Abstract
Bartonella are vector-borne hemotropic bacteria that infect a wide variety of hosts, including people. While there are PCR assays that can identify individual or groups of Bartonella, there is no reliable molecular method to simultaneously detect all species while maintaining genus specificity and sensitivity. By comparing highly conserved 16S rRNA sequences of the better-recognized Bartonella spp. on GenBank, we selected primers and probes for a genus-specific pan-Bartonella FRET-qPCR. Then, a gltA-based Bartonella PCR was established by selecting primers for a highly variable region of gltA, of which the sequenced amplicons could identify individual Bartonella spp. The pan-Bartonella FRET-qPCR did not detect negative controls (Brucella spp., Anaplasma spp., Rickettsia spp., Coxiella burnetii, and Wolbachia) but reliably detected as few as two copies of the positive control (Bartonella henselae) per reaction. There was complete agreement between the pan-Bartonella FRET-qPCR and the gltA-based Bartonella PCR in detecting Bartonella in convenience test samples from China and St. Kitts: cats (26%; 81/310), Ctenocephalides felis (20%; 12/60), cattle (24%; 23/98), and donkeys (4%; 1/20). Sequencing of the gltA-based Bartonella PCR products revealed B. henselae (70%; 57/81) and B. clarridgeiae (30%; 24/81) in cats and C. felis (67%; 8/12, and 33%; 4/12, respectively) and B. bovis in cattle (23.5%; 23/98) and donkeys (4.0%; 1/24). The pan-Bartonella FRET-qPCR and gltA-based Bartonella PCR we developed are highly sensitive and specific in detecting recognized Bartonella spp. in a single reaction. The pan-Bartonella FRET-qPCR is convenient requiring no gel electrophoresis and providing copy numbers, while the gltA-based Bartonella PCR reliably differentiates individual Bartonella species. The use of these PCRs should greatly facilitate large-scale surveillance studies and the diagnosis of infections in clinical samples.
1. Introduction
There are now over 40 species and subspecies of Bartonella which are small, intracellular, vector-borne hemotropic Gram-negative bacteria. High prevalences of the organisms have been reported around the world in a wide range of insect vectors and domestic and wild animal hosts including rodents, felines, canines, ruminants, and even bats [1–4]. In China, Bartonella species have been detected in a wide range of animals [5–8] with at least 10 Bartonella species having been implicated in human diseases that range from self-limiting regional lymphadenitis to severe endocarditis [9–11]. To the best of our knowledge, there has been only a single report of Bartonella infecting people in the Caribbean [12] although infections have been described in cats [13], mongooses [14], bats [15, 16], dogs [17, 18], rodents [19], cat and rodent fleas [20, 21], and bat flies [22].
Detecting Bartonella species in their vectors and mammalian hosts is often not easy as it is difficult and time-consuming to culture them and serology may be unreliable. Although numerous molecular methods to detect Bartonella have been described [17–28], in most cases, a single PCR only detects individual or closely related species of Bartonella [29]. Although multiplex PCRs have been described to detect Bartonella, they require a wide range of primers and probes to detect all species. Furthermore, the PCRs have poor sensitivity (with up to 8 × 107 bacteria per g being undetectable in feces) [30, 31] because of inhibitors in environmental and clinical samples. Recently, a highly specific and quantitative qPCR has been established to specifically amplify nuoG of the Bartonella genus and identified B. henselae and B. clarridgeiae in cats in Brazil [32].
We used the available 16S rRNA sequences of 20 Bartonella species in GenBank to develop and validate a highly sensitive and genus-specific pan-Bartonella FRET-qPCR. We also used available sequences for the citrate synthase gene (gltA) and considered a reliable tool for distinguishing genotypes [33], to develop a gltA-based Bartonella PCR to identify and differentiate the species. The development of these PCRs and their use on convenience samples from China and St. Kitts is described below.
2. Materials and Methods
2.1. Whole Blood Samples and Fleas
This study was reviewed and approved by the Institutional Animal Care and Use Committee of the Yangzhou University College of Veterinary Medicine, China (YZU-13-58Wang), and of the Ross University School of Veterinary Medicine, St. Kitts (07/02/3MOURA). Convenience whole blood samples in EDTA were collected from cats in China and St. Kitts. Between April 2013 and June 2014, 164 cat whole blood samples were collected from five cities in four provinces in China and stored at −80°C until DNA extraction (see below). The cats sampled in Yangzhou were kept in a shelter, while those from Beijing, Shanghai, and Guangdong were pet cats presenting to veterinary clinics with a variety of conditions. Cat fleas (n = 60) were collected from the cats used for blood sampling, and the flea species were identified according to the morphological criteria as described in previous studies [34, 35]. Frozen (−80°C) whole blood samples were also available from apparently healthy stray cats from St. Kitts (n = 146) sampled during a Trap, Neuter, Release program conducted in 2012. Archived whole blood samples from cattle (n = 98), sheep (n = 30), donkeys (n = 24), and horses (n = 24) collected as described in previous studies [35–38] were also used in this project. Storage and laboratory analysis of all the samples were performed in Yangzhou University, Yangzhou, China.
2.2. DNA Extraction
Samples were thawed at room temperature, and DNA was extracted from whole blood and homogenized arthropods [33] with the QIAamp® DNA Blood Mini Kit (Qiagen, Valencia, CA, USA), following the manufacturer's protocol. DNA was frozen at −80°C until PCRs were performed. The concentration and quality of the samples in this study were validated by running PCR endogenous control of the universal hydroxymethylbilane synthase-based PCR as described [39].
