Abstract
Migraine is a prevalent neurological disorder which causes a huge economic burden on society. It is thought to be a neurovascular disease with oxidative stress might be involved. Curcumin, one of the major ingredients of turmeric, has potent antioxidative and anti-inflammatory properties, but whether it could be used as a potential treatment for migraine remains to be explored. In the present study, human umbilical vein endothelial cells (HUVECs) were pretreated with various concentrations of curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h, thereby exposed to H2O2 (100 μM) for another 12 h. The viability of HUVECs was tested by the CCK-8 assay, and the activities of antioxidant enzymes including superoxide dismutase (SOD) and glutathione (GSH) were also examined. Intracellular reactive oxygen species (ROS) and malondialdehyde (MDA) were assayed to determine H2O2-induced oxidative stress. In addition, several cell death-related genes (p53, p21, Bax, and Bcl-2) were detected by PCR, and an apoptosis-related protein (caspase3) was evaluated by western blotting. Our results showed that curcumin improved the H2O2-induced decrease of cell viability and antioxidative enzyme activities and decreased the level of oxidative stress. As a conclusion, curcumin could mitigate H2O2-induced oxidative stress and cell death in HUVECs and may be a potential therapeutic drug for migraine.
1. Introduction
Migraine is a widespread neurological disorder with the typical clinical symptom being recurrent headache [1]. The global prevalence of migraine was reported to be 15%, and 10% will progress to chronic migraine [2]. Migraine-associated functional disability affects patients' work capacity and productivity, thus leading to a huge economic burden on society [3]. Currently, long-term use of migraine drugs may cause some adverse events, such as abuse, addiction, and dependence [4]. As a result, it is of value to conduct more research to promote the understanding of the molecular mechanism of migraine, thus developing novel approaches with improved efficacy and safety.
Curcumin, one of the major ingredients of turmeric, attracted much attention because of its antioxidative, anticarcinogenic, antitumor, and anti-inflammatory properties [5, 6].
A growing body of evidences reports curcumin may have a beneficial antioxidative and neuroprotective potential for neurological diseases such as Alzheimer's disease and Parkinson's disease [7]. As reviewed by Shameemah, 17 studies have revealed the protective effect of curcumin in different cellular models of neurodegenerative disorders [8]. However, to the best of our knowledge, curcumin's treatment potential in migraine has not yet been evaluated.
It is believed that migraine is a neurovascular disorder caused by chronic sensitization of central pain pathways [9]. However, the mechanism of migraine could not be explained by a single theory.
In recent years, oxidative stress has attracted growing interest in the pathogenesis of this disease [10]. For instance, the research conducted by Geyik et al. revealed that oxidative stress marker 8-OHdG was higher in the plasma of migraine patients than that in the control group [11]. Oxidative stress may be caused by a lower activity of certain antioxidant enzymes and a higher activity of oxidant-generating factors [12]. Angiotensin, endothelin-1, and urotensin-2 have been reported to be implicated in this process [13]. Migraines may also be associated with mitochondrial defects, resulting in a much higher metabolic rate. Magnesium, Coenzyme Q10 (CoQ10), and vitamins B2 and B12 have been revealed to have the potential for the treatment of migraine because of their antioxidant abilities [14].
In the present study, we investigated curcumin's effect on H2O2-induced oxidative stress in HUVECs in vitro and aimed to explore the potential use of curcumin in migraine treatment.
2. Materials and Methods
2.1. Cell Culture
HUVECs were obtained from Cell Bank in the Shanghai Institute for Biological Sciences of the Chinese Academy of Sciences and were cultured in Dulbecco's modified Eagle's Medium (DMEM) medium with 10% fetal bovine serum, 1% penicillin/streptomycin in 37°C, and 5% CO2 [15]. All reagents were purchased from Gibco Thermo Fisher Scientific Inc. (MA, USA). HUVECs were treated with various concentrations of curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) and H2O2 (0 μM, 25 μM, 50 μM, 75 μM, and 100 μM, Sigma-Aldrich, St. Louis, MO, USA) 12 h later.
