63; 0.85) | 7.05 | 19.54 | 2.50 |
| Function | 57 | 4 | 85 (17) | 92 (36–100) | 87 (14) | 92 (42–100) | −2 (11) | −33; 29 | 1.5 | 0.74 (0.60; 0.84) | 7.80 | 21.63 | 2.86 |
| Bother | 61 | 6 | 83 (16) | 88 (33–100) | 85 (16) | 92 (38–100) | −2 (11) | −29; 26 | 1.4 | 0.76 (0.63; 0.85) | 7.98 | 22.12 | 2.83 |
| Sexual domain | 19 | 12 | 40 (16) | 42 (3–65) | 38 (17) | 35 (8–69) | 2 (11) | −11; 26 | 2.5 | 0.78 (0.53; 0.91) | 7.55 | 20.92 | 4.80 |
| Function | 14 | 7 | 26 (16) | 29 (0–48) | 29 (16) | 29 (4–54) | −3 (5) | −14; 8 | 1.4 | 0.93 (0.79; 0.98) | 4.17 | 11.56 | 3.09 |
| Bother | 42 | 5 | 62 (28) | 60 (6–100) | 55 (26) | 50 (13–100) | 7 (28) | −59; 80 | 4.3 | 0.47 (0.20; 0.67) | 20.07 | 55.63 | 8.58 |
| FACT-Bl-Cys | |||||||||||||
| FACT-PWB | 58 | 7 | 25 (4) | 26 (13–28) | 25 (3) | 26 (16–28) | 0 (2) | −7; 8 | 0.3 | 0.74 (0.59; 0.83) | 1.76 | 4.88 | 0.64 |
| FACT-SWB | 57 | 7 | 22 (4) | 22 (0–28) | 22 (3) | 23 (13–28) | −1 (4) | − 19; 5 | 0.6 | 0.43 (0.19; 0.62) | 3.01 | 8.34 | 1.10 |
| FACT-EWB | 54 | 6 | 21 (3) | 21 (11–24) | 21 (3) | 22 (12–24) | 0 (2) | −4; 7 | 0.3 | 0.72 (0.56; 0.83) | 1.57 | 4.36 | 0.59 |
| FACT-FWB | 54 | 7 | 20 (5) | 21 (6–28) | 20 (5) | 22 (9–28) | −1 (3) | −8; 8 | 0.5 | 0.78 (0.64; 0.86) | 2.35 | 6.52 | 0.89 |
| Bl-Cys domain | 54 | 15 | 45 (7) | 44 (24–57) | 46 (8) | 47 (20–59) | 0 (4) | −10; 13 | 0.6 | 0.82 (0.71; 0.89) | 3.14 | 8.69 | 1.18 |
| EQ-5D-5 L | |||||||||||||
| 5D-5 L | 55 | 5 | 0.87 (0.1) | 0.91 (0.49–0.95) | 0.85 (0.1) | 0.88 (0.54–0.95) | 0 (0.1) | −0.1;0.2 | 0.01 | 0.82 (0.71; 0.89) | 0.05 | 0.13 | 0.02 |
| EQ-VAS | 62 | 1 | 75 (22) | 81 (0–100) | 73 (26) | 81 (0–100) | 2 (30) | −85;87 | 4 | 0.23 (−0.26; 0.45) | 21.21 | 58.79 | 7.47 |
aICC Intraclass Correlation Coefficient (two-way random effects model, absolute agreement, single measures); bSEM Standard Error of Measurement (√(σ2o + σ2residual)); cSDCindividual Smallest Detectable Change on individual level (=1.96*√ (2)*SEM); dSDCgroup Smallest Detectable Change on group level, i.e. the smallest difference in score that can be distinguished from measurement errors (=SDCindividual/√(n))
For BCI, Cronbach’s α of the domains ranged from 0.70 (“bowel function”) to 0.91 (sexual domain), which indicates a good internal consistency. All ICCs were above 0.70, except for “sexual bother” which showed an ICC of 0.47. The SEM ranged from 4.17 (“sexual function”) to 20.07 (“sexual bother”), which resulted in a SDC individual score ranging from 11.56 to 55.63. The SDC group ranged from 1.90 (urinary domain) to 8.58 (“sexual bother”). Thus for the domain “sexual bother”, an average group difference in score ≥ 8.58 cannot be attributed to only measurement error, but actual change will have taken place.
For FACT-Bl-Cys, Cronbach’s α of the domains ranged from 0.77 (Bl-Cys) to 0.85 (FWB), which indicates a good internal consistency. All ICCs were above 0.70, except for SWB which showed an ICC of 0.43. The SEM ranged from 1.57 (EWB) to 3.14 (Bl-Cys domain), which resulted in a SDC individual score ranging from 4.36 to 8.69. Our SDC individual values show that, to determine a treatment effect a difference of at least 4.36 to 8.69 points between two scores from an individual patient is required to ensure that the difference is not attributed to measurement error. The SDC group ranged from 0.59 (EWB) to 1.18 (Bl-Cys). See Additional file 1 for separate variance components.
