Abstract
Background:
Hepatitis B virus (HBV) infection is a major cause of morbidity and mortality; however, little is known about the prevalence and distribution of HBV in some populations and regions.
Methods:
A total of 9791 participants, 15–49 years old, were enrolled in a household survey in KwaZulu-Natal, South Africa. Peripheral blood samples were tested for markers of HBV (hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), antibody to HBeAg (anti-HBe)) and analysed, accounting for multilevel sampling and weighted to represent the population.
Results:
Overall HBsAg prevalence was 4.0% (95% confidence interval (CI) 3.4–4.5%): 4.8% (95% CI 3.8–5.8%) in men and 3.2% (95% CI 2.5–3.9%) in women (p = 0.01). Among HBsAg-positive participants, 35.2% (95% CI 29.2–41.2%) were HBeAg-positive and 66.3% (95% CI 60.1–72.4%) were anti-HBe-positive. HBsAg prevalence was 6.4% (95% CI 5.3–7.5%) among HIV-positive participants compared to 2.6% (95% CI 1.9–3.2%) among HIV-negative participants (p < 0.01), and was higher among HIV-positive men (8.7%, 95% CI 6.3–11.2%) than among HIV-positive women (5.0%, 95% CI 3.8–6.2%) (p < 0.01).
Conclusions:
HBV infection among HIV-positive men remains an important public health problem in communities in KwaZulu-Natal, South Africa. The prevalence of HBsAg and HBeAg highlight the importance of surveillance and an important missed opportunity for the scale-up of programmes to achieve the goal of controlling HBV for public health benefit.
Keywords: Hepatitis B virus (HBV) prevalence, HBsAg, HBeAg, Anti-HBe, HBV-HIV co-infection, South Africa
Introduction
Hepatitis B virus (HBV) infection is a common cause of viral hepatitis and affects more than 257 million people worldwide. Almost 8% of this global burden is in Sub-Saharan Africa, with over 80 000 new infections occurring each year (World Health Organisation, 2017). In South Africa, HBV infections account for an estimated 3.5 million infected individuals (Schweitzer et al., 2015). HBV remains endemic in the region. Viral hepatitis from HBV infection is a potentially life-threatening liver infection resulting in acute hepatitis and chronic liver disease, leading to complications of cirrhosis and hepatocellular carcinoma, and contributing to high rates of morbidity and mortality (World Health Organisation, 2017).
The transmission of HBV is known to occur through multiple routes. The most common is mother-to-child transmission at birth or through exposure to infected blood (Kiire, 1996). Other routes of transmission include sexual transmission and percutaneous or mucosal exposure to infected blood and various body fluids. Transmission can also occur through saliva, menstrual, vaginal, and seminal fluids, and iatrogenic spread through the re-use of contaminated needles. Equipment in healthcare settings or among persons who inject drugs, and the use of razors or similar objects during ritual scarification may be other routes of transmission (Kew, 1996; Mphahlele et al., 2002; Heiberg et al., 2010).
South Africa has been at the forefront of new vaccine introduction; the country introduced the HBV vaccine as part of the Expanded Programme on Immunization as early as 1995. By 2015, South Africa had become the first African country to phase out the pentavalent vaccine, and this vaccine was replaced with the more baby-friendly hexavalent vaccine (Dlamini and Maja, 2016). Despite the introduction of these vaccines, no nationwide surveillance programme has measured and monitored trends in HBV (Mayaphi et al., 2012). Thus, data on the burden of HBV, including the clinical manifestations of advancing disease, are limited and are based on cohort studies undertaken more than a decade ago. Prior to the immunization programme, approximately 6.5 million South Africans were HBV carriers, with prevalence being highest among school-aged children, people residing in rural areas, and among sexually active individuals (Botha et al., 1984; Di Bisceglie et al., 1986; Burnett et al., 2005). The prevalence of HBV infection in rural areas has been estimated to be between 5% and 16% among men and between 4% and 12% among women, while in urban areas, rates of between 8% and 9% among men and between 3% and 4% among women have been estimated (Kew et al., 1976; Vos et al., 1980; Prozesky et al., 1983; Kew et al., 1987; Abdool Karim et al., 1988; Dusheiko et al., 1989). Currently, there are an estimated three to four million HBV carriers in South Africa (Kew, 2008).
Given that the World Health Organization (WHO) strategy is to eliminate HBV as a public health threat by the year 2030 (World Health Organisation, 2017; Perz et al., 2006; World Health Organisation, 2016), it is important to establish the HBV seroprevalence through surveillance. The purpose of this study was to assess the HBV seroprevalence as part of a household survey in rural and peri-urban areas in KwaZulu-Natal, South Africa.
