Abstract
Background and Aim
The progression of liver fibrosis in chronic hepatitis B (CHB) patients is currently insufficiently controlled worldwide. The Yi Guan Jian decoction (YGJD) has been widely used in the treatment of liver fibrosis in CHB cases. Although animal studies have reported the antifibrotic effects of the decoction, the active ingredients of the YGJD remain unknown. This study aimed at identifying the potential active ingredients and exploring the mechanisms of action (MOA) of the decoction when treating CHB patients with fibrosis.
Methods
Using data mining techniques and a structural clustering analysis, the potential active ingredients were determined. A network analysis of the differentially expressed genes was conducted to identify the potential targets. Selected compounds were docked to the potential targets for the compound-target interaction simulation. In vitro validation, including a cell proliferation assay and Western blot analysis, was conducted to evaluate the prediction results.
Results
In the microarray data, 224 differentially expressed genes related to liver fibrosis were considered to be potential targets. Thirty active ingredients of the YGJD and 15 main targets and relevant pathways were identified. Among them, two active ingredients, methylophiopogonone A and 8-geranyloxypsoralen, were validated as exhibiting antifibrotic effects on hepatic stellate cells.
Conclusions
We identified the potential active ingredients of the YGJD and proposed the possible explanation for the MOA in the treatment of CHB patients with liver fibrosis. Moreover, this study provides a methodological reference for the systematic investigation of the bioactive compounds and related MOA of a traditional Chinese medicine formula in a clinical context.
1. Introduction
The hepatitis B virus (HBV) is a major health problem worldwide. Indeed, the World Health Organization has estimated that some 257 million people are infected with the HBV globally, with the virus having been associated with nearly one million deaths in 2015 alone, mainly due to complications such as cirrhosis and liver cancer [1]. In the absence of curative treatment, the most common treatments for the HBV include IFN-α and nucleotide analogues, which often lead to severe side effects and drug resistance issues, while having little effect in terms of treating liver fibrosis, cirrhosis, or liver cancer [2].
Due to the issues associated with mainstream treatments, the use of herbal products as a form of complementary medicine has long been widely practiced in Asian countries. In China, over 90% of chronic hepatitis B (CHB) patients received the traditional Chinese medicine (TCM) therapy [3]. Studies have shown various types of TCM to target the HBV [4], liver fibrosis [5], or hepatocellular carcinoma [6]. Further, a meta-analysis of clinical trials found that TCM had an equivalent or better effect than interferon or lamivudine in the treatment of CHB [7]. Moreover, randomized controlled clinical studies have reported the effects of different TCM formulae with regard to improving the clinical parameters of patients with liver fibrosis [8].
As a TCM formula listed in the “Catalogue of Classical TCM Prescriptions” issued by the State Administration of TCM of the People's Republic of China, the Yi Guan Jian decoction (YGJD) is known to have hepatoprotective effects [9]. The YGJD consists of six herbal medicines, namely, Rehmanniae Radix (Rehmannia glutinosa), Glehniae Radix (Glehnia littoralis), Ophiopogonis Radix (Ophiopogon japonicus), Angelica Sinensis Radix (Angelica sinensis), Lycii Fructus (Lycium barbarum), and Toosendan Fructus (Melia toosendan). Three possible mechanisms of action (MOA) have been suggested for the antifibrotic effects of the YGJD: first, the inhibition of hepatic stellate cell (HSC) activation and hepatocytes apoptosis [10]; second, antiangiogenesis via the HIF-1α/VEGF signaling pathway [11]; and finally, anti-inflammation [12]. However, the bioactive ingredients of the YGJD remain unknown, while the MOA at the molecular level require further investigation.
In clinical practice involving TCM, the YGJD has been widely used as a basic formula in the treatment of liver-kidney Yin deficiency syndrome (LKYDS) in patients with CHB [13]. LKYDS is mainly seen in CHB patients with severe liver fibrosis [14]. We hypothesized that the beneficial clinical effect of the YGJD in terms of reducing liver fibrosis stems from the oral bioavailable chemical compounds found in the decoction, which are related to the changes in the gene transcription of the blood leukocytes seen in CHB patients with LKYDS. This study hence had two key aims: first, to identify the potential active ingredients of the YGJD with regard to reducing liver fibrosis, and second, to delineate the MOA of the YGJD in relation to the effective treatment of CHB patients with liver fibrosis.
2. Materials and Methods
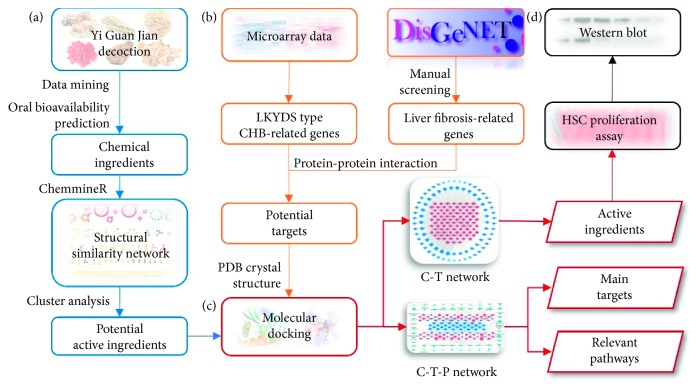
To achieve the abovementioned research aims, this study gathered relevant bioinformatic data concerning both the YGJD and CHB patients with LKYDS. Further, the study comprehensively employed various methods of analysis, including network pharmacology [15], microarray analysis, molecular docking, and in vitro experiments, to analyze the collected data. The research roadmap is shown in Figure 1, while detailed descriptions of the utilized materials and methods are given below.
Figure 1.
Research roadmap. (a) Collection and screening of the ingredients in the YGJD. (b) Identification of the targets for the YGJD. (c) Network analysis of the active ingredients and the mechanisms of action. (d) Experimental validation.
2.1. Compound Collection and Oral Bioavailability Prediction
The relevant chemical information concerning the YGJD was retrieved from four TCM databases in December 2017, including the Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) [16], the Herbal Ingredients Targets Database (HIT) [17], the Psoriasis Database of Traditional Chinese Medicine (PDTCM) [18], and the Potential Target Database of Traditional Chinese Medicine (TCM-PTD). The three-dimensional structures were downloaded from the corresponding database or PubChem.
We used the two parameters proposed by Veber for the preliminary screening of the collected compounds. Veber's filter was used for the oral bioavailability prediction [19], which meant that the number of rotatable bonds of a given compound had to be ≤10, while the topological polar surface area had to be ≤140 Å2 [20]. Further, DataWarrior was employed to calculate the molecular properties of the compounds.
2.2. Structural Clustering Analysis
The ChemmineR package run in Rstudio was used to compute the fingerprints and the Tanimoto coefficients among the compounds. The obtained cross-similarity matrix was subsequently applied to build the structural similarity network of the compounds with Tanimoto coefficients ≥0.6 [21]. The MCODE plugin in Cytoscape was used to partition the clusters for the network.