Responsiveness
Our total study population did not show major changes between T0 and T3 for the BCI and FACT-Bl-Cys (see Table 2). When evaluating the occurrence of complications, it appeared that our dataset at the time contained too few registered complications to assess responsiveness, see Additional file 2. Only 42 patients had at least one registered complication (Clavien-Dindo classification grade 1 or higher). The number of patients that completed enough items to calculate a total score ranged from 0 to 27, depending on the domain.
Additionally, the change score between T0 and T3 of BCI and FACT-Bl-Cys in comparison to the change score of the EQ-VAS was studied. As presented in Additional file 3, mean change scores between T0 and T3 were calculated for patients who showed an improvement in the EQ-VAS score of ≥10 points (n = 55, mean change:25, SD:16) or a deterioration in this score of ≥10 points (n = 89, mean change:-50, SD:30).
For BCI, the number of patients with an improved EQ-VAS ranged from 17 to 47, depending on the domain. We hypothesized to find a larger effect size in the improved group compared to the no-change group, but only 1 of 9 hypotheses was confirmed (see Table 7). We only found a larger effect size for the urinary domain in the improved group, in comparison to the no-change group (Cohens d: 0.36 > 0.06). Regarding hypotheses on correlations, only 22% of all a priori hypotheses (2 of 9) were confirmed. We only found the expected moderate correlations for the change score of the bowel domain (r = 0.42) and the “bowel bother” domain (r = 0.39).
Table 7.
Responsiveness properties of the BCI and FACT-Bl-Cys
| Domains | Valid population (n) a | Observed correlation changes f | Hypotheses on correlations: confirmed? g | Observed effect size e | Hypotheses on effect sizes: confirmed? h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EQ-VAS no change group b | EQ-VAS improved group c | EQ-VAS deteriorated group d | EQ-VAS no change group b | EQ-VAS improved group c | EQ-VAS deteriorated group d | EQ-VAS improved group c | EQ-VAS deteriorated group d | EQ-VAS no change group b | EQ-VAS improved group c | EQ-VAS deteriorated group d | EQ-VAS improved group c | EQ-VAS deteriorated group d | |
| EQ-VAS | 68 | 55 | 89 | 1.00 | 1.00 | 1.00 | NA. | NA. | 0.00 | 1.32 | −2.94 | NA. | NA. |
| BCI | |||||||||||||
| Urinary domain | 55 | 42 | 32 | 0.10 | 0.17 | −0.12 | No | No | 0.06 | 0.36 | −0.44 | Yes | Yes |
| Function | 51 | 43 | 29 | 0.00 | −0.13 | − 0.19 | No | No | −0.45 | − 0.44 | − 0.56 | No | Yes |
| Bother | 52 | 38 | 31 | 0.04 | 0.29 | −0.05 | No | No | 0.35 | 0.35 | −0.05 | No | No |
| Bowel domain | 57 | 47 | 40 | 0.32 | 0.42 | 0.11 | Yes | No | −0.90 | −0.06 | −0.44 | No | No |
| Function | 53 | 46 | 38 | 0.24 | 0.25 | −0.18 | No | No | −0.43 | 0.05 | −0.36 | No | No |
| Bother | 57 | 47 | 39 | 0.29 | 0.39 | 0.18 | Yes | No | −0.75 | −0.11 | −0.42 | No | No |
| Sexual domain | 15 | 20 | 10 | −0.01 | 0.23 | −0.14 | No | No | −0.85 | −0.50 | −1.10 | No | Yes |
| Function | 14 | 17 | 12 | 0.01 | 0.25 | −0.36 | No | Yes | −0.68 | −0.48 | −1.25 | No | No |
| Bother | 43 | 34 | 28 | 0.06 | 0.04 | 0.28 | No | No | −0.35 | −0.29 | −0.72 | No | No |
| FACT-Bl-Cys | |||||||||||||
| FACT-PWB | 66 | 52 | 42 | 0.54 | 0.51 | 0.44 | Yes | Yes | 0.00 | 0.80 | −0.40 | Yes | Yes |
| FACT-SWB | 65 | 52 | 41 | 0.26 | 0.20 | 0.24 | No | No | −0.50 | −0.17 | 0.00 | No | No |
| FACT-EWB | 61 | 49 | 40 | 0.30 | 0.40 | 0.38 | Yes | Yes | 0.60 | 0.80 | 0.25 | Yes | No |
| FACT-FWB | 64 | 51 | 40 | 0.42 | 0.47 | 0.31 | Yes | Yes | 0.20 | 0.67 | −0.40 | Yes | Yes |
| Bl-Cys domain | 64 | 50 | 41 | 0.13 | 0.44 | 0.42 | Yes | Yes | 0.22 | 0.55 | −0.22 | Yes | Yes |
| FACT-Bl-Cys total | 59 | 45 | 40 | 0.39 | 0.62 | 0.53 | Yes | Yes | 0.17 | 0.75 | −0.18 | Yes | Yes |
a The number of patients that completed enough items to calculate a total score; b No change between T0 and T3 for patients in EQ-VAS score (plus or minus ≤5 points); c Change between T0 and T3 for patients with an improved EQ-VAS score of ≥10 points; d Change between T0 and T3 for patients with a deteriorated EQ-VAS score of ≥10 points; e Formula assessed for Effect Size (Cohens d): ChangeT3T0Mean/T0SD; f Spearman correlation (r) between change score EQ-VAS and change score BCI and FACT-Bl-Cys (T0 and T3);
