Abstract
Background
Uric acid is a powerful free-radical scavenger in humans, but hyperuricemia may induce insulin resistance and beta-cell dysfunction. The study aimed to evaluate the association between hyperuricemia and hyperglycemia, considering the confounding factors in a Vietnamese population.
Methods
A population-based cross-sectional study recruited 1542 adults aged 50 to 70 years to collect data on socioeconomic status, lifestyle factors, and clinical patterns. Associations between hyperuricemia and hyperglycemia (isolated impaired fasting glucose (IFG), isolated impaired glucose tolerance (IGT), combined IFG-IGT, and type 2 diabetes (T2D)) were evaluated by multinomial logistic regression analysis in several models, adjusting for the confounding factors including socioeconomic status, lifestyle factors, and clinical measures.
Results
Uric acid values were much higher in IFG, IFG-IGT, and T2D groups compared to those in the normal glucose tolerance (NGT) group. The significant association of hyperuricemia with IFG, IFG-IGT, and T2D was found in the model unadjusted and remained consistently in several models adjusted for socioeconomic status, lifestyle factors, and clinical patterns. In the final model, the consistent hyperglycemia risk was found in total sample (OR = 2.23 for IFG, OR = 2.29 for IFG-IGT, and 1.75 for T2D, P ≤ 0.006) and in women (OR = 2.90 for IFG, OR = 3.96 for IFG-IGT, and OR = 2.49 for T2D, P < 0.001) but not in men.
Conclusions
It is the first report in Vietnamese population suggesting the significant association of hyperuricemia with IFG, IFG-IGT, and T2D; and the predominant association was found in women than in men, taken into account the confounding factors.
1. Introduction
Uric acid is produced during the exogenous metabolic breakdown of purines from dietary intake, and it is also a product from the endogenous degradation from dead cells. Most serum uric acid (SUA) is freely filtered, and approximately 90% of filtered uric acid is reabsorbed, showing its essential role in human body [1]. Uric acid plays a considerable physiological role as strong reactive oxygen species, a powerful free radical and peroxynitrite scavenger [2, 3] and a major plasma antioxidant [4]. However, there has been also a controversial opinion about prooxidative and antioxidant properties of uric acid [5]. Uric acid represents a marker for high levels of damaging oxidative stress [6]. Moreover, hyperuricemia induces insulin resistance [7], while hyperinsulinemia caused by insulin resistance increases SUA concentration by both reducing renal uric acid secretion [8] and accumulating substrates for uric acid production [9]. The relation between elevated SUA and type 2 diabetes remains controversial. Elevated SUA was an independent risk factor for type 2 diabetes in some studies in Sweden [10], China [11], Netherlands [12], and Brazil [13]. However, the inverse association with diabetes was found in several reports. SUA levels tended to increase with increasing fasting plasma glucose levels in nondiabetic individuals but decrease in diabetic individuals in a general Chinese population [14]. Higher SUA levels were inversely associated with type 2 diabetes in a representative sample of adults in United States [15]. Furthermore, in Japanese men, uric acid was negatively associated with diabetes in a cross-sectional study [16], while prospective studies reported inconsistent findings that SUA level was not associated [17] or associated [18] with an increased risk for type 2 diabetes. The inconsistent association between elevated SUA and type 2 diabetes may be explained by confounding factors including socioeconomic status, lifestyle-related factors, and clinical measures (body mass index, blood pressures, and dyslipidemia). Recently, a recommendation that the correlation between SUA and type 2 diabetes requires further evaluation has been noted from a systematic review and meta-analysis including 970 studies in 61,714 participants [19]. Therefore, we conducted a population-based cross-sectional study to investigate the association between hyperuricemia and hyperglycemia including impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes, considering the confounding factors in a Vietnamese population. The gender difference in the association was also reported.
2. Methods
2.1. Setting and Study Subjects
The cross-sectional study was a part of the DiaMetS-VN population-based prospective study designed to conduct in Ha Nam province, Vietnam, from July to December 2016. Ha Nam province locates in the southwest of the Red River Delta and 50 km far from Hanoi City. It has a population of about 800,000 inhabitants living in 108 rural communes and 6 urban wards [20]. The Ethics Committee of the National Institute of Hygiene and Epidemiology, Vietnam, approved the protocol of the survey (IRB-VN01057-34/2016). All the participants belonged to the Viet ethnic group, and they provided written informed consent before taking part in the study.