2.3. Development of a 16S rRNA-Based Pan-Bartonella FRET-qPCR
2.3.1. Primers and Probes
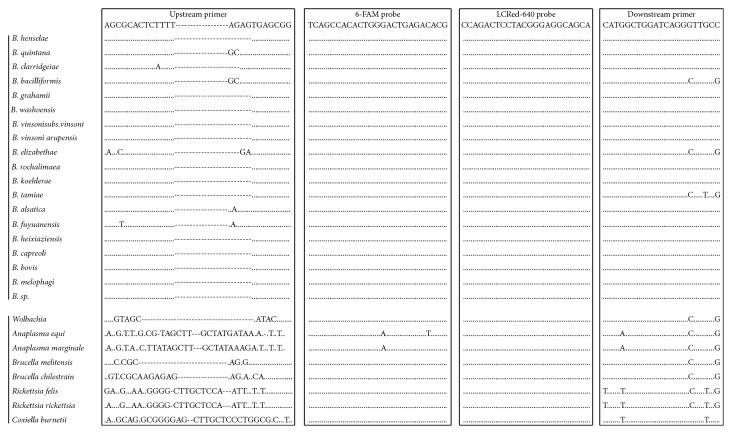
The 16S rRNA sequences for the 18 better-recognized Bartonella spp. on GenBank and eight closely related species were obtained from GenBank: B. quintana (NR_044748), B. henselae (AJ223778), B. clarridgeiae (NR_03696), B. elizabethae (AB246807), B. bacilliformis (NR_044743), B. bovis (NR_025121), B. capreoli (NR_025120), B. koehlerae (NR_024932), B. alsatica (NR_025272), B. grahamii (NR_029366), B. vinsonii subsp. berkhoffii (DQ2281315), B. vinsonii subsp. vinsonii (NR_037056), B. vinsonii subsp. arupensis (AF214558), B. doshiae (NR_029368), B. queenslandensis (EU111758), B. washoensis (AB519066), B. rattaustraliani (EU111753), B. tamiae (DQ395176), B. rochalimae (DQ683196), B. melophagi (AY724770), Bartonella sp. (U71322), Anaplasma phagocytophilum (AF172167), Anaplasma marginale (AF414873), Wolbachia (KF249715), Brucella melitensis (CP002932), Brucella chilestrain (AY513509), Rickettsia felis (NR_074483), R. rickettsii (L36217), and Coxiella burnetii (D89798) (Figure 1). These sequences were aligned using Clustal multiple sequence alignment in VectorNTI (Informax Inc., North Bethesda, MD, USA) to identify a highly conserved region of the 16S rRNA common to all the above Bartonella spp., but significantly different from the other species (Figure 1). The primers and probes we developed were situated within the conserved region and synthesized by Integrated DNA Technologies (Coralville, IA, USA). The pan-Bartonella FRET-qPCR we established amplifies a 304 bp target with the positions of primers and probes shown in Figure 1: forward primer: 5′-AGCGCACTCTTTTAGAGTGAGCGG-3′; reverse primer: 5′-CATGGCTGGATCAGGGTTGCC-3′; anchor probe: 5′-TCAGCCACACTGGGACTGAGACACG-(6-FAM)-3′; and reporter probe: 5′-(LCRed640)-CCAGACTCCTACGGGAGGCAGCA-phosphate-3′.
Figure 1.
Alignment of oligonucleotides for the pan-Bartonella FRET-qPCR based on the 16S rRNA used in this study. Primers and probes are shown at the top of the boxes. Dots indicate nucleotides identical to primers and probes, and dashes denote absence of the nucleotide. The upstream primers and two probes are used as the indicated sequences, while the downstream primer is used as the antisense oligonucleotide. The designed oligonucleotides show minimum mismatching with Bartonella spp. (0 mismatches with 14 species, 1 mismatch with 3 species, 2 mismatches with 1 species, and 4 mismatches with 2 species) but 11–29 nucleotide mismatches with the related non-Bartonella species. The 6-FAM label is directly attached to the 3-terminal nucleotide of the fluorescein probe, and the LCRed-640 fluorescein label is added via a linker to the 5′ end of the LCRed-640 probe.
2.3.2. Thermal Cycling
The pan-Bartonella FRET-qPCR was performed in a LightCycler 480® II real-time PCR platform with 20 μl final volumes containing 10 μl extracted DNA. The primers and probes were 1 μM and 0.2 μM in the final reaction system, respectively. The qPCR buffer was established with a final concentration of 20 mM Tris-HCl, 4.5 mM MgCl2, 0.05% Tween 20, and 0.05% Nonidet P-40. Thermal cycling consisted of a 2 min denaturation step at 95°C followed by 18 high-stringency step-down thermal cycles and 40 low-stringency fluorescence acquisition cycles for 1 sec at 95°C, 12 sec at 58°C, 30 sec at 67°C, and 10 sec at 72°C. Melting curve analysis was performed by monitoring fluorescence between 38°C and 80°C. All the PCRs were run in duplicate in this study.
