Abstract
Background:
Germline DNA damage repair gene mutation (gDDRm) is found in >10% of metastatic prostate cancer (mPC). Their prognostic and predictive impact relating to standard therapies is unclear.
Objective:
To determine whether gDDRm status impacts benefit from established therapies in mPC.
Design, setting, and participants:
This is a retrospective, international, observational study. Medical records were reviewed for 390 mPC patients with known gDDRm status. All 372 patients from Royal Marsden (UK), Weill-Cornell (NY), and University of Washington (WA) were previously included in a prevalence study (Pritchard, NEJM 2016); the remaining 18 were gBRCA1/2m carriers, from the kConFab consortium, Australia.
Outcome measurements and statistical analysis:
Response rate (RR), progression-free survival (PFS), and overall survival (OS) data were collected. To account for potential differences between cohorts, a mixed-effect model (Weibull distribution) with random intercept per cohort was used.
Results and limitations:
The gDDRm status was known for all 390 patients (60 carriers of gDDRm [gDDRm +], including 37 gBRCA2m, and 330 cases not found to carry gDDRm [gDDRm–]); 74% and 69% were treated with docetaxel and abiraterone/enzalutamide, respectively, and 36% received PARP inhibitors (PARPi) and/or platinum. Median OS from castration resistance was similar among groups (3.2vs 3.0 yr, p = 0.73). Median docetaxel PFS for gDDRm+ (6.8 mo) was not significantly different from that for gDDRm- (5.1 mo), and RRs were similar (gDDRm+ = 61%; gDDRm- = 54%). There were no significant differences in median PFS and RR on first-line abiraterone/enzalutamide (gDDRm+ = 8.3 mo, gDDRm- = 8.3 mo; gDDRm+ = 46%, gDDRm- = 56%). Interaction test for PARPi/platinum and gDDRm+ resulted in an OS adjusted hazard ratio of 0.59 (95% confidence interval 0.28–1.25; p = 0.17). Results are limited by the retrospective nature of the analysis.
Conclusions:
mPC patients with gDDRm appeared to benefit from standard therapies similarly to the overall population; prospective studies are ongoing to investigate the impact of PARPi/platinum.
Patient summary:
Patients with inherited DNA repair mutations benefit from standard therapies similarly to other metastatic prostate cancer patients.
Keywords: Biomarkers, BRCA, DNA repair, Genomics, Germline, Prostate cancer, Precision medicine
1. Introduction
Inherited mutations in DNA damage repair (DDR) genes associate with an increased risk of developing prostate, breast, ovarian, and other cancers [1,2]. We previously described enrichment of such mutations in metastatic prostate cancer (mPC), with 11.8% of these men harbouring germline DNA damage repair gene mutation (gDDRm) [3]. Mutations in BRCA2 were most prevalent (5.3%), with these data leading to a change in National Comprehensive Cancer Network (NCCN) guidelines, now recommending germline testing for all men with mPC [4]. Studies in mPC as well as in other diseases support tailored therapeutic approaches for this molecularly defined subset of patients [5–8].
Characterisation of the genomic landscape of prostate cancer has led to the identification of clinically actionable molecular alterations [9,10]. This renders an opportunity for a new classification of this common disease, beyond traditional anatomical and histological considerations, based on the prognostic and predictive significance of some of these alterations for treatment stratification.
Prior studies stated the role of germline BRCA2 mutations are an independent poor prognostic factor for localised prostate cancer, associated with a more aggressive phenotype, increased rates of developing metastatic disease, and shorter survival from the disease [11,12]. However, when focusing on patients with mPC, the prognostic and predictive roles of gDDRm are unclear. Prior case series have reported conflicting data with regard to the relative benefit derived for patients carrying gDDRm from standard of care therapies (taxanes, abiraterone acetate, enzalutamide) [13–15].
Herein, we retrospectively reviewed the clinical outcome of mPC patients with and without gDDRm. We included 372 patients from three institutions enrolled in a previously published prevalence study of gDDRm (Royal Marsden, UK; Weill-Cornell, NY; University of Washington, WA); in order to increase the number of gDDRm carriers in this analysis, we included an additional cohort of 18 known gBRCA1/2m carriers with mPC from the kConFab consortium (Australia).