2.2. Serum Biochemical Analysis
Serum uric acid was measured by the uricase-based methods using a fully automatic biochemistry analyzer (FACA-401 autoanalyzer, Labomed Inc., USA) with a commercial kit (Erbra, Germany). In the procedure, uric acid is converted by uricase to allantoin and hydrogen peroxide, and the amount of hydrogen peroxide produced is measured. Hyperuricemia was classified as follows: for women, uric acid level ≥360 μmol/L (6 mg/dL) and for men ≥420 μmol/L (7.0 mg/dL) [21].
Serum glucose and lipid profile were quantified using laboratory methods as reported previously [22]. Dyslipidemia [23] is defined as low-density lipoprotein cholesterol level <40 mg/dL for men and <50 mg/dL for women, and total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels ≥200, ≥130, and ≥130 mg/dL, respectively. The glycemic status of subjects was determined using fasting plasma glucose (FPG) and 2h plasma glucose (2h-PG) by the oral glucose tolerance test with 75 g glucose. The glycemic status was classified as normal glucose tolerance (NGT) when FPG < 5.6 mmol/L and 2h-PG < 7.8 mmol/L, as isolate impaired fasting glucose (IFG) when 5.6 ≤ FPG ≤ 6.9 mmol/L and 2h-PG < 7.8 mmol/L, as isolated impaired glucose tolerance (IGT) when FPG < 5.6 mmol/L and 7.8 ≤ 2h-PG ≤ 11.0 mmol/L, as combined IFG and IGT (IFG−IGT) when 5.6 ≤ FPG ≤ 6.9 mmol/L and 7.8 ≤ 2h-PG ≤ 11.0 mmol/L, and as type 2 diabetes (T2D) when FPG ≥ 7.0 mmol/L and/or 2h-PG ≥ 11.1 mmol/L or previous diagnosis of diabetes and current use of drug for its treatment, according to guidelines of the World Health Organization and International Diabetes Federation [24].
2.3. Socioeconomic, Lifestyle, and Clinical Covariates
All participants were directly interviewed by trained surveyors to complete a structured questionnaire as presented previously [22]. Socioeconomic variables included age, gender, residence, marital status, education level (elementary, intermediate, secondary, and postsecondary), occupation, and income level. Lifelong occupation was defined as the occupation that the subject engaged in most frequently in the life. It was categorized as heavy occupation (farmer and manual worker) and nonheavy occupation (office clerks, teacher, retired worker, and houseworker).
Lifestyle factors were composed of consumption of wine and beer (none, <1 drink/mo, ≥1 drink/mo to <1 drink/wk, 1 drink/wk to ≤ 1 drink/d, and ≥2 drink/d, one drink was defined as a 50 ml cup of rice wine at about 30%), smoking (never, current, and former), consumption of sugary drinks (<1 drink/mo, ≥1 drink/mo to <1 drink/wk, 1 drink/wk to <1 drink/d, and ≥1 drink/d, one drink was defined as a 330 ml cup), sporting habit (none, light, heavy), time spent for night's sleep (7 h, ≤6 h, and ≥8 h), siesta (per 30 min), watching television (3 h and >3 h), and leisure sitting (4 h and >4 h).
Clinical patterns were characterized by body mass index (BMI), systolic blood pressure, diastolic blood pressure, and dyslipidemia. Body mass index was calculated as weight per square of height (kg/m2).
2.4. Statistical Analysis
Quantitative variables were checked for normal distribution and compared using One-Way ANOVA or independent-sample T test. Kruskal–Wallis or Mann–Whitney U test was used to compare quantitative variables without normal distribution. Frequencies of category variables were compared by Pearson's chi-squared test.
Multinomial logistic regression analysis was performed to test several models for the associations between hyperuricemia and hyperglycemia, adjusting for confounding factors including (i) socioeconomic status: age, sex, residence, education level, occupation, marital status, and income level; (ii) lifestyle-related factors: consumption of wine and beer, smoking, consumption of sugary drinks, sporting habit, time spent for night's sleep, siesta, watching television, and leisure sitting; and (iii) clinical measures: BMI, systolic blood pressure, diastolic blood pressure, and dyslipidemia. Data are expressed as odds ratios with 95 percent confidence intervals (CI). Associations were considered statistically significant at two-sided P values of less than 0.05 for all the analyses. The above statistical procedures were performed using SPSS version 16.0.
3. Results
3.1. Characteristics of the Study Cohort
Table 1 shows the characteristics of the subjects according to blood glucose levels. Mean (±SD) of age and BMI of the subjects were 56.5 (±6.7) years and 21.9 (±2.7) kg/m2, respectively. The diabetes group had significant higher age, weight, BMI, and blood pressures than the NGT group. There was no significant difference of weight, height, and BMI among NGT, IFG, IGT, and IFG-IGT groups.