2.2. CCK-8 Assay for Cell Viability
The effects of curcumin and H2O2 on HUVECs viability were detected by the CCK-8 assay. In brief, cells were cultured on a 96-well plate at a density of 1 × 104 per well for 24 h and then administrated with curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h or with H2O2 (0 μM, 25 μM, 50 μM, 75 μM, 100 μM) for another 12 h. Then, the HUVECs were incubated at 37°C for 2 h. Thereafter, a multifunctional microplate reader (SpectraMax M5, Sunnyvale, CA, USA) was adopted to read the absorbance values at 450 nm [16].
2.3. LDH, GSH, and SOD Assay
In order to estimate the level of oxidative damage, we used a colorimetric assay kit (Beyotime, Nanjing, China) to measure the activity of lactate dehydrogenase (LDH) release [17], superoxide dismutase (SOD), and glutathione (GSH). In brief, HUVECs were seeded in 6-well plates at a density of 1 × 105/well. The HUVECs were then treated for 12 h with various concentrations of curcumin (10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) followed by H2O2 (25 μM, 50 μM, 75 μM, and 100 μM) for another 12 h.
2.4. Analysis of Oxidative Stress
The generation of reactive oxygen species (ROS) was measured using the fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH-DA). The level of intracellular ROS was detected using a BD FACSCalibur Flow Cytometer (Becton, Dickinson and Company, USA). Another indicator of oxidative stress malondialdehyde (MDA) was also detected with commercial kits.
2.5. RNA Isolation and Real-Time Quantitative PCR
HUVECs were harvested, and RNA samples were exacted with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The primer sequences used were as follow: p53: 5ʹ-CTTTGAGGTGCGTGTTTGTGC-3ʹ (forward), 5ʹ-TGTTGTTGGGCAGTGCTCG-3ʹ (reverse); p21: 5′-TAGCAGCGGAACAAGGAG-3′ (forward), 5′-AAACGGGAACCAGGACAC-3′ (reverse); Bcl-2: 5′-GTAGTGAATGAACTCTTCCG-3′ (forward), 5′-GTATCCCAGCCGCCGTTCTC-3′ (reverse); Bax: 5′-GACGTGGGCATTTTTCTTAC-3′ (forward), 5′-GTGTCCCGAAGGAGGTTTAT-3′ (reverse); and ß-actin: 5′-TGGCACCCAGCACAATGAA-3′ (forward), 5′-CTAAGTCATAGTCCGCCTAG AAGCA-3′ (reverse).
2.6. Western Blot
The protein was extracted using the RIPA buffer and measured using the BCA Protein Assay Kit (Beyotime, P0013B, Shanghai, China) according to the instruction. Equal amounts of proteins were separated and transferred to the PVDF membrane (Merk Millipore, Billerica, MA) and incubated with the primary antibodies at 4°C overnight.
This was followed by incubation with the goat anti-rabbit IgG antibody (1 : 10000, Cell Signaling Technology) at room temperature for 2 h. Membranes were scanned by using a chemiluminescent detective system (Amersham Biosciences UK Ltd., Little Chalfont, UK). The primary antibodies anti-cleaved caspase3 (1 : 1000, Abcam, MA, USA) and GAPDH (1 : 1000, Cell Signaling Technology) were used in this study.
2.7. Statistical Analysis
SPSS 18.0 for Windows (IBM Corp, Armonk, NY, USA) was used for the statistical analyses. Statistical analyses were performed using one-way analysis of variance (ANOVA) and Student's t-test for comparisons between groups. The data were expressed as mean ± SEM, and p < 0.05 was regarded as significant differences.