2. Patients and methods
All patients included had previously been tested for gDDRm. Germline mutations were called based on a panel of 20 genes summarised in Supplementary Table 1. For all the 372 cases from the three UK and US sites, these data had been published in a prior report, including sequencing and bioinformatics methodology [3]. In the original study, patients were not selected on the basis of family history, age, or any knowledge of genetic background. The remaining 18 patients were an independent cohort of known germline BRCA1/2 germline mutation carriers from Australia. Patient medical records were retrospectively reviewed, and patients had received treatment according to local guidelines. Baseline characteristics (demographic characteristics, age, Gleason score, prostate-specific antigen [PSA] and presence of metastatic disease at diagnosis, treatment exposure, and survival data) were collected. Response data (defined as a 50% PSA fall from baseline and/or radiological response according to RECIST) and progression-free survival (PFS; defined as the time from start of a treatment to RECIST/PSA progression or start of a new therapy for clinical progression) for abiraterone, enzalutamide, and docetaxel were annotated.
To account for potential differences between the cohorts, a mixed-effect parametric survival model (Weibull distribution) with random intercept per cohort was used to study correlations with clinical outcome. Multivariate analyses adjusted for age, Gleason score, metastatic disease at diagnosis, and prior radical treatment at diagnosis (either radical prostatectomy or radiotherapy). Fisher’s exact test was used to study response rates to each therapy. A test for interaction was pursued for an exploratory subgroup analysis assessing the impact of PARP inhibitors (PARPi) and/or platinum therapy on patient outcome. Kaplan–Meier curves were used to represent time to event data.
3. Results
3.1. Baseline characteristics and treatment exposure
Clinical data were available for 390 patients including 330 not found to carry gDDRm (gDDRm–) and 60 cases with presence of gDDRm (gDDRm+). The distribution of genes mutated per case within the gDDRm+ group was as follows: BRCA2: 37; ATM: seven; CHEK2: four; BRCA1, PALB2, RAD51D: two each; others: seven (one patient had both ATM and CHEK2 mutations; Supplementary Tables 2 and 3). There were no significant differences in baseline characteristics based on gDDRm status (Table 1), including age at diagnosis (median of 62.6 vs 64.9 yr for gDDRm+ vs gDDRm–). Overall, 74% and 69% of patients received, respectively, docetaxel and novel androgen receptor signalling inhibitors (ARSIs: abiraterone acetate, enzalutamide) for metastatic castration-resistant prostate cancer (mCRPC). Based on the cross resistance demonstrated between abiraterone acetate and enzalutamide, in this analysis we considered only the first exposure to either abiraterone acetate or enzalutamide. Of note, 28/60 (47%) gDDRm+ and 113/330 (34%) gDDRm–patients also received treatment with PARPi and/or platinum chemotherapy, treatments that are not currently routinely used for prostate cancer care, reflecting the research focus of the involved academic groups.
Table 1 -.
Baseline characteristics of the study population (n = 390)
| Patients with any germline mutation (n = 60) | Patients without germline mutation (n = 330) | p valuea | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Gleason score | |||||
| 5–7 | 15 | 28.9 | 105 | 37.5 | 0.27 |
| 8–10 | 37 | 71.2 | 175 | 62.5 | |
| Metastatic disease at diagnosis | |||||
| No | 34 | 58.6 | 173 | 53.7 | 0.57 |
| Yes | 24 | 41.4 | 149 | 46.3 | |
| Received radical treatment | |||||
| No | 22 | 36.7 | 140 | 42.4 | 0.48 |
| Yes | 38 | 58.5 | 190 | 57.6 | |
| Docetaxel | |||||
| No | 16 | 26.7 | 88 | 26.7 | 1.00 |
| Yes | 44 | 73.3 | 242 | 73.3 | |
| Abiraterone and/or enzalutamide | |||||
| No | 18 | 30 | 101 | 30.6 | 1.00 |
| Yes | 42 | 70 | 229 | 69.4 | |
| PARPi and/or platinum | |||||
| No | 32 | 53.3 | 217 | 65.8 | 0.08 |
| Yes | 28 | 46.7 | 113 | 34.2 | |
| Radium-223 | |||||
| No | 52 | 86.7 | 296 | 90.2 | 0.37 |
| Yes | 8 | 13.3 | 32 | 9.8 | |
| Median | Q1–Q3 | Median | Q1–Q3 | p valueb | |
| Age at diagnosis (yr) | 62.6 | 55.3–66.2 | 62.4 | 57.7–68.5 | 0.24 |
| PSA (ng/dl) | 17.2 | 7.7–109.6 | 33.0 | 9.8–148.3 | 0.34 |
PARPi = PARP inhibitors; PSA = prostate-specific antigen.