Table 1.
Characteristics of the studied subjects according to blood glucose levels.
| Variables | NGT (n = 998) | IFG (n = 176) | IGT (n = 121) | IFG + IGT (n = 57) | Diabetes (n = 190) | P value |
|---|---|---|---|---|---|---|
| Gender (male) | 318 (31.9) | 54 (30.7) | 49 (40.5)∗# | 24 (42.1)∗# | 77 (40.5)∗# | 0.033 |
| Age (year) | 56.0 ± 6.7 | 56.2 ± 6.6 | 57.5 ± 6.6 | 58.3 ± 6.1∗# | 58.3 ± 6.7∗# | <0.001 |
| Height (cm) | 155.9 ± 7.3 | 156.2 ± 7.3 | 156.3 ± 6.9 | 156.0 ± 7.2 | 156.5 ± 7.7 | 0.825 |
| Weight (kg) | 52.9 ± 8.1 | 54.0 ± 8.4 | 54.1 ± 7.9 | 54.9 ± 9.1 | 55.4 ± 8.4∗ | 0.001 |
| BMI (kg/m2) | 21.8 ± 2.6 | 22.1 ± 2.6 | 22.1 ± 2.9 | 22.5 ± 3.0 | 22.6 ± 2.8∗ | 0.001 |
| SBP (mmHg) | 122.5 (112.5–137.7) | 126.8∗ (114.5–137.5) | 129.0∗# (117.3–143.8) | 139.0∗# (120.8–149.5) | 134.5∗# (121.0–147.1) | <0.001 |
| DBP (mmHg) | 80.0 (71.0–87.5) | 80.0 (70.0–84.8) | 82.5∗# (75.0–91.3) | 85.5∗# (75.3–92.3) | 82.0∗# (73.9–90.0) | <0.001 |
Data are expressed as the mean with standard deviation and median (interquartile range), except for gender as number (percentage). P value for difference between the groups was calculated from the one-way ANOVA or Kruskal–Wallis test or chi-squared test: ∗vs. NGT group and #vs. IFG group. NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
3.2. Uric Acid Concentrations by Gender and Glucose Levels
Table 2 shows the comparison of SUA concentration among 5 glucose levels (NGT, IFG, IGT, IFG-IGT, and diabetes) in men, women, and total sample. SUA values were much higher in IFG, IFG-IGT, and diabetes groups compared to the NGT group. There was no significant difference of SUA value between NGT and IGT groups. Men had higher SUA concentration than women in NGT, IFG, and diabetes groups.
Table 2.
Uric acid concentration among glucose levels of subjects.
| Glucose level | Total | Men | Women | P 1 value | |||
|---|---|---|---|---|---|---|---|
| n | Median (interquartile range) | n | Median (interquartile range) | n | Median (interquartile range) | ||
| NGT | 998 | 294.3 (241.6–357.9) | 319 | 331.8 (265.0–410.7) | 679 | 276.7 (230.8–337.7)ϕ | <0.001 |
| IFG | 176 | 358.7 (244.6–406.1)∗ | 54 | 391.2 (343.1–454.5)∗ | 122 | 337.3 (225.2–392.0)∗ϕ | <0.001 |
| IGT | 121 | 295.6 (236.8–379.9)# | 49 | 315.3 (240.5–420.9)# | 72 | 285.5 (215.1–354.7)# | 0.073 |
| IFG and IGT | 57 | 370.4 (279.3–418.0)∗† | 24 | 389.5 (331.0–425.8)∗† | 33 | 359.7 (256.7–410.0)∗† | 0.056 |
| Diabetes | 190 | 343.9 (252.3–410.0)∗† | 77 | 371.7 (307.9–426.7)∗ | 113 | 304.4 (238.5–398.0)∗ϕ | 0.001 |
| P 2 value | <0.001 | ≤0.001 | <0.001 | ||||
P 1 value for difference between men and women was calculated from the Mann–Whitney test: ϕvs. men. P2 value for difference among glucose levels (NGT, IFG, IGT, IFG-IGT, and diabetes) in men, women, and total sample was calculated from the Kruskal–Wallis test: ∗vs. NGT group, #vs. IFG group, and †vs. IGT group. NGT, normal glucose tolerance; IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
3.3. Association between Elevated Uric Acid and Hyperglycemia
Table 3 shows the association of hyperuricemia with hyperglycemia including IFG, IGT, IFG-IGT, and T2D in several models considering the influence of socioeconomic conditions (age, sex, residence, education level, occupation, marital status, and income level) in the Model 2, adding lifestyle-related factors (consumption of wine and beer, smoking, consumption of sugary drinks, sporting habit, time spending for night's sleep, siesta, watching TV, and leisure sitting) in the Model 3, and further clinical measures (BMI, blood pressures, and dyslipidemia) in the Model 4. The significant association of hyperuricemia with IFG, IFG-IGT, and diabetes was found in the Model 1 unadjusted and remained consistently in several models adjusted for socioeconomic status (Model 2), lifestyle-related factors (Model 3), and clinical patterns (Model 4). In the final Model 4, after adjustments for socioeconomic status, lifestyle factors, and clinical pattern, the hyperuricemia was found to be an independent risk factor for hyperglycemia in the total sample (OR = 2.23 for IFG, OR = 2.29 for IFG-IGT, and 1.75 for T2D, P ≤ 0.006) and in women (OR = 2.90 for IFG, OR = 3.96 for IFG-IGT, and OR = 2.49 for T2D, P < 0.001). Such an association was not observed for IGT. When analyzing by stratum of gender, the associations remained significantly in females, but not in males.