g Hypotheses correlations = in patients with an improved or declined EQ-VAS score (≥10 points), we hypothesized to find moderate correlations of ≥0.30 between change in EQ-VAS and the change in BCI and FACT-Bl-Cys values;
h Hypotheses effect sizes = in patients with an improved or declined EQ-VAS score (≥10 points), we hypothesized to find a larger effect size, in comparison to patients that did not show a change in EQ-VAS (≤5 points)
For BCI, the number of patients with a deteriorated EQ-VAS ranged from 10 to 40, depending on the domain. Regarding hypotheses on effect sizes for the deterioration group, only 3 out of 9 hypotheses were confirmed. We found larger effect sizes in the urinary domain (Cohens d: 0.44 > 0.06), “urinary function” (Cohens d: − 0.56 > − 0.45), and the sexual domain (Cohens d: − 1.10 > − 0.85). Regarding hypotheses on correlations, only 11% of all a priori hypotheses (1 of 9) were confirmed. We only found a moderate correlation for the change score of the “sexual function” domain.
For FACT-Bl-Cys, the number of patients with an improved EQ-VAS ranged from 45 to 52, depending on the domain. For both effect sizes and correlations, 83% of all a priori hypotheses (5 of 6) were confirmed. Only for FACT-SWB we found a smaller effect size (Cohens d: − 0.17 < − 0.50) and a low correlation (r = 0.20).
For FACT-Bl-Cys, the number of patients with a deteriorated EQ-VAS ranged from 40 to 42, depending on the domain. Regarding hypotheses on effect sizes for the deterioration group, 67% of all a priori hypotheses (4 of 6) were confirmed (see Table 7). We found lower effect sizes in the FACT-SWB (Cohens d: 0.00 < -0.50) and FACT-EWB (Cohens d: 0.25 < 0.60). Regarding hypotheses on correlations, 83% of all a priori hypotheses (5 of 6) were confirmed. We only found a low correlation for the change in FACT-SWB (r = 0.24), which was contrary to our expectations. Overall, these results indicate that the FACT-Bl-Cys total score seems responsive to change in generic HRQOL.
Discussion
The aim of this study was to translate two disease-specific measures (BCI and FACT-Bl-Cys) into Dutch and to evaluate their measurement properties according to the COSMIN criteria. Both measures were found to be reliable and have good content validity, but limited structural validity. This study showed that the Dutch version of the BCI was not very responsive to changes in generic HRQOL, and showed a ceiling effect. In the Dutch version of the FACT-Bl-Cys no floor or ceiling effects were observed and the FACT-Bl-Cys total score was responsive to changes in generic HRQOL.
The structural validity of the BCI was assessed as moderate, as the hypothesized domains based on the original validation [13] and translational study [16] could partially be confirmed. For BCI, we found a better fit for a six-factor than a three-factor structure, which could indicate that BCI distinguishes function and bother. The structural validity of the FACT-Bl-Cys was assessed as moderate as the majority of hypothesized domains could not be confirmed. This limited fit could be caused by the heterogeneous characteristics of BCa disease, as many different topics are covered in the domains. The FACT-Bl-Cys domains contain a wide range of complaints that do not necessarily relate to each other. For instance, the Bl-Cys domain contains items related to body image, physical functioning, satisfaction, and socials aspects. Future exploration of whether there is a more adequate component solution is recommended for further research.
Although experts debate on the minimum number of patients needed for a factor analysis (ranging from 100 to 300 patients) [49–54], COSMIN advises to use a sample size of 7 patients per item, with a minimum number of 100 patients [38]. Though we handled the missing data with multiple imputation in order to perform the CFA with an adequate sample size, when interpreting the results it should be considered that due to multiple imputation, in which missing values are imputed with the assumption that the distribution of the missing values is similar to the observed data (i.e. predictive mean matching approach), indices might be overfitted.