Fisher’s exact test.
Wilcoxon rank sum test.
3.2. Prognosis of patients with gDDRm
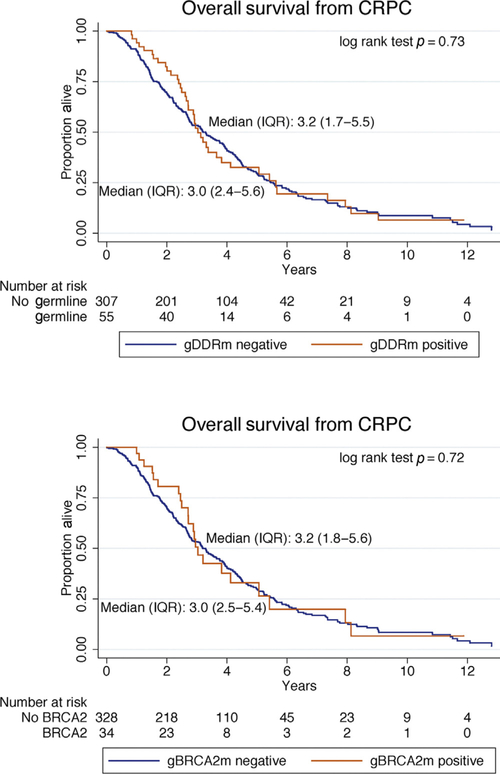
Overall survival (OS) was similar in the two subgroups, with 296 death events (75% of the study population), median OS from castration resistance was 3.0 yr for gDDRm+ (inter-quartile range [IQR] 2.4–5.6), 3.0 yr for gBRCA2+ (IQR 2.5–5.4), and 3.2 yr for gDDRm- (IQR 1.7–5.5; log-rank test p = 0.73). In multivariate analysis, age at diagnosis (per 10 yr older, adjusted hazard ratio [aHR] 1.45, 95% confidence interval [CI] 1.21–1.73; p < 0.001), and Gleason score ≥8 (aHR 1.54, 95% CI 1.16–2.04; p = 0.003), but not germline mutations (aHR 0.93, 95% CI 0.63–1.37; p = 0.72) were associated with worse survival. When looking specifically at the impact of germline BRCA2 mutations, these were also not associated with a significantly different prognosis (aHR 0.83, 95% CI 0.50–1.36, p = 0.45; Table 2 and Fig. 1).
Table 2 -.
Overall survival from castration resistance and progression-free survival to standard therapies
| aHR (MVA) | 95% CI | p value | |
|---|---|---|---|
| OS from castration resistance | |||
| Any gDDRm+ | 0.93 | 0.63–1.37 | 0.72 |
| Age at diagnosis (per 10 yr) | 1.45 | 1.22–1.73 | <0.001 |
| Gleason 8–10 | 1.54 | 1.16–2.04 | 0.003 |
| Metastatic disease | 1.22 | 0.84–1.75 | 0.30 |
| Radical treatment | 1.50 | 1.03–2.18 | 0.03 |
| HR | 95% CI | p value | |
| PFS docetaxel | |||
| Any gDDRm + | 0.86 | 0.61–1.20 | 0.37 |
| Only gBRCA2m+ | 0.96 | 0.64–1.43 | 0.83 |
| PFS first line of ARS therapy | |||
| Any gDDRm + | 0.96 | 0.69–1.35 | 0.83 |
| Only gBRCA2m+ | 1.10 | 0.72–1.67 | 0.67 |
aHR = adjusted hazard ratio; ARS = androgen receptor signal; CI = confidence interval; gDDRm = germline DNA damage repair gene mutation; MVA = multivariate analysis; PSA = prostate-specific antigen. Results from a mixed-effect survival model (Weibull distribution) with random intercept per cohort.
