Abstract
Objectives
Unknown onset stroke (UOS) is usually excluded from intravenous thrombolysis concerning the unclear symptom onset time. Attempts have been done to use thrombolytic therapy in these patients. The current meta-analysis was done to examine the efficacy and safety of intravenous thrombolysis in UOS.
Methods
PubMed, Web of Science, and Cochrane Library were searched for studies comparing thrombolysis with conservative therapy among UOSs. Data of good outcome (mRS, 0-2), mortality, and intracerebral hemorrhage (ICH) and symptomatic ICH (sICH) were extracted and analyzed using the Revman 5.2 software.
Results
In total, 8 studies with 1271 subjects (542 with thrombolysis and 729 with conservative therapy) were included in this meta-analysis. The data showed that patients receiving thrombolysis had a higher incidence of 90-day good outcome (P = 0.0005) than conservative therapy. The comparison of discharge (P = 0.89) and 90-day mortality (P = 0.10) in both groups did not find any significances. The incidences of ICH (P = 0.42) and sICH (P = 0.06) were relatively comparable between the two therapies.
Conclusions
Intravenous thrombolysis is a better choice for UOS patients for its efficacy and safety. In addition, pretreatment imaging assessment is beneficial for improving the efficacy of thrombolytic therapy. However, it needs more supporting evidences for clinical use in the future.
1. Introduction
Ischemic stroke is one of the most common causes of death globally [1, 2]. Intravenous tissue plasminogen activator (rtPA) is recommended for acute ischemic stroke within the time window [3, 4]. It is proven to be effective to save neurological functions against stroke in clinical practice, and it has become a keystone of acute stroke treatment [5, 6].
However, in a certain proportion of stroke sufferers, the clear time point of symptom onset cannot be known. Patients with unknown onset stroke (UOS) may wake up with stroke (WUS) symptoms or cannot state the exact time for unconsciousness [7]. This kind of stroke then poses a challenge for neurological physicians to make appropriate therapeutic decisions for these patients. The unclear symptom onset time may lead to the exclusion of many patients from the first-line thrombolytic therapy. However, efforts have been done to investigate the clinical features and the possibility to apply thrombolytic agents in these patients. Aoki et al. [8] and Schwamm et al. [9] found that thrombolytic therapy was safe and effective in patients with UOS who had diffusion-weighted imaging (DWI)/fluid-attenuated inversion recovery (FLAIR) mismatch. Some studies indicated the similar imaging and clinical characteristics between UOS and stroke with known onset time [10]. And intravenous rtPA may still be beneficial for these patients [11, 12]. But there is still a controversy facing the therapeutic selection for this kind of stroke. Therefore, we conduct this meta-analysis to summarize the current evidences in this field.
2. Methods
2.1. Search Strategy
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [13] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) [14] recommendations. PubMed, Web of Science, and Cochrane Library were searched up to September 1, 2018, using the following terms with various combinations: (i) unknown onset stroke or unclear onset stroke or UOS or wake-up stroke or WUS and (ii) thrombolysis or thrombolyticor fibrinolysis or urokinase or alteplase or rt-PA or rtPA or t-PA or tPA. The articles yielded were then analyzed by two independent researchers for potential studies comparing intravenous thrombolysis with conservative therapy in UOS patients. Studies without clear description of patient characteristics or treatment details or outcomes were excluded. Since the study was done based on the published articles, no ethical approval and patient consent were needed.
2.2. Data Extraction
Data in each study were extracted by two authors independently. Any disagreement was resolved by consensus-based discussion among the authors and determined by the senior author. UOS means strokes with discordant last-known normal time and first-found abnormal time [15, 16]. Data, including authors, publication year, trial design, study period, patient number, age, gender, disease history, Toast classification [17], time parameters, neuroimaging methods, National Institutes of Health Stroke Scale (NIHSS) [18], modified Rankin Scale (mRS), and the incidence of intracerebral hemorrhage (ICH) and symptomatic ICH (sICH), were included. For imaging details, technics applied in each study were collected, including noncontrast CT, CT perfusion, CT angiography, and magnetic resonance imaging (MRI) sequences. Time parameters included time from last seen normal (LSN) to symptom onset, LSN to door, LSN to thrombolysis, symptom to door, symptom onset to thrombolysis, and door to treatment. Treatment efficacy was measured at two levels: discharge and 90-day post charge, defined as good outcome (mRS 0-2) and mortality [19]. Therapy safety was assessed using the development of ICH and sICH. sICH was defined as any ICH detected on noncontrast computed tomography (CT) associated with a greater than or equal to a 4-point increase in NIHSS within 48 hours after treatment [20, 21].
2.3. Quality Assessment, Sensitivity Analysis, and Publication Bias Assessment
The quality assessment of observational studies was done according to the Newcastle-Ottawa Quality Assessment Scale [22, 23] in terms of patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 was used for each study. Studies that achieved six or more stars were considered to be of high quality. The Cochrane Risk of Bias Tool [24, 25] was adopted to explore the risk of bias for each randomized controlled trial (RCT). The following items were analyzed: generation of allocation sequence, allocation concealment, blinding (participants and personnel), blinding (outcome assessment), incomplete outcome data, selective reporting, and other sources of bias. Sensitivity analysis was done using the leave-one-out method to test the stability of the results and the source of heterogeneity if necessary. Publication bias analysis was done with funnel plots if the number of included studies exceeded 10.