Table 3.
Association of elevated serum uric acid with hyperglycemia in multinomial logistic regression adjusted for confounding factors.
| Model | IFG | IGT | IFG-IGT | Diabetes | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Total (n = 1542) | ||||||||
| Model 1 | 2.58 (1.833.62) | <0.001 | 1.18 (0.75–1.85) | 0.487 | 2.57 (1.48–4.48) | 0.001 | 2.08 (1.48–2.92) | <0.001 |
| Model 2 | 2.33 (1.63.3.32) | <0.001 | 1.15 (0.73–1.83) | 0.544 | 2.50 (1.42–4.38) | 0.001 | 1.96 (1.38–2.78) | <0.001 |
| Model 3 | 2.31 (1.61–3.32) | <0.001 | 1.12 (0.71–1.79) | 0.624 | 2.76 (1.55–4.90) | 0.001 | 1.99 (1.39–2.83) | <0.001 |
| Model 4 | 2.23 (1.54–3.23) | <0.001 | 1.08 (0.67–1.74) | 0.744 | 2.29 (1.27–4.11) | 0.006 | 1.75 (1.22–2.52) | 0.002 |
|
| ||||||||
| Women (n = 1019) | ||||||||
| Model 1 | 3.27 (2.17–4.93) | <0.001 | 1.14 (0.61–2.10) | 0.685 | 4.43 (2.18–9.02) | <0.001 | 2.78 (1.81–4.28) | <0.001 |
| Model 2 | 2.72 (1.77–4.19) | <0.001 | 1.14 (0.62–2.13) | 0.672 | 3.91 (1.87–8.16) | <0.001 | 2.57 (1.65–4.02) | <0.001 |
| Model 3 | 2.87 (1.84–4.50) | <0.001 | 1.21 (0.64–2.27) | 0.559 | 4.44 (2.08–9.47) | <0.001 | 2.79 (1.76–4.41) | <0.001 |
| Model 4 | 2.90 (1.83–4.58) | <0.001 | 1.07 (0.56–2.03) | 0.837 | 3.96 (1.83–8.54) | <0.001 | 2.49 (1.55–3.99) | <0.001 |
|
| ||||||||
| Men (n = 523) | ||||||||
| Model 1 | 1.57 (0.84–2.93) | 0.154 | 1.14 (0.57–2.25) | 0.717 | 1.05 (0.40–2.73) | 0.924 | 1.26 (0.72–2.19) | 0.421 |
| Model 2 | 1.85 (0.96–3.55) | 0.065 | 1.14 (0.57–2.28) | 0.712 | 1.05 (0.40–2.79) | 0.917 | 1.36 (0.77–2.41) | 0.297 |
| Model 3 | 1.72 (0.88–3.36) | 0.114 | 1.06 (0.52–2.16) | 0.879 | 1.10 (0.40–3.04) | 0.859 | 1.36 (0.76–2.45) | 0.304 |
| Model 4 | 1.55 (0.78–3.08) | 0.207 | 1.10 (0.53–2.29) | 0.808 | 0.79 (0.27–2.29) | 0.667 | 1.19 (0.65–2.19) | 0.576 |
Model 1: unadjusted. Model 2: adjusted for socioeconomic status (age, gender, residence, marital status, education level, occupation, and income level). Model 3: Model 2 adjusted for lifestyle factors (consumption of wine and beer, smoking, consumption of sugary drinks, sporting habit, time spent for night's sleep, siesta, watching television, and leisure sitting). Model 4: Model 3 adjusted for clinical patterns (body mass index, systolic blood pressure, diastolic blood pressure, and dyslipidemia). IFG, impaired fasting glucose; IGT, impaired glucose tolerance.