Fig. 1 -.
Kaplan–Meier curves for survival from date of castration resistance and from initial diagnosis based on the presence of gDDRm and specifically for gBRCA2m carriers. CRPC = castration-resistant prostate cancer; gDDRm = germline DNA damage repair gene mutation; IQR = interquartile range.
3.3. gDDRm and docetaxel
On docetaxel chemotherapy, gDDRm did not associate with significantly different PFS (HR 0.86, 95% CI 0.61–1.20, p = 0.37); similar results were observed when evaluating germline BRCA2 mutation carriers alone (HR 0.96, 95% CI 0.64–1.43, p = 0.83). Kaplan–Meier curves for PFS on docetaxel are shown in Figure 2. Response rate to docetaxel was 61% and 54% for gDDR+ and gDDR- patients, respectively (Fisher’s exact p = 0.48, Supplementary Table 4); this resulted in an odds ratio of response to docetaxel of 1.33 (95% CI 0.66–2.69; p = 0.43) for patients carrying gDDRm compared with gDDRm- patients.
Fig. 2 -.
Kaplan–Meier curves for progression-free survival on docetaxel and first-line ARSI therapy based on the presence of any gDDRm. ARSI = androgen receptor signalling inhibitor; gDDRm = germline DNA damage repair gene mutation; IQR = interquartile range; PFS = progression-free survival.
3.4. gDDRm and ARSIs (abiraterone, enzalutamide)
PFS on first ARSI (either abiraterone or enzalutamide) for mCRPC was not significantly different for patients with or without gDDRm (HR 0.93, 95% CI 0.65–1.32, p = 0.67), with similar median PFS for gDDRm+ (8.3 mo) and gDDRm- (8.3 mo; Fig. 2). Patients with BRCA2 mutations also had similar PFS to the overall population (HR 1.09, 95% CI 0.72–1.67, p = 0.66). Response rates to the first ARSI were 46% and 56% for gDDRm+ and gDDRm- patients, respectively (Fisher’s exact p = 0.28, Supplementary Table 4), resulting in a nonsignificant trend towards a lower chance of response for gDDRm+ (odds ratio 0.65, 95% CI 0.32–1.32, p = 0.23).
3.5. PARPi and platinum in patients with gDDRm
In this cohort, 141 (36%) patients had received PARPi and/or platinum chemotherapy, including 28/60 (47%) gDDRm+ cases. We explored the potential interaction of these treatments and gDDRm on survival from castration resistance in this cohort.
There was no statistically significant impact from PARPi/platinum on OS for the overall population (aHR 0.97, 95% CI 0.73–1.31; p = 0.88). The hazard of death based on the presence of gDDR mutations once adjusted for exposure to PARPi/platinum indicated no statistically significant difference in risk of death (aHR 1.23; 95% CI 0.73–2.07; p = 0.44).
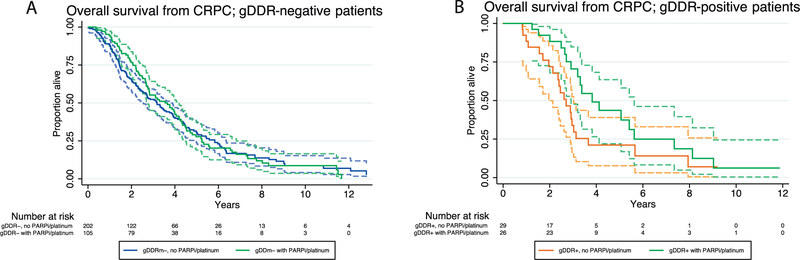
An interaction test between gDDRm+ and PARPi/platinum therapy revealed an aHR of 0.59 (95% CI 0.28–1.25; p = 0.17). These data suggest that the association of gDDRm status and survival could have been impacted by the exposure to PARPi/platinum. Nevertheless, with this size of the gDDRm+ subgroup, no statistically significant differences were observed in this cohort and the null hypothesis could not be excluded. Survival curves illustrating the impact of PARPi/platinum by gDDRm status are shown in Figure 3.