2.3.3. Specificity
Bartonella henselae DNA from a cat in St. Kitts [21] was used as the positive control, and the PCR product was confirmed by electrophoresis (1.5% MetaPhor agarose gels) and by purification using QIAquick PCR Purification Kit (Qiagen, Valencia, CA, USA) and genomic sequencing (GenScript, Nanjing, Jiangsu, China). We used BLAST to compare the sequencing data from the positive Bartonella samples with the available Bartonella sequences in GenBank. The specificity of the PCR was further verified using the related species Brucella melitensis, Brucella chilestrain, Wolbachia, Coxiella burnetii, Rickettsia felis, Rickettsia rickettsii, Anaplasma phagocytophilum, and Anaplasma marginale (provided by the Parasitological Laboratory of Yangzhou University College of Veterinary Medicine).
2.3.4. Sensitivity
For quantitative standards, we used amplified DNA of B. henselae identified in this study. The amplification products were gel purified using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA, USA) and quantified using the PicoGreen DNA fluorescence assay (Molecular Probes, Eugene, OR, USA). The molarity of the DNA was estimated using the calculated molecular mass of the amplicons and dilutions made to give solutions containing 10,000, 1,000, 100, 10, and 1 gene copies/μl in T10E0.1 buffer. These were used as quantitative standards in the FRET-qPCR surveys to enable standard curves to be developed for the calculation of the gene copy numbers in positive samples. Twofold dilutions of 10 and 1 gene copies/μl solution were used to determine the minimal detection limit.
2.4. Development of a Citrate Synthase Gene- (gltA-) Based PCR to Differentiate Bartonella spp
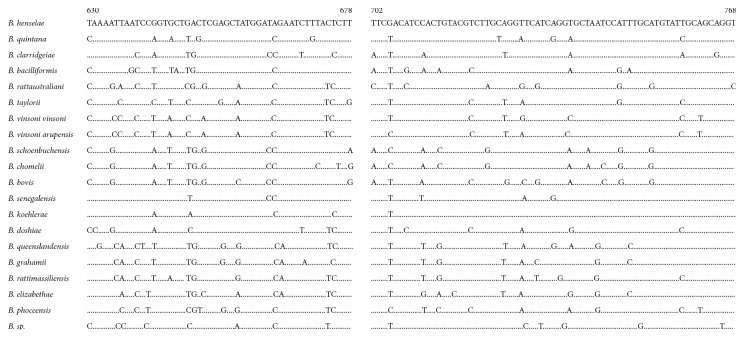
The amplicon of the pan-Bartonella FRET-qPCR we established was based on a region of the 16S rRNA that is highly conserved among different Bartonella spp. and is thus ideal for screening samples for the organisms. To differentiate Bartonella spp. that gave positive reactions in screening pan-Bartonella FRET-qPCR, we used a standard PCR that amplifies a relatively highly polymorphic region of the citrate synthase gene (gltA) (439 nucleotides for different Bartonella spp.) and sequenced the products (GenScript, Nanjing, Jiangsu, China). For the gltA-based Bartonella PCR, we designed, as above for the pan-Bartonella FRET-qPCR, a forward primer (5′-CGTAATGATCTYAGTTAYGCTGCAAA-3′) and a reverse primer (5′-AGAAGTGGATCATTTTGAATRTTBARYTC-3′) that amplify all the 18 better-recognized Bartonella spp. available in GenBank (Figure 2). The sensitivity and specificity of the gltA-based PCR were assessed as described above for the pan-Bartonella FRET-qPCR. The PCRs were performed in a LightCycler 480® II real-time PCR platform with 20 μl final volumes containing 10 μl extracted DNA, and 1 μM primers with the same PCR buffer as described above. Thermal cycling of gltA-PCRs was performed the same as pan-Bartonella FRET-qPCR except removing the melting curve analysis and revising annealing temperature to 56°C. All the PCRs were run in duplicate.
Figure 2.
Alignment of oligonucleotides for Bartonella PCR based on gltA used in this study. Two partial amplicons of polymorphic regions of Bartonella spp. based on gltA are shown. The base of B. henselae (L38987.1) shown at the top of the figure was complete with the coding sequences from 630 to 678 and 702 to 768. Dots indicate identical nucleotide sequence to that of B. henselae. Through the polymorphic region of Bartonella spp., different Bartonella species could be determined by genomic sequencing in this study. The number of nucleotide mismatches between B. henselae and other Bartonella species in the 116 polymorphic coding sequences is also noted (mismatch in coding sequences from 630 to 678 and 702 to 768 with about 5 and 23 nucleotides). In this polymorphic region, none of Babesia species had identical sequences.
2.5. Phylogenetic Analysis
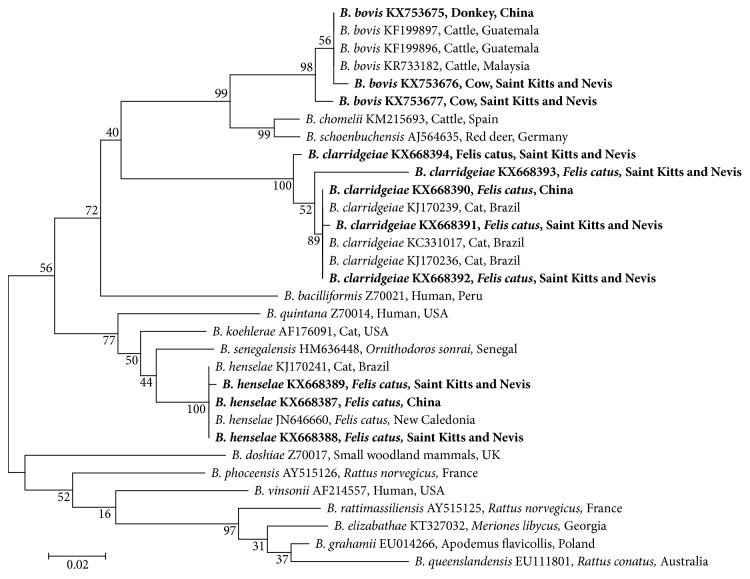
The retrieved Bartonella sequences of the present study and the reference sequences from GenBank were aligned using the MEGA 6.0 software (Figure 3). Based on these alignments, phylogenetic trees were constructed by the maximum likelihood and Bayesian inference method using MEGA 6.0. Bootstrap values were calculated using 500 replicates.