3. Results
3.1. Cell Viability
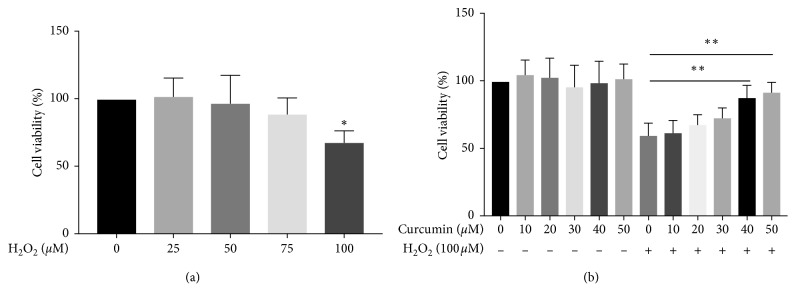
To examine the cytotoxicity of curcumin and H2O2 on HUVECs, cell viability was detected by the CCK-8 assay. As shown in Figure 1, treatment with H2O2 (0 μM, 25 μM, 50 μM, and 75 μM) for 12 h had no effect on cell viability of HUVECs. However, cell viability of HUVECs decreased when the concentration of H2O2 increased to 100 μM (p < 0.05). Meanwhile, curcumin treatment at the concentrations of 0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM showed no cytotoxicity on HUVECs when compared with the control group (p > 0.05). Therefore, the concentrations of 0 μM, 10 μM, 20 μM, 30 μM, 40 μM, 50 μM, and 100 μM were chosen for curcumin and H2O2, respectively, for the subsequent experiments. Furthermore, the decreased cell viability of HUVECs induced by H2O2 was improved with curcumin treatment (40 μM and 50 μM) (p < 0.05), suggesting that curcumin rescued H2O2-induced cell injury in HUVECs.
Figure 1.
Cell viability of HUVECs treated with curcumin and H2O2. (a) Treatment with H2O2 (0 μM, 25 μM, 50 μM, and 75 μM) for 12 h had no effect on cell viability of HUVECs, while H2O2 (100 μM) decreased cell viability which was tested by the CCK-8 assay. ∗p < 0.05 versus the control group. (b) HUVECs treated with curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h showed no change of cell vitality. The cell vitality after H2O2 (100 μM) treatment increased after curcumin (40 μM and 50 μM) administration compared with curcumin (0 μM). ∗∗p < 0.01 (n = 3).
3.2. The Effect of Curcumin on the Level of LDH Release and GSH and SOD Activity in H2O2-Exposed HUVECs
Superoxide dismutase (SOD), glutathione (GSH), and lactate dehydrogenase (LDH) have been widely used as indicators for oxidative injury. As a result, the production of LDH and activities of SOD and GSH were measured to evaluate the effects of curcumin on H2O2-induced injury in HUVECs. Cells were pretreated with curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h and with H2O2 (100 μM) treatment for another 12 h. As shown in Figure 2, H2O2 administration increased the levels of LDH when compared with the control group (p < 0.05). However, the productions of LDH decreased significantly upon treatment with curcumin at the concentration of 40 μM (p < 0.05) and 50 μM (p < 0.05), even though there was no significant change at the concentrations of 10 μM, 20 μM, and 30 μM (p > 0.05). Conversely, the activities of GSH and SOD were enhanced by curcumin (40 μM and 50 μM). Taken together, our result indicated that curcumin showed protective capacity in a dose-dependent manner in H2O2-induced cell injury in HUVECs.
Figure 2.
The effect of curcumin on LDH release and GSH and SOD activity in H2O2-exposed HUVECs. HUVECs were pretreated with curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h and with H2O2 (100 μM) treatment for another 12 h. The LDH release and GSH and SOD activity were measured, respectively. ∗p < 0.05 and ∗∗p < 0.01, n = 3.
3.3. Curcumin Inhibited H2O2-Induced Oxidative Stress in HUVECs
In order to test whether curcumin could influence oxidative stress in HUVECs, we examined the generation of the intracellular ROS and MDA level. As is shown in Figure 3(a), intracellular ROS was increased in HUVECs under H2O2 treatment (p < 0.05). In addition, H2O2 treatment also caused a significant induction of another oxidative stress marker, malondialdehyde (MDA) (Figure 3(b)) (p < 0.05). Notably, a significant reduction of both the intracellular ROS and MDA level was observed with curcumin pretreatment (40 μM and 50 μM) for 12 h. In contrast, the curcumin concentration of 10 μM, 20 μM, and 30 μM had no effect on in H2O2-induced HUVECs in terms of either intracellular ROS or MDA level (p > 0.05). Our data suggested that the effect of H2O2 on the intracellular ROS and MDA levels in HUVECs could be blocked by curcumin at a certain concentration.
Figure 3.