2.4. Statistical Analysis
The meta-analysis was done using the Revman 5.2 software. The odds ratio (OR) was used to compare dichotomous variables. All results were displayed with 95% confidence intervals (CIs). Heterogeneity was quantified by the estimated I2 with a Cochrane Q test. When the level of I2 was ≥50% or P ≤ 0.10, the results were considered by the application of the random effects model. Otherwise, it was considered using the fixed effects model.
3. Results
3.1. Study Search and Study Characteristics
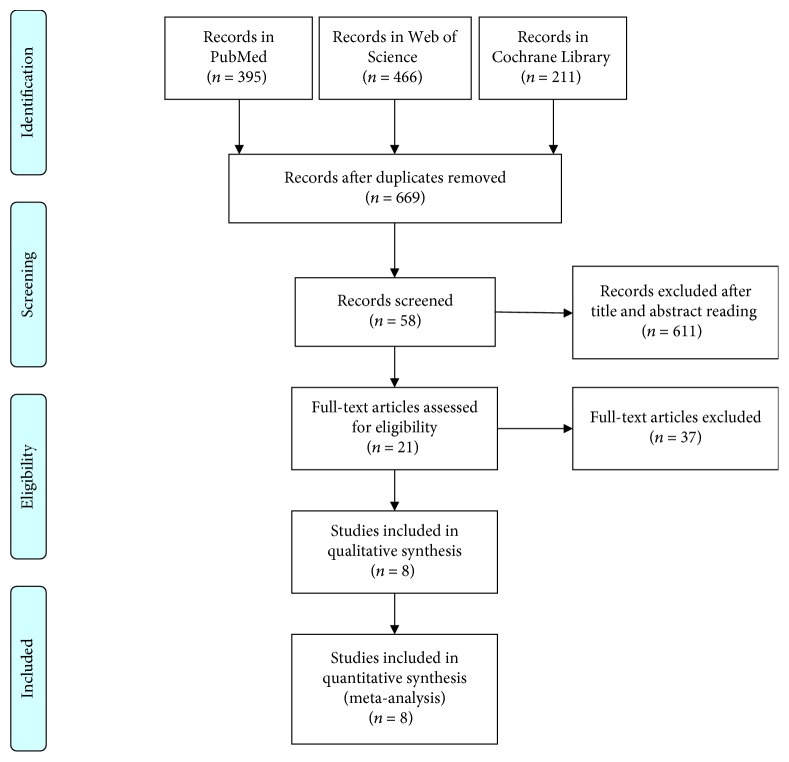
The search diagram was shown in Figure 1. A systematic search in PubMed, Web of Science, and Cochrane Library yielded 1072 articles. Then 403 duplicates were removed. After screening by going through titles, abstracts, and whole texts, there were 8 studies [12, 15, 16, 25–29] with 1271 subjects (542 with thrombolysis and 729 with conservative therapy) after screening (Table 1). Six countries contributed to the production of the studies: USA [15, 16], Germany [12, 28], Switzerland [29], Korea [16], UK [27], and Canada [25]. Among them, 3 were retrospective studies [15, 25, 26], 3 were prospective studies [16, 27, 28], and 2 were RCTs [12, 29]. The Toast classification was available in 7 studies [15, 16, 25–27, 29], and one single study only enrolled those patients with proximal large artery occlusion [25]. All the studies used rtPA as the thrombolytic agent.
Figure 1.
Flow diagram of the study inclusion process in this meta-analysis.
Table 1.
The baseline characteristics of the included studies in the meta-analysis (study quality of non-RCTs was shown).
| Study | Barreto 2009 [15] | Breuer 2010 [28] | Michel 2011 | Kang 2012 [16] | Manawadu 2013 [27] | Bal 2014 [25] | Anaissie 2016 [26] | Thomalla 2018 [12] |
|---|---|---|---|---|---|---|---|---|
| Region | USA | Germany | USA | Korea | UK | Canada | USA | Germany |
| Design | Retrospective | Prospective | RCT | Prospective | Prospective | Retrospective | Retrospective | RCT |
| Period | 2003.03-2008.01 | 2006.10-2008.05 | 2004.06-2007.12 | 2006.09-2009.07 | 2009.01-2010.12 | 2003.01-2010.03 | 2008.07-2014.05 | 2012.09-2017.06 |
| Outcome assessment time | NA | 90 days | 90 days | 90 days | 90 days | 90 days | 90 days | 90 days |
| Admission time (h) | 3 | 6 | 3 | 3 | 4.5 | NA | 4.5 h | 4.5 h |
| Preexamination | All NCT, 10 CT perfusion, 6 MRI | All NCT, 43 MRI, 2 CTA | All NCT+CT perfusion | All NCT+MRI | All NCT, 64 CTP | All NCT, CTA | All NCT | All NCT+MRI |
| No. of patients | ||||||||
| Thrombolysis | 46 | 10 | 6 | 83 | 68 | 29 | 46 | 254 |
| Conservative | 34 | 35 | 6 | 156 | 54 | 41 | 154 | 249 |
| Age (y) | ||||||||
| Thrombolysis | 62 ± 14a | 73 (57-92)b | 69.5 (57-78)b | NA | 73.9 ± 15.6a | 68 (23)c | 69 (42-98)b | 65.3 ± 11.2a |
| Conservative | 64 ± 13a | 66 (39-87)b | 49 (44-78)b | NA | 70.6 ± 16.7a | 74 (21)c | 63 (22-93)b | 65.2 ± 11.9a |
| Gender | ||||||||
| Thrombolysis | 39 | 5 | 3 | 55 | 23 | 12 | 22 | 165 |
| Conservative | 44 | 23 | 3 | 88 | 28 | 17 | 85 | 160 |
| NIHSS | ||||||||