Materials and methods
Study design, setting, and population
This secondary analysis was based on the testing of samples from participants enrolled in the HIV Incidence Provincial Surveillance System (HIPSS). The HIPSS study design, source population, and recruitment and enrolment procedures have been described previously (Kharsany et al., 2015; Kharsany et al., 2018). Briefly, HIPSS was a population-based household study conducted in the Vulindlela (rural) and the Greater Edendale (peri-urban) areas in the uMgungundlovu District of KwaZulu-Natal, South Africa. The study used a multi-stage cluster sampling method to randomly select enumeration areas, households, and individuals, resulting in a total of 9812 men and women (15–49 years old) enrolled between June 2014 and June 2015. All enrolled participants provided written informed consent and/or parental consent/child assent for those participants below the age of 18 years for study participation and for long-term storage of clinical samples for future testing. All completed interviewer administered questionnaires, had peripheral blood samples collected, and were allocated a unique identification number with a linking number to link the household, participant’s questionnaire, and laboratory data.
The Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal reviewed and approved the HBV seroprevalence protocol (BE057/17), the HIPSS study protocol, and informed consent and questionnaire forms (BF269/13). The US Centers for Disease Control and Prevention and the Provincial Department of Health (KwaZulu-Natal; HRKM 08/14) approved the HIPSS study protocol. All participants were referred to their local primary healthcare clinics to access their study-related laboratory test results and for individualized HIV testing services. Prior to any interaction with household members and participants, community engagement processes were established with key community stakeholders including health-service providers and traditional leadership and key members from community structures.
Laboratory tests
Peripheral blood samples were shipped to the laboratory within 6 h of collection. The blood samples of 21 of the 9812 participants enrolled in HIPSS (0.2%) were insufficient for any HBV testing and were excluded.
Testing for HBV
Testing for the detection of markers of HBV infection was performed using the automated ADVIA Centaur System (Siemens, Tarrytown, NY, USA), which utilizes magnetic particle separation technology with direct chemiluminescence. The HBV assay panel included the qualitative detection of hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and antibody to HBeAg (anti-HBe). HBsAg-positive samples were further tested for HBeAg and anti-HBe. All results were reported as positive or negative. The diagnostic specificity is reported to be 99.4% for the HBsAg assay, 99.5% for the HBeAg assay, and 98.2% for the anti-HBe assay (van Helden et al., 2004; van Helden et al., 2008).
Interpretation of HBV test results
In this study HBV status was defined as follows (North Carolina Hepatitis B Public Health Program Manual, 2012): (1) HBsAg-positive: the presence of HBsAg in serum indicates that the individual has a current infection. (2) HBeAg-positive: the presence of HBV nucleocapsid gene (HBeAg) in serum indicates active viral replication in individuals with high levels of viraemia who are more likely to transmit the virus. (3) Anti-HBe-positive: the presence of antibodies to HBV nucleocapsid gene (HBeAb) indicates that the individual is recovering as a result of an immune system response to HBeAg and has a reduced level of infectivity.
HBV and HIV-positive indicated that the individual was co-infected with HBV (HBsAg-positive) and HIV-1.
Testing for HIV
HIV testing was performed using the fourth-generation HIV enzyme bioMerieux Vironostika Uniform 11 Antigen/Antibody Microelisa system (bioMerieux, Marcy l’Etoile, France) and HIV 1/2 Combi Roche Elecsys (Roche Diagnostics, Penzberg, Germany). Samples testing indeterminate for HIV were further tested using the ADVIA Centaur HIV Antigen/Antibody Combo (CHIV) Assay (Siemens, Tarrytown, NY, USA).
Statistical analysis
Each participant’s laboratory and questionnaire data were merged using a unique linking number. All analyses were performed using SAS 9.4 survey procedures (SAS Institute Inc., Cary, NC, USA). Sampling weights were calculated taking into account the probabilities of selecting the enumeration area, the household in the enumeration area, and the individual in the household, weighted for sampling, participation bias, and non-response, and rescaled to the size of the population in the survey area with the StatsSA 2011 Census population (Kharsany et al., 2018). Descriptive statistics with counts and population-weighted percentages with 95% confidence intervals (CI) are presented for key demographic, psychosocial, and behavioral factors. The Taylor series linearization method was used to estimate standard errors of estimates, from which Wald confidence limits were derived. The Rao-Scott Chi-square test was used to test for the association between prevalence and socio-demographic, behavioral, and clinical characteristics.
Result
Demographic characteristics of the study participants
Among the 9791 participants tested for HBV infection, 3541 (36.2%) were men (median age 27 years, interquartile range (IQR) 21–35 years) and 6250 (63.8%) were women (median age 28 years, IQR 21–37 years). The majority of participants were single (92.1% of men and 85.8% of women), reported having one to five lifetime sexual partners (39.0% men and 51.3% women), and lived in rural areas (57.3% men and 58.7% women). In addition, the prevalence of HIV (28.0% men and 44.0% women) and sexually transmitted infections (STIs) (52.3% men and 78.3% women) was high. Over a third of participants had an incomplete secondary education (38.1% of men and 41.3% of women) and earned a monthly income of between 501 and 2500 South African Rand (ZAR) (44.5% men and 46.3% women). Overall, consistent condom use during sex in the last 12 months was low (Table 1).
