Key Points
Question
How long is the prodromal phase of multiple sclerosis?
Findings
In this nested case-control study, we found that serum levels of neurofilament light chain were elevated in case patients with multiple sclerosis compared with matched control individuals 6 years before the clinical onset. This difference between cases and controls increased with decreasing time to the case clinical onset, and clinical onset was associated with a marked increase in neurofilament light chain levels.
Meaning
Multiple sclerosis may have a prodromal phase lasting several years, and neuroaxonal damage may occur already during this phase.
Abstract
Importance
Unrecognized demyelinating events often precede the clinical onset of multiple sclerosis (MS). Identification of these events at the time of occurrence would have implications for early diagnosis and the search of causal factors for the disease.
Objective
To assess whether serum neurofilament light chain (sNfL) levels are elevated before the clinical MS onset.
Design, Setting, and Participants
Nested case-control study among US military personnel who have serum samples stored in the US Department of Defense Serum Repository. Serum samples were collected from 2000 to 2011; sNfL assays and data analyses were performed from 2018 to 2019. We selected 60 case patients with MS who either had 2 samples collected before onset (mean follow-up, 6.3 years) or 1 sample collected before and 1 after onset (mean follow-up, 1.3 years), among 245 previously identified case patients. For each case, we randomly selected 1 of 2 previously identified control individuals matched by age, sex, race/ethnicity, and dates of sample collection. The sample size was chosen based on the available funding.
Exposures
Serum NfL concentrations measured using an ultrasensitive single-molecule array assay (Simoa).
Main Outcomes and Measurements
Log-transformed sNfL concentrations in case patients and control individuals compared using conditional logistic regression and linear mixed models.
Results
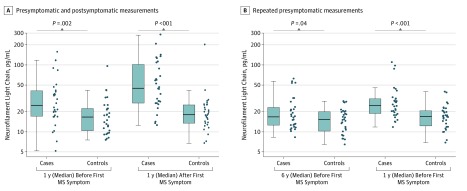
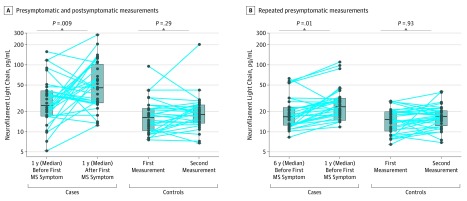
Mean age at baseline was 27.5 years, and 92 of 120 participants (76.7%) were men. Serum NfL levels were higher in case patients with MS compared with their matched control individuals in samples drawn a median of 6 years (range, 4-10 years) before the clinical onset (median, 16.7 pg/mL; interquartile range [IQR], 12.6-23.1 pg/mL vs 15.2 pg/m; IQR, 10.3-19.9 pg/mL; P = .04). This difference increased with decreasing time to the case clinical onset (estimated coefficient for interaction with time = 0.063; P = .008). A within-person increase in presymptomatic sNfL levels was associated with higher MS risk (rate ratio for ≥5 pg/mL increase, 7.50; 95% CI, 1.72-32.80). The clinical onset was associated with a marked increase in sNfL levels (median, 25.0; IQR, 17.1-41.3 vs 45.1; IQR, 27.0-102.7 pg/mL for presymptomatic and postonset MS samples; P = .009).
Conclusions and Relevance
The levels of sNfL were increased 6 years before the clinical MS onset, indicating that MS may have a prodromal phase lasting several years and that neuroaxonal damage occurs already during this phase.