Figure 3.
Molecular phylogenetic analysis of Bartonella spp. in this study. Distances and groupings of Bartonella spp. detected in this study (bold font) and reference Bartonella sequences from NCBI were determined by the maximum likelihood and Bayesian inference method with the MEGA version 6 software based on the gltA gene (439 bp). Bootstrap values were calculated using 500 replicates. Scale bar indicates a genetic distance of 0.02 nt substitutions per position.
3. Results
3.1. Testing of the Pan-Bartonella FRET-qPCR and Survey of Animals and Fleas for Bartonella spp. DNA
The probes we designed for the pan-Bartonella FRET-qPCR were highly conserved, while the primers only had between zero and four nucleotide mismatches with the 18 representative Bartonella spp. we used in the study (Figure 1). In contrast, the primers and probes had 11, 26, 27, 10, 17, 23, 22, and 29 mismatches with A. phagocytophilum, A. marginale, Wolbachia, B. melitensis, B. chilestrain, R. felis, R. rickettsia, and C. burnetii, respectively (Figure 1). The pan-Bartonella FRET-qPCR was positive with B. henselae DNA, but no products were obtained with DNAs from Wolbachia, A. equi, A. marginale, B. melitensis, B. chilestrain, R. felis, R. rickettsia, and C. burnetii. Using the gel-purified PCR products as quantitative standards [20], we determined the detection limit of the pan-Bartonella FRET-qPCR was two copies of the Bartonella 16S rRNA per reaction.
To confirm accuracy and reproducibility of real-time PCR, the intra-assay precision was determined in three repeats within one LightCycler run. Interassay variation was investigated in three different experimental runs, while the quantitative standards (1,000, 100, 10, and 1 copies/reaction) were diluted in 1 × T10E0.1, and with the diluents containing lambda DNA (New England Biolabs, Inc.) at the concentration of 1 ng/μl. The calculation of test precision and test variability is based on the CP variation from the CP mean value. Test reproducibility for all investigated quantitative standards was lower in intratest experiments (<3.8%) and was higher in intertest experiments (<6.1%). The reproducibility dropped in lower copies of standards (1 and 10 copies per reaction) in comparison with the high copies of standards (100 copies and 1,000 copies).
Of the 310 cat blood samples we examined, 78 (25.2%) were positive in our pan-Bartonella FRET-qPCR. Cats from all the sites we studied were positive, mainly from Beijing (11.1%), Shanghai (5.6%), Guangdong (15.0%), Yangzhou (19.4%), and St. Kitts (39.7%) (Table 1). The 60 fleas collected from the Chinese cats were identified as Ctenocephalides felis based on their morphology, and 20.0% (12/60) were positive in the pan-Bartonella FRET-qPCR, while only one donkey (5.0%) from China was positive. Horses, sheep, and donkeys from St. Kitts were negative, but cattle from the island were commonly positive (54.8%) in the pan-Bartonella FRET-qPCR, although cattle from China were negative.
Table 1.
Molecular prevalence of Bartonella in mammals.
| Mammal | Source | Positive pan-Bartonella FRET-qPCR (N, %) | Bartonella sp. from gltA-based PCR | Average copies/ml blood, or per flea |
|---|---|---|---|---|
| Cat (n = 310) | Beijing | 4/36, 11.1 | 4 B. henselae | 105.60 |
| Shanghai | 2/36, 5.6 | 1 B. henselae | 108.35 | |
| 1 B. clarridgeiae | 105.48 | |||
| Guangdong | 3/20, 15.0 | 3 B. henselae | 104.60 | |
| Yangzhou | 14/72, 19.4 | 11 B. henselae | 106.36 | |
| 3 B. clarridgeiae | 107.86 | |||
| St. Kitts | 58/146, 39.7 | 38 B. henselae | 106.33 | |
| 20 B. clarridgeiae | 106.65 | |||
|
| ||||
| Cattle (n = 98) | St. Kitts | 23/42, 54.8 | 23 B. bovis | 106.03 |
| Yangzhou | 0/56, 0 | — | ||
|
| ||||
| Horse (n = 24) | St. Kitts | 0/24, 0 | — | |
|
| ||||
| Sheep (n = 30) | St. Kitts | 0/30, 0 | — | |
|
| ||||
| Donkey (n = 24) | St. Kitts | 0/4, 0 | — | |
| Hebei | 1/20, 5.0 | 1 B. bovis | 105.44 | |
|
| ||||
| Ctenocephalides felis (n = 60) | Yangzhou | 12/60, 20.0 | 8 B. henselae | 102.88 |
| 4 B. clarridgeiae | 107.01 | |||
In general, the copies detected in the pan-Bartonella FRET-qPCR were considerably higher in the positive blood samples we collected (106.20/ml whole blood). Fleas positive for B. henselae generally had low copy numbers, but those positive for B. clarridgeiae had considerably higher copy numbers (107.01 vs. 102.88).