Fig. 3 -.
Kaplan–Meier curves depicting survival from detection of castration resistance, with patients grouped by exposure to PARP inhibitors and/or platinum therapy for (A) gDDRm+ or (B) gDDRm- metastatic castration-resistant prostate cancers. Dashed lines indicate the 95%CI limits. CI = confidence interval; CRPC = castration-resistant prostate cancer; gDDR = germline DNA damage repair; gDDRm = germline DNA damage repair gene mutation; IQR = interquartile range; PARPi = PARP inhibitor.
4. Discussion
In this study, we retrospectively reviewed clinical outcome of lethal prostate cancer patients according to their gDDRm status [3]. Overall, we did not observe significant differences in response rate and PFS from docetaxel and ARSIs based on gDDRm status, suggesting that gDDRm+ carriers derive benefit from these therapies similarly to the overall population. These data are of major interest to the clinical community at this time in view of changes in NCCN guidelines in 2018 recommending germline testing for all men suffering from mPC [4].
Prior analyses interrogating this question have reported conflicting results. A recent retrospective study including 319 patients (22 gDDRm+, 16 being germline BRCA2 mutation carriers) reported shorter OS and worse outcome from abiraterone/enzalutamide treatment, but not from docetaxel for mCRPC patients with gDDRm [13]. Preliminary results of a prospective clinical trial of abiraterone and the PARP inhibitor veliparib suggested conversely that prostate cancer patients with DDR defects (here including germline and somatic alterations) may actually be more likely to respond to abiraterone acetate therapy [16]. Differences in the baseline characteristics, genes included in each analysis, and distribution and prevalence of mutations between study populations may have accounted for these differences. The retrospective nature of ours and other studies is a significant limitation, and prospective validation is required in ongoing studies [15]. Data from breast cancer studies also suggest that patients with germline BRCA1/2 mutations derive significant benefit from taxane-based chemotherapy [17].
A notable distinction of our patient cohort was the substantial proportion of patients treated with PARP inhibitors and/or platinum chemotherapy, which are not part of the standard of care for prostate cancer. This has to be taken into account when comparing the survival analysis in this study to others, since the introduction of these treatments may have impacted outcome. The use of such therapies should not, however, have impacted response data to the specific standard therapies presented here, since these were largely administered prior to the PARP inhibitor or platinum therapy. We observed a trend towards prolonged OS in gDDRm+ patients receiving PARPi/platinum. This interaction was not, however, statistically significant in this small gDDRm+ cohort, and may be a chance finding or have been impacted by other unrecognised confounding factors [7,8].
Another limitation of our study is the focus on germline, to the exclusion of somatic only, mutations [9,18,19]. It is estimated that 20–25% mPC have somatic inactivation of a DNA repair gene, but just less than half of these carry a germline mutation. Hence, it is likely that a substantial proportion of our cases in the gDDRm- group harboured somatic DDR defects and that some but not all the gDDRm+ cases would have had somatic inactivation of the second allele. Moreover, the lack of somatic DNA data for this cohort also prevented us from analysing the impact of other concurrent genomic events influencing prostate cancer progression, such as AR, TP53, or RB1 aberrations. Studies assessing clinical outcome to specific therapies incorporating somatic genomic data are ongoing and will be fundamental to shape precision medicine strategies in mCRPC and complement ongoing clinical trials of DNA repair targeting agents in CRPC. These studies and prospective clinical trials will also need to control for other potential prognostic factors not assessed in this retrospective study.
5. Conclusions
The data presented here suggest that mPC patients with inherited mutations in DDR genes, including those with BRCA2 mutations, can derive similar benefit from standard of care therapies in terms of both response rate and PFS. Based on the limitations described, we acknowledge that this study may not be sufficient to fully inform clinical decisions; in view of the discrepancies identified among different retrospective analyses, prospective studies are now needed evaluating the impact of germline DNA repair mutations in advanced prostate cancer, beyond their clear importance to prompt family cascade counselling. Nevertheless, our overall data indicate that detection of gDDRm should not preclude mPC patients from receiving taxanes, abiraterone, and enzalutamide as standards of care. Pivotal clinical trials of PARPi are ongoing for prostate cancer sufferers with germline and somatic DDRm, and may offer additional therapy options for this group of patients.