| Thrombolysis | 16 (3-24)b | 10.5 (1-22)b | 17 (13-21)b | 14 (10-18)c | 11.5 (8-17)c | 14 (11)c | 9.5 (1-27)b | 6 (4–9)c |
| Conservative | 10.5 (2-26)b | 6 (1-21)b | 14.5 (12-19)b | 12 (6.25-17)c | 9 (5-15)c | 13 (11)c | 5 (0-33)b | 6 (4–9)c |
| HT | ||||||||
| Thrombolysis | 30 | 9 | 4 | 54 | 45 | 18 | 36 | 135 |
| Conservative | 22 | 29 | 1 | 94 | 32 | 27 | 119 | 131 |
| DM | ||||||||
| Thrombolysis | 10 | 4 | 1 | 23 | 14 | 3 | 20 | 43 |
| Conservative | 11 | 13 | 0 | 57 | 13 | 7 | 45 | 39 |
| CAD | ||||||||
| Thrombolysis | 8 | 3 | 0 | NA | NA | NA | NA | NA |
| Conservative | 5 | 6 | 0 | NA | NA | NA | NA | NA |
| Smoking | ||||||||
| Thrombolysis | NA | 1 | 0 | 30 | 8 | 13 | NA | NA |
| Conservative | NA | 9 | 2 | 54 | 7 | 19 | NA | NA |
| AF | ||||||||
| Thrombolysis | NA | NA | 2 | NA | 21 | 10 | 6 | 30 |
| Conservative | NA | NA | 0 | NA | 9 | 18 | 21 | 29 |
| Previous stroke | ||||||||
| Thrombolysis | NA | 5 | NA | 19 | NA | NA | NA | 37 |
| Conservative | NA | 9 | NA | 31 | NA | NA | NA | 31 |
| Hyperlipidemia | ||||||||
| Thrombolysis | 12 | 8 | 4 | 24 | 22 | 9 | 19 | 93 |
| Conservative | 9 | 27 | 3 | 40 | 25 | 6 | 60 | 85 |
| TOAST classification | ||||||||
| Cardioembolic | ||||||||
| Thrombolysis | 20 | 4 | 2 | 31 | 33 | NA | 11 | NA |
| Conservative | 14 | 8 | 0 | 57 | 21 | NA | 34 | NA |
| Large artery atherosclerosis | ||||||||
| Thrombolysis | 15 | 2 | 2 | 46 | 14 | NA | 8 | NA |
| Conservative | 7 | 10 | 0 | 77 | 11 | NA | 27 | NA |
| Small vessel | ||||||||
| Thrombolysis | 1 | 1 | 0 | 0 | 4 | NA | 3 | NA |
| Conservative | 5 | 7 | 4 | 0 | 12 | NA | 53 | NA |
| Unknown | ||||||||
| Thrombolysis | 5 | 2 | 2 | NA | 13 | NA | 17 | NA |
| Conservative | 5 | 8 | 2 | NA | 13 | NA | 25 | NA |
| Other | ||||||||
| Thrombolysis | 5 | 1 | 0 | 6 | 4 | NA | 7 | NA |
| Conservative | 2 | 2 | 0 | 22 | 2 | NA | 14 | NA |
| LSN to symptom onset | ||||||||
| Thrombolysis | NA | NA | NA | NA | NA | NA | NA | 7.2 (4.7–8.7) hc |
| Conservative | NA | NA | NA | NA | NA | NA | NA | 7.0 (5.0–9.0) hc |
| LSN to door | ||||||||
| Thrombolysis | NA | 508 (200-691) minb | NA | 8.6 (5.4-11.1) hc | NA | NA | NA | NA |
| Conservative | NA | 577 (182-849) minb | NA | 7.8 (4.9-11.7) hc | NA | NA | NA | NA |
| LSN to thrombolysis | ||||||||
| Thrombolysis | 10.7 ± 4.3 ha | NA | 564 (390–805) minb | NA | NA | NA | NA | 10.3 (8.1–12.0) hc |
| Conservative | NA | NA | 437.5 (330–656) minb | NA | NA | NA | NA | 10.4 (8.1–12.1) hc |
| Symptom onset to door | ||||||||
| Thrombolysis | 2.0 ± 1.9 ha | 93 (20-287) minb | NA | 1.7 (0.9-2.7) hc | NA | 526 ± 112 mina | NA | 2.6 (1.9–3.3) hc |
| Conservative | 3.7 ± 3.6 ha | 95 (38-360) minb | NA | 2.0 (1.0-3.6) hc | NA | 540 ± 140 mina | NA | 2.6 (2.1–3.3) hc |
| Symptom onset to thrombolysis | ||||||||
| Thrombolysis | 4.3 ± 3.3 ha | NA | NA | 4.6 (2.8-6.0) hc | NA | 516 ± 160 mina | NA | 3.1 (2.5–3.8) hc |
| Conservative | NA | NA | NA | NA | NA | NA | NA | 3.2 (2.6–3.9) hc |
| Door to treatment | ||||||||
| Thrombolysis | 2.4 ± 1.9 ha | 80 (45–127) minb | 109.5 (85–131) minb | 155 (100–195) minc | NA | NA | NA | 25 (16–35) minc |
| Conservative | NA | NA | 113 (75–205) minb | NA | NA | NA | NA | 26 (18–37) minc |