Adjusting for covariates improved the area under ROC curve significantly (Figure 1) from 0.570 (P=0.002) in the unadjusted model to 0.687, 0.738, and 0.763 (P < 0.001), respectively, in models adjusted for socioeconomic status (Model 2), lifestyle factors (Model 3), and clinical patterns (Model 4). Adding the hyperuricemia variable in the final Model 4 improved the area under the receiver-operating characteristic curve slightly (from 0.758 to 0.763, P < 0.001).
Figure 1.
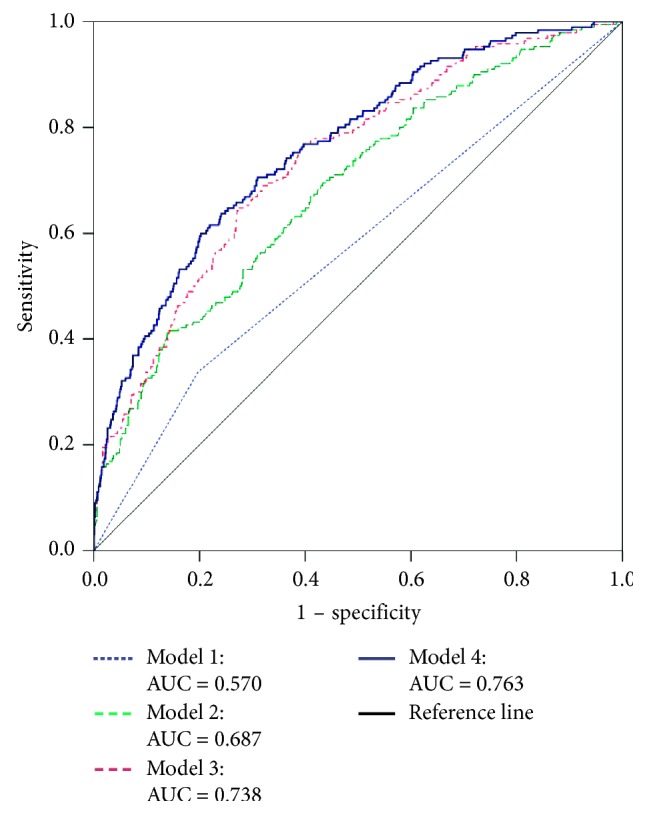
Serum uric acid receiver-operating characteristic curve for type 2 diabetes. Model 1: unadjusted. Model 2: adjusted for socioeconomic status (age, gender, residence, marital status, education level, occupation, and income level). Model 3: Model 2 adjusted for lifestyle factors (consumption of wine and beer, smoking, consumption of sugary drinks, sporting habit, time spending for night's sleep, siesta, watching TV, and leisure sitting). Model 4: Model 3 adjusted for clinical patterns (body mass index, systolic blood pressure, diastolic blood pressure, and dyslipidemia).
4. Discussion
In this study, we have found the significant association of hyperuricemia acid with IFG, IFG-IGT, and diabetes, independent of the confounding factors. Given the multifactorial pattern of type 2 diabetes, the association of SUA with type 2 diabetes varies among populations depending on the socioeconomic status, lifestyle factor, genetic background, and clinical risk factor profile of each population [25]. Our finding is consistent with several reports [10–13]. The inconsistent findings on the association between SUA and T2D even in one population, i.e., Japanese men [16–18], may be resulted from the confounding factors which are not considered in the analysis of the association. Our analysis considered the important T2D risk factors including gender, age, alcohol consumption, smoking, consumption of sugary drinks, BMI, blood pressures, and lipid profile as well as other socioeconomic and lifestyle-related factors.
In line with a report in Japan [18], the present study showed the association of SUA with IFG and T2D in Vietnamese population with the BMI mean <25 (21.9 ± 2.7) kg/m2, whereas the other reported that the association between SUA and incident prediabetes was not significant among Caucasian population with BMI <25 kg/m2 [26]. This indicates the important difference in BMI between Vietnamese and Caucasians in the pathogenesis of type 2 diabetes [27], and Vietnamese may develop type 2 diabetes with smaller increases in BMI than Taiwanese [28].