Table 1.
Socio-demographic, behavioral, and clinical characteristics of participants 15–49 years old, enrolled between June 2014 and June 2015 in rural and peri-urban areas in KwaZulu-Natal, South Africa.
| Men (n = 3541) | Women (n = 6250) | |
|---|---|---|
| Age, years, median (IQR) | 27 (21–35) | 28 (21–37) |
| Age groups in years, number in sample (weighted %) | ||
| 15–19 | 657 (19.6) | 956 (18.1) |
| 20–24 | 813 (20.9) | 1262 (19.5) |
| 25–29 | 602 (18.3) | 1085 (17.9) |
| 30–34 | 459 (13.9) | 831 (13.7) |
| 35–39 | 404 (12.3) | 757 (12.2) |
| 40–44 | 319 (8.6) | 660 (9.6) |
| 45–49 | 287 (6.5) | 699 (8.9) |
| Relationship status, number in samples (weighted %) | ||
| Single (includes divorced, separated, widowed) | 3300 (92.1) | 5288 (85.8) |
| Married | 180 (5.9) | 681 (11.7) |
| Living with someone | 61 (2.0) | 175 (2.5) |
| Education, number in samples (weighted %)a | ||
| Incomplete secondary or less | 1929 (56.5) | 3306 (53.4) |
| Complete secondary | 1403 (38.1) | 2597 (41.3) |
| Tertiary | 207 (5.4) | 344 (5.3) |
| Income per household per month, number in samples (weighted %)b | ||
| No income | 523 (13.2) | 764 (10.4) |
| <ZAR 500 | 293 (6.2) | 610 (7.3) |
| ZAR 501–2500 | 1434 (44.5) | 2713 (46.3) |
| ZAR 2501–6000 | 914 (35.5) | 1558 (34.6) |
| >ZAR 6000 | 30 (0.9) | 70 (1.5) |
| Geographic location, number in samples (weighted %) | ||
| Peri-urban | 2296 (42.8) | 4047 (41.3) |
| Rural | 1245 (57.3) | 2203 (58.7) |
| Lifetime number of sexual partners, number in samples (weighted %)c | ||
| 0 partners | 691 (21.2) | 816 (15.6) |
| 1 partner | 420 (13.8) | 1543 (28.9) |
| 1–5 partners | 1196 (39.0) | 2848 (51.3) |
| >5 partners | 758 (26.1) | 280 (4.3) |
| HIV status, number in samples (weighted %) | ||
| Negative | 2531 (72.1) | 3305 (56.0) |
| Positive | 1010 (28.0) | 2945 (44.0) |
| Self-reported to be on ART (HIV-positive only), number in samples (weighted %) | ||
| No | 669 (63.1) | 1695 (54.3) |
| Yes | 341 (36.9) | 1250 (45.7) |
| STId present, number in samples (weighted %) | ||
| No | 1689 (47.7) | 1354 (21.7) |
| Yes | 1852 (52.3) | 4896 (78.3) |
| Condom use during sex in the last 12 months for those sexually active in the last 12 months, number in samples (weighted %) | ||
| Always | 593 (25.2) | 992 (22.8) |
| Sometimes | 1290 (54.9) | 2317 (53.4) |
| Never | 468 (19.9) | 1032 (23.8) |
%, population weighted percentage; IQR, interquartile range, ZAR, South African Rand; ART, antiretroviral therapy; STI, sexually transmitted infection.
Two men and three women were missing education data.
Income data were missing for 347 men and 535 women.
A total of 476 men and 763 women refused to provide their number of lifetime sex partners.
Any laboratory diagnosis of Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis, and/or Mycoplasma genitalium DNA from self-collected swabs (women) and first-pass urine (men) samples and antibodies to herpes simplex virus type 2 and Treponema pallidum (syphilis).