2.3. Microarray Data Analysis
The microarray data concerning the leukocyte samples obtained from three CHB patients with LKYDS and three healthy donors were provided by the Research Center for TCM Complexity System, Shanghai University of TCM [22]. The differentially expressed genes were identified under the conditions of a fold-change >2 and a p value <0.05. The collected genes were then submitted to the STRING database to establish the protein-protein interaction (PPI) network, while the edges with confidence scores greater than 0.7 were retained for further analysis.
The liver fibrosis-related genes were collected from DisGeNET. Using the search term of “Fibrosis, Liver” (UMLS CUI: C0239946), a total of 354 genes were selected from among the results. The CHB with LKYDS genes that exhibited high correlations (a confidence score greater than 0.9 in the STRING database) with the liver fibrosis-related genes were considered to be potential targets for the YGJD. PPI network analysis via pathway enrichment and topological parameter calculation was performed to verify the importance of the potential targets [23].
2.4. Molecular Docking
A cross-docking analysis involving sets of compounds and targets was conducted using AutoDock Vina. The crystal structures of the proteins were downloaded from the Research Collaboratory for Structural Bioinformatics Protein Data Bank, and only those X-ray structures with a resolution of less than 3 Å were selected for the analysis (). The binding sites were obtained from the original ligand binding locations of the crystal structures, or the reported pockets, or else were predicted by the CASTp 3.0 web server [24]. The gird boxes were adjusted to cover the entire pocket, the majority of which were 22 × 22 × 22 Å3. To eliminate any bias caused by the properties of the protein pockets, all the docking scores for each protein were normalized by the median number, as shown in equation (1) [25]. The receiver operating characteristic (ROC) curves between the normalized scores of the active ligands and the ingredients in the YGJD were used to determine the cutoff values [26]:
| (1) |
where Sij is the original docking score and Sij′ is the normalized score, i and j are the indexes of the corresponding ligand and protein, respectively, and mj and σj are the median and standard deviation, respectively, of the docking scores of protein j.
2.5. C-T and C-T-P Network Analysis
Cytoscape was used to visualize the compound-target (C-T) network and the compound-target-pathway (C-T-P) network for the YGJD in the treatment of fibrosis caused by CHB. Two main parameters, namely, the degree centrality (DC) and the betweenness centrality (BC), were used to determine the key nodes in the network [27]. The pathway enrichment of the targets was performed using the STRING database with a false discover rate (FDR) <0.05. In the C-T-P network, the number of shortest paths from all the active ingredients to a biological pathway was exploited to rank the pathways, and the utilized calculation method was as shown in the following equation [28]:
| (2) |
where Pm is the number of shortest paths from the active ingredients to pathway m, IDn is the in-DC of the target n in the C-T-P network, N represents the number of targets in the network, and kmn is a dummy variable. Further, kmn=1 when there is a path between the target m and the pathway n; otherwise, kmn=0.
2.6. Cell Proliferation Assay
The LX-2 immortalized human hepatic stellate cell line was purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) [29]. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA), which was supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 U/mL penicillin, and 100 U/mL streptomycin and then incubated at 37°C in humidified air containing 5% CO2.
After being seeded overnight in 96-well plates with DMEM containing 2% FBS, the LX-2 cells were treated with serially diluted compounds for 24 h, 48 h, and 72 h. The cell number was measured by means of a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) assay. The absorbance was monitored at 450 nm using a microplate reader (PE Envision). Further, the viability percentage was calculated using the following equation:
| (3) |
where T and C refer to the absorbance of the treatment group and the control group, respectively.
2.7. Western Blot Analysis
The cellular content of the collagen I and the α-smooth muscle actin (α-SMA) in the LX-2 cells was determined by means of Western blot analysis. The LX-2 cells were treated under different conditions with the active ingredients in 5 ng/mL of TGF-β1 for 24 h. The lysates of the denatured cells were separated on NuPAGE 4–12% Bis-Tris gel (Thermo Fisher Scientific), and the proteins were transferred to Hybond ECL membranes (GE Healthcare). The membranes were incubated overnight at 4°C with the primary antibodies against collagen type I (Abcam), α-SMA (Abcam), and β-actin (Sigma) as the endogenous controls, followed by the corresponding secondary peroxidase-coupled antibodies (Sigma). After the chemiluminescence had been enhanced (ECL, Pierce), the digital detection was evaluated using Image Lab Software (ChemiDoc, Bio-Rad).
3. Results
3.1. Identification of the Representative Compounds of the YGJD
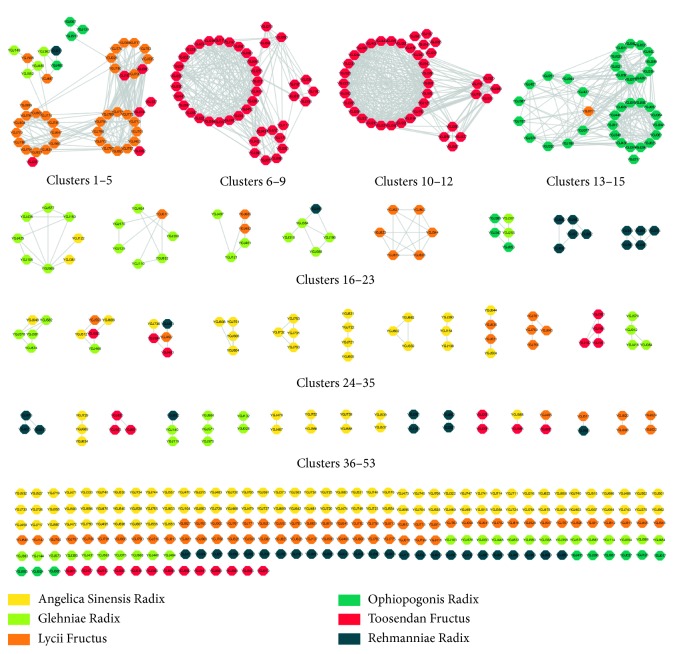
A total of 836 compounds contained within the YGJD were acquired from the databases, with 526 being predicted to exhibit better oral bioavailability. A structural similarity network was constructed to identify the representative compounds among the 526 ingredients (Figure 2). The network was divided into 53 clusters. Further investigation of the network showed that the compounds contained within clusters 1–3 were mainly cholestanes from Lycii Fructus, while those within clusters 6–12 were mostly limonoids from Toosendan Fructus, and those within clusters 13–15 were mostly isoflavones from Ophiopogonis Radix.
Figure 2.
Structural similarity network of the compounds within the YGJD with potential oral bioavailability. The colours of the nodes represent the origin of a compound. The edges in the network represent the connected compounds with a Tanimoto coefficient of no less than 0.6.