The effect of curcumin on oxidative stress in H2O2-exposed HUVECs. HUVECs were pretreated with curcumin (0 μM, 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM) for 12 h and with H2O2 (100 μM) treatment for another 12 h. The DCFH-DA assay was adopted to detect endogenous ROS, and the commercial MDA kit was used to examine the MDA level. ∗p < 0.05, n = 3.
3.4. The Effect of Curcumin on Cell Death-Related Genes in H2O2-Exposed HUVECs
In order to elucidate the effect of curcumin on cell apoptosis, the expression of cell apoptosis-related genes, including p53, p21, Bax, and Bcl-2, was examined by PCR. The result showed that H2O2 induced a significant increase in apoptosis-related genes (p53, p21, and Bax) and a significant decrease of antiapoptotic gene (Bcl-2) (Figure 4) (p > 0.05). Notably, after curcumin (40 μM, 50 μM) treatment, these effects were partly reversed. Moreover, the expression of apoptosis-related protein was evaluated by western blot. As shown in Figure 4(e), the caspase3 level was significantly reduced by curcumin treatment (50 μM). Therefore, we suggested that curcumin had a protective effect on HUVECs against oxidative stress.
Figure 4.
The effect of curcumin on cell death-related genes in H2O2-exposed HUVECs. HUVECs were pretreated with curcumin (40 μM and 50 μM) for 12 h and with H2O2 (100 μM) treatment for another 12 h. The mRNA level of cell death-related genes including p53, p21, Bax, and Bcl-2 was detected by PCR. The expression of apoptosis protein caspase3 was evaluated by western blot. ∗p < 0.05 and ∗∗p < 0.01, n = 3.
4. Discussion
In the present research, our results showed that curcumin could mitigate H2O2-induced decrease of cell vitalities and antioxidative enzyme activities and decrease the level of oxidative stress in HUVECs. Curcumin, an active compound isolated from turmeric, has been demonstrated to have protective effect on neurological disorders [18]. However, as we know, there is still no published paper reported on the role of curcumin in model of migraine.
The safety of curcumin has been well proved [19]. Our result also showed that curcumin at the concentration of up to 50 μM had no effect on the cell viabilities of HUVECs. Notably, the concentration of 40 μM and 50 μM showed a property to inhibit H2O2-induced oxidative stress (Figure 1). Furthermore, our results also showed that curcumin suppressed H2O2-induced induction of p53, p21, and Bax while enhanced the expression of antiapoptotic gene Bcl-2 (Figure 4).
The oxidative stress is mostly caused by the imbalance of ROS production and the clearance system [20]. It has been reported that curcumin rescued 6-OHDA-induced reduction of the antioxidant enzymes GSH, GPx, GR, and SOD [21]. Harish et al. exposed curcumin-pretreated N27 cells to buthionine sulfoximine and observed the upregulation of GSH and glutathione S-transferase [22]. The effect on GSH levels was also observed in a lipopolysaccharide-induced mouse model with curcumin treatment for seven days [23]. Consistently, we also observed an upregulation of SOD and GSH levels, suggesting that curcumin could regulate oxidative homeostasis in HUVECs by stimulating the activities of antioxidant enzymes.
Increasing evidences have reported the role of oxidative stress in migraine [11]. As shown in Figure 3, curcumin could ameliorate H2O2-mediated oxidative stress by reducing ROS and MDA levels. This is similar to the experiment performed by Cui et al. who applied rotenone to induce oxidative stress in the dopaminergic neuron [24]. In addition, another study investigated the antioxidant properties of curcumin also used H2O2 to stimulate oxidative stress. The authors suggested that curcumin was capable of improving both DNA damage and cell apoptosis in PC12, which is an established cell model of PD [25]. However, the effect of curcumin against oxidative damage depends on the cell line. van Meeteren et al. observed poor efficacy of curcumin in H2O2-stimulated oligodendrocytes and macrophages cells [26]. In the present study, we firstly showed that curcumin had the protective effect on H2O2-induced oxidized proteins and lipid radicals in HUVECs.