Seroprevalence of HBsAg
Among the 9791 participants, 361 were HBsAg-positive, with an overall weighted seroprevalence of 4.0% (95% CI 3.4–4.5%) (Table 2). Seroprevalence in women was 3.2% (95% CI 2.5–3.9%) and was higher in men at 4.8% (95% CI 3.8–5.8%) (p = 0.01). Seroprevalence varied with age among men (p < 0.01) and women (p < 0.01). Seroprevalence was 1.1% (95% CI 0–2.3%) among men in the 15–19 years age group and increased steadily to reach a peak of 9.6% (95% CI 5.0–14.1%) in the 40–44 years age group. In contrast, seroprevalence was consistently lower in each of the age groups among women and was 0.9% (95% CI 0–1.8%) among those 15–19 years old and peaked at 6.0% (95% CI 3.1–9.0%) in the 30–34 years age group. Seroprevalence increased with an increasing number of lifetime sex partners in women (p < 0.01), with HIV-positive status among men (p < 0.01) and women (p < 0.01), and with positive STIs (other than HIV) among men (p < 0.01). There was no difference in seroprevalence among rural and peri-urban men (p = 0.69) or between rural and peri- urban women (p = 0.92).
Table 2.
Seroprevalence of HBsAg and association with socio-demographic, behavioral, and clinical characteristics of 9791 participants (15–49 years of age), enrolled between June 2014 and June 2015 in rural and peri-urban KwaZulu-Natal, South Africa.
| Variable | Men | Women | ||||
|---|---|---|---|---|---|---|
| n/N | HBsAg seroprevalence Weighted % (95% CI) | n/N | HBsAg seroprevalence Weighted % (95% CI) | |||
| Seroprevalence of HBsAg | ||||||
| Overall | 165/3541 | 4.8 | (3.8–5.8) | 196/6250 | 3.2 | (2.5–3.9) |
| By age group in years | ||||||
| 15–19 | 7/657 | 1.1 | (0–2.3) | 8/956 | 0.9 | (0–1.8) |
| 20–24 | 26/813 | 3.6 | (1.7–5.4) | 32/1262 | 2.7 | (1.5–3.8) |
| 25–29 | 30/602 | 4.5 | (2.4–6.6) | 39/1085 | 3.8 | (2.2–5.4) |
| 30–34 | 40/459 | 7.8 | (4.5–11) | 40/831 | 6.0 | (3.1–9) |
| 35–39 | 23/404 | 5.6 | (3.1–8.1) | 27/757 | 2.9 | (1.4–4.5) |
| 40–44 | 24/319 | 9.6 | (5–14.1) | 26/660 | 4.1 | (1.9–6.3) |
| 45–49 | 15/287 | 6.6 | (2.3–10.8) | 24/699 | 2.5 | (1.3–3.8) |
| p <0.01a | p <0.01a | |||||
| Relationship status | ||||||
| Single (includes divorced, separated, widowed) | 150/330 | 4.7 | (3.7–5.7) | 173/5394 | 3.1 | (2.4–3.9) |
| Married | 12/180 | 5.7 | (2.3–9.2) | 16/681 | 2.9 | (1.1–4.7) |
| Living with someone | 3/61 | 8.6 | (0–20.3) | 7/175 | 4.3 | (0.6–8.1) |
| Other (divorced, separated, widowed) | 0/13 | 0 | (0–0) | 5/106 | 5 | (0–10.4) |
| p = 0.52a | p = 0.79a | |||||
| Educationb | ||||||
| Incomplete secondary or less | 96/1929 | 5.3 | (4–6.7) | 116/3306 | 3.3 | (2.6–4.0) |
| Complete secondary | 60/1403 | 4.1 | (2.6–5.6) | 75/2597 | 3.3 | (2.1–4.5) |
| Tertiary | 9/207 | 4.3 | (0.4–8.3) | 5/344 | 1 | (0–2.1) |
| p = 0.49a | p = 0.16a | |||||
| Income per household per monthc,d | ||||||
| No income | 28/523 | 5.5 | (2.9–8.1) | 23/764 | 3 | (1.2–4.8) |
| <ZAR 500 | 19/293 | 5.5 | (2.6–8.3) | 24/610 | 4.5 | (2.2–6.8) |
| ZAR 501–2500 | 66/1434 | 4.9 | (3.2–6.5) | 96/2713 | 3.3 | (2.5–4.1) |
| ZAR 2501–6000 | 37/914 | 4.2 | (2.6–5.9) | 39/1558 | 2.8 | (1.3–4.3) |
| >ZAR 6000 | 1/30 | 5.4 | (0–15.9) | 3/70 | 7 | (0–15.5) |
| p = 0.91a | p = 0.48a | |||||
| Geographic location | ||||||
| Peri-urban | 104/2296 | 4.6 | (3.5–5.7) | 133/4047 | 3.2 | (2.6–3.8) |
| Rural | 61/1245 | 5.0 | (3.4–6.5) | 63/2203 | 3.1 | (2–4.2) |