Regarding BCI at T0, high ceiling effects were observed for both urinary and bowel domains. The maximum scores indicate good function and no bother, which might be when patients are recently diagnosed and have minor symptoms (e.g. painless hematuria). Similar findings are reported in previous studies [13, 16, 17].
Regarding FACT-Bl-Cys, there were uncertainties concerning the calculation of total scores, as we excluded the items BL4 ‘I am interested in sexual activity’ and BL5 ‘I am able to have and maintain an erection’, according to FACIT instructions. These items were also not included in the CFA. Previously published studies each handled these items differently [11, 12, 18, 19].
In comparison to previous BCI [13, 16, 17] and FACT-Bl-Cys [11, 18, 19] studies, we found similar internal consistency values for all domains. Reliability of both measures in terms of test-retest scores was good. To our knowledge, the SEM and SDC have not yet been assessed before for both measures. It should be noticed that our design methodologically differs from previous conducted studies. Previous FACT-Bl-Cys studies [11, 12, 18, 19] assessed test-retest reliability using an interval of 4 weeks, while COSMIN advises to use an interval of 2 weeks [38]. Additionally, FACT-Bl-Cys was completed at different moments (e.g. 7 months to 3 years post-operatively) [11, 12, 18, 19]. Anderson et al. did not show an ICC, but reported a test-retest Spearman correlation of 0.89 for the Bl-Cys domain [11]. Three other studies reported an ICC of 0.79 [12], 0.75 [19], and 0.82 [18] for the Bl-Cys domain, which is similar to our ICC of 0.82.
Regarding construct validity of the BCI, our results confirm the suggestion that the BCI captures additional information which is not covered by generic instruments. Schmidt et al. found similar results as they reported low to moderate correlations (range: 0.16–0.48) between BCI and the SF-36 [17]. Regarding construct validity of the FACT-Bl-Cys, only Kim et al. correlated FACT-Bl-Cys with a generic measure [18]. They found moderate correlations (range: 0.29–0.69) between FACT-Bl-Cys and the SF-36 [18]. These results are comparable to our moderate correlations between FACT-Bl-Cys and the generic measures. In contrast to our results, three previous studies showed a low correlation (range: 0.16–0.24) between SWB and Bl-Cys domain [12, 18, 19]. Cookson et al. suggested that this low correlation reflects the predominant focus on physical/functional aspects of HRQOL in the FACT-Bl-Cys [12]. We found moderate correlations between all four domains (PWB, SWB, EWB, FWB) and the Bl-Cys domain, which indicates an equal share in HRQOL.
Only one BCI study mentioned responsiveness [17]. In 2014, Schmidt et al. reported an improvement 12 months after treatment for the urinary domain (Cohens d: 0.38) and “urinary bother” (Cohens d: 0.53). Based on these findings, the authors concluded that the BCI is responsive [17]. In 2018, a systematic review performed by Mason et al., however, rated the BCI responsiveness as unknown because Schmidt et al. only calculated effect sizes and did not construct hypotheses a priori which is not conform COSMIN recommendations [4].
We found one study that reported on longitudinal changes of FACT-Bl-Cys [11]. Anderson et al. evaluated change in 190 patients who underwent a radical cystectomy (pre-operative and 12 months post-operative) [11]. Authors did not calculate an effect size or correlations regarding responsiveness according to COSMIN, but they reported that patients with an ileal conduit diversion had an average 5 points higher score at 12 months, than those with an orthotopic neobladder diversion.
Strengths and limitations
As far as we are aware, this is the first study determining the measurement properties of BCI and FACT-Bl-Cys, according to COSMIN guidelines, in a large study population. COSMIN enhances clarity and stimulates uniform usage of terminology, enables researchers to evaluate each measurement property individually, and improves comparability with other studies.
This study also has some limitations. First, our study population comprised only patients treated with radical cystectomy. Therefore, we were unable to determine whether both measures will be valid and reliable in a more generic BCa population. Second, due to our study design test-retest and measurement error had to be determined at 3 months (T3, post-operatively) rather than at baseline (T0, pre-operatively). For FACT-Bl-Cys this is an adequate moment, but for BCI this might result in a bias since this measure is originally intended for BCa patients in general. Third, to explore responsiveness we studied the correlation and effect size of change score of both measures with the change score of the EQ-VAS between T0 and T3. Although we expected that radical cystectomy impacts HRQOL, the BCI and FACT-Bl-Cys did not detect large changes between T0 and T3. Two previous studies did also not find major changes between T0 and T3 for FACT-Bl-Cys [55, 56]. This small change could indicate that radical cystectomy does not impact HRQOL largely, but it is also possible that the BCI and FACT-Bl-Cys do not adequately detect the actual impact and change over time. Particularly since we did find a large change in EQ-VAS scores: the population with an improved EQ-VAS score (n = 55) showed a mean increase of 25 points, and the population with a deteriorated EQ-VAS score (n = 89) showed a mean decrease of 50 points. Previous studies in other patient populations reported MIC values varying from 6.9 to 8.9 [45–48], but comparability between these different populations should be considered. The FACT-Bl-Cys appeared to be responsive to changes in generic HRQOL, though limited structural validity must be considered as this could negatively impact the responsiveness of specific domains. The possibility remains that in some cases responsiveness was not detected, because there was no meaningful change. In our study population we found too few registered complications to assess responsiveness based on the occurrence of complications. This study was a first exploration of responsiveness of both measures, further research is warranted to evaluate whether the BCI and FACT-Bl-Cys are responsive to objective measures of change (e.g. occurrence of complications).