This nested case-control study evaluates the levels of serum neurofilament light chain in presymptomatic individuals with multiple sclerosis to determine a prodromal phase of the disease.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease whose etiology is unknown. While most patients experience their first clinical symptoms of the disease around age 30 years,1 the underlying pathophysiologic processes often occur long before these symptoms become apparent. The existence of this prodromal phase is revealed by the fact that at the time of the first demyelinating event, patients often present with evidence of central nervous system lesions in different stages of evolution,2 indicating the past occurrence of subclinical demyelinating events. Further, one-third of individuals with incidental white-matter lesions consistent with MS but with no other clinical signs of the disease (radiologically isolated syndrome) go on to develop neurologic symptoms consistent with MS within 5 years,3 suggesting that the initial lesions were manifestations of MS. The unrecognized demyelinating events that precede the clinical disease onset could be asymptomatic or could cause symptoms too vague to suggest MS as a possible cause. The later occurrence is consistent with the observations in electronic databases that patients with MS more frequently sought medical care in the 5 years before the first demyelinating event compared with control individuals4 and more often reported a number of clinical symptoms and disturbances to their general practitioner up to 10 years before their MS diagnosis than control individuals.5
The early identification of MS has implications not only for the implementation of interventions to prevent demyelinating events and the progression of neurodegeneration but also for the search of causal factors of the disease because it will allow more accurate identification of the relevant period of exposure to potential etiological factors. Therefore, we conducted a study to assess whether the levels of serum neurofilament light chain (sNfL), a sensitive biomarker of ongoing neuroaxonal degeneration,6 were elevated in the years before and around the time of clinical onset and could thus be a direct marker of the prodromal phase of MS.
Methods
Study Population
The source population of the study includes more than 10 million active-duty US military personnel who have at least 1 serum sample stored in the US Department of Defense Serum Repository (DoDSR).7 The DoDSR catalogs and stores more than 60 million serum samples remaining after routine HIV type 1 antibody testing and deployment-related blood tests. Personnel generally provide 1 serum sample at entry into the military and, on average, every 2 years after that. The samples are stored at −30°C.
Standard Protocol Approvals, Registrations, and Patient Consents
The research protocol was reviewed and approved by the institutional review boards of the Uniformed Services University of the Health Sciences and the Harvard T. H. Chan School of Public Health, both of which determined that a waiver of informed consent was appropriate for the use of already existing medical record data and biological samples.
Case and Control Ascertainment
The case patients with MS in this study were identified among active-duty military personnel with at least 1 presymptomatic serum sample stored in the DoDSR, as previously described.8,9 In short, the MS diagnoses were confirmed by review of the medical record and required an MS diagnosis made by a neurologist in addition to clinical history of 2 or more attacks and magnetic resonance imaging results consistent with MS. The date of clinical MS onset was defined as the date of the first neurologic symptoms attributable to MS documented in the medical record. Two control individuals were randomly selected from the DoDSR and matched to each case patient by age (±1 year), sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), and dates of sample collection (±30 days, except for the sample collected after the date of MS onset). Control individuals had to be on active duty on the date of onset of the matched case. Information on all matching, demographic, and serum sample characteristics was provided by the Armed Forces Health Surveillance Branch (data from the Defense Medical Surveillance System, The Armed Forces Health Surveillance Branch, Defense Health Agency; Silver Spring, Maryland; year of data, 2011-2013; release date of data, September 2016).
In this sNfL study, we selected 2 groups, each consisting of 30 case patients and 30 control individuals, among 245 previously identified case patients with MS (eFigure in the Supplement). We included only a subset of the 245 cases to reduce cost; further, we restricted the selection to cases with available serum samples collected at the specific times of interest for the study. In the first group, we included case patients who had provided at least 1 serum sample before MS onset and 1 sample soon after MS onset (defined as ≤2 years). The purpose of this group was to determine whether there was evidence of sNfL elevation around the time of onset of the first neurological symptoms. The second group was composed of cases with at least 2 presymptomatic serum samples, one sample collected more than 5 years before the date of MS diagnosis and the other collected between 2 and 5 years before the date of diagnosis, to determine whether sNfL elevation could be detected years before the onset or diagnosis of MS. In this group, we included participants who were diagnosed as having MS after age 30 years to maximize follow-up. The median interval from serum collection to MS onset was 6 years for the first sample and 1 year for the second sample. All of the samples in the second group were collected before MS onset even if they were selected by years they were collected prior to the date of diagnosis. Two MS cases were, by chance, included in both groups, but different samples were used for the 2 groups.
Assessment of sNfL
Pairs of serum samples (from 1 case and its matched control) were handled identically and assayed in the same batch. The order of the samples within each case-control pair was arranged at random to ensure that all assays were conducted without the knowledge of the case-control status.