The molecular mechanism of the protective effect of curcumin on HUVECs under oxidative stress still remains to be explored. By exploring the antioxidant effect of curcumin in amyloid-β exposed SH-SY5Y and IMR-32 cells, Sarkar et al. proposed that it might be associated with the activation of antioxidant element and nuclear factor erythroid 2-related factor 2 (Nrf2) pathways [27]. In another study, the reduction of oxidative stress by curcumin only occurred in cells with mutant α-synuclein, suggesting that the oxidative stressor might be essential for the antioxidant effect of curcumin [18]. Another study with a model PC12 cells showed that mitogen-activated protein kinases (MAPKs) and serine/threonine protein kinase (Akt) pathways might play a vital role in the antioxidant effect of curcumin [25]. However, whether the mechanism is implicated in HUVECs is unclear.
In the present experiment, DMSO was adopted as the solvent for curcumin. Previous published papers reported that DMSO might change the conformation of curcumin [28]. However, according to the published paper, the working concentration of DMSO we used in this research (0.1%) is not likely to cause effect on curcumin [29]. With high lipophilicity, curcumin is free to pass through cellular membranes, which might be the basis of the effect in HUVECs [30]. However, the potential application of curcumin in clinical trials is largely hindered by its poor bioavailability [31]. Its low intestinal absorption, poor structural stability, and rapid metabolism, especially the poor permeability across the blood-brain barrier, are major obstacles for the use of curcumin as a therapeutic agent for migraine [19]. As a result, various drug delivery approaches have been used to increase curcumin's bioavailability [8]. It has been demonstrated that structural alteration and bioconjugates enhanced the curcumin's protective property against oxidative stress [23]. In addition, lots of researches showed that nanocarriers including liposomes, isomerization, polymeric nanoparticles, and polymeric micelles rendered a larger effect size for curcumin [32, 33]. For instance, Khalil et al. illustrated that the PLGA-PEG nanoparticles increased the bioavailability of curcumin by up to 55.4-fold by decreasing the degradation [34]. With the improvement of nanotechnology, curcumin would be more likely to be used to treat migraine.
To the best of our knowledge, there is no widely accepted in vitro model for migraine. Both C2C12 myoblasts and meningeal mast cells [35] have been used as model for migraine research [36]. Harriott et al. also suggested the spreading depression model to be used as a preclinical model of migraine [37]. Since migraine is a vascular disorder which was thought to be related to the trigeminovascular pathway, we used HUVECs as a model to study the mechanism (oxidative stress) that may be associated with migraine. There are limitations to the approach of using HUVECs as a migraine model. The model we used is just one component part of the complex heterogeneous pathogenesis of migraine; however, we think it could be helpful for the examination of alterations in vascular dysfunction. The effect of curcumin on migraine remained to be confirmed in an in vivo experiment and clinical trial.
5. Conclusion
In the present study, we report the effect of curcumin on H2O2-induced oxidative stress in HUVECs, which might be a potential therapy for migraine.
Acknowledgments
This study was funded by the Guangdong Medical Science and Technology Research Foundation (to Jipeng Ouyang, B2018117).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Lipton R. B., Tepper S. J., Reuter U., et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250–e2260. doi: 10.1212/wnl.0000000000007452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buse D. C., Lipton R. B., Hallström Y., et al. Migraine-related disability, impact, and health-related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38(10):1622–1631. doi: 10.1177/0333102418789072. [DOI] [PubMed] [Google Scholar]
- 3.Jain S., Silberstein S. D. Invited commentary on preventive anti-migraine therapy (PAMT) Current Treatment Options in Neurology. 2019;21(4) doi: 10.1007/s11940-019-0555-4. [DOI] [PubMed] [Google Scholar]
- 4.Tassorelli C., De Icco R. Getting closer to a cure for migraine. Nature Reviews Neurology. 2019;15(2):64–65. doi: 10.1038/s41582-019-0134-z. [DOI] [PubMed] [Google Scholar]