| p = 0.69a | p = 0.92a | |||||
| Lifetime number of sexual partnerse | ||||||
| 0 partners | 16/691 | 3.2 | (1.1–5.2) | 10/816 | 1.7 | (0.3–3.1) |
| 1 partner | 18/420 | 3.4 | (1.6–5.2) | 30/1543 | 1.9 | (1–2.9) |
| 1–5 partners | 64/1196 | 5.2 | (3.4–7.1) | 103/2848 | 3.7 | (2.7–4.8) |
| >5 partners | 28/758 | 4.5 | (2.4–6.6) | 17/280 | 6.8 | (2.8–10.9) |
| p = 0.41a | p <0.01a | |||||
| HIV status | ||||||
| Negative | 74/2531 | 3.3 | (2.3–4.3) | 58/3305 | 1.7 | (1.1–2.3) |
| Positive | 91/1010 | 8.7 | (6.3–11.2) | 138/2945 | 5.0 | (3.8–6.2) |
| p <0.01a | p <0.01a | |||||
| Self-reported to be on ART (HIV-positive only) | ||||||
| No | 57/669 | 6.9 | (4.5–9.4) | 78/1695 | 4.9 | (3.5–6.4) |
| Yes | 34/341 | 11.9 | (6.8–16.9) | 60/1250 | 5.2 | (3.6–6.8) |
| p = 0.05a | p = 0.78a | |||||
| STIf present | ||||||
| No | 49/1689 | 2.8 | (1.7–3.8) | 20/1354 | 1.9 | (0.7–3.1) |
| Yes | 116/1852 | 6.7 | (5.2–8.3) | 176/4896 | 3.6 | (2.8–4.4) |
| p <0.01a | p = 0.06a | |||||
| Condom use in the last 12 months (sexually active in last 12 months only) | ||||||
| Always | 24/593 | 3.6 | (1.9–5.4) | 34/992 | 2.9 | (1.5–4.3) |
| Sometimes | 66/1290 | 5.3 | (3.5–7.1) | 73/2317 | 3.7 | (2.3–5.1) |
| Never | 27/468 | 6.1 | (3.1–9.0) | 35/1032 | 3.5 | (2.1–4.9) |
| p = 0.33a | p = 0.72a | |||||
HBsAg, hepatitis B surface antigen; CI, confidence interval; %, population weighted percentages; ZAR, South African Rand; ART, antiretroviral therapy; STI, sexually transmitted infection.
p-Value for the association of the variable with HBsAg status.
Data on education missing for two men and three women.
ZAR 20 approximately equal to GBP 1.
Income data were missing for 347 men and 535 women.
A total of 476 men and 763 women refused to provide their number of lifetime sex partners.
Any laboratory diagnosis of Neisseria gonorrhoeae, Chlamydia trachomatis, Trichomonas vaginalis and/or Mycoplasma genitalium DNA from self-collected swabs (women) and first-pass urine (men) samples and antibodies to herpes simplex virus type 2 and Treponema pallidum (syphilis).
Seroprevalence of HBeAg
Of the 361 participants with HBsAg-positive samples, 357 had sufficient sample remaining for HBeAg testing. Of these, 133 were HBeAg-positive, with an overall weighted seroprevalence of 35.2% (95% CI 29.2–41.2%) (Table 3). HBeAg seroprevalence in men was 36.9% (95% CI 27.5–46.2%) and in women was 32.8% (95% CI 32.3–41.9%) (p = 0.58). Among men 15–19 years old, HBeAg seroprevalence was 92.2% (95% CI 75.8–100%) compared to 4.4% (95% CI 0–13.7%) in women in the same age group (p < 0.01). Similarly, HBeAg seroprevalence was higher among men aged 40–44 years (55.5%, 95% CI 30.6–80.4%) than among women in the same group (27.7%, 95% CI 10.4–45.0% p = 0.05). However, seroprevalence of HBeAg among men and women across the other age groups was similar. There was no difference in seroprevalence of HBeAg among men in rural (34.0%, 95% CI 24.0–44.0%) and peri-urban areas (38.9%, 95% CI 24.6–53.2%) (p = 0.58), or between women in rural (36.7%, 95% CI 26.9–45.9%) and peri-urban areas (30.0%, 95% CI 16.1–43.9%) (p = 0.43).
Table 3.
Seroprevalence of hepatitis B virus markers (HBsAg, HBeAg, and anti-HBe) among 9791 participants (15–49 years of age), enrolled between June 2014 and June 2015, in rural and peri-urban areas in KwaZulu-Natal, South Africa.