We used an ultrasensitive single-molecule array assay (Simoa; UmanDiagnostics) to measure sNfL as previously described.10 The sNfL levels measured by the ultrasensitive single molecule array assay correlate strongly with NfL levels in the cerebrospinal fluid.10 Serum NfL has been found to be stable after 5 thaw-and-refreeze cycles and after 8 days at room temperature.11 One of the sNfL measurements failed for 1 control individual. The mean intraassay coefficient of variation based on 12 quality control samples was 10.1%; all coefficients of variation of concentrations of duplicate determinations were less than 20%. Measurements were performed on coded samples. The laboratory personnel had no access to clinical data and diagnosis.
Statistical Analyses
The sNfL levels were log transformed to improve normality. To account for the matched study design, we used paired t tests to compare sNfL levels in cases and controls at each time. Further, we used linear mixed-effects models with random intercepts for individual participants to compare repeatedly measured sNfL levels over time while adjusting for age (continuous) because sNfL levels increase approximately 2% per year in healthy control individuals.10 We used conditional logistic regression to estimate odds ratios (ORs) and 95% confidence intervals for the association of a within-person increase in sNfL levels with MS risk. Because multiple thresholds of within-person increase were compared, we adjusted for multiple comparisons using the Bonferroni correction. Because the control individuals in our study were selected from the DoDSR using risk-set sampling, the ORs estimate incidence rate ratios (RRs).12 To test whether the difference in sNfL levels between cases and controls varied based on the time until the clinical MS onset, we used a multilevel linear mixed-effects model with random intercepts for both matched case-control pairs and individual participants and included an interaction term between case-control status and time. Only participants in group 2 (with 2 presymptomatic samples) were included in this analysis. For the linear mixed models, we tested whether random slopes improved the model fit by comparing a model with random slopes with a model without random slopes using the likelihood-ratio test. Because this did not significantly improve the model fit (P from likelihood-ratio test greater than .05 and similar Akaike information criterion and Bayesian information criterion values), we did not include random slopes in the final models. The P values from the linear mixed models were calculated using the Kenward-Roger approximation. To illustrate differences in NfL levels in case patients and matched control individuals according to time to MS onset, we estimated the ratio of NfL levels in each case-control pair at each point and plotted these according to time to MS onset. All of the statistical analyses were conducted using STATA, version 15.1 (StataCorp) and R, version 3.6.0 (the R Foundation) using the survival, lme4, and afex packages. The figures were made using the ggplot2 and ggpol packages. The α level was set at .05, and all tests were 2-sided. The data sets analyzed in this study are not publicly available because of restricted access, but further information about the data sets is available from the corresponding author on reasonable request.
Results
Selected characteristics of the participants in the study are shown in Table 1 and were, owing to the matched design, similarly distributed in case patients and control individuals. In cases with both a presymptomatic serum sample (n = 30), which was collected at a median of 1 year (range, 0-3) before the first clinical symptoms, and a serum sample collected after the clinical onset, which was collected at a median 1 year (range, 0-2 years) after the first clinical symptoms, the sNfL levels were higher than in their matched control individuals at both points (Figure 1A). Most cases converting from the presymptomatic to the symptomatic phase experienced a marked increase in sNfL levels, from a median level of 25.0 pg/mL (interquartile range [IQR], 17.1-41.3 pg/mL) to a median level of 45.1 pg/mL (IQR, 27.0-102.7 pg/mL) (Figure 2A). There was no significant difference in the sNfL levels measured at the 2 points in the controls. A within-person increase in sNfL between the presymptomatic and the symptomatic measurement was associated with an increased MS risk (Table 2).
Table 1. Selected Characteristics of Case Patients With MS and Matched Control Individualsa.