The significant difference of association by gender was found in our cohort. In agreement with the study in German population [29], the present study reported the significant association of hyperuricemia with hyperglycemia risk in females (OR = 2.90 for IFG, OR = 3.96 for IFG-IGT, and OR = 2.49 for T2D, P < 0.001) but not in males. In addition, the association was found in the IFG group but not in the IGT group. These findings may be explained by that SUA affects women more strongly in the early stages of glucose intolerance development, whereas it affects men more strongly in more advanced stages [26]. Previous studies supported the similar finding that SUA may be a useful predictor of type 2 diabetes in older adults with impaired fasting glucose [13] and a strong positive association between SUA and incident prediabetes in females rather than in males [30].
Both insulin resistance and β-cell dysfunction play determinate roles in the pathogenesis of type 2 diabetes. High SUA directly induces insulin resistance by impairing glucose tolerance and inhibiting insulin signaling in vivo and by inducing oxidative stress in vitro [31]. Higher SUA is associated with greater insulin secretion ability at the early stage of the disease, but it seems to reduce residual β-cell function more rapidly [32]. Increased SUA has a direct negative effect on β-cell function, which could cause β-cell death and dysfunction by activation of the NF-κB and iNOS-NO signal axis [33]. Collectively, elevated SUA has a direct effect on both insulin resistance and β-cell dysfunction in the pathogenesis of type 2 diabetes. These evidences support the significant association of hyperuricemia with IFG, IFG-IGT, and diabetes in the Vietnamese population.
There has been an inconsistent relation between hyperuricemia and IGT. In agreement with other reports [29], the present study showed no association between hyperuricemia and IGT status, whereas SUA was related to IGT in the Chinese adults, independent of other conventional metabolic risk factors [34]. Based on the oral glucose tolerance test, the previous study indicated that IFG was due to impaired basal insulin secretion and preferential resistance of glucose production to suppression by insulin (as reflected by fasting hyperglycemia despite normal plasma insulin concentrations and increased HOMA-IR), whereas IGT mainly resulted from reduced second-phase insulin release and peripheral insulin resistance (as reflected by reduced clamp-determined insulin sensitivity) [35]. The aetiologies of IFG and IGT also seem to differ, with IFG being predominantly related to genetic factors, smoking, and male sex, whereas IGT is predominantly related to physical inactivity, unhealthy diet, and short stature [36]. Both IFG and IGT had inappropriately elevated glucagon secretion. Subjects with IFG had predominant reduced hepatic insulin sensitivity and normal skeletal muscle insulin sensitivity, while subjects with IGT had near-normal hepatic and moderate to severe skeletal muscle insulin resistance [37]. IFG induced dysfunction and/or chronic low mass of beta cell and altered glucagon-like peptide-1 secretion, whereas IGT induced progressive loss of beta-cell function and reduced secretion of glucose-dependent insulinotropic polypeptide. It is necessary to conduct further studies to elucidate the relation between uric acid concentrations and IGT status.
Using medications with potential effect on SUA levels may be the confounding factor for the association between hyperuricemia and hyperglycemia. This study design as a population-based study can reduce the confounding factor of drugs, which usually happens in a hospital-based study. In the total of 1542 participants, there were 46 (3%) patients with previously diagnosed diabetes. Only 15 patients recalled the antidiabetic drugs: insulin (n = 8), metformin (n = 6), and gliclazide (n = 1). Among antidiabetic drugs, SGLT2 inhibitors have a potential effect of reducing SUA levels. In the study conducted in 2016, SGLT2 inhibitors were not recommended for patients with diabetes in Ha Nam province because these drugs were costly and not paid by Vietnam Health Insurance. Therefore, using antidiabetic drugs may not be the confounding factor for the observed association.
The present findings must be interpreted in the context of several limitations. First, the findings obtained from a cross-sectional study cannot give any causative relation. Next, insulin resistance and beta-cell function were not evaluated, limiting the analysis of the most important pathogenesis of type 2 diabetes and other hyperglycemic status. Third, the study did not clarify antihypertensive drugs as confounding factors in 128 (8.3%) previously diagnosed patients with hypertension. Lastly, genetic factors were not included in the analysis, so the findings could not explained by genetic variances in the population.
5. Conclusions
In summary, the study showed that there was a significant association of hyperuricemia with IFG, IFG-IGT, and diabetes in the Vietnamese population, and the predominant association was found in females than in males, taken into account the confounding factors.