According to the theory of structure-activity relationship, structurally similar compounds tend to perform similar biological activities [30]. The representative compounds were identified after ranking all the compounds based on their DC and BC scores. The compounds with the top scores in each cluster were selected, giving a total of 57 representative compounds. The representative compounds, in addition to the compounds shown as isolated nodes within the network, were chosen for further docking analysis. In total, 278 compounds () were docked to the selected targets.
3.2. The Pool of Potential Targets for the YGJD
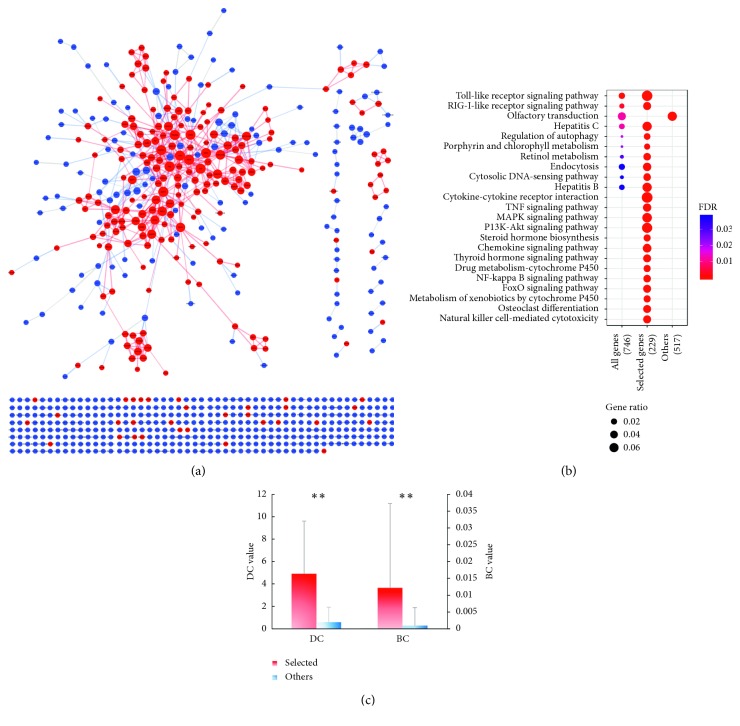
The CHB with LKYDS genes were mapped by means of analyzing the microarray data concerning the CHB with LKYDS patients who had been diagnosed by senior TCM doctors at the Shanghai Longhua Hospital. A PPI network for the differently expressed genes was constructed (Figure 3(a)). In order to obtain the genes highly associated with liver fibrosis and to reduce the potential bias of the microarray data, liver fibrosis-related genes were collected from the DisGeNET database. Some 224 CHB with LKYDS genes that exhibited a high correlation with liver fibrosis were selected as potential targets.
Figure 3.
(a) PPI network of the CHB with LKYDS genes. The red nodes represent the genes associated with liver fibrosis, which were selected as potential targets. The sizes of the nodes are proportional to the values of their corresponding DC scores. (b) Results of the pathway enrichment analysis of the CHB with LKYDS genes, the potential targets, and others (genes not selected as potential targets). The sizes of the bubbles represent the gene ratios, which were calculated using the ratio of the pathway-related genes to the total number of gene sets. Only the top 20 enrichment items of the potential targets are shown in the figure. (c) Comparison of the mean values of the DC scores and the BC scores between the selected and the other genes in the network. ∗∗P < 0.01 in the t-test for the two independent samples. The standard deviation is shown as the error bar in the chart.
To verify the importance of the selected targets, the different gene sets were subjected to a pathway enrichment analysis (Figure 3(b)) and a topological parameter analysis (Figure 3(c)). After manually deleting those pathways that were apparently unrelated diseases, the potential targets were found to be involved in 64 pathways, while all 740 genes were involved in ten pathways, and those genes not selected as potential targets were only correlated with one pathway. Further, it was found that the selected targets exhibited significantly higher DC and BC scores than the unselected ones. Taken together, the network analysis results indicated the successful exclusion of interfering genes as well as the high correlation between LKYDS and liver fibrosis in CHB patients.
3.3. Compound-Target Interaction Simulation Using In Silico Molecular Docking
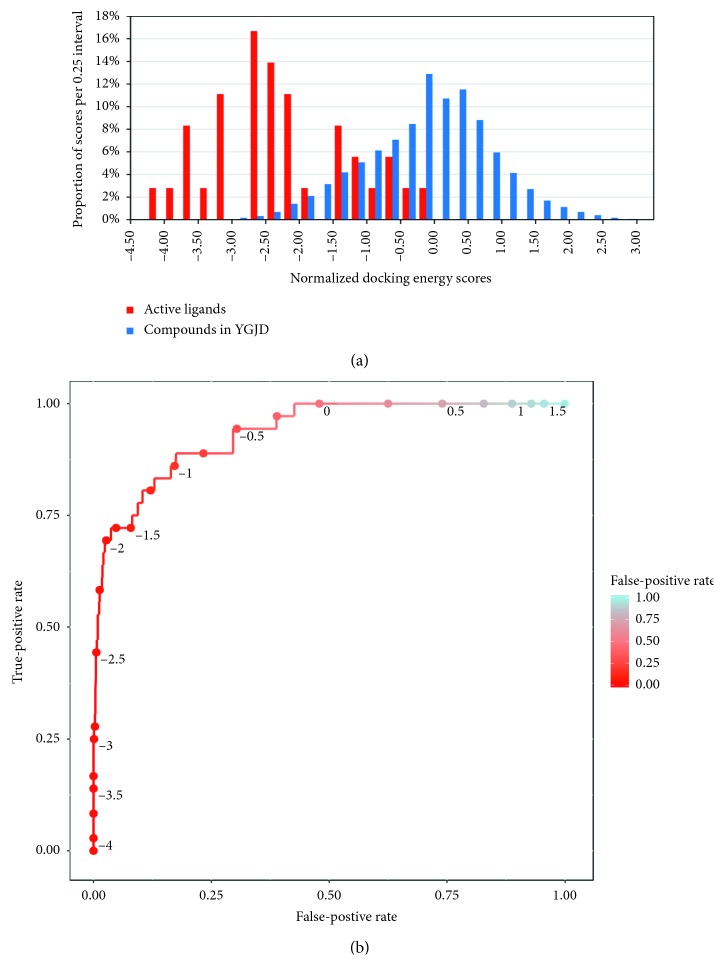
The selected compounds from Figure 2 were docked with the potential targets one to one with AutoDock Vina. The active ligands collected from the protein crystal structures, the DrugBank, or the Therapeutic Target Database were used to determine the cutoff values for the docking scores. The distribution of the normalized docking scores of the active ligands (), as well as the selected compounds from the YGJD, is illustrated in Figure 4(a).
Figure 4.
(a) The normalized docking energy score distribution for the active ligands and selected compounds in the YGJD. (b) The ROC curve illustrating the sensitivity/specificity trade-off.