Clinical implications
Based on this study, both measures seem suitable for cross-sectional use in clinical practice and for research purposes. Consulted urologists mentioned that all topics of both measures are usually discussed with patients in clinical practice, and usage of measures could be valuable for clinicians in order to provide more personalized care. Additionally, urologists noticed that the measures seem quite long for daily practice which limits the suitability and feasibility, but removing or combining items influences validity.
When using the BCI, it should be considered that the largest proportion of missing values was found in the sexual domain, which is also noticed by two previous studies [16, 17]. During cognitive interviews, some patients mentioned that the sexual domain items were clear but ‘irrelevant’ as they had no sexual life. Thus a ‘never’ response or a missing value could indicate no sexual bother or function, or the lack of activity. Therefore, it is questionable whether BCI is suitable to measure “sexual function” and “sexual bother”. Patients also indicated that BCI items about “urine loss” and FACT-Bl-Cys items about “controlling my urine” could be interpreted in different ways, as patients might have different bladder diversions (e.g. stoma, neobladder of bladder replacement). We recommend that future users of the measures provide an instruction which states that patients should answer these items according to their own specific situation.
Conclusion
The Dutch versions of the BCI and FACT-Bl-Cys were shown to be reliable and have good content validity. Structural validity was limited for both measures. Only the FACT-Bl-Cys total score was responsive to changes in generic HRQOL. Despite some limitations, both measures are suitable for cross-sectional use in clinical practice and for research purposes. Exploration of a more adequate component solution and whether both PROMs are responsive when objective measures (e.g. occurrence of complications) are used as an indicator of change, needs to be further evaluated in future research.
Additional files
Variance components of the measures. (DOCX 22 kb)
Change in scores between T0 (baseline) and T3 (90 days post-operative) of the measures in relation to registered complications. (DOCX 44 kb)
Change in scores between T0 (baseline) and T3 (90 days post-operative) of the measures in relation responsiveness properties. (DOCX 26 kb)
Acknowledgements
The authors would like to thank all patients who participated in the cognitive interviews. Many thanks to Gerjon Hannink for his valuable input regarding the multiple imputation and analyses. Authors would like to thank all translators for their contribution to translation procedure of the measures. Analyses of measurement properties were conducted using data from our concurrent comparative effectiveness study (i.e. RACE, Radical Cystectomy Evaluation) on effectiveness of robot-assisted and open radical cystectomy.
Abbreviations
- BCa
Bladder Cancer
- BCI
Bladder Cancer Index
- CFA
Confirmatory Factor Analyses
- CFI
Comparative Fit Index
- COSMIN
COnsensus-based Standards for the selection of health Measurement Instruments
- EFA
Exploratory Factor Analyses
- EQ-5D-5 L
EuroQol-5 Dimensions-5 Levels
- EQ-VAS
EuroQol Visual Analogue Scale
- ES
Effect size
- EWB
Emotional well-being domain
- FACIT
Functional Assessment of Chronic Illness Therapy
- FACT-Bl-Cys
Functional Assessment of Cancer Therapy–Bladder cancer Cystectomy
- FWB
Functional well-being domain
- HRQOL
Health-related quality of life
- ICC
Intraclass Correlation Coefficient
- LRC
Laparoscopic-assisted Radical Cystectomy
- MCAR
Missing Completely At Random
- MIC
Minimal Important Change
- NTR
Nederlands Trial Register (Dutch Trial Registry)
- ORC
Open Radical Cystectomy
- PRO
Patient Reported Outcomes
- PWB
Physical well-being domain
- QALY
Quality Adjusted Life Years
- RACE
Radical Cystectomy Evaluation
- RARC
Robot-assisted Radical Cystectomy
- RMSEA
Root Means Square Error of Approximation
- SDC
Smallest Detectable Change
- SEM
Standard Error of Measurement
- SRMR
Standardized Root Mean Square Residual
- SWB
Social/family well-being domain
- VAS
Visual Analogue Scale
Authors’ contributions
All authors were involved in the conception of the research and research design. CM, CW, JG were closely involved with the translation procedure. FW and CW approached patients for cognitive interviews. CM analyzed the data and together with IA, JG, MR she interpreted the results. CM drafted the manuscript, all authors critically revised it. All authors read and approved the final manuscript.