- 5.Méndez-García L. A., Martínez-Castillo M., Villegas-Sepúlveda N., Orozco L., Córdova E. J. Curcumin induces p53-independent inactivation of Nrf2 during oxidative stress-induced apoptosis. Human & Experimental Toxicology. 2019;38(8):951–961. doi: 10.1177/0960327119845035. [DOI] [PubMed] [Google Scholar]
- 6.Salehi B., Jornet P. L., López E. P., et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, green tea and coffee properties. Biomolecules. 2019;9(3):p. 106. doi: 10.3390/biom9030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang M., Taghibiglou C. The mechanisms of action of curcumin in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2017;58(4):1003–1016. doi: 10.3233/jad-170188. [DOI] [PubMed] [Google Scholar]
- 8.Abrahams S., Haylett W. L., Johnson G., Carr J. A., Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: a review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Tepper S. J., Diener H. C., Ashina M., et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology. 2019;92(20):e2309–e2320. doi: 10.1212/WNL.0000000000007497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripathi G. M., Kalita J., Misra U. K. A study of oxidative stress in migraine with special reference to prophylactic therapy. International Journal of Neuroscience. 2018;128(4):318–324. doi: 10.1080/00207454.2017.1374959. [DOI] [PubMed] [Google Scholar]
- 11.Geyik S., Altunisik E., Neyal A. M., Taysi S. Oxidative stress and DNA damage in patients with migraine. The Journal of Headache and Pain. 2016;17(1):p. 10. doi: 10.1186/s10194-016-0606-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borkum J. M. The migraine attack as a homeostatic, neuroprotective response to brain oxidative stress: preliminary evidence for a theory. Headache: The Journal of Head and Face Pain. 2018;58(1):118–135. doi: 10.1111/head.13214. [DOI] [PubMed] [Google Scholar]
- 13.Gupta R. M., Hadaya J., Trehan A., et al. A genetic variant associated with five vascular diseases is a distal regulator of endothelin-1 gene expression. Cell. 2017;170(3):522–533. doi: 10.1016/j.cell.2017.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parohan M., Sarraf P., Javanbakht M. H., Ranji-Burachaloo S., Djalali M. Effect of coenzyme Q10 supplementation on clinical features of migraine: a systematic review and dose-response meta-analysis of randomized controlled trials. Nutritional Neuroscience. 2019:1–8. doi: 10.1080/1028415X.2019.1572940. [DOI] [PubMed] [Google Scholar]
- 15.Xiong Y., Chang L. L., Tran B., et al. ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacologica Sinica. 2019 doi: 10.1038/s41401-019-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao W., Zheng Y., Fang W., et al. Dual specificity phosphatase 6 protects neural stem cells from β-amyloid-induced cytotoxicity through ERK1/2 inactivation. Biomolecules. 2018;8(4):p. 181. doi: 10.3390/biom8040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao W., Jiang M., Li M., et al. Magnesium elevation promotes neuronal differentiation while suppressing glial differentiation of primary cultured adult mouse neural progenitor cells through ERK/CREB activation. Frontiers in Neuroscience. 2017;11:p. 87. doi: 10.3389/fnins.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z., Yu Y., Li X., Ross C. A., Smith W. W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for parkinsonism. Pharmacological Research. 2011;63(5):439–444. doi: 10.1016/j.phrs.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Shi L.-Y., Zhang L., Li H., et al. Protective effects of curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacological Reports. 2018;70(5):1040–1046. doi: 10.1016/j.pharep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Huang X., Liao W., Huang Y., et al. Neuroprotective effect of dual specificity phosphatase 6 against glutamate-induced cytotoxicity in mouse hippocampal neurons. Biomedicine & Pharmacotherapy. 2017;91:385–392. doi: 10.1016/j.biopha.2017.04.096. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal S. S., Gullaiya S., Dubey V., et al. Neurodegenerative shielding by curcumin and its derivatives on brain lesions induced by 6-OHDA model of Parkinson’s disease in albino wistar rats. Cardiovascular Psychiatry and Neurology. 2012;2012:8. doi: 10.1155/2012/942981.942981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harish G., Venkateshappa C., Mythri R. B., et al. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: implications for Parkinson’s disease. Bioorganic & Medicinal Chemistry. 2010;18(7):2631–2638. doi: 10.1016/j.bmc.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Jangra A., Kwatra M., Singh T., et al. Piperine augments the protective effect of curcumin against lipopolysaccharide-induced neurobehavioral and neurochemical deficits in mice. Inflammation. 2016;39(3):1025–1038. doi: 10.1007/s10753-016-0332-4. [DOI] [PubMed] [Google Scholar]