| Characteristic | No. (%) | |
|---|---|---|
| Case Patients (n = 30) | Control Individuals (n = 30) | |
| Group 1b | ||
| Sex | ||
| Male | 23 (76.7) | 23 (76.7) |
| Female | 7 (23.3) | 7 (23.3) |
| Race/ethnicity | ||
| White | 18 (60.0) | 18 (60.0) |
| Black | 9 (30.0) | 9 (30.0) |
| Hispanic | 3 (10.0) | 3 (10.0) |
| Other | 0 | 0 |
| Age, mean (SD) [range], y | ||
| First serum sample collection | 27.8 (6.5) [18-42] | 27.9 (6.5) [19 -42] |
| Second serum sample collection | 29.1 (6.6) [20-44] | 29.3 (6.8) [20-44] |
| MS onset | 28.5 (6.4) [19-42] | NA |
| Group 2c | ||
| Sex | ||
| Male | 23 (76.7) | 23 (76.7) |
| Female | 7 (23.3) | 7 (23.3) |
| Race/ethnicity | ||
| White | 18 (60.0) | 18 (60.0) |
| Black | 8 (26.7) | 8 (26.7) |
| Hispanic | 1 (3.3) | 1 (3.3) |
| Other | 3 (10.0) | 3 (10.0) |
| Age, mean (SD) [range], y | ||
| First serum sample collection | 27.2 (4.5) [22-41] | 27.1 (4.4) [22-41] |
| Second serum sample collection | 31.8 (4.7) [26-45] | 31.9 (4.8) [26-45] |
| MS onset | 33.5 (5.0) [27-48] | NA |
Abbreviations: MS, multiple sclerosis; NA, not applicable.
Percentages may not add up to 100% owing to missing data or rounding.
Case patients with 1 presymptomatic and 1 postonset sample and matched control individuals.
Case patients with only presymptomatic samples and matched control individuals.
Figure 1. Boxplots of Serum Neurofilament Light Chain (sNfL) Levels in Presymptomatic and Symptomatic Case Patients With Multiple Sclerosis (MS) and Matched Control Individuals.
The figure illustrates levels of sNfL in cases with MS with 1 presymptomatic and 1 postonset measurement (A) and with repeated presymptomatic measurements (B). Log-transformed sNfL levels were compared using paired 2-tailed t tests.
Figure 2. Boxplots and Within-Person Variation of Serum Neurofilament Light Chain (sNfL) Levels in Presymptomatic and Symptomatic Case Patients With Multiple Sclerosis (MS) and Matched Control Individuals.
The figure illustrates levels of sNfL in case patients with MS with 1 presymptomatic and 1 postonset measurement (A) and with repeated presymptomatic measurements (B). Log-transformed sNfL levels were compared using linear mixed-effects models adjusted for age.
Table 2. Rate Ratio of Multiple Sclerosis According to Within-Person Increase in sNfL.
| Increase in sNfLa | No. (%) | RR (95% CI) | P Valueb | P Valuec | |
|---|---|---|---|---|---|
| Cases | Controls | ||||
| Within-person increase from presymptomatic to postonset samples | |||||
| ≥5 pg/mL | 16 (53.3) | 10 (33.3) | 2.25 (0.69-7.31) | .18 | .71 |
| ≥10 pg/mL | 14 (46.7) | 3 (10.0) | 6.00 (1.34-26.81) | .02 | .08 |
| ≥15 pg/mL | 14 (46.7) | 3 (10.0) | 6.00 (1.34-26.81) | .02 | .08 |
| ≥20 pg/mL | 13 (43.3) | 1 (3.3) | 12.00 (1.56-92.29) | .02 | .07 |
| Within-person increase in presymptomatic samples | |||||
| ≥5 pg/mL | 19 (63.3) | 6 (20.0) | 7.50 (1.72-32.80) | .007 | .03 |
| ≥10 pg/mL | 11 (36.7) | 2 (6.7) | 10.00 (1.28-78.12) | .03 | .11 |
| ≥15 pg/mL | 7 (23.3) | 1 (3.3) | 11.25 (1.15-110.04)d | .04d | .15 |
| ≥20 pg/mL | 6 (20.0) | 1 (3.3) | 9.03 (0.90-90.66)d | .06d | .25 |
Abbreviations: RR, rate ratio; sNfL, serum neurofilament light.
The categories are not mutually exclusive.
Raw P value.
P value adjusted for multiple comparisons using the Bonferroni correction.
Estimated using unconditional logistic regression owing to no discordant pairs in cases and matched controls that experienced an increase in sNfL. The model was adjusted for age, sex, and race/ethnicity.