| Good outcome | ||||||||
| Thrombolysis | 13 | 5 | 4 | 37 | 25 | 14 | 22 | 188 |
| Conservative | 4 | 21 | 1 | 51 | 14 | 17 | 66 | 162 |
| Mortality | ||||||||
| Thrombolysis | 7 | 2 | 0 | NA | 10 | 7 | 2 | 10 |
| Conservative | 0 | 0 | 0 | NA | 14 | 3 | 6 | 3 |
| NIHSS at 24 h | ||||||||
| Thrombolysis | NA | 7.5 (0-18)b | 10.5 (7-19)b | NA | 6 (2, 13.5)c | 10 (13)c | 4 (0-24)b | NA |
| Conservative | NA | 5 (0-21)b | 19.5 (6-24)b | NA | 5 (3, 10)c | 10.5 (15.5)c | 3 (0-29)b | NA |
| NIHSS change at 24 h | ||||||||
| Thrombolysis | NA | NA | NA | NA | -4 (-8, 0)c | NA | -2 (-16, 19)b | NA |
| Conservative | NA | NA | NA | NA | -3 (-4, 0)c | NA | -1 (-13, 17)b | NA |
| NIHSS at discharge | ||||||||
| Thrombolysis | NA | 6 (0–21)b | NA | NA | NA | NA | 3 (2-42)b | NA |
| Conservative | NA | 3 (0–15)b | NA | NA | NA | NA | 3 (0-42)b | NA |
| sICH | ||||||||
| Thrombolysis | 2 | 0 | 2 | NA | 2 | 2 | 1 | 20 |
| Conservative | 0 | 0 | 2 | NA | 0 | 2 | 1 | 12 |
| ICH | ||||||||
| Thrombolysis | NA | 1 | 2 | NA | 15 | 5 | NA | NA |
| Conservative | NA | 0 | 2 | NA | 2 | 1 | NA | NA |
| Study quality | 7 | 6 | NA | 8 | 8 | 7 | 6 | NA |
RCT: randomized controlled trial; CT: computed tomography; NCT: noncontrast CT; MRI: magnetic resonance imaging; a: mean ± SD; b: median (minimum-maximum); c: mean (interquartile); y: year; h: hour; min: minute; NA: not applicable; HT: hypertension; DM: diabetes mellitus; CAD: cardiovascular disease; AF: arterial fibrillation; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; LSN: last seen normal; ICH: intracerebral hemorrhage; sICH: symptomatic ICH.
One single study [26] only used noncontrasted CT as the diagnostic method, 2 used MRI-based approach for pretreatment screening [12, 16], 3 studies applied CT-based method for screening [25, 27, 29], and 2 studies used MRI- plus CT-based technics [15, 28]. All studies indicated 90-day mRS, except 1 study only indicating the scale at discharge level [26]. All non-RCTs were with relatively high quality (Table 1). Two RCTs kept a good control in each domain (Table 2).
Table 2.
Quality assessment of RCT in this meta-analysis. RCT: randomized controlled trial.
| Study | Random sequence generation | Allocation concealment | Blinding (participants and personnel) | Blinding (outcome assessment) | Incomplete outcome data | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| Michel | Low | Low | Low | Low | Low | Low | Low |
| Thomalla | Low | Low | Low | Low | Low | Low | Low |
3.2. Outcome Assessment
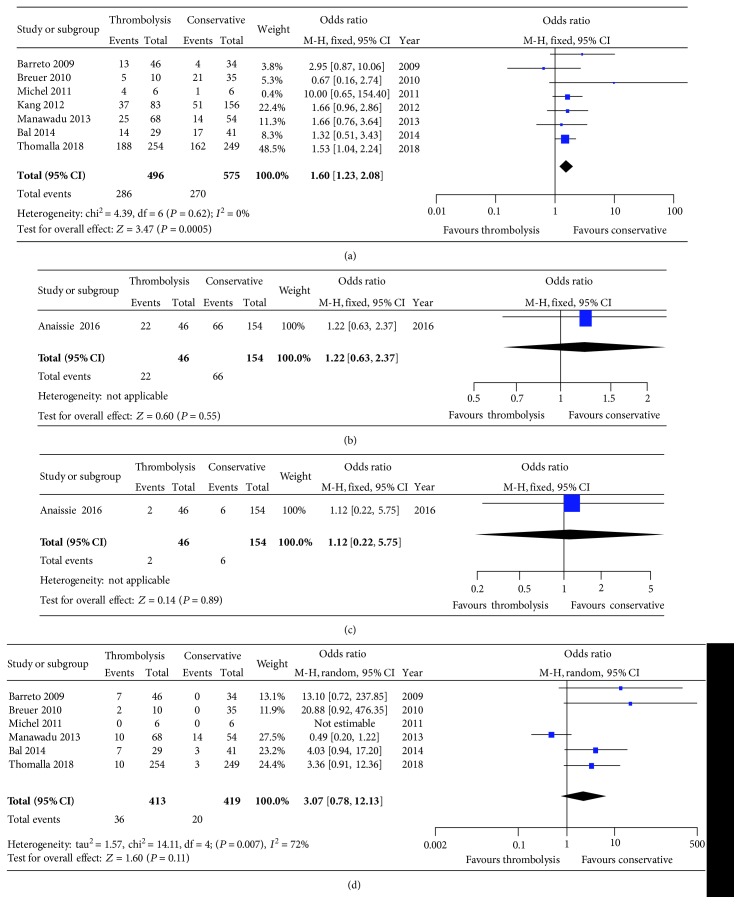
All the outcomes were shown in Table 3. The pooling of good outcome showed that patients receiving thrombolysis intended to have a higher rate in 90 days than conservative therapy after treatment (57.66% vs. 46.96%; P = 0.0005) (Figure 2(a)). But we did not found any differences with respect to discharge good outcome (47.82% vs. 42.85%; P = 0.55) (Figure 2(b)). And the comparison of discharge (4.35% vs. 3.90%; P = 0.89) (Figure 2(c)) and 90-day mortality (8.72% vs. 4.77%; P = 0.11) (Figure 2(d)) did not find any significances.