Acknowledgments
This study was supported by Vietnam's National Foundation for Science and Technology Development (NAFOSTED), grant no. 106-YS.01-2015.10, from the Ministry of Science and Technology, Vietnam. The authors would like to thank Mrs. Thai N. M., Dr. Thoang D. D., and Dr. Thang P. V. for kind help and support. The authors are grateful to all the participants and the health staff of the Ha Nam Center for Preventive Medicine for their cooperation and assistance.
Abbreviations
- BMI:
Body mass index
- NGT:
Normal glucose tolerance
- IFG:
Impaired fasting glucose
- IGT:
Impaired glucose tolerance
- FPG:
Fasting plasma glucose level
- OGTT:
Oral glucose tolerance test
- SBP:
Systolic blood pressure
- DBP:
Diastolic blood pressure
- SUA:
Serum uric acid.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
TQB conceptualized and designed the study, analysed data, and discussed and edited the final draft for publication. PTP, BTN, NTC, and DDT conceptualized the study, collected data, and discussed the final draft for publication. TQT, DTL, BTTN, and NAN entered, analysed, and interpreted the data. LDT supervised the project and discussed the findings. All authors approved the final draft of this article prior to submission.
References
- 1.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. International Journal of Cardiology. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 2.Waring W. S. Uric acid: an important antioxidant in acute ischaemic stroke. QJM. 2002;95(10):691–693. doi: 10.1093/qjmed/95.10.691. [DOI] [PubMed] [Google Scholar]
- 3.Glantzounis G., Tsimoyiannis E., Kappas A., Galaris D. Uric acid and oxidative stress. Current Pharmaceutical Design. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 4.Ames B. N., Cathcart R., Schwiers E., Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sautin Y. Y., Johnson R. J. Uric acid: the oxidant-antioxidant paradox. Nucleosides, Nucleotides And Nucleic Acids. 2008;27(6-7):608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higgins P., Ferguson L. D., Walters M. R. Xanthine oxidase inhibition for the treatment of stroke disease: a novel therapeutic approach. Expert Review of Cardiovascular Therapy. 2011;9(4):399–401. doi: 10.1586/erc.11.29. [DOI] [PubMed] [Google Scholar]
- 7.Khosla U. M., Zharikov S., Finch J. L., et al. Hyperuricemia induces endothelial dysfunction. Kidney International. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.Quiñones Galvan A., Natali A., Baldi S., et al. Effect of insulin on uric acid excretion in humans. American Journal of Physiology-Endocrinology and Metabolism. 1995;268(1):E1–E5. doi: 10.1152/ajpendo.1995.268.1.e1. [DOI] [PubMed] [Google Scholar]
- 9.Fox I. H. Metabolic basis for disorders of purine nucleotide degradation. Metabolism. 1981;30(6):616–634. doi: 10.1016/0026-0495(81)90142-6. [DOI] [PubMed] [Google Scholar]
- 10.Ohlson L.-O., Larsson B., Björntorp P., et al. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988;31(11):798–805. doi: 10.1007/bf00277480. [DOI] [PubMed] [Google Scholar]
- 11.Chou P., Li C.-L., Wu G.-S., Tsai S.-T. Progression to type 2 diabetes among hign-risk groups in Kin-Chen, Kinmen: exploring the natural history of type 2 diabetes. Diabetes Care. 1998;21(7):1183–1187. doi: 10.2337/diacare.21.7.1183. [DOI] [PubMed] [Google Scholar]
- 12.Dehghan A., van Hoek M., Sijbrands E. J. G., Hofman A., Witteman J. C. M. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care. 2008;31(2):361–362. doi: 10.2337/dc07-1276. [DOI] [PubMed] [Google Scholar]
- 13.Kramer C. K., von Muhlen D., Jassal S. K., Barrett-Connor E. Serum uric acid levels improve prediction of incident type 2 diabetes in individuals with impaired fasting glucose: the Rancho Bernardo study. Diabetes Care. 2009;32(7):1272–1273. doi: 10.2337/dc09-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nan H., Dong Y., Gao W., Tuomilehto J., Qiao Q. Diabetes associated with a low serum uric acid level in a general Chinese population. Diabetes Research and Clinical Practice. 2007;76(1):68–74. doi: 10.1016/j.diabres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Bandaru P., Shankar A. Association between serum uric acid levels and diabetes mellitus. International Journal of Endocrinology. 2011;2011:6. doi: 10.1155/2011/604715.604715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oda E., Kawai R., Sukumaran V., Watanabe K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabetes in Japanese men. Internal Medicine. 2009;48(20):1785–1791. doi: 10.2169/internalmedicine.48.2426. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi Y., Hayashi T., Tsumura K., Endo G., Fujii S., Okada K. Serum uric acid and the risk for hypertension and type 2 diabetes in Japanese men: the Osaka Health Survey. Journal of Hypertension. 