| Variable | Men | Women | p-Valuea | ||||
|---|---|---|---|---|---|---|---|
| n/N | Weighted % (95% CI) | n/N | Weighted % (95% CI) | ||||
| Seroprevalence of HBsAg | |||||||
| Overall | 165/3541 | 4.8 | (3.8–5.8) | 196/6250 | 3.2 | (2.5–3.9) | 0.01 |
| By age group in years | |||||||
| 15–19 | 7/657 | 1.1 | (0–2.3) | 8/956 | 0.9 | (0–1.8) | 0.66 |
| 20–24 | 26/813 | 3.6 | (1.7–5.4) | 32/1262 | 2.7 | (1.5–3.8) | 0.40 |
| 25–29 | 30/602 | 4.5 | (2.4–6.6) | 39/1085 | 3.8 | (2.2–5.4) | 0.59 |
| 30–34 | 40/459 | 7.8 | (4.5–11) | 40/831 | 6.0 | (3.1–9.0) | 0.47 |
| 35–39 | 23/404 | 5.6 | (3.1–8.1) | 27/757 | 2.9 | (1.4–4.5) | 0.08 |
| 40–44 | 24/319 | 9.6 | (5–14.1) | 26/660 | 4.1 | (1.9–6.3) | <0.01 |
| 45–49 | 15/287 | 6.6 | (2.3–10.8) | 24/699 | 2.5 | (1.3–3.8) | 0.02 |
| p <0.01b | p <0.01b | ||||||
| Seroprevalence of HBeAgc,d | |||||||
| Overall | 65/164 | 36.9 | (27.5–46.2) | 68/193 | 32.8 | (23.7–41.9) | 0.58 |
| By age group in years | |||||||
| 15–19 | 6/7 | 92.2 | (75.8–100) | 1/8 | 4.4 | (0–13.7) | <0.01 |
| 20–24 | 10/26 | 43.3 | (18.3–68.4) | 17/32 | 56.7 | (32.6–80.8) | 0.45 |
| 25–29 | 11/30 | 29.8 | (11.1–48.5) | 12/39 | 30.2 | (9.6–50.7) | 0.98 |
| 30–34 | 15/39 | 24.8 | (9.4–40.1) | 14/39 | 31.0 | (11.5–50.5) | 0.62 |
| 35–39 | 7/23 | 24.7 | (6.2–43.1) | 8/27 | 24.6 | (7.0–42.3) | 1.00 |
| 40–44 | 11/24 | 55.5 | (30.6–80.4) | 9/25 | 27.7 | (10.4–45.0) | 0.05 |
| 45–49 | 5/15 | 23.3 | (0.7–45.9) | 7/23 | 34.0 | (12.4–55.5) | 0.51 |
| p = 0.09b | p = 0.18b | ||||||
| Seroprevalence of anti-HBec,e | |||||||
| Overall | 104/163 | 64.5 | (54.7–74.3) | 133/193 | 68.8 | (59.3–78.3) | 0.51 |
| By age group in years | |||||||
| 15–19 | 1/7 | 7.8 | (0–24.2) | 7/8 | 81.0 | (45.5–100) | <0.01 |
| 20–24 | 17/26 | 64.7 | (40.3–89.2) | 20/32 | 65.9 | (44.1–87.8) | 0.94 |
| 25–29 | 20/30 | 69.7 | (50.5–88.8) | 27/39 | 62.9 | (42.9–82.9) | 0.63 |
| 30–34 | 23/39 | 70.0 | (52–87.9) | 25/39 | 65.4 | (40.2–90.6) | 0.77 |
| 35–39 | 18/22 | 83.0 | (66.4–99.5) | 20/27 | 78.5 | (60.0–97.1) | 0.72 |
| 40–44 | 15/24 | 47.1 | (21.6–72.6) | 17/25 | 76.8 | (60.9–92.7) | 0.03 |
| 45–49 | 10/15 | 75.2 | (51.5–98.8) | 17/23 | 68.3 | (46.7–90.0) | 0.68 |
| p = 0.11b | p = 0.91b | ||||||
HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; anti-HBe, antibody to hepatitis B e antigen; %, population weighted percentages; CI, confidence interval.
p-Value for the association of HBsAg/HBeAg/anti-HBe status and sex by age category.
p-Value for the association of age with HBsAg/HBeAg/anti-HBe status by sex.
Based on HBsAg-positive samples.
Four samples insufficient for HBeAg testing.
Five samples insufficient for anti-HBe testing.
Seroprevalence of anti-HBe
Of the 361 participants with HBsAg-positive samples, 356 had sufficient remaining sample for anti-HBe testing. Of these, 237 samples were positive for anti-HBe, with an overall weighted seroprevalence of 66.3% (95% CI 60.1–72.4%) (Table 3). Seroprevalence in men was 64.5% (95% CI 54.7–74.3%) and was similar in women at 68.8% (95% CI 59.3–78.3%) (p = 0.51). Anti-HBe seroprevalence was high among men and women across most age groups. More women than men in the age group 15–19 years were anti-HBe-positive (81.0%, 95% CI 45.5–100% vs. 7.8%, 95% CI 0–24.2%) (p < 0.01). Similarly, among women in the age group 40–44 years, seroprevalence was 76.8% (95% CI 60.9–92.7%) compared to 47.1% (95% CI 21.6–72.6%) in men in the same age group (p = 0.03). There was no difference in seroprevalence of anti-HBe between men in rural areas (61.4%, 95% CI 46.7–76.1%) and peri-urban areas (68.9%, 95% CI 57.4–80.3%) (p = 0.43) or between women in rural areas (69.3%, 95% CI 54.4–84.2%) and peri-urban areas (68.1%, 95% CI 58.6–77.6%) (p = 0.89).