Table 3.
Overall and subgroup meta-analysis of the included studies.
| Study heterogeneity | |||||||
|---|---|---|---|---|---|---|---|
| Outcomes | No. | OR [95% CI] | P | χ 2 | df | I 2 (%) | P-Q test |
| Overall analysis | |||||||
| 90-day good outcome | 7 | 1.60 [1.23, 2.08] | 0.0005 | 4.39 | 6 | 0 | 0.62 |
| Discharge good outcome | 1 | 1.22 [0.63, 2.37] | 0.55 | NA | NA | NA | NA |
| 90-day mortality | 6 | 3.07 [0.78, 12.13] | 0.11 | 14.11 | 4 | 72 | 0.007 |
| By discharge mortality | 1 | 1.12 [0.22, 5.75] | 0.89 | NA | NA | NA | NA |
| ICH | 4 | 2.06 [0.35, 12.04] | 0.42 | 7.38 | 3 | 59 | 0.06 |
| sICH | 7 | 1.73 [0.98, 3.05] | 0.06 | 1.29 | 5 | 0 | 0.94 |
| Subgroup analysis | |||||||
| MRI | |||||||
| 90-day good outcome | 2 | 1.57 [1.15, 2.15] | 0.005 | 0.05 | 1 | 0 | 0.82 |
| 90-day mortality | 1 | 3.36 [0.91, 12.36] | 0.07 | NA | NA | NA | NA |
| sICH | 1 | 1.63 [0.82, 3.27] | 0.17 | NA | NA | NA | NA |
| CT | |||||||
| 90-day good outcome | 3 | 1.68 [0.93, 3.01] | 0.08 | 1.88 | 2 | 0 | 0.39 |
| 90-day mortality | 3 | 1.30 [0.17, 10.18] | 0.8 | 5.82 | 2 | 83 | 0.02 |
| ICH | 3 | 1.41 [0.17, 11.49] | 0.75 | 6.46 | 2 | 69 | 0.04 |
| sICH | 3 | 1.56 [0.50, 4.84] | 0.44 | 0.68 | 2 | 0 | 0.71 |
| MRI+CT | |||||||
| 90-day good outcome | 2 | 1.47 [0.34, 6.30] | 0.61 | 2.44 | 1 | 59 | 0.12 |
| 90-day mortality | 2 | 16.25 [1.94, 136.20] | 0.01 | 0.05 | 1 | 0 | 0.82 |
| ICH | 1 | 11.21 [0.42, 297.84] | 0.15 | NA | NA | NA | NA |
| sICH | 2 | 3.72 [0.18, 75.14] | 0.39 | NA | NA | NA | NA |
OR: odds ratio; P: percentage; NA: not applicable; ICH: intracerebral hemorrhage; sICH: symptomatic ICH; MRI: magnetic resonance imaging; CT: computed tomography.
Figure 2.
Forest plots of good outcome and mortality between thrombolysis and conservative therapy. (a) 90-day good outcome; (b) discharge good outcome; (c) discharge mortality; (d) 90-day mortality.
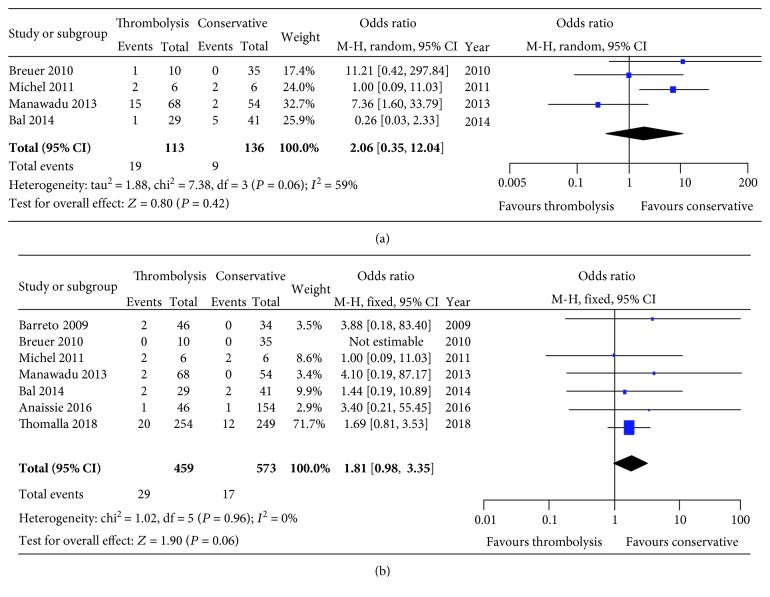
For safety analysis, the incidences of ICH (16.81% vs. 6.62%; P = 0.42) (Figure 3(a)) and sICH (6.32% vs. 2.97%; P = 0.06) (Figure 3(b)) were relatively comparable between the thrombolysis and conservative therapy.
Figure 3.
Forest plots of ICH (a) and sICH (b) incidences between thrombolysis and conservative therapy. ICH: intracerebral hemorrhage; sICH: symptomatic intracerebral hemorrhage.