The ROC curve presented in Figure 4(b) depicts the sensitivity/specificity trade-off for the threshold values ranging from −4 to 1.5. A cutoff value of −1.75 was selected, with a sensitivity of 0.722 and a specificity of 0.952, and 4.79% of the compound-target interactions were determined to fall within that range. The C-T network was subsequently constructed and visualized based on the predicted compound-target interactions.
3.4. Identification of the Active Ingredients in the YGJD That Act on CHB-Related Liver Fibrosis
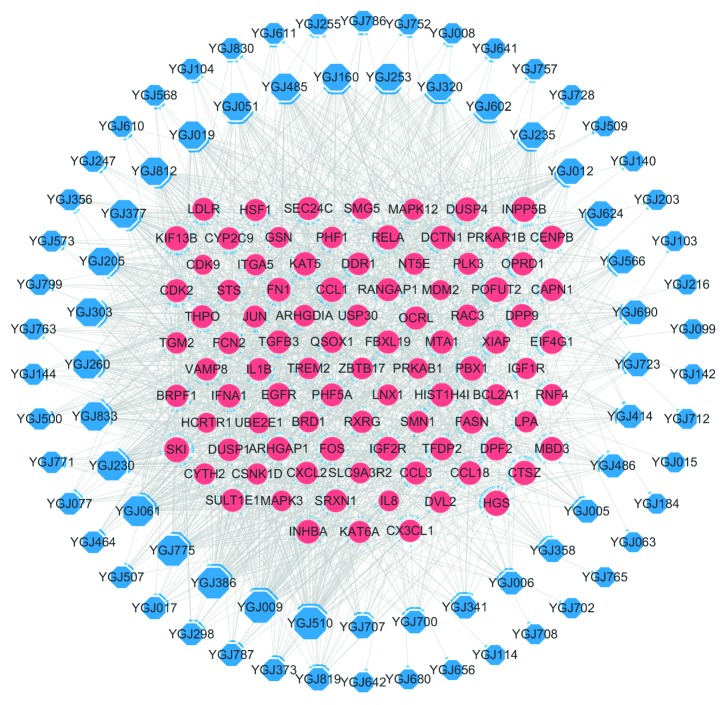
The C-T network consisted of selected compounds and their respective targets, as visualized in Figure 5. The compounds that exhibited the top 30 out-DC scores were considered to be the active ingredients, and they are listed in Table 1.
Figure 5.
The C-T network based on the results of the molecular docking analysis. The blue octagonal nodes represent the compounds, while the pink elliptical nodes represent the targets.
Table 1.
Active ingredients of the YGJD in the treatment of CHB-related liver fibrosis.
| ID | Out DC | Compound name | Origin | Molecular formula | Cluster |
|---|---|---|---|---|---|
| YGJ510 | 65 | Diosgenin | Ophiopogonis Radix | C27H42O3 | 3 |
| YGJ009 | 60 | Belladonnine | Lycii Fructus | C34H42N2O4 | Isolated |
| YGJ386 | 59 | 6-Deacetyloxy-7-deacetylchisocheton | Toosendan Fructus | C26H36O4 | Isolated |
| YGJ775 | 55 | 24-Methylenecycloartanol ferulate | Lycii Fructus | C41H60O4 | Isolated |
| YGJ061 | 54 | Methylophiopogonanone A | Ophiopogonis Radix | C19H16O6 | 15 |
| YGJ230 | 50 | Mesendanin M | Toosendan Fructus | C30H44O4 | 6 |
| YGJ833 | 46 | Marmesinin | Glehniae Radix | C20H24O9 | Isolated |
| YGJ260 | 44 | Meliasenin X | Toosendan Fructus | C30H48O5 | 7 |
| YGJ303 | 43 | 3,3′Z-6.7′,7.6′-diligustilide | Angelica Sinensis Radix | C24H28O4 | Isolated |
| YGJ205 | 42 | Mesendanin J | Toosendan Fructus | C28H40O8 | 48 |
| YGJ377 | 42 | Trichilinin E | Toosendan Fructus | C35H42O8 | 12 |
| YGJ812 | 41 | Lantadene A | Lycii Fructus | C35H54O5 | Isolated |
| YGJ019 | 40 | Ophiopogonone A | Ophiopogonis Radix | C18H14O6 | 14 |
| YGJ485 | 39 | Lupeol | Glehniae Radix; Lycii Fructus |
C30H50O | 2 |
| YGJ051 | 39 | N-p-coumaroyltyramine | Ophiopogonis Radix | C18H15NO4 | Isolated |
| YGJ253 | 38 | Meliasenin W | Toosendan Fructus | C30H50O4 | 9 |
| YGJ160 | 38 | Meliasenin P | Toosendan Fructus | C31H50O4 | 8 |
| YGJ320 | 35 | Nimbolinin C | Toosendan Fructus | C38H46O9 | 11 |
| YGJ602 | 32 | Levistolide A | Angelica Sinensis Radix | C24H28O4 | 30 |
| YGJ235 | 31 | Mesendanin U | Toosendan Fructus | C32H50O6 | Isolated |
| YGJ012 | 30 | 8-Geranyloxypsoralen | Glehniae Radix | C21H22O4 | 35 |
| YGJ624 | 28 | Campesteryl ferulate | Lycii Fructus | C38 H56 O4 | 53 |
| YGJ566 | 22 | 24-Methylenecycloartanol | Lycii Fructus | C31H52O | 5 |
| YGJ690 | 21 | 12-O-nicotinoylisolineolone | Lycii Fructus | C27H35NO6 | Isolated |
| YGJ723 | 20 | Isotetandrine | Angelica Sinensis Radix | C38H42N2O6 | Isolated |
| YGJ486 | 18 | Sitogluside | Rehmanniae Radix; Glehniae Radix; Angelica Sinensis Radix |
C35H60O6 | 51 |
| YGJ414 | 18 | Toosendanic acid A | Toosendan Fructus | C30H48O4 | 6 |
| YGJ005 | 17 | 2′-Hydroxymatteucinol | Ophiopogonis Radix | C18H18O6 | Isolated |
| YGJ006 | 16 | Taurochenodeoxycholic acid | Lycii Fructus | C26H45NO6S | Isolated |
| YGJ358 | 16 | 2-Hydroxy-3-(3-methylbut-2-enyl) furo[3,2 g] chromen-7-one | Glehniae Radix | C16H14O4 | Isolated |
3.5. MOA for the YGJD When Treating CHB-Related Liver Fibrosis
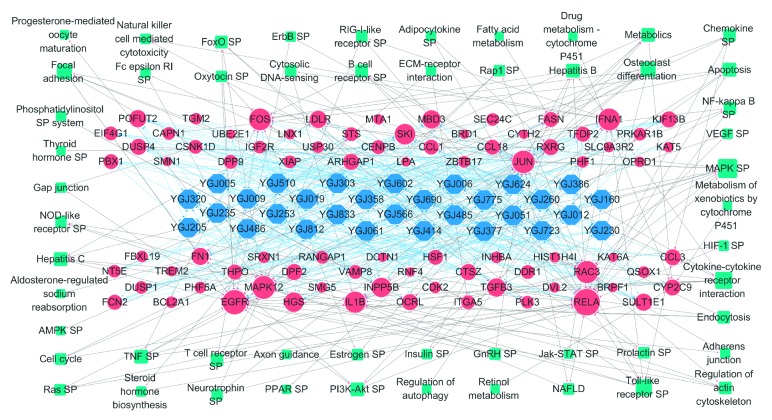
The C-T-P network was constructed (Figure 6) to further explore the MOA of the YGJD in the treatment of liver fibrosis caused by CHB. For each active ingredient, the targets that exhibited the top ten highest binding affinities to the ingredients were retained and shown in the graph. The links between the targets and the pathways were drawn based on the results obtained in the pathway enrichment analysis described above.