Supplementary Material
Acknowledgements:
This work was supported by a Stand Up To Cancer-Prostate Cancer Foundation Prostate Dream Team Translational Cancer Research Grant. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research (SU2C-AACR-DT0712). We also acknowledge support from Movember and Prostate Cancer UK, and from the KConFab consortium (Kathleen Cuningham Foundation Consortium for research into familial breast cancer; Australia) for providing data for this report. J. Mateo, H. Cheng, H. Beltran, and C. Pritchard were supported by Prostate Cancer Foundation Young Investigator Awards. We also acknowledge funding support from NIH/NCI (P50CA097186), the Institute for Prostate Cancer Research (Seattle, WA, USA), a Medical Research Council-Prostate Cancer UK fellowship (J. Mateo), an Experimental Cancer Medical Centre grant, and a Biomedical Research Centre grant to the ICR/Royal Marsden. S. Sandhu was supported by a Prostate Cancer Foundation of Australia Young Investigator Award.
Financial disclosures: Johann S. de Bono certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: J. Mateo, D. Dolling, H. Mossop, P. Rescigno, R. Perez-Lopez, M. Kolinsy, A. Balasopoulou, C. Bertan, S. Carreira, and J. de Bono are employees of the Institute of Cancer Research, which is a joint applicant in patents involving PARP inhibitors and receive royalties from the development of abiraterone. S.T. Tagawa declares honoraria from Sanofi and Janssen. M.A. Rubin is a cofounder of Thucydx LLC. J. de Bono has served as an advisor for AZ, Janssen, Pfizer, Qiagen, Sanofi-Aventis.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eururo.2018.01.010.
References
- [1].Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer 2011;105:1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eeles R, Goh C, Castro E, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol 2014;11:18–31. [DOI] [PubMed] [Google Scholar]
- [3].Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016;375:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian. 2018. https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf
- [5].Fong PCP, Boss DDS, Yap TA, et al. Inhibition of poly (ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–34. [DOI] [PubMed] [Google Scholar]
- [6].Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382–92. [DOI] [PubMed] [Google Scholar]
- [7].Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cheng HH, Pritchard CC, Boyd T, Nelson PS, Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol 2016;69:992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robinson D, Van Allen EM, Wu Y- M, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abeshouse A, Ahn J, Akbani R, et al. The molecular taxonomy of primary prostate cancer. Cell 2015;163:1011–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Castro E, Goh C, Leongamornlert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. Eur Urol 2015;68:186–93. [DOI] [PubMed] [Google Scholar]
- [13].Annala M, Struss WJ, Warner EW, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair–deficient prostate cancer. Eur Urol 2017;72:34–42. [DOI] [PubMed] [Google Scholar]
- [14].Gallagher DJ, Cronin AM, Milowsky MI, et al. Germline BRCA mutation does not prevent response to taxane-based therapy for the treatment of castration-resistant prostate cancer. BJU Int 2012;109:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Castro E, Romero-Laorden N, Piulats J, et al. PROREPAIR-B: a prospective cohort study of DNA repair defects in metastatic castration resistant prostate cancer. Ann Oncol 2017;28(Suppl 5):LBA32. [Google Scholar]
- [16].Hussain M, Daignault S, Twardowski P, et al. Co-targeting androgen receptor (AR) and DNA repair: a randomized ETS gene fusion-stratified trial of abiraterone + prednisone (Abi) +/− the PARP1 inhibitor veliparib for metastatic castration-resistant prostate cancer (mCRPC) patients (pts) (NCI9012). J Clin Oncol 2016;34 (Suppl):5010. [Google Scholar]
- [17].Hahnen E, Lederer B, Hauke J, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer. JAMA Oncol 2017;3:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beltran H, Yelensky R, Frampton GM, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol 2013;63:920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abida W, Armenia J, Gopalan A, Brennan R. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol 2017. 10.1200/PO.17.00029, Epub 2017 May 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.