Funding
This study was financially supported by a research grant (project number 843002602) from the Dutch Organization for Health Research and Development (ZonMw). The funding organization had no role in the design, analysis, or presentation of this research.
Availability of data and materials
When the RACE study is completed, data will be made available in accordance with ZonMw regulations.
Ethics approval and consent to participate
The study protocol has been reviewed and approved by the sponsor (ZonMw, The Netherlands Organization for Health Research and Development) and the accredited Medical Research Ethics Committee (MREC, Commission for Research in Human Subjects, in Dutch: Commissie Mensgebonden Onderzoek (CMO), region Arnhem – Nijmegen, the Netherlands). The MREC “CMO Regio Arnhem-Nijmegen” determined that the RACE study does not fall under the scope of the Medical Research Involving Human Subjects Act (WMO). Informed consent was obtained from all individual participants included in the study.
Consent for publication
N.A.
Competing interests
The authors declare that they do not have any competing interests. CM, JG, CW, FW, and MR are currently conducting a comparative effectiveness study on the (cost-)effectiveness of radical cystectomy, named the RACE study (trial identifying number NTR5362, Dutch Trial Registry, www.trialregister.nl), in which the PROMs under study are being administered (among other outcome measures). However, in the authors’ opinion this does not pose a conflict of interest, since results of the current study will not have any consequences for the measurement instruments used in the RACE study nor will future results of the RACE study affect the current manuscript. All primary and secondary outcomes of the RACE study have been predefined in the registered and published trial protocol.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Charlotte T. J. Michels, Phone: +31 (0)6 13 11 56 34, Email: cmichels@rijnstate.nl
Carl J. Wijburg, Email: cwijburg@rijnstate.nl
Inger L. Abma, Email: inger.abma@radboudumc.nl
J. Alfred Witjes, Email: fred.witjes@radboudumc.nl.
Janneke P. C. Grutters, Email: janneke.grutters@radboudumc.nl
Maroeska M. Rovers, Email: maroeska.rovers@radboudumc.nl
References
- 1.Witjes JA, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder Cancer. European Urology. 2017;71(3):462–475. doi: 10.1016/j.eururo.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Leal J, Luengo-Fernandez R, Sullivan R, Witjes JA. Economic burden of bladder Cancer across the European Union. European Urology. 2016;69(3):438–447. doi: 10.1016/j.eururo.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 3.Botteman MF, Pashos CL, Hauser RS, Laskin BL, Redaelli A. Quality of life aspects of bladder cancer: A review of the literature. Quality of life Research. 2003;12(6):675–688. doi: 10.1023/A:1025144617752. [DOI] [PubMed] [Google Scholar]
- 4.Mason SJ, Catto JWF, Downing A, Bottomley SE, Glaser AW, Wright P. Evaluating patient-reported outcome measures (PROMs) for bladder cancer: A systematic review using the COnsensus-based standards for the selection of health measurement INstruments (COSMIN) checklist. BJU International. 2018;90(5):760–773. doi: 10.1111/bju.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed NE, Gilbert F, Lee CT, et al. Pursuing quality in the application of bladder Cancer quality of life research. Bladder Cancer. 2016;2(2):139–149. doi: 10.3233/BLC-160051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black N. Patient reported outcome measures could help transform healthcare. Bmj. 2013;346:f167. doi: 10.1136/bmj.f167. [DOI] [PubMed] [Google Scholar]
- 7.Soreide K, Soreide AH. Using patient-reported outcome measures for improved decision-making in patients with gastrointestinal cancer - the last clinical frontier in surgical oncology? Frontiers in Oncology. 2013;3:157. doi: 10.3389/fonc.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. Jama. 2013;309(8):814–822. doi: 10.1001/jama.2013.879. [DOI] [PubMed] [Google Scholar]
- 9.Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. American Journal of Clinical Oncology. 2012;30(34):4249–4255. doi: 10.1200/JCO.2012.42.5967. [DOI] [PubMed] [Google Scholar]
- 10.Danna BJ, Metcalfe MJ, Wood EL, Shah JB. Assessing symptom burden in bladder Cancer: An overview of bladder Cancer specific health-related quality of life instruments. Bladder Cancer. 2016;2(3):329–340. doi: 10.3233/BLC-160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson CB, Feurer ID, Large MC, et al. Psychometric characteristics of a condition-specific, health-related quality-of-life survey: The FACT-Vanderbilt cystectomy index. Urology. 2012;80(1):77–83. doi: 10.1016/j.urology.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 12.Cookson MS, Dutta SC, Chang SS, Clark T, Smith JA, Jr, Wells N. Health related quality of life in patients treated with radical cystectomy and urinary diversion for urothelial carcinoma of the bladder: Development and validation of a new disease specific questionnaire. The Journal of Urology. 