We also did further analysis based on imaging methods (Table 3). In studies using MRI, a higher incidence of 90-day good outcome (P = 0.005) was seen in the thrombolysis group. And no changes of trends in 90-day mortality and sICH were seen. When analyzing CT-based approaches in 3 studies [25, 27, 29], no favorable results were seen in each outcome. Two studies used CT and MRI methods in preexamination. And no differences between the two groups in the outcomes of 90-day good outcome, ICH, and sICH were revealed, except a lower rate of 90-day mortality in conservative therapy (P = 0.01).
3.3. Heterogeneity, Sensitivity Analysis, and Publication Bias Assessment
There were relatively high heterogeneity in 90-day mortality (I2 = 74%) and ICH incidence (I2 = 59%). We then used the leave-one-out method to analyze these results. The results of 90-day mortality turned significant (P = 0.0003) with a robust change of heterogeneity (I2 = 0%) when the study by Manawadu et al. [27] was extracted. Also, without the study by Bal et al. [25], ICH incidence is relatively higher in the thrombolysis group than in the conservative therapy group (P = 0.0006, I2 = 9%). As there were less than 10 studies in this meta-analysis, the publication bias assessment cannot be performed accurately.
4. Discussion
The current study is aimed at comparing intravenous thrombolysis with conservative therapy in UOS patients. We found that intravenous thrombolysis induced a higher incidence of 90-day good outcome without increased mortality, compared with conservative therapy.
UOS patients are unable to provide accurate symptom onset time for some reasons, for example, awakening with stroke symptoms, nonwitnessed stroke with aphasia or unconsciousness [30, 31]. They are usually considered as a contraindication for intravenous thrombolysis for the uncertain symptom onset. There is an increasing attention to determine the potential role of thrombolytic therapy for patients in this situation [32–34]. A trial in 2009 [15] indicated that thrombolysis-treated WUS had higher rates of excellent and favorable outcome but higher mortality than those receiving conservative treatment. But some studies pointed that thrombolysis may be as safe as conservative therapy in WUS [25], with an even better outcome [26]. The present analysis found a higher 90-day good outcome rate in thrombolysis than conservatives, pointing out that intravenous thrombolysis should be beneficial for UOS. Although it indicated comparable effects between the two therapies at discharge level, only one single study [26] was included in the outcome. More trials should be done at this time point. And the mortality in both groups did not differ in the short and long term, implying the comparable safety between thrombolysis and conservative therapy. ICH is a severe situation in acute ischemic stroke following thrombolytic agent use [35]. It is also confirmed in this meta-analysis that ICH and sICH incidences were similar in patients receiving the two therapies, ensuring again the safety of thrombolysis.
The pretreatment neuroimaging evaluation is becoming an important factor for UOS. Noncontrast CT is the common method for stroke patients after initial admission. CT perfusion and MRI are methods with increasing application for screening, which have unique roles for patient selection. The mismatch in noncontrast CT with CT perfusion and MRI indicated that some UOS patients are eligible for intravenous thrombolysis [16]. The inconsistent use of neuroimaging methods was seen in the current study. Here, we did subgroup analysis on this basis. Only one study used noncontrast CT for neuroimaging [26]. Two studies selected patients for thrombolysis according to the mismatch between DWI and FLAIR. Positive DWI and negative FLAIR changes identify stroke within 4.5 hours [12]. Obviously, patients using MRI for screening had an increased incidence with 90-day good outcome. Some researchers believe that CT perfusion and CT angiography are also able to define suitable patients for rtPA use [25, 27]. However, the pooling of data from CT perfusion or CT angiography did not indicate the potential benefits of thrombolysis in recovery. Also, data with mixed use of MRI and CT perfusion or CT angiography also did not reveal any differences in 90-day good outcome. Then, it seems that MRI should be a better choice for patient selection into thrombolytic treatment. But the fact that there is a lack of MRI in some small centers and the long duration of imaging may impair the wide use of MRI in the clinical practice. The inconsistent use of neuroimaging methods may hinder the reliability of the results. And studies in these comparisons were limited, calling for more trials.
A higher heterogeneity was found in the results of 90-day mortality and ICH. And the sensitivity analysis demonstrated the stable results of 90-day good outcome and sICH. But 90-day mortality turned significant (P = 0.0003) with a robust reduction of heterogeneity from 75% to 0% by excluding the study by Manawadu et al. [27]. We found that not all the subjects in this study received CTP in the inclusion process. Some patients may be not suitable for thrombolysis, but they still received thrombolysis. Then, a higher rate of 90-day mortality (7.54%) than conservative therapy (1.37%) may also explain it. Without the study by Bal et al. [25], ICH incidence is relatively higher in the thrombolysis group than in the conservative therapy group (P = 0.0006, I2 = 9%). The review of this article found a higher hemorrhagic infarction in the conservative group. It is believed that hemorrhagic infarction is likely to induce an occurrence of ICH [36]. Also, the admission time from symptom onset was not available. These should demonstrate the high heterogeneity caused by the study.
Two clinical trials, EXTEND [37] and THAWS [38], are aimed at exploring the safety and efficacy of rtPA in the treatment of UOS and WUS patients and may provide additional evidence.