3.2. Testing of the gltA-Based Bartonella PCR and Differentiation of the Bartonella spp. in the Survey Animals and Fleas
The gltA-based Bartonella PCR did not amplify the negative controls but did amplify the positive control B. henselae with an amplicon sequence identical to those for this species in GenBank. It also amplified all the test samples that were positive in the pan-Bartonella FRET-qPCR, indicating the tests had similar sensitivity. All of the cats that were positive in the pan-Bartonella FRET-qPCR were also positive in the gltA-based Bartonella PCR. Sequencing of the products of the gltA-based Bartonella PCR revealed B. henselae was most prevalent (64.1%; 50/78), followed by B. clarridgeiae (24.3%; 19/78) (Table 1). Both species were detected in cats from China and from St. Kitts. In the pan-Bartonella FRET-qPCR positive C. felis we collected from the Chinese cats, both B. Bartonella henselae (13.3%, 8/60) and B. clarridgeiae (6.7%, 4/60) were identified with the gltA-based Bartonella PCR. Each of the positive fleas was found on a cat positive for the same Bartonella sp.
Sequencing of amplicons from the gltA-based Bartonella PCR that were positive with the cattle from St. Kitts showed only B. bovis was present; this organism was also present in the one positive Chinese donkey (Table 1). The phylogenic comparison shows the identity or highly similar nucleotide sequences between Bartonella spp. identified in this work and those of the reference strains from GenBank (Table 2; Figure 3).
Table 2.
Comparison of isolates identified in this study and similar sequences in GenBank by BLAST.
| Isolates identified in this study | Highly similar sequences in GenBank | ||||
|---|---|---|---|---|---|
| Bartonella spp. | Gene accession # | Source/origin | Gene accession # | Source/origin | Mismatches |
|
| |||||
| B. henselae | KX668387 | Cats from China | JN646660 | Animals from New Caledonia | 0/405 |
| KX668389 | Cats from St. Kitts | KJ170241 | Domestic cats from Brazil | 1/397 | |
| KX668388 | Cats from St. Kitts | JN646660 | Animals from New Caledonia | 0/405 | |
|
| |||||
| B. clarridgeiae | KX668391 | Cats from St. Kitts | KJ170239 | Domestic cats from Brazil | 1/401 |
| KX668390 | Cats from China | KJ170239 | Domestic cats from Brazil | 0/401 | |
| KX668392 | Cats from St. Kitts | KJ170236 | Cats from animal shelters in Brazil | 0/401 | |
| KX668393 | Cats from St. Kitts | KC331017 | Cats from animal shelters in Brazil | 14/401 | |
| KX668394 | Cats from St. Kitts | KC331017 | Cats from animal shelters in Brazil | 4/402 | |
|
| |||||
| B. bovis | KX753675 | Donkey from China | KR733182 | Cattle from Malaysia | 0/409 |
| KX753676 | Cows from St. Kitts | KF199896 | Cattle from Guatemala | 0/409 | |
| KX753677 | Cows from St. Kitts | KF199897 | Cattle from Guatemala | 0/409 | |
4. Discussion
As knowledge on the wide diversity and host ranges of Bartonella species increases, it becomes ever more important to develop reliable molecular assays that can detect all Bartonella species and thereby facilitate epidemiological and ecological studies. The pan-Bartonella FRET-qPCR we developed proved to be specific, not giving reactions with closely related organisms. This was expected as, by systematically aligning the sequences of 18 better-recognized Bartonella spp. in GenBank and other species, we were able to identify a highly conserved and specific region of the 16S rRNA for the PCR (Figure 1). The pan-Bartonella FRET-qPCR was also very sensitive, detecting as few as two Bartonella 16S rRNA copies per reaction. Further advantages were that it was quantitative and relatively rapid to perform as gel electrophoresis was not needed to demonstrate amplicons.
Although the pan-Bartonella FRET-qPCR was very sensitive (2 copies per reaction) and specific, quantitative, and rapid (normally 1.5 hours including DNA extraction), because it was designed against a highly conserved region of the 16S rRNA, it did not enable us to determine the Bartonella spp. in the samples. This was possible, however, with the gltA-based Bartonella PCR which was as accurate and specific as the pan-Bartonella FRET-qPCR in identifying Bartonella spp. in our study. Scola et al. first reported that, of the commonly used genetic targets for Bartonella identification, only gltA and rpoB sequences provide sufficient discriminatory power and interspecies diversity to allow discrimination of Bartonella spp. [33]. We therefore used gltA as the target of our PCR and selected our primers to ensure that, although they only amplified Bartonella spp., they covered a 439 bp hypervariable area of the gene that enabled differentiation of the recognized and poorly characterized Bartonella spp. recorded in GenBank (Figure 2). Our gltA-based PCR clearly differentiated the control B. henselae and identified three common species in our survey, B. henselae, B. clarridgeiae, and B. bovis, which were the species expected to be present in the animals surveyed.
Applying our pan-Bartonella FRET-qPCR to a number of convenience samples available enabled us to rapidly test a variety of samples from different geographic areas for Bartonella. We obtained positive results with all the samples tested, mainly mammalian whole blood, and vector insects (fleas) which comprise the most commonly used samples to date in epidemiological studies. Although the gltA-based Bartonella PCR was more time-consuming, requiring gel electrophoresis to reveal amplicons, it identified all the samples that were positive in the pan-Bartonella FRET-qPCR.