In cases with 2 presymptomatic samples (n = 30), sNfL levels were statistically significantly higher than in their matched controls, both in samples collected at a median time of 6 years (range, 4-10 years) and 1 year (range, 0-4 years) before the first clinical symptoms (Figure 1B). The sNfL levels increased significantly in the case patients between the 2 points, with a median increase of 1.3 pg/mL per year (IQR, 0.5-3.7 pg/mL), while there was no significant difference in sNfL between the 2 points for the matched control individuals (Figure 2B). A within-person increase in presymptomatic sNfL levels was associated with a higher risk of MS (Table 2).
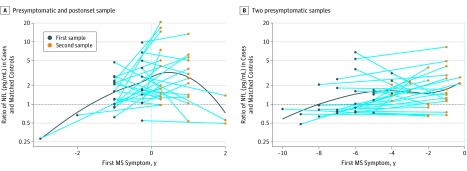
In analyses including participants with only presymptomatic samples (30 cases), sNfL levels increased closer to the time of clinical MS (P for interaction with time = .008). When we plotted the ratio (ie, the relative difference) of sNfL levels in each matched case-control pair according to time to MS onset, we observed that the ratio was highest around the time of clinical onset for the group with 1 presymptomatic and 1 postonset sample (Figure 3A); in the group with 2 presymptomatic samples, this ratio was higher than 1 in 6 of 6 pairs 4 years before MS onset and in 4 of 5 pairs 5 years before MS onset (Figure 3B). This supports the results from our main analyses indicating that sNfL elevations often precede the clinical onset of MS by several years.
Figure 3. Ratio in Serum Neurofilament Light Chain (sNfL) Levels in Case Patients With Multiple Sclerosis (MS) and Matched Control Individuals According to Time to the First Clinical Symptoms.
The figure illustrates the relative difference (ratio) in sNfL levels in case patients with MS and their matched control individuals in a group with 1 presymptomatic and 1 postonset sample (A) and in a group with 2 presymptomatic samples (B). Each dot represents 1 case-control pair. A locally estimated scatterplot smoothing curve illustrates the changes in the ratio over time.
Discussion
In this study, we found that sNfL levels were increased in case patients with MS compared with matched control individuals a median of 6 years before the first clinical symptoms. The levels of sNfL increased closer to the clinical onset, and the onset itself was associated with a marked increase in sNfL levels. These results are consistent with the notion that neuroaxonal degeneration often starts several years before the clinical MS onset.
Our study differs from previous studies on the prodromal phase of MS because these have used indirect markers of this phase, which included unspecific symptoms or disturbances occurring before the clinical onset4,5,13,14,15,16,17 compared with a marker of neurodegeneration. Overall, our findings are consistent with those of previous investigations on the prodromal phase of MS based on health care databases.4,5,15,16 Another potential sign of prodromal MS is a subtle decline in cognition. In a 2016 prospective study18 of men entering the military service, the mean cognitive performance, assessed by neurophysiological testing, was lower among men who developed symptoms of MS onset within the following 2 years, although not among those who developed MS after a longer interval.18
Serum NfL is a sensitive biomarker for disease activity in MS19 and is therefore useful to characterize the prodromal phase of the disease. As a specific marker of neuroaxonal degeneration,6 increasing serum levels are seen in patients with MS with more disease activity, such as gadolinium-enhancing magnetic resonance imaging lesions and relapses,10 and in patients with a higher degree of disability independently of ongoing relapses.10 Initiation of disease-modifying therapy is associated with a reduction in sNfL levels,20 and in 1 study,21 the levels in patients with MS were reduced to the levels of healthy control individuals 6 to 12 months after the treatment initiation. This could explain why some patients in our study had higher sNfL levels before their clinical onset compared with after. However, this also likely reflects the relapsing-remitting nature of MS because sNfL levels tend to increase in periods with increased disease activity and decrease thereafter.22 In patients with radiologically isolated syndrome, higher levels of NfL are associated with increased risk of both clinically isolated syndrome and MS,23 which is consistent with our findings indicating that sNfL levels increase already at the presymptomatic stage of MS.23 Serum NfL levels assessed by the fourth-generation immunoassay used in our study are highly associated with cerebrospinal fluid levels,10 indicating that serum levels capture ongoing neuroaxonal degeneration in the central nervous system. Thus, our findings of a presymptomatic increase in sNfL not only suggest the presence of a prodromal phase in MS but also that this phase is associated with neurodegeneration.