HBV-HIV co-infection
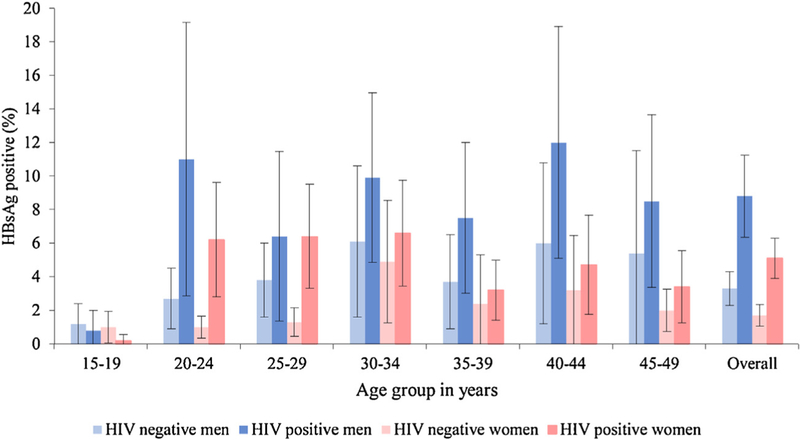
Of the 3955 HIV-positive participants, 229 were also infected with HBV, of whom 91 were men and 138 were women. HBV seroprevalence was 6.4% (95% CI 5.3–7.5%) among HIV-positive participants compared to 2.6% (95% CI 1.9–3.2%) among HIV-negative participants (n = 5836) (p < 0.01). Among HIV-positive men, seroprevalence of HBsAg was 8.8% (95% CI 6.3–11.2%) compared to 3.3% (95% CI 2.3–4.3%) among HIV-negative men (p < 0.01). Among HIV-positive women, seroprevalence of HBsAg was 5.1% (95% CI 3.8–6.3%) compared to 1.7% (95% CI 1–2.3%) among HIV-negative women (p < 0.01). The difference in HBsAg prevalence among HIV-positive and negative individuals was greatest in age groups 20–24 years and 25–29 years. Overall, seroprevalence of HBsAg was higher among HIV-positive participants in the 20–24 years and 25–29 years age groups (p < 0.01) (Figure 1). Among HIV-positive men in the age group 20–24 years, HBsAg seroprevalence was 11% (95% CI 2.8–19.2%) compared to 2.7% (95% CI 1.0–4.5%) among HIV-negative men in the same age group (p < 0.01). Among HIV-positive women in the age group 20–24 years, HBsAg seroprevalence was 6.2% (95% CI 2.8–9.7%) compared to 1.0% (95% CI 0.3–1.6%) among HIV-negative women in the same age group (p < 0.01). Similarly, among HIV-positive women in the 25–29 years age group, HBsAg seroprevalence was 6.4% (95% CI 3.3–9.5%) compared to 1.3% (95% CI 0.4–2.1%) among HIV-negative women in the same age group (p < 0.01).
Figure 1.
Seroprevalence of HBV (HBsAg)-HIV co-infection by sex and age group.
Discussion
In this population-based survey in rural and peri-urban areas of KwaZulu-Natal, South Africa, the overall HBsAg seroprevalence among men and women in the age group 15–49 years was 4.0%, with a seroprevalence of 4.8% in men and 3.2% in women. While lower than the HBsAg seroprevalence of 6.1% reported for Sub-Saharan Africa (Spearman et al., 2017), and between 5.3% and 9.7% in recent cohort studies among pregnant women (Thumbiran et al., 2014; Matthews et al., 2015), this still represents a substantial HBV burden. In fact, worldwide, despite the availability of hepatitis B vaccine, the overall prevalence of HBV infection has declined little in recent years (Ocama et al., 2005; O’Hara et al., 2017). South Africa has been reported to be at the forefront of the implementation of the Expanded Programme on Immunization, which includes HBV immunization (Dlamini and Maja, 2016); however, the prevalence of HBV infection remains moderate to high, especially in the age group 15–19 years, as this age group should have benefitted from the immunization programme. In the absence of an active HBV surveillance programme, it is unlikely that HBV immunization and treatment programmes are easily accessible and reach individuals where the need is the greatest. These findings emphasize that HBV remains an important public health problem and all efforts to achieve the WHO targets for the elimination of viral hepatitis are urgently needed (World Health Organisation, 2016). In 2016, the WHO set ambitious targets of a 90% reduction in new cases of HBV infection and an associated 65% decline in HBV-related mortality to be achieved by the year 2030. These measures rely on 80% of treatment-eligible individuals with chronic HBV infection to be on treatment (World Health Organisation, 2016).