2003;170(5):1926–1930. doi: 10.1097/01.ju.0000092830.03247.ef. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert SM, Dunn RL, Hollenbeck BK, et al. Development and validation of the bladder Cancer index: A comprehensive, disease specific measure of health related quality of life in patients with localized bladder cancer. The Journal of Urology. 2010;183(5):1764–1769. doi: 10.1016/j.juro.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert SM, Wood DP, Dunn RL, et al. Measuring health-related quality of life outcomes in bladder cancer patients using the bladder Cancer index (BCI) Cancer. 2007;109(9):1756–1762. doi: 10.1002/cncr.22556. [DOI] [PubMed] [Google Scholar]
- 15.Gaunez N, Larre S, Pires C, et al. French translation and linguistic validation of the questionnaire bladder Cancer index (BCI) Progrès en Urologie. 2010;22(6):350–353. doi: 10.1016/j.purol.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Hever NV, Pentek M, Ballo A, et al. Health related quality of life in patients with bladder cancer: A cross-sectional survey and validation study of the Hungarian version of the bladder Cancer index. Pathology and Oncology Research. 2015;21(3):619–627. doi: 10.1007/s12253-014-9866-7. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt S, Riel R, Frances A, et al. Bladder cancer index: Cross-cultural adaptation into Spanish and psychometric evaluation. Health and Quality of Life Outcomes. 2014;12:20. doi: 10.1186/1477-7525-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim M, Lee HE, Kim SH, et al. Korean version of the functional assessment of cancer therapy (FACT)-vanderbilt cystectomy index (VCI): Translation and linguistic. Urology Journal. 2014;11(6):1961–1967. [PubMed] [Google Scholar]
- 19.Stenzelius K, Lind AK, Wanegard J, Liedberg F. Patient-reported outcome after radical cystectomy: Translation and psychometric validation of the Swedish version of the functional assessment of Cancer therapy scale Vanderbilt cystectomy index. Scandinavian Journal of Urology. 2016;50(5):374–379. doi: 10.1080/21681805.2016.1201857. [DOI] [PubMed] [Google Scholar]
- 20.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. Journal of Clinical Epidemiology. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Quality of life research. 2012;21(4):651–657. doi: 10.1007/s11136-011-9960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25(24):3186–3191. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Eremenco SL, Cella D, Arnold BJ. A comprehensive method for the translation and cross-cultural validation of health status questionnaires. Evaluation & the health professions. 2005;28(2):212–232. doi: 10.1177/0163278705275342. [DOI] [PubMed] [Google Scholar]
- 25.Wijburg CJ, Michels CTJ, Oddens JR, Grutters JPC, Witjes JA, Rovers MM. Robot assisted radical cystectomy versus open radical cystectomy in bladder cancer (RACE): Study protocol of a non-randomized comparative effectiveness study. BMC Cancer. 2018;18(1):861. doi: 10.1186/s12885-018-4779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novara G, Catto JW, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. European urology. 2015;67(3):376–401. doi: 10.1016/j.eururo.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Allareddy V, Kennedy J, West MM, Konety BR. Quality of life in long-term survivors of bladder cancer. Cancer. 2006;106(11):2355–2362. doi: 10.1002/cncr.21896. [DOI] [PubMed] [Google Scholar]
- 28.Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer. 1996;78(5):1089–1097. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1089::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Hedgepeth RC, Gilbert SM, He C, Lee CT, Wood DP., Jr Body image and bladder cancer specific quality of life in patients with ileal conduit and neobladder urinary diversions. Urology. 2010;76(3):671–675. doi: 10.1016/j.urology.2010.01.087. [DOI] [PubMed] [Google Scholar]
- 30.Mansson A, Davidsson T, Hunt S, Mansson W. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder substitution: Is there a difference? BJU internationaL. 2002;90(4):386–390. doi: 10.1046/j.1464-410X.2002.02899.x. [DOI] [PubMed] [Google Scholar]
- 31.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Quality of life research. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of Cancer therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 33.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45(3):67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 34.Jorgensen TD, Pornprasertmanit S, Schoemann AM, Rosseel Y. R - SemTools package: Useful tools for structural equation modeling. R package version 0.5–1. 2018. [Google Scholar]
- 35.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: Are available health status surveys adequate? Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1995;4(4):293–307. doi: 10.1007/BF01593882. [DOI] [PubMed] [Google Scholar]
- 36.Mokkink LB, Terwee CB, Patrick DL, et al. COSMIN checklist manual. 2012. [Google Scholar]