Tenecteplase, a third-generation thrombolytic, compared with rtPA, has a stronger binding ability to fibrin and is more resistant to the inactivation of plasminogen activator inhibitor-1 [39]. Single doses may be administered rather than sustained administration due to longer half-life. In the treatment of myocardial infarction, tenecteplase is equivalent to rtPA, but the risk of bleeding is reduced [40]. Burgos and Saver [41] found in a meta-analysis involving 5 RCTs that tenecteplase is not inferior to rtPA in the treatment of acute ischemic stroke. Kheiri et al. [42] conducted a network meta-analysis and found that tenecteplase had a higher recanalization rate and more favorable early neurological function improvement than rtPA in the treatment of acute ischemic stroke, and there was no difference in safety. Based on the above, whether tenecteplase is safer and more effective in the treatment of UOS needs to be further studied.
There were some limitations in this meta-analysis. First, most of the studies included were observational studies, in which intravenous rtPA was given based on clinical decision and imaging assessment. This lays a potential bias in the analysis. Enrollment of RCTs can help solve this, but only 2 studies were found in this study. Also, it encouraged high-quality large-sample RCTs focusing on this point to evaluate the role of intravenous rtPA.
And the number of the included studies was limited. This made it difficult to assess publication bias accurately. Also, the sample size in each study was small and they came from different regions. Moreover, the preexamination imaging methods were not consistent in all the studies, which indicated the variety in patient enrollment. And the high heterogeneity meant the unstable results of certain variables. This hindered the credibility of our results.
Intravenous thrombolysis is a better choice for UOS patients for its efficacy and safety. And pretreatment MRI assessment is beneficial for improving the efficacy of thrombolytic therapy. However, it needs more supporting evidences for clinical use in the future.
Acknowledgments
This study was supported by grants from the Talent Scientific Research Start-up Foundation of the First Affiliated Hospital of Wannan Medical College (Grant No. YR201604) and the Scientific Research Foundation of Wannan Medical College (Grant No. WK2019F20).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Di Luan and Yuanxiang Zhang contributed equally to this work.
References
- 1.Benjamin E. J., Blaha M. J., Chiuve S. E., et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crichton S. L., Bray B. D., McKevitt C., Rudd A. G., Wolfe C. D. A. Patient outcomes up to 15 years after stroke: survival, disability, quality of life, cognition and mental health. Journal of Neurology, Neurosurgery, and Psychiatry. 2016;87(10):1091–1098. doi: 10.1136/jnnp-2016-313361. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W., Kaste M., Bluhmki E., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England Journal of Medicine. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W., Kaste M., Fieschi C., et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) The Lancet. 1998;352(9136):1245–1251. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 5.Muruet W., Rudd A., Wolfe C. D. A., Douiri A. Long-term survival after intravenous thrombolysis for ischemic stroke: a propensity score-matched cohort with up to 10-year follow-up. Stroke. 2018;49(3):607–613. doi: 10.1161/STROKEAHA.117.019889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W., Ou Z., Luo R. Solitaire stent in the treatment of acute ischemic stroke with large cerebral artery occlusion. Translational Neuroscience. 2017;8(1) doi: 10.1515/tnsci-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wouters A., Lemmens R., Dupont P., Thijs V. Wake-up stroke and stroke of unknown onset: a critical review. Frontiers in Neurology. 2014;5:p. 153. doi: 10.3389/fneur.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoki J., Kimura K., Iguchi Y., et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovascular Diseases. 2011;31(5):435–441. doi: 10.1159/000323850. [DOI] [PubMed] [Google Scholar]
- 9.Schwamm L. H., Wu O., Song S. S., et al. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Annals of Neurology. 2018;83(5):980–993. doi: 10.1002/ana.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denny M. C., Boehme A. K., Dorsey A. M., et al. Wake-up strokes are similar to known-onset morning strokes in severity and outcome. Journal of Neurology and Neurological Disorders. 2014;1(1) doi: 10.15744/2454-4981.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C. C., Yeh H. L., Hsiao C. Y., Lien L. M. 10-Point CT-ASPECTS-based reperfusion therapy for unknown onset stroke. Journal of the Formosan Medical Association. 2018;117(7):640–645. doi: 10.1016/j.jfma.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Thomalla G., Simonsen C. Z., Boutitie F., et al. MRI-guided thrombolysis for stroke with unknown time of onset. The New England Journal of Medicine. 2018;379(7):611–622. doi: 10.1056/NEJMoa1804355. [DOI] [PubMed] [Google Scholar]
- 13.Panic N., Leoncini E., de Belvis G., Ricciardi W., Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8(12, article e83138) doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D. F., Berlin J. A., Morton S. C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Barreto A. D., Martin-Schild S., Hallevi H., et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40(3):827–832. doi: 10.1161/STROKEAHA.108.528034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang D.-W., Sohn S.-I., Hong K.-S., et al. Reperfusion therapy in unclear-onset stroke based on MRI evaluation (RESTORE) a prospective multicenter study. Stroke. 2012;43(12):3278–3283. doi: 10.1161/STROKEAHA.112.675926. [DOI] [PubMed] [Google Scholar]
- 17.Adams H. J., Biller J. Classification of subtypes of ischemic stroke: history of the trial of org 10172 in acute stroke treatment classification. Stroke. 2015;46(5):e114–e117. doi: 10.1161/STROKEAHA.114.007773. [DOI] [PubMed] [Google Scholar]