Bartonella henselae and B. clarridgeiae were the species most commonly identified by the gltA-based Bartonella PCR in our study. These are the organisms most commonly associated with cats and their fleas and have already been described to be prevalent in the West Indies [13] and China [40] where they are often associated with persistent subclinical bacteremia and transmitted by C. felis [2]. Although we found B. bovis at a high level in cattle from St. Kitts, we failed to identify the organism in cattle from China. We did, however, find B. bovis in a donkey in China, indicating the organism is present in the country. Bartonella bovis is a recently described species [41] that is generally the most prevalent of the three species that infect cattle, the others being Bartonella schoenbuchensis and Bartonella chomelii. The latter have mainly been described in Europe or in cattle originating from Europe [42], while B. bovis has been described from widely around the world with prevalences in cattle varying from 0% in Kenya and Japan [43] to up to 96% in the USA [44]. Although high levels of bacteremia can be found in cattle, persisting for up to seven months [45], most infections seem subclinical although cases of bovine endocarditis have been described [46]. There are little data on B. bovis in equids although horses seropositive against the organism have been described and experimental infections generally cause no clinical signs with no bacteremia and a low-grade and short-duration antibody response [47]. The vector of B. bovis has not been determined but is suspected to be a blood-sucking arthropod [48].
The specificity of the 16S rRNA-based pan-Bartonella FRET-qPCR and the gltA-based Bartonella PCR we developed was based on the alignment of the available recognized Bartonella spp. sequences in GenBank. For completeness, the newly established PCRs should be tested on known positive control samples of DNA of all the recognized and poorly characterized Bartonella spp., but it is no simple matter for one laboratory to obtain all of these samples. Similarly, with the rapid growth in the recognized and suspected Bartonella species, it will be important for ongoing testing of the PCR to ensure it recognizes the newly recognized strains and species. Ideally, the established Bartonella PCRs in this work should be compared with other existing molecular approaches for their sensitivity and specificity and validated fully following the MIQE guidelines for publication of quantitative real-time PCR experiments [49].
In conclusion, in this report, we describe what we believe to be the first FRET-qPCR that specifically detects all the currently recognized Bartonella species. We also describe a gltA-based PCR that enables the differentiation of the recognized Bartonella spp. The pan-Bartonella FRET-qPCR enabled us to rapidly and quantitatively screen a variety of samples from a number of sources and different geographical areas for the presence of Bartonella spp., while the gltA-based PCR provided a convenient method to differentiate the species involved using only a single reaction.
Acknowledgments
This work was supported by Alabama Agricultural Experiment Station and the USDA National Institute of Food and Agriculture through the Hatch project (ALA052-1-17026) and by the National Natural Science Foundation of China (32472225).
Data Availability
Whole data including the nucleotide sequence used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Chang C.-C., Chomel B. B., Kasten R. W., et al. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerging Infectious Diseases. 2000;6(3):306–311. doi: 10.3201/eid0603.000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomel B. B., Kasten R. W. Bartonellosis, an increasingly recognized zoonosis. Journal of Applied Microbiology. 2010;109(3):743–750. doi: 10.1111/j.1365-2672.2010.04679.x. [DOI] [PubMed] [Google Scholar]
- 3.Lilley T. M., Veikkolainen V., Pulliainen A. T. Molecular detection of Candidatus Bartonella hemsundetiensis in bats. Vector-borne and Zoonotic Diseases. 2015;15(11):706–708. doi: 10.1089/vbz.2015.1783. [DOI] [PubMed] [Google Scholar]
- 4.Malania L., Bai Y., Osikowicz L. M., et al. Prevalence and diversity of Bartonella species in rodents from Georgia (caucasus) The American Journal of Tropical Medicine and Hygiene. 2016;95(2):466–471. doi: 10.4269/ajtmh.16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han H. J., Wen H. L., Zhao L., et al. Novel Bartonella species in insectivorous bats, Northern China. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0167915.e0167915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li D.-M., Hou Y., Song X.-P., et al. High prevalence and genetic heterogeneity of rodent-borne Bartonella species on Heixiazi island, China. Applied and Environmental Microbiology. 2015;81(23):7981–7992. doi: 10.1128/aem.02041-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao H. X., Yu J., Guo P. Bartonella species detected in the plateau pikas (Ochotona curzoiae) from Qinghai plateau in China. Biomedical Environmental Science. 2015;28(9):674–678. doi: 10.3967/bes2015.094. [DOI] [PubMed] [Google Scholar]
- 8.Liu Q., Sun J., Lu L., et al. Detection of Bartonella species in small mammals from Zhejiang province, China. Journal of Wildlife Diseases. 2010;46(1):179–185. doi: 10.7589/0090-3558-46.1.179. [DOI] [PubMed] [Google Scholar]