Better characterization of the prodromal phase is important for etiological research in MS. An exposure must precede the outcome to be causal,24 and with a prodromal phase that could last up to a decade, it may be challenging to separate risk factors from consequences of presymptomatic disease processes. If a range of unspecific symptoms characterizes the prodromal phase, as previously suggested,5 then these may lead to behavioral changes, such as changes in diet, that may be misinterpreted as risk factors if they are assessed too close to the clinical onset. An example of this is heat sensitivity, a common symptom in patients with MS,25 that may lead to sun avoidance and thus lower levels of vitamin D. If the prodromal phase of MS lasts several years and vitamin D is assessed during this period, it could lead to a spurious association between low vitamin D and higher MS risk induced by reverse causation. While low vitamin D has consistently been associated with higher MS risk, both in prospective studies where vitamin D was assessed many years before MS onset26,27 as well as in mendelian randomization studies,28,29 and thus is likely a risk factor, a long prodromal phase could influence the interpretation of other novel risk factors, especially when the exposure is assessed close to MS onset. Thus, more knowledge about the prodromal phase will also point to a time window when environmental risk factors are most likely to play a role for future MS risk.
Strengths and Limitations
Our study has several strengths. We included participants from a well-defined population, which minimizes the risk of selection bias when selecting the controls. Further, the study included repeated measures of sNfL and used medical records to confirm the MS diagnosis. The serum samples from cases and controls were analyzed at the same time, blindly, and in random order, which eliminates any artifactual difference in the sNfL concentrations. Our study also has some limitations to consider. First, owing to the small sample size, we could not evaluate specific cutoffs in sNfL that may indicate the start of the prodromal phase, which would be important for use of sNfL in a clinical setting, and the estimates have wide confidence intervals illustrating the uncertainty in the estimates. Further, we were not able to evaluate whether the presymptomatic sNfL levels varied with age at clinical onset, sex, or race/ethnicity. Larger and more extensive studies are thus warranted because a more detailed characterization of the prodromal phase is needed. Second, owing to the small number of women in our study, our findings largely reflect those of male participants, and further studies are needed to determine whether the findings can be generalized to women. Third, while sNfL is a specific marker of neuroaxonal damage, it is not a specific biomarker of MS. Thus, other conditions could potentially have caused increased levels. However, given the age distribution of our study population, the prevalence of other neurodegenerative diseases is very low, and while other conditions, such as traumatic brain injury, which occurs at a higher rate in the military population,30 could cause an increase in sNfL,31 we observed little within-person variation in the 2 repeated measurements in the control individuals, suggesting that there were few such incidences in our study. Further, because the length of the prodromal phase is uncertain, it is possible that some of the presymptomatic samples may overlap with the early symptomatic phase. Still, we made every effort to establish the time of onset of MS symptoms by a thorough review of medical records. Our US military study includes a longitudinal case ascertainment, and at the time of this study, we did not have information on potential confounders including serum levels of 25-hydroxyvitamin D or Epstein-Barr virus antibody levels; thus, we cannot exclude the possibility that the results may be affected by residual or unmeasured confounding that we cannot account for.
Conclusions
In summary, levels of sNfL were increased before the first clinical symptoms of MS, indicating that the clinical disease may have a prodromal phase that can last more than 5 years. This phase is associated with neurodegeneration, emphasizing the importance of early diagnosis and treatment.