- 18.Fischer U., Arnold M., Nedeltchev K., et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121–2125. doi: 10.1161/01.STR.0000182099.04994.fc. [DOI] [PubMed] [Google Scholar]
- 19.Patel R. D., Starkman S., Hamilton S., et al. The Rankin focused assessment-ambulation: a method to score the modified Rankin scale with emphasis on walking ability. Journal of Stroke and Cerebrovascular Diseases. 2016;25(9):2172–2176. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Wahlgren N., Ahmed N., Dávalos A., et al. Thrombolysis with alteplase 3–4• 5 h after acute ischaemic stroke (SITS-ISTR): an observational study. The Lancet. 2008;372(9646):1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.-I., Gliem M., Gerdes G., et al. Safety of bridging antiplatelet therapy with the gpIIb-IIIa inhibitor tirofiban after emergency stenting in stroke. PLoS One. 2017;12(12, article e190218):p. e0190218. doi: 10.1371/journal.pone.0190218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA SBOC. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2001. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23.Zeng X., Zhang Y., Kwong J. S. W., et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. Journal of Evidence-Based Medicine. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J. P. T., Altman D. G., Gotzsche P. C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343, article d5928 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bal S., Bhatia R., Shobha N., et al. Stroke- on- awakening: safety of CT-CTA based selection for reperfusion therapy. The Canadian Journal of Neurological Sciences. 2014;41(2):182–186. doi: 10.1017/S0317167100016553. [DOI] [PubMed] [Google Scholar]
- 26.Anaissie J. E., Monlezun D. J., Siegler J. E., et al. Intravenous tissue plasminogen activator for wake-up stroke: a propensity score-matched analysis. Journal of Stroke and Cerebrovascular Diseases. 2016;25(11):2603–2609. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Manawadu D., Bodla S., Keep J., Jarosz J., Kalra L. An observational study of thrombolysis outcomes in wake-up ischemic stroke patients. Stroke. 2013;44(2):427–431. doi: 10.1161/STROKEAHA.112.673145. [DOI] [PubMed] [Google Scholar]
- 28.Breuer L., Schellinger P. D., Huttner H. B., et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: initial single-centre experience. International Journal of Stroke. 2010;5(2):68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Michel P., Ntaios G., Reichhart M., et al. Perfusion-CT guided intravenous thrombolysis in patients with unknown-onset stroke: a randomized, double-blind, placebo-controlled, pilot feasibility trial. Neuroradiology. 2012;54(6):579–588. doi: 10.1007/s00234-011-0944-1. [DOI] [PubMed] [Google Scholar]
- 30.Thomalla G., Gerloff C. Treatment concepts for wake-up stroke and stroke with unknown time of symptom onset. Stroke. 2015;46(9):2707–2713. doi: 10.1161/STROKEAHA.115.009701. [DOI] [PubMed] [Google Scholar]
- 31.Dekker L., Hund H., Lemmens R., Boiten J., van den Wijngaard I. Unknown onset ischemic strokes in patients last-seen-well> 4.5 h: differences between wake-up and daytime-unwitnessed strokes. Acta Neurologica Belgica. 2017;117(3):637–642. doi: 10.1007/s13760-017-0830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dorado L., Ahmed N., Thomalla G., et al. Intravenous thrombolysis in unknown-onset stroke: results from the safe implementation of treatment in stroke-international stroke thrombolysis registry. Stroke. 2017;48(3):720–725. doi: 10.1161/STROKEAHA.116.014889. [DOI] [PubMed] [Google Scholar]
- 33.Stern G. M., van Hise N., Urben L. M., Korobey M. J., Pitlick J. M., Crannage A. J. Thrombolytic therapy in wake-up stroke patients. Clinical Neuropharmacology. 2017;40(3):140–146. doi: 10.1097/WNF.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 34.Kurz M. W., Advani R., Behzadi G. N., Eldøen G., Farbu E., Kurz K. D. Wake-up stroke—amendable for thrombolysis-like stroke with known onset time? Acta Neurologica Scandinavica. 2017;136(1):4–10. doi: 10.1111/ane.12686. [DOI] [PubMed] [Google Scholar]
- 35.Lansberg M. G., Albers G. W., Wijman C. A. C. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovascular Diseases. 2007;24(1):1–10. doi: 10.1159/000103110. [DOI] [PubMed] [Google Scholar]
- 36.Christensen M. C., Mayer S., Ferran J. M. Quality of life after intracerebral hemorrhage: results of the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2009;40(5):1677–1682. doi: 10.1161/STROKEAHA.108.538967. [DOI] [PubMed] [Google Scholar]
- 37.Churilov L., Ma H., Campbell B. C. V., Davis S. M., Donnan G. A. Statistical analysis plan for EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND) trial. International Journal of Stroke. 2018 doi: 10.1177/1747493018816101. [DOI] [PubMed] [Google Scholar]
- 38.Koga M., Toyoda K., Kimura K., et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0.6 mg/kg (THAWS) Trial. International Journal of Stroke. 2014;9(8):1117–1124. doi: 10.1111/ijs.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verstraete M. Third-generation thrombolytic drugs. The American Journal of Medicine. 2000;109(1):52–58. doi: 10.1016/S0002-9343(00)00380-6. [DOI] [PubMed] [Google Scholar]
- 40.Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators, Van De Werf F. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. The Lancet. 1999;354(9180):716–722. doi: 10.1016/S0140-6736(99)07403-6. [DOI] [PubMed] [Google Scholar]
- 41.Burgos A. M., Saver J. L. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156–2162. doi: 10.1161/STROKEAHA.119.025080. [DOI] [PubMed] [Google Scholar]
- 42.Kheiri B., Osman M., Abdalla A., et al. Tenecteplase versus alteplase for management of acute ischemic stroke: a pairwise and network meta-analysis of randomized clinical trials. Journal of Thrombosis and Thrombolysis. 2018;46(4):440–450. doi: 10.1007/s11239-018-1721-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.