- 9.Kosoy M., Peruski L. F., Maloney S. A., et al. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. The American Journal of Tropical Medicine and Hygiene. 2010;82(6):1140–1145. doi: 10.4269/ajtmh.2010.09-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainardi J. L., Figliolini C., Goldstein F. W. Cat scratch disease due to Bartonella henselae serotype Marseille (Swiss cat) in a seronegative patient. Journal of Clinical Microbiology. 1998;36(9):p. 2800. doi: 10.1128/jcm.36.9.2800-2800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson W., Palmer N. O. A. Guideline comment: infective endocarditis. British Dental Journal. 2015;219(7):p. 303. doi: 10.1038/sj.bdj.2015.761. [DOI] [PubMed] [Google Scholar]
- 12.Donnio A., Jean-Charles A., Merle H. Macular hole following Bartonella henselae neuroretinitis. European Journal of Ophthalmology. 2008;18(3):456–458. doi: 10.1177/112067210801800324. [DOI] [PubMed] [Google Scholar]
- 13.Kelly P. J., Moura L., Miller T., et al. Feline immunodeficiency virus, feline leukemia virus and Bartonella species in stray cats on St. Kitts, West Indies. Journal of Feline Medical Surgery. 2010;12:447–450. doi: 10.1016/j.jfms.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaffe D. A., Chomel B. B., Kasten R. W., et al. Bartonella henselae in small Indian mongooses (Herpestes auropunctatus) from Grenada, West Indies. Veterinary Microbiology. 2018;216:119–122. doi: 10.1016/j.vetmic.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Olival K. J., Dittmar K., Bai Y., et al. Bartonella spp. in a Puerto Rican bat community. Journal of Wildlife Diseases. 2015;51(1):274–278. doi: 10.7589/2014-04-113. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda P., Seki M. C., Carrasco A. O. T., et al. Evidence and molecular characterization of Bartonella spp. and hemoplasmas in neotropical bats in Brazil. Epidemiology and Infection. 2017;145(10):2038–2052. doi: 10.1017/s0950268817000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yabsley M. J., McKibben J., Macpherson C. N., et al. Prevalence of Ehrlichia canis, Anaplasma platys, Babesia canis vogeli, Hepatozoon canis, Bartonella vinsonii berkhoffii, and Rickettsia spp. in dogs from Grenada. Veterinary Parasitology. 2008;151(2–4):279–285. doi: 10.1016/j.vetpar.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Müller A., Soto F., Sepúlveda M., et al. Bartonella vinsonii subsp. berkhoffii and B. henselae in dogs. Epidemiology and Infection. 2018;146(9):1202–1204. doi: 10.1017/s0950268818001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves L. R., Favacho A. R. D. M., Roque A. L. R., et al. Association of Bartonella species with wild and synanthropic rodents in different Brazilian biomes. Applied and Environmental Microbiology. 2016;82(24):7154–7164. doi: 10.1128/aem.02447-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sousa K. C. M., do Amaral R. B., Herrera H. M., et al. Genetic diversity of Bartonella spp. in wild mammals and ectoparasites in Brazilian Pantanal. Microbial Ecology. 2018;76(2):544–554. doi: 10.1007/s00248-017-1138-0. [DOI] [PubMed] [Google Scholar]
- 21.Müller A., Rodríguez E., Walker R., et al. Occurrence and genetic diversity of Bartonella spp. (Rhizobiales: bartonellaceae) and Rickettsia spp. (Rickettsiales: Rickettsiaceae) in cat fleas (Siphonaptera: pulicidae) from Chile. Journal of Medical Entomology. 2018;55(6):1627–1632. doi: 10.1093/jme/tjy124. [DOI] [PubMed] [Google Scholar]
- 22.do Amaral R. B., Lourenço E. C., Famadas K. M., et al. Molecular detection of Bartonella spp. and Rickettsia spp. in bat ectoparasites in Brazil. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198629.e0198629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz M. H., Bai Y., Malania L., Winchell J. M., Kosoy M. Y. Development of a novel genus-specific real-time PCR assay for detection and differentiation of Bartonella species and genotypes. Journal of Clinical Microbiology. 2012;50(5):1645–1649. doi: 10.1128/jcm.06621-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciervo A., Ciceroni L. Rapid detection and differentiation of Bartonella spp. by a single-run real-time PCR. Molecular and Cellular Probes. 2004;18(5):307–312. doi: 10.1016/j.mcp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Staggemeier R., Pilger D. A., Spilki F. R., Cantarelli V. V. Multiplex SYBR green-real time PCR (qPCR) assay for the detection and differentiation of Bartonella henselae and Bartonella clarridgeiae in cats. Revista do Instituto de Medicina Tropical de São Paulo. 2014;56(2):93–95. doi: 10.1590/s0036-46652014000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y.-Y., Zhao L.-S., Song X.-P., et al. Development of fluorogenic probe-based and high-resolution melting-based polymerase chain reaction assays for the detection and differentiation of Bartonella quintana and Bartonella henselae. Journal of Microbiological Methods. 2017;138:30–36. doi: 10.1016/j.mimet.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Diederen B. M. W., Vermeulen M. J., Verbakel H., Zee A., Bergmans A., Peeters M. F. Evaluation of an internally controlled real-time polymerase chain reaction assay targeting the groEL gene for the detection of Bartonella spp. DNA in patients with suspected cat-scratch disease. European Journal of Clinical Microbiology & Infectious Diseases. 2007;26(9):629–633. doi: 10.1007/s10096-007-0353-x. [DOI] [PubMed] [Google Scholar]