- 24.Cui Q., Li X., Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Molecular Medicine Reports. 2016;13(2):1381–1388. doi: 10.3892/mmr.2015.4657. [DOI] [PubMed] [Google Scholar]
- 25.Fu X.-Y., Yang M.-F., Cao M.-Z., et al. Strategy to suppress oxidative damage-induced neurotoxicity in PC12 cells by curcumin: the role of ROS-mediated DNA damage and the MAPK and AKT pathways. Molecular Neurobiology. 2016;53(1):369–378. doi: 10.1007/s12035-014-9021-1. [DOI] [PubMed] [Google Scholar]
- 26.van Meeteren M. E., Hendriks J. J. A., Dijkstra C. D., van Tol E. A. F. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochemical Pharmacology. 2004;67(5):967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar B., Dhiman M., Mittal S., Mantha A. K. Curcumin revitalizes amyloid beta (25-35)-induced and organophosphate pesticides pestered neurotoxicity in SH-SY5Y and IMR-32 cells via activation of APE1 and Nrf2. Metabolic Brain Disease. 2017;32(6):2045–2061. doi: 10.1007/s11011-017-0093-2. [DOI] [PubMed] [Google Scholar]
- 28.Slabber C. A., Grimmer C. D., Robinson R. S. Solution conformations of curcumin in DMSO. Journal of Natural Products. 2016;79(10):2726–2730. doi: 10.1021/acs.jnatprod.6b00726. [DOI] [PubMed] [Google Scholar]
- 29.Schultze E., Coradini K., Dos Santos Chaves P., et al. Drug-loaded nanoemulsion as positive control is an alternative to DMSO solutions for in vitro evaluation of curcumin delivery to MCF-7 cells. Pharmacological Reports. 2017;69(6):1408–1412. doi: 10.1016/j.pharep.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Hu S., Maiti P., Ma Q., et al. Clinical development of curcumin in neurodegenerative disease. Expert Review of Neurotherapeutics. 2015;15(6):629–637. doi: 10.1586/14737175.2015.1044981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motaghinejad M., Motevalian M., Fatima S., Faraji F., Mozaffari S. The neuroprotective effect of curcumin against nicotine-induced neurotoxicity is mediated by CREB-BDNF signaling pathway. Neurochemical Research. 2017;42(10):2921–2932. doi: 10.1007/s11064-017-2323-8. [DOI] [PubMed] [Google Scholar]
- 32.Pieretti S., Ranjan A. P., Di Giannuario A., et al. Curcumin-loaded Poly (d, l-lactide-co-glycolide) nanovesicles induce antinociceptive effects and reduce pronociceptive cytokine and BDNF release in spinal cord after acute administration in mice. Colloids and Surfaces B: Biointerfaces. 2017;158:379–386. doi: 10.1016/j.colsurfb.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Del Prado-Audelo M. L., Caballero-Florán I. H., Meza-Toledo J. A., et al. Formulations of curcumin nanoparticles for brain diseases. Biomolecules. 2019;9(2):p. 56. doi: 10.3390/biom9020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalil N. M., Nascimento T. C. F. D., Casa D. M., et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids and Surfaces B: Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 35.Koroleva K., Gafurov O., Guselnikova V., et al. Meningeal mast cells contribute to ATP-induced nociceptive firing in trigeminal nerve terminals: direct and indirect purinergic mechanisms triggering migraine pain. Frontiers in Cellular Neuroscience. 2019;13:p. 195. doi: 10.3389/fncel.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren Y., Li Y., Lv J., et al. Parthenolide regulates oxidative stress-induced mitophagy and suppresses apoptosis through p53 signaling pathway in C2C12 myoblasts. Journal of Cellular Biochemistry. 2019;120(9):15695–15708. doi: 10.1002/jcb.28839. [DOI] [PubMed] [Google Scholar]
- 37.Harriott A. M., Takizawa T., Chung D. Y., Chen S. P. Spreading depression as a preclinical model of migraine. The Journal of Headache and Pain. 2019;20(1):p. 45. doi: 10.1186/s10194-019-1001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.