Figure 6.
The C-T-P network. The cyan rectangular nodes represent the pathways frequently involved with the potential targets. The sizes of the nodes are proportional to the values of the DC scores. SP: signaling pathway.
The BC was used to measure the importance of the targets within the C-T-P network. Those targets with high BC scores were the intermediaries that controlled the flow of interactions within the network. Based on the BC score, the top 15 targets, which are listed in Table 2, were screened as the main targets of YGJD treatment.
Table 2.
Main targets of the YGJD in the treatment of CHB-related liver fibrosis.
| Symbol | Entrez ID | Protein name | BC (10−3) |
|---|---|---|---|
| RELA | 5970 | Transcription factor p65, NF-κB subunit | 5.28 |
| EGFR | 1956 | Epidermal growth factor receptor | 2.66 |
| RAC3 | 5881 | Ras-related C3 botulinum toxin substrate 3 | 1.44 |
| FOS | 2353 | Proto-oncogene c-Fos, AP-1 transcription factor subunit | 1.25 |
| IFNA1 | 3439 | Interferon-alpha 1/13 | 1.05 |
| JUN | 3725 | Transcription factor AP-1 | 0.89 |
| MAPK12 | 6300 | Mitogen-activated protein kinase 12 | 0.61 |
| FN1 | 2335 | Fibronectin | 0.59 |
| INPP5B | 3633 | Type II inositol 1,4,5-trisphosphate 5-phosphatase | 0.56 |
| TGFB3 | 7043 | Transforming growth factor-beta 3 | 0.47 |
| RXRG | 6258 | Retinoic acid receptor RXR-gamma | 0.45 |
| CYP2C9 | 1559 | Cytochrome P450 2C9 | 0.43 |
| OCRL | 4952 | Inositol polyphosphate 5-phosphatase OCRL-1 | 0.36 |
| IL1B | 3553 | Interleukin-1-beta | 0.35 |
| HGS | 9146 | Hepatocyte growth factor-regulated tyrosine kinase substrate | 0.32 |
Next, based on the C-T-P network, we calculated the shortest paths from the active ingredients to each pathway. By ranking the scores for this indicator, the key biological pathways influenced by the YGJD were identified. The top ten pathways included MAPK signaling pathway, cytokine-cytokine receptor interaction, Toll-like receptor signaling pathway, hepatitis C, endocytosis, PI3K-Akt signaling pathway, hepatitis B, metabolic pathways, chemokine signaling pathway, and osteoclast differentiation. Details concerning these key pathways are provided in .
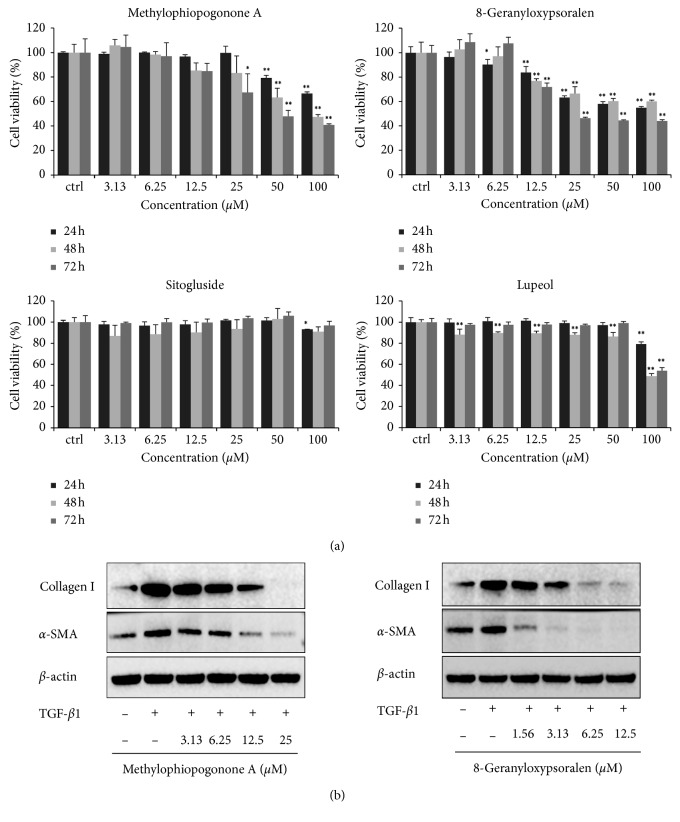
3.6. Antifibrotic Effects of Methylophiopogonone A and 8-Geranyloxypsoralen on LX-2 Cells
In the 30 active ingredients in Table 1, the 17 representative compounds were selected based on their topological features in Figure 2. The 13 compounds in 17 were considered for the further experimental validation, except for 4 compounds reported to show antifibrotic or hepatoprotective effects in existing literature. In consideration of the availability of these sample compounds, 4 representative compounds in Table 1 were selected for further pharmacological experiments. As shown in Figure 7(a), two ingredients in four, namely, methylophiopogonone A from cluster 15 and 8-geranyloxypsoralen from cluster 35, were found to inhibit the proliferation of the LX-2 cells in a time- and dose-dependent manner. However, there was inadequate evidence to confirm the role of sitogluside and lupeol in inhibiting the proliferation of the LX-2 cells, which indicates the need for further validation of the other potential MOA of the YGJD predicted using our method.
Figure 7.
Experimental validation of the antifibrotic effects of the active ingredients in the LX-2 cell line. (a) The viability of the LX-2 cells after treatment with the four active ingredients for 24, 48, and 72 hours. ∗P < 0.05 and ∗∗P < 0.01 in Dunnett's test. (b) The protein levels of collagen I and α-SMA induced by TGF-β1 (5 ng/ml) in the LX-2 cells after the treatment with methylophiopogonone A and 8-geranyloxypsoralen.
Additionally, two of the main markers of HSC activation, namely, collagen I and α-SMA, were determined by means of Western blot analysis after the cells were treated with the compounds for 24 h. As shown in Figure 7(b), the methylophiopogonone A and 8-geranyloxypsoralen inhibited the TGF-β1-induced collagen I and α-SMA expression.