- 37.de Vet HC, Terwee CB, Mokkink LB, Knol DL. Measurement in medicine: A practical guide (practical guides to biostatistics and epidemiology) Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 38.Mokkink LB, Prinsen CA, Patrick DL, et al. COSMIN methodology for systematic reviews of Patient-Reported Outcome Measures (PROMs): user manual. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeve BB, Hays RD, Bjorner JB, et al. Psychometric evaluation and calibration of health-related quality of life item banks: Plans for the patient-reported outcomes measurement information system (PROMIS) Medical Care. 2007;45(5 Suppl 1):S22–S31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 40.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the functional assessment of Cancer therapy-breast quality-of-life instrument. Journal of Clinical Oncology. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 41.Bland JM, Altman DG. Cronbach's alpha. Bmj. 1997;314(7080):572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vet HC, Terwee CB, Knol DL, Bouter LM. When to use agreement versus reliability measures. Journal of Clinical Oncology. 2006;59(10):1033–1039. doi: 10.1016/j.jclinepi.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. Journal of Strength and Conditioning Research. 2005;19(1):231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 44.de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: Distinction between minimally detectable change and minimally important change. Health and Quality of Life Outcomes. 2006;4:54. doi: 10.1186/1477-7525-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC. Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Quality of life research. 2016;25(6):1585–1596. doi: 10.1007/s11136-015-1196-z. [DOI] [PubMed] [Google Scholar]
- 46.Hoehle LP, Phillips KM, Speth MM, Caradonna DS, Gray ST, Sedaghat AR. Responsiveness and minimal clinically important difference for the EQ-5D in chronic rhinosinusitis. Rhinology. 2019;57(2):110–116. doi: 10.4193/Rhin18.122. [DOI] [PubMed] [Google Scholar]
- 47.Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: Validity, responsiveness and minimum important difference. Thorax. 2016;71(6):493–500. doi: 10.1136/thoraxjnl-2015-207782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zanini A, Aiello M, Adamo D, et al. Estimation of minimal clinically important difference in EQ-5D visual analog scale score after pulmonary rehabilitation in subjects with COPD. Respiratory Care. 2015;60(1):88–95. doi: 10.4187/respcare.03272. [DOI] [PubMed] [Google Scholar]
- 49.Edelen MO, Reeve BB. Applying item response theory (IRT) modeling to questionnaire development, evaluation, and refinement. Quality of life research. 2007;16(Suppl 1):5–18. doi: 10.1007/s11136-007-9198-0. [DOI] [PubMed] [Google Scholar]
- 50.Floyd F, Widaman K. Factor Analysis in the Development and Refinement of Clinical Assessment Instruments. Psychological Assessment. 1995;7:286–299. doi: 10.1037/1040-3590.7.3.286. [DOI] [Google Scholar]
- 51.Hurley AE, Scandura TA, Schriesheim CA, et al. Exploratory and confirmatory factor analysis: Guidelines, issues, and alternatives. Journal of Organizational Behavior. 1997;18(6):667–683. doi: 10.1002/(SICI)1099-1379(199711)18:6<667::AID-JOB874>3.0.CO;2-T. [DOI] [Google Scholar]
- 52.MacCallum RC, Widaman KF, Preacher KJ, Hong S. Sample size in factor analysis: The role of model error. Multivariate Behavioral Research. 2001;36(4):611–637. doi: 10.1207/S15327906MBR3604_06. [DOI] [PubMed] [Google Scholar]
- 53.Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: A clear and user-friendly guideline. Journal of Evaluation in Clinical Practice. 2011;17(2):268–274. doi: 10.1111/j.1365-2753.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 54.Streiner DL. Figuring out factors: The use and misuse of factor analysis. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 1994;39(3):135–140. doi: 10.1177/070674379403900303. [DOI] [PubMed] [Google Scholar]
- 55.Messer JC, Punnen S, Fitzgerald J, Svatek R, Parekh DJ. Health-related quality of life from a prospective randomised clinical trial of robot-assisted laparoscopic vs open radical cystectomy. BJU International. 2014;114(6):896–902. doi: 10.1111/bju.12818. [DOI] [PubMed] [Google Scholar]
- 56.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): An open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391(10139):2525–2536. doi: 10.1016/S0140-6736(18)30996-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variance components of the measures. (DOCX 22 kb)
Change in scores between T0 (baseline) and T3 (90 days post-operative) of the measures in relation to registered complications. (DOCX 44 kb)
Change in scores between T0 (baseline) and T3 (90 days post-operative) of the measures in relation responsiveness properties. (DOCX 26 kb)
Data Availability Statement
When the RACE study is completed, data will be made available in accordance with ZonMw regulations.