- 28.Pennisi M. G., La Camera E., Giacobbe L., et al. Molecular detection of Bartonella henselae and Bartonella clarridgeiae in clinical samples of pet cats from Southern Italy. Research in Veterinary Science. 2010;88(3):379–384. doi: 10.1016/j.rvsc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Doring G., Unertl K., Heininger A. Validation criteria for nucleic acid amplification techniques for bacterial infections. Clinical Chemistry Laboratory Medicine. 2008;46(7):909–918. doi: 10.1515/cclm.2008.152. [DOI] [PubMed] [Google Scholar]
- 30.Monteiro L., Bonnemaison D., Vekris A., et al. Complex polysaccharides as PCR inhibitors in feces: Helicobacter pylori model. Journal of Clinical Microbiology. 1997;35(4):995–998. doi: 10.1128/jcm.35.4.995-998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schrader C., Schielke A., Ellerbroek L., Johne R. PCR inhibitors-occurrence, properties and removal. Journal of Applied Microbiology. 2012;113(5):1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 32.André M. R., Dumler J. S., Herrera H. M., et al. Assessment of a quantitative 5′ nuclease real-time polymerase chain reaction using the nicotinamide adenine dinucleotide dehydrogenase gamma subunit (nuoG) for Bartonella species in domiciled and stray cats in Brazil. Journal of Feline Medicine and Surgery. 2016;18(10):783–790. doi: 10.1177/1098612x15593787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scola B. L., Zeaiter Z., Khamis A., Raoult D. Gene-sequence-based criteria for species definition in bacteriology: the Bartonella paradigm. Trends in Microbiology. 2003;11(7):318–321. doi: 10.1016/s0966-842x(03)00143-4. [DOI] [PubMed] [Google Scholar]
- 34.CDC. Pictorial Keys to Arthropods, Reptiles, Birds, Mammals of Public Health Significance. Atlanta, GA, USA: CDC; 2013. http://www.cdc.gov/nceh/ehs/Publications/Pictorial_Keys.htm. [Google Scholar]
- 35.Zhang J., Lu G., Kelly P., et al. First report of Rickettsia felis in China. BMC Infectious Disease. 2014;14:p. 682. doi: 10.1186/s12879-014-0682-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y., Fan W., Mao Y., et al. Bovine leukemia virus infection in cattle of China: association with reduced milk production and increased somatic cell score. Journal of Dairy Science. 2016;99(5):3688–3697. doi: 10.3168/jds.2015-10580. [DOI] [PubMed] [Google Scholar]
- 37.Qiu H., Kelly P. J., Zhang J., et al. Molecular detection of Anaplasma spp. and Ehrlichia spp. in ruminants from twelve provinces of China. Canadian Journal of Infectious Diseases and Medical Microbiology. 2016;2016:9. doi: 10.1155/2016/9183861.9183861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y., Mao Y., Kelly P., et al. A pan-Theileria FRET-qPCR survey for Theileria spp. in ruminants from nine provinces of China. Parasites & Vectors. 2014;7(1):p. 413. doi: 10.1186/1756-3305-7-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei L., Kelly P., Zhang J., et al. Use of a universal hydroxymethylbilane synthase (HMBS)-based PCR as an endogenous internal control and to enable typing of mammalian DNAs. Applied Microbiology and Biotechnology. 2014;98(12):5579–5587. doi: 10.1007/s00253-014-5659-x. [DOI] [PubMed] [Google Scholar]
- 40.Liu Q., Eremeeva M. E., Li D. Bartonella and Bartonella infections in China: from the clinic to the laboratory. Comparative Immunology, Microbiology and Infectious Diseases. 2012;35(2):93–102. doi: 10.1016/j.cimid.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Bermond D., Piémont Y., Heller R., et al. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. International Journal of Systematic and Evolutionary Microbiology. 2002;52(2):383–390. doi: 10.1099/00207713-52-2-383. [DOI] [PubMed] [Google Scholar]
- 42.Mediannikov O., Davoust B., Cabre O., Rolain J. M., Raoult D. Bartonellae in animals and vectors in New Caledonia. Comparative Immunology and Microbiology of Infectious Diseases. 2011;34(6):497–501. doi: 10.1016/j.cimid.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Bai Y., Malania L., Alvarez Castillo D., et al. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080894.e80894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherry N. A., Maggi R. G., Cannedy A. L., et al. PCR detection of Bartonella bovis and Bartonella henselae in the blood of beef cattle. Veterinary Microbiology. 2009;135(3-4):308–312. doi: 10.1016/j.vetmic.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 45.Maillard R., Petit E., Chomel B., et al. Endocarditis in cattle caused by Bartonella bovis. Emerging Infectious Diseases. 2007;13(9):1383–1385. doi: 10.3201/eid1309.070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erol E., Jackson C., Bai Y., Sells S., Locke S., Kosoy M. Bartonella bovis isolated from a cow with endocarditis. Journal of Veterinary Diagnostic Investigation. 2013;25(2):288–290. doi: 10.1177/1040638713477408. [DOI] [PubMed] [Google Scholar]
- 47.Palmero J., Pusterla N., Cherry N. A., et al. Experimental infection of horses with Bartonella henselae and Bartonella bovis. Journal of Veterinary Internal Medicine. 2012;26(2):377–383. doi: 10.1111/j.1939-1676.2012.00890.x. [DOI] [PubMed] [Google Scholar]
- 48.Gutiérrez R., Cohen L., Morick D., Mumcuoglu K. Y., Harrus S., Gottlieb Y. Identification of different Bartonella species in the cattle tail louse (Haematopinus quadripertusus) and in cattle blood. Applied and Environmental Microbiology. 2014;80(17):5477–5483. doi: 10.1128/aem.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bustin S. A., Benes V., Garson J. A., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Whole data including the nucleotide sequence used to support the findings of this study are included within the article.