4. Discussion
The YGJD is an herbal formula that is widely used in clinical practice involving TCM for the treatment of CHB patients with LKYDS, a syndrome known to be related to liver fibrosis. Modern pharmacological studies have confirmed the antifibrotic and anti-inflammatory effects of YGJD [31]. In this study, we explored the effects of YGJD on liver fibrosis of CHB by using the LKYDS as a clue to collect microarray data in the specific patient pool of CHB. The microarray data analysis further provided a potential target set in the treatment of liver fibrosis with YGJD from a clinical perspective. The interactions between the compounds of YGJD and the potential targets were revealed by means of molecular docking. Moreover, the method of network pharmacology was used to predict the bioactive ingredients and biological basis of YGJD acting on CHB patients with liver fibrosis.
For the 30 active ingredients predicted by our method, some have been reported to exhibit effects against liver fibrosis. Diosgenin from Ophiopogonis Radix and levistolide A from Angelica Sinensis Radix were observed to inhibit the HSC activation and proliferation [32, 33]. Lupeol, lantadene A, and two trans-ferulic acids from Lycii Fructus, namely, 24-methylenecycloartanol ferulate and campesteryl ferulate, were reported to exhibit hepatoprotective effects in mice with toxically induced liver injuries [34–36]. N-p-coumaroyltyramine from Ophiopogonis Radix, 8-geranyloxypsoralen from Glehniae Radix, and sitogluside from Rehmanniae Radix were all reported to be involved in anti-inflammatory activities [37–39]. In addition, several limonoids from Toosendan Fructus, such as mesendanin M, trichilinin E, and nimbolinin C, exhibited high out-DC scores in the C-T network. It has previously been reported that limonoids extracted from Meliaceae possess hepatoprotective functions and could hence alleviate liver fibrosis by reducing the TGF-β1 and collagen levels [40, 41]. Thus, the limonoids listed in Table 1 might be worthy of further investigation.
Interestingly, 17 of the 30 active ingredients were found to belong to 57 representative compounds in Figure 2, whereas only 13 of the 221 isolated nodes performed well in the docking analysis, which indicated that the highly connected nodes in the compound similarity network had greater opportunity to be biologically active. The compounds with similar structures might represent the driving force within the YGJD, since they could work synergistically to achieve the identified therapeutic effects.
To reveal the MOA of the YGJD in relation to the effective treatment of CHB patients with liver fibrosis, the main targets and the relevant pathways were obtained using the C-T-P network analysis. The findings reported in the literature regarding these principal targets support the suggestion that the YGJD attenuated or even reversed the progression of liver fibrosis among CHB patients using three main approaches. The first approach was the inhibition of the fibrosis-related HSC activation and proliferation. RELA (also known as nuclear factor-κB p65), which has previously been reported to be downregulated in YGJD treatment [42], was found to be a significant factor against the apoptosis of the HSC [43]. Additionally, it has been reported that the inhibition of EGFR could decrease the activation of the HSC [44]. Further, OCRL and INPP5B could regulate HSC proliferation by modulating the AKT signaling pathway [45]. HGS might affect HSC activation via the modulation of both Smad2 and PDGFR [46]. The second approach concerned the fact that some of the main targets were involved in preventing excessive extracellular matrix (ECM) deposition. Fibronectin was part of the ECM [5]. JUN and FOS were reported to influence several genes related to the ECM in the HSC [47]. Moreover, MAPK12 might be involved in regulating α1(I) collagen, a key part of the ECM in fibrosis, via the p38 MAPK signaling pathway [48]. The third approach involved regulating the inflammatory response to the HBV by regulating both IL-1B and IFNA1. It has previously been reported that the YGJD could decrease the levels of IL-1B so as to exert anti-inflammatory effects [12]. Further, interferon-alpha encoded by IFNA1 can inhibit the replication of the HBV, thereby leading to the remission of liver diseases [49]. All of the three summarized approaches are consistent with the antifibrotic effects of YGJD reported in the existing literature. This further confirms the accuracy of the target prediction in this study.
The key pathways were also confirmed to be involved in the above-mentioned three approaches accordingly. The MAPK signaling pathway and the PI3K-Akt signaling pathway both played an important role in the entire pathological process of liver fibrosis. Moreover, it was found that the active substances targeting the two pathways could hinder the HSC activation and exert an antifibrotic effect [50, 51]. Cytokine-cytokine receptor interaction, the chemokine signaling pathway, and the Toll-like receptor signaling pathway were all highly related to the immunological process associated with the hepatitis B infection [52]. As for the metabolic pathways, both the retinol metabolism and fatty acid metabolism pathways shown in the C-T-P network were found to be associated with HSC activities as well as with antifibrotic effects [53, 54].
However, a research limitation should be noted. In this study, only two active ingredients were verified to exhibit antifibrotic effects on the HSC by significantly inhibiting the cell proliferation. Further animal experiments and clinical trials on the two ingredients and more potential active ingredients may be performed to explore the scientific nature of YGJD.
5. Conclusions
This study predicted 224 potential targets for treating CHB-caused liver fibrosis using microarray data analysis and PPI network analysis and also identified 278 candidates of active ingredients of YGJD through network cluster analysis. The interaction between the compounds and potential targets was revealed by molecular docking analysis and led to a total of 30 active ingredients. Among these active ingredients, methylophiopogonone A and 8-geranyloxypsoralen were found to inhibit HSC proliferation in LX-2 cells in vitro. And almost half of the active ingredients have been reported to have antifibrotic or anti-inflammatory effects.
As for the MOA, fifteen genes related to the liver fibrosis caused by the CHB were predicted to be the main targets of YGJD. Literature findings regarding these main targets support YGJD attenuated or even reversed the progression of liver fibrosis among CHB patients by inhibiting fibrosis-related HSC activation and proliferation via RELA, EGFR, OCRL, INPP5B, and HGS, preventing excessive ECM deposition via FN1, JUN, FOS, and MAPK12, and regulating inflammatory response to HBV by regulating both IL-1B and IFNA1. Furthermore, the network analysis revealed the pathways involved in the YGJD treatment, including MAPK signaling, PI3K-Akt signaling, cytokine-cytokine receptor interaction, Toll-like receptor signaling, endocytosis, and metabolism pathway.
In summary, we identified the active ingredients in the YGJD and proposed the possible MOA for the YGJD in the treatment of liver fibrosis among CHB patients. Our study not only provided a direction for the development of new drugs to treat liver fibrosis in CHB patients, but also offered an alternative approach for the systematic investigation of the MOA of a TCM formula by using the YGJD as an example.
Acknowledgments
We thank the Science and Technology Development Fund of Macao SAR and the University of Macau and Guangdong-Macao Traditional Chinese Medicine Technology Industrial Park for financial support for the projects 013-2015-A1, MYRG2016-00144-ICMS-QRCM and D-Pro-02742017 for this research.