eFigure. Flowchart of the Study
References
- 1.Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology. 2010;74(24):2004-2015. [DOI] [PubMed] [Google Scholar]
- 2.Rovira A, Swanton J, Tintoré M, et al. . A single, early magnetic resonance imaging study in the diagnosis of multiple sclerosis. Arch Neurol. 2009;66(5):587-592. [DOI] [PubMed] [Google Scholar]
- 3.Okuda DT, Siva A, Kantarci O, et al. ; Radiologically Isolated Syndrome Consortium (RISC); Club Francophone de la Sclérose en Plaques (CFSEP) . Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijnands JMA, Kingwell E, Zhu F, et al. . Health-care use before a first demyelinating event suggestive of a multiple sclerosis prodrome: a matched cohort study. Lancet Neurol. 2017;16(6):445-451. [DOI] [PubMed] [Google Scholar]
- 5.Disanto G, Zecca C, MacLachlan S, et al. . Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol. 2018;83(6):1162-1173. [DOI] [PubMed] [Google Scholar]
- 6.Khalil M, Teunissen CE, Otto M, et al. . Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577-589. [DOI] [PubMed] [Google Scholar]
- 7.Russell KL. The Department of Defense Serum Repository (DoDSR): a study of questions. Mil Med. 2015;180(10)(suppl):1-2. [DOI] [PubMed] [Google Scholar]
- 8.Levin LI, Munger KL, Rubertone MV, et al. . Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293(20):2496-2500. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838. [DOI] [PubMed] [Google Scholar]
- 10.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaiottino J, Norgren N, Dobson R, et al. . Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One. 2013;8(9):e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case-control studies estimate? survey of methods and assumptions in published case-control research. Am J Epidemiol. 2008;168(9):1073-1081. [DOI] [PubMed] [Google Scholar]
- 13.Berger JR, Pocoski J, Preblick R, Boklage S. Fatigue heralding multiple sclerosis. Mult Scler. 2013;19(11):1526-1532. [DOI] [PubMed] [Google Scholar]
- 14.Byatt N, Rothschild AJ, Riskind P, Ionete C, Hunt AT. Relationships between multiple sclerosis and depression. J Neuropsychiatry Clin Neurosci. 2011;23(2):198-200. [DOI] [PubMed] [Google Scholar]
- 15.Högg T, Wijnands JMA, Kingwell E, et al. . Mining healthcare data for markers of the multiple sclerosis prodrome. Mult Scler Relat Disord. 2018;25:232-240. [DOI] [PubMed] [Google Scholar]
- 16.Wijnands JMA, Zhu F, Kingwell E, et al. . Prodrome in relapsing-remitting and primary progressive multiple sclerosis. Eur J Neurol. 2019;26(7):1032-1036. [DOI] [PubMed] [Google Scholar]
- 17.Gout O, Lebrun-Frenay C, Labauge P, Le Page GE, Clavelou P, Allouche S; PEDIAS Group . Prior suggestive symptoms in one-third of patients consulting for a “first” demyelinating event. J Neurol Neurosurg Psychiatry. 2011;82(3):323-325. [DOI] [PubMed] [Google Scholar]
- 18.Cortese M, Riise T, Bjørnevik K, et al. . Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol. 2016;80(4):616-624. [DOI] [PubMed] [Google Scholar]
- 19.Kuhle J, Kropshofer H, Haering DA, et al. . Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novakova L, Zetterberg H, Sundström P, et al. . Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89(22):2230-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunnarsson M, Malmeström C, Axelsson M, et al. . Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann Neurol. 2011;69(1):83-89. [DOI] [PubMed] [Google Scholar]
- 22.Varhaug KN, Barro C, Bjørnevik K, et al. . Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. 2017;5(1):e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matute-Blanch C, Villar LM, Álvarez-Cermeño JC, et al. . Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain. 2018;141(4):1085-1093. [DOI] [PubMed] [Google Scholar]
- 24.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295-300. [PMC free article] [PubMed] [Google Scholar]
- 25.Flensner G, Ek AC, Söderhamn O, Landtblom AM. Sensitivity to heat in MS patients: a factor strongly influencing symptomology: an explorative survey. BMC Neurol. 2011;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology. 2017;89(15):1578-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79(21):2140-2145. [DOI] [PubMed] [Google Scholar]
- 28.Mokry LE, Ross S, Ahmad OS, et al. . Vitamin D and risk of multiple sclerosis: a mendelian randomization study. PLoS Med. 2015;12(8):e1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhead B, Bäärnhielm M, Gianfrancesco M, et al. . Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agimi Y, Regasa LE, Stout KC. Incidence of traumatic brain injury in the U.S. military, 2010-2014. Mil Med. 2019;184(5-6):e233-e241. [DOI] [PubMed] [Google Scholar]
- 31.Shahim P, Gren M, Liman V, et al. . Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of the Study