2001;19(7):1209–1215. doi: 10.1097/00004872-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Nakanishi N., Okamoto M., Yoshida H., Matsuo Y., Suzuki K., Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. European Journal of Epidemiology. 2003;18(6):523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y.-L., Xu K.-F., Bai J.-L., et al. Elevation of serum uric acid and incidence of type 2 diabetes: a systematic review and meta-analysis. Chronic Diseases and Translational Medicine. 2016;2(2):81–91. doi: 10.1016/j.cdtm.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.General Statistics Office of Vietnam. The 2009 Vietnam Population and Housing Census: Completed Results. Hanoi, Vietnam: Statistical Publishing House; 2010. http://www.gso.gov.vn/ [Google Scholar]
- 21.Chizyński K., Rózycka M. Hyperuricemia. Polski Merkuriusz Lekarski. 2005;19(113):693–696. in Polish. [PubMed] [Google Scholar]
- 22.Quang Binh T., Tran Phuong P., Thi Nhung B., et al. Prevalence and correlates of hyperglycemia in a rural population, Vietnam: implications from a cross-sectional study. BMC Public Health. 2012;12(1):939. doi: 10.1186/1471-2458-12-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA: The Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Geneva, Switzerland: World Health Organization; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO consultation. [Google Scholar]
- 25.Adeyemo A., Rotimi C. Genetic variants associated with complex human diseases show wide variation across multiple populations. Public Health Genomics. 2010;13(2):72–79. doi: 10.1159/000218711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Schaft N., Brahimaj A., Wen K.-X., Franco O. H., Dehghan A. The association between serum uric acid and the incidence of prediabetes and type 2 diabetes mellitus: the Rotterdam study. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179482.e0179482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son L. N. T. D., Hanh T. T. M., Kusama K., Ichikawa Y., Hung N. T. K., Yamamoto S. Vietnamese type 2 diabetic subjects with normal BMI but high body fat. Diabetes Care. 2003;26(6):1946–1947. doi: 10.2337/diacare.26.6.1946-a. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto S., Le D. S., Hsu T. F., et al. Vietnamese may develop type 2 diabetes with smaller increases in body mass index and waist circumference than Taiwanese. International Journal of Diabetology & Vascular Disease Research. 2013;1(1):1–4. doi: 10.19070/2328-353x-130001. [DOI] [Google Scholar]
- 29.Meisinger C., Döring A., Stöckl D., Thorand B., Kowall B., Rathmann W. Uric acid is more strongly associated with impaired glucose regulation in women than in men from the general population: the KORA F4-Study. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037180.e37180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Zhao Z., Mu Y., et al. Gender differences in the association between serum uric acid and prediabetes: a six-year longitudinal cohort study. International Journal of Environmental Research and Public Health. 2018;15(7):1560. doi: 10.3390/ijerph15071560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y., Hu Y., Huang T., et al. High uric acid directly inhibits insulin signalling and induces insulin resistance. Biochemical and Biophysical Research Communications. 2014;447(4):707–714. doi: 10.1016/j.bbrc.2014.04.080. [DOI] [PubMed] [Google Scholar]
- 32.Tang W., Fu Q., Zhang Q., et al. The association between serum uric acid and residual β-cell function in type 2 diabetes. Journal of Diabetes Research. 2014;2014:9. doi: 10.1155/2014/709691.709691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia L., Xing J., Ding Y., et al. Hyperuricemia causes pancreatic β-cell death and dysfunction through NF-κB signaling pathway. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0078284.e78284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Q., Gong Y., Liu X., et al. Serum uric acid and impaired glucose tolerance: the Cardiometabolic Risk in Chinese (CRC) study. Cell Biochemistry and Biophysics. 2015;73(1):155–162. doi: 10.1007/s12013-015-0597-5. [DOI] [PubMed] [Google Scholar]
- 35.Meyer C., Pimenta W., Woerle H. J., et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29(8):1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 36.Færch K., Borch-Johnsen K., Holst J. J., Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52(9):1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Ghani M. A., Tripathy D., DeFronzo R. A. Contributions of -cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29(5):1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