Contributor Information
Shibing Su, Email: shibingsu07@163.com.
Yuanjia Hu, Email: yuanjiahu@umac.mo.
Data Availability
The data that support the findings of this study are available in the Supplementary Materials and openly available in the TCMSP (http://lsp.nwu.edu.cn/tcmsp.php), the HIT (http://lifecenter.sgst.cn/hit/), the PDTCM (http://cadd.gdhtcm.com:2180/PDTCM/), the TCMPTD (http://pharminfo.zju.edu.cn/ptd), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the DisGeNET (http://www.disgenet.org/).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Guangyao Li and Yuan Zhou contributed equally to this work.
Supplementary Materials
Table S1: potential targets for docking analysis. Table S2: selected compounds for docking analysis. Table S3: active ligands as reference for the docking analysis. Table S4: key pathways involved with the YGJD acting on CHB-related liver fibrosis. Table S5: raw data of the docking results.
References
- 1.WHO. WHO Guidelines Approved by the Guidelines Review Committee. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2.Terrault N. A., Bzowej N. H., Chang K.-M., Hwang J. P., Jonas M. M., Murad M. H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63(1):261–283. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X., Wang J., Yang D. Antiviral therapy for chronic hepatitis B in China. Medical Microbiology and Immunology. 2015;204(1):115–120. doi: 10.1007/s00430-014-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui X., Wang Y., Kokudo N., Fang D., Tang W. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. BioScience Trends. 2010;4(2):39–47. [PubMed] [Google Scholar]
- 5.Bataller R., Brenner D. A. Liver fibrosis. Journal of Clinical Investigation. 2005;115(2):209–218. doi: 10.1172/jci24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B., Wang S. S., Du Q. Traditional Chinese medicine for prevention and treatment of hepatocarcinoma: from bench to bedside. World Journal of Hepatology. 2015;7(9):1209–1232. doi: 10.4254/wjh.v7.i9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Wang G., Hou W., Li P., Dulin A., Bonkovsky H. L. Contemporary clinical research of traditional Chinese medicines for chronic hepatitis B in China: an analytical review. Hepatology. 2010;51(2):690–698. doi: 10.1002/hep.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Schuppan D. Traditional Chinese medicine (TCM) for fibrotic liver disease: hope and hype. Journal of Hepatology. 2014;61(1):166–168. doi: 10.1016/j.jhep.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Weiskirchen R., Mahli A., Weiskirchen S., Hellerbrand C. The hop constituent xanthohumol exhibits hepatoprotective effects and inhibits the activation of hepatic stellate cells at different levels. Frontiers in Physiology. 2015;6:p. 303. doi: 10.3389/fphys.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mu Y., Liu P., Du G., et al. Action mechanism of Yi Guan Jian decoction on CCl4 induced cirrhosis in rats. Journal of Ethnopharmacology. 2009;121(1):35–42. doi: 10.1016/j.jep.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y. N., Mu Y. P., Fu W. W., et al. Yiguanjian decoction and its ingredients inhibit angiogenesis in carbon tetrachloride-induced cirrhosis mice. BMC Complementary and Alternative Medicine. 2015;15(1):p. 342. doi: 10.1186/s12906-015-0862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shui S., Shen S., Huang R., Xiao B., Yang J. Metabonomic analysis of biochemical changes in the plasma and urine of carrageenan-induced rats after treatment with Yi-Guan-Jian decoction. Journal of Chromatography B. 2016;1033-1034:80–90. doi: 10.1016/j.jchromb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Jia W., Gao W.-Y., Yan Y.-Q., et al. The rediscovery of ancient Chinese herbal formulas. Phytotherapy Research. 2004;18(8):681–686. doi: 10.1002/ptr.1506. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X.-X., Bian Z.-X., Wu T.-X., Fu S.-F., Ziea E., Woon W. T. C. Traditional Chinese medicine syndrome distribution in chronic hepatitis B populations: a systematic review. The American Journal of Chinese Medicine. 2011;39(6):1061–1074. doi: 10.1142/s0192415x11009408. [DOI] [PubMed] [Google Scholar]
- 15.Li S. Mapping ancient remedies: applying a network approach to traditional Chinese medicine. Science. 2015;350(6262):S72–S74. [Google Scholar]
- 16.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye H., Ye L., Kang H., et al. HIT: linking herbal active ingredients to targets. Nucleic Acids Research. 2011;39:D1055–D1059. doi: 10.1093/nar/gkq1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Gu J., Zhu W., et al. PDTCM: a systems pharmacology platform of traditional Chinese medicine for psoriasis. Annals of Medicine. 2017;49(8):652–660. doi: 10.1080/07853890.2017.1364417. [DOI] [PubMed] [Google Scholar]
- 19.Lipinski C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Veber D. F., Johnson S. R., Cheng H.-Y., Smith B. R., Ward K. W., Kopple K. D. Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry. 2002;45(12):2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 21.Delaney J. S. Assessing the ability of chemical similarity measures to discriminate between active and inactive compounds. Molecular Diversity. 1996;1(4):217–222. doi: 10.1007/bf01715525. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y. Y., Chen Q. L., Guan Y., et al. Study of ZHENG differentiation in hepatitis B-caused cirrhosis: a transcriptional profiling analysis. BMC Complementary and Alternative Medicine. 2014;14(1):p. 371. doi: 10.1186/1472-6882-14-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He X., Zhang J. Why do hubs tend to be essential in protein networks? PLoS Genetics. 2006;2(6):p. e88. doi: 10.1371/journal.pgen.0020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian W., Chen C., Lei X., Zhao J., Liang J. CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Research. 2018;46(W1):W363–w367. doi: 10.1093/nar/gky473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Q., Zhao L., Hu J., Jin H., Liu Z., Zhang L. The scoring bias in reverse docking and the score normalization strategy to improve success rate of target fishing. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171433.e0171433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M. W., Lindstrom W., Olson A. J., Belew R. K. Analysis of HIV wild-type and mutant structures via in silico docking against diverse ligand libraries. Journal of Chemical Information and Modeling. 2007;47(3):1258–1262. doi: 10.1021/ci700044s. [DOI] [PubMed] [Google Scholar]
- 27.Newman M. E. J. The structure of scientific collaboration networks. Proceedings of the National Academy of Sciences. 2001;98(2):404–409. doi: 10.1073/pnas.021544898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang Z., Sun H., Cai X., Chen D., Zheng X. The study on the material basis and the mechanism for anti-renal interstitial fibrosis efficacy of rhubarb through integration of metabonomics and network pharmacology. Molecular BioSystems. 2015;11(4):1067–1078. doi: 10.1039/c4mb00573b. [DOI] [PubMed] [Google Scholar]
- 29.Xu L., Hui A. Y., Albanis E., et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54(1):142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezey P. G. Functional groups in quantum chemistry. Advances in Quantum Chemistry. 1996;27:163–222. doi: 10.1016/s0065-3276(08)60252-x. [DOI] [Google Scholar]
- 31.Yao W. L., Fan W. W., Chen X. L., et al. Researching prospect of correspondence between prescription and syndromes on the pathologic basis of yin deficiency syndrome of liver and kidney and effective mechanism of decoction of Yiguan Jian. Journal of Developing Drugs. 2017;06(02):1–6. doi: 10.4172/2329-6631.1000176. [DOI] [Google Scholar]
- 32.Xie W. L., Jiang R., Shen X. L., Chen Z. Y., Deng X. M. Diosgenin attenuates hepatic stellate cell activation through transforming growth factor-beta/Smad signaling pathway. International Journal of Clinical and Experimental Medicine. 2015;8(11):20323–20329. [PMC free article] [PubMed] [Google Scholar]
- 33.Lee T.-F., Lin Y.-L., Huang Y.-T. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Medica. 2007;73(6):527–534. doi: 10.1055/s-2007-981520. [DOI] [PubMed] [Google Scholar]
- 34.Preetha S. P., Kanniappan M., Selvakumar E., Nagaraj M., Varalakshmi P. Lupeol ameliorates aflatoxin B1-induced peroxidative hepatic damage in rats. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2006;143(3):333–339. doi: 10.1016/j.cbpc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Grace-Lynn C., Chen Y., Latha L., et al. Evaluation of the hepatoprotective effects of Lantadene A, a pentacyclic triterpenoid of Lantana plants against acetaminophen-induced liver damage. Molecules. 2012;17(12):13937–13947. doi: 10.3390/molecules171213937. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Chotimarkorn C., Ushio H. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse. Phytomedicine. 2008;15(11):951–958. doi: 10.1016/j.phymed.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Lin L.-C., Shen C.-C., Shen Y.-C., Tsai T.-H. Anti-inflammatory neolignans from Piper kadsura. Journal of Natural Products. 2006;69(5):842–844. doi: 10.1021/np0505521. [DOI] [PubMed] [Google Scholar]
- 38.Shen D.-Y., Chan Y.-Y., Hwang T.-L., et al. Constituents of the roots of Clausena lansium and their potential anti-inflammatory activity. Journal of Natural Products. 2014;77(5):1215–1223. doi: 10.1021/np500088u. [DOI] [PubMed] [Google Scholar]
- 39.Ding Y., Liang C., Kim J. H., et al. Triterpene compounds isolated from Acer mandshuricum and their anti-inflammatory activity. Bioorganic & Medicinal Chemistry Letters. 2010;20(5):1528–1531. doi: 10.1016/j.bmcl.2010.01.096. [DOI] [PubMed] [Google Scholar]
- 40.Germanò M. P., D’Angelo V., Sanogo R., Morabito A., Pergolizzi S., De Pasquale R. Hepatoprotective activity of Trichilia roka on carbon tetrachloride-induced liver damage in rats. Journal of Pharmacy and Pharmacology. 2001;53(11):1569–1574. doi: 10.1211/0022357011777954. [DOI] [PubMed] [Google Scholar]
- 41.Fan S., Chen H.-N., Wang C.-J., Tseng W.-C., Hsu H.-K., Weng C.-F. Toona sinensis Roem (Meliaceae) leaf extract alleviates liver fibrosis via reducing TGFβ1 and collagen. Food and Chemical Toxicology. 2007;45(11):2228–2236. doi: 10.1016/j.fct.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Xiang Y., Pang B.-Y., Zhang Y., et al. Effect of Yi Guan Jian decoction on differentiation of bone marrow mesenchymalstem cells into hepatocyte-like cells in dimethylnitrosamine-induced liver cirrhosis in mice. Molecular Medicine Reports. 2017;15(2):613–626. doi: 10.3892/mmr.2016.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinzani M., Rombouts K. Liver fibrosis: from the bench to clinical targets. Digestive and Liver Disease. 2004;36(4):231–242. doi: 10.1016/j.dld.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs B. C., Hoshida Y., Fujii T., et al. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59(4):1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bohdanowicz M., Balkin D. M., De Camilli P., Grinstein S. Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Molecular Biology of the Cell. 2012;23(1):176–187. doi: 10.1091/mbc.e11-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farooqi A. A., Waseem S., Riaz A. M., et al. PDGF: the nuts and bolts of signalling toolbox. Tumor Biology. 2011;32(6):1057–1070. doi: 10.1007/s13277-011-0212-3. [DOI] [PubMed] [Google Scholar]
- 47.Poulos J. E., Weber J. D., Bellezzo J. M., et al. Fibronectin and cytokines increase JNK, ERK, AP-1 activity, and transin gene expression in rat hepatic stellate cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1997;273(4):G804–G811. doi: 10.1152/ajpgi.1997.273.4.g804. [DOI] [PubMed] [Google Scholar]
- 48.Parsons C. J., Takashima M., Rippe R. A. Molecular mechanisms of hepatic fibrogenesis. Journal of Gastroenterology and Hepatology. 2007;22(s1):S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 49.Lok A. S. F., McMahon B. J. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 50.He W., Shi F., Zhou Z. W., et al. A bioinformatic and mechanistic study elicits the antifibrotic effect of ursolic acid through the attenuation of oxidative stress with the involvement of ERK, PI3K/Akt, and p38 MAPK signaling pathways in human hepatic stellate cells and rat liver. Drug Design, Development and Therapy. 2015;9:3989–4104. doi: 10.2147/dddt.s85426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Chu E. S. H., Chen H. Y., et al. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget. 2015;6(9):7325–7338. doi: 10.18632/oncotarget.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jung M.-C., Pape G. R. Immunology of hepatitis B infection. The Lancet Infectious Diseases. 2002;2(1):43–50. doi: 10.1016/s1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 53.Blomhoff R., Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis. The FASEB Journal. 1991;5(3):271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- 54.Gou X., Tao Q., Feng Q., et al. Urine metabolic profile changes of CCl4-liver fibrosis in rats and intervention effects of Yi Guan Jian decoction using metabonomic approach. BMC Complementary and Alternative Medicine. 2013;13(1):p. 123. doi: 10.1186/1472-6882-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: potential targets for docking analysis. Table S2: selected compounds for docking analysis. Table S3: active ligands as reference for the docking analysis. Table S4: key pathways involved with the YGJD acting on CHB-related liver fibrosis. Table S5: raw data of the docking results.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary Materials and openly available in the TCMSP (http://lsp.nwu.edu.cn/tcmsp.php), the HIT (http://lifecenter.sgst.cn/hit/), the PDTCM (http://cadd.gdhtcm.com:2180/PDTCM/), the TCMPTD (http://pharminfo.zju.edu.cn/ptd), the PubChem (https://pubchem.ncbi.nlm.nih.gov/), and the DisGeNET (http://www.disgenet.org/).