Whilst vertical transmission of HBV is the most common route of transmission, the age-specific HBV data highlight several important findings. Firstly, men had higher seroprevalence compared to women in every age group. Secondly, the data showed a bimodal distribution of seroprevalence of HBsAg among men in the 30–34 years and 40–44 years age groups, whilst in women, peak seroprevalence occurred in the 30–34 years age group. These findings show that the peak prevalence occurred among men and women in the age groups that are likely to sustain the transmission of HBV. Thirdly, overall estimates are useful to understand the burden of HBV infection in the general population; however, disaggregation by sex and age provides a more precise understanding of the age group that might be affected and allows for prioritization and targeted interventions to further reduce this burden. Several studies have shown urban-rural differences, with the highest prevalence of HBsAg found in men residing in rural areas, placing a greater burden on rural healthcare services (Vos et al., 1980; Abdool Karim et al., 1988; Abdool Karim et al., 1991; Lodenyo et al., 2000; Hoffmann et al., 2008; Firnhaber et al., 2008; Di Bisceglie et al., 2010). However, this study found no difference in the seroprevalence of HBsAg among men and women, irrespective of their area of residence being rural or peri-urban. It is possible that extensive in- and out-migration in the study community and cultural practices of scarification (Abdool Karim et al., 1988) may not clearly delineate urban-rural differences.
This study found that at least a third of men (36.9%) and women (32.6%) were HBeAg-positive and therefore more likely to be infectious. These prevalence values are higher than the 16.7–30.0% observed in cohorts of pregnant women in South Africa (Thumbiran et al., 2014). Whilst men in the 15–19 years age group and women in the 20–24 years age group had low seroprevalence of HBsAg, more than 90% of these men and about 60% of these women were positive for HBeAg, suggesting that these individuals are likely to have a high viral load (Spearman et al., 2017) and are therefore highly infectious. The high prevalence of HBeAg among both men and women highlights the sustained transmission in this community. Reaching out to men and women at high risk of transmitting HBV with messaging on immunization and treatment options, could further guide and scale-up targeted programming and strategies to further reduce the spread of HBV and the opportunity for a catch-up immunization programme targeting young boys (Stabinski et al., 2011).
An overall seroprevalence of HBsAg of 6.4% among HIV-positive individuals was observed, with a higher seroprevalence amongst HIV-positive men (8.8%) compared to HIV-positive women (5.1%). Since, HBV and HIV share similar routes of transmission, co-infections are likely to be common and are reported to range from 5% to 30% (Abdool Karim et al., 1991). Although co-infections were low at 0.8% in the 15–19 years age group, co-infections were high in all other age groups, ranging from 6.4% to 11.9%. Identifying co-infections is important, as HBV infections are associated with an increased risk of liver disease. Antiretroviral therapy (ART) use in HIV-positive individuals may be compromised by drug-associated hepatotoxicity, leading to the use of complex regimens (Di Bisceglie et al., 2010). In addition, this study showed that among women, HBV-HIV co-infections were above 6% in the age groups belonging to the reproductive years and those already disproportionately burdened with HIV (Kharsany et al., 2018). This could potentially increase the risk of HBV and HIV transmission. Expanding treatment for HBV and HIV-positive individuals could limit onward transmission of both infections and reduce the risk of mother-to-child transmission of HBV (Di Bisceglie et al., 2010; Seremba et al., 2017). Even initiating ART in the absence of HBV reduces the risk of new HBV cases. Therefore, public sector ART and antenatal care programmes are missed opportunities to screen for HBV at the time of testing for HIV and when initiating ART. These individuals would benefit from immunization if HBV-negative or from treatment to prevent the onward and mother-to-child transmission of HBV if positive (Cui et al., 2018).
This study has several strengths and limitations. A key strength is the measurement of HBV seroprevalence in a large randomly selected study sample from a population-based household survey. Furthermore, the sex and age disaggregated data provide a nuanced understanding of the segments of the population that might be affected, although the sample was not representative of all age groups. The results are from cross-sectional data and are limited to the Greater Edendale and Vulindlela areas of KwaZulu-Natal, South Africa and are not necessarily generalizable to other settings. It is important to note that this study was not able to fully assess the impact of the HBV immunization programme on the seroprevalence of HBV markers, as the benefit would be in the <15 years age groups rather than those included in the sample (Tsebe et al., 2001). The testing strategy did not include the full HBV testing panel to comprehensively classify individuals; for example, no testing was performed for occult HBV infection, HBV viral load, or for hepatitis B core antibody (anti-HBc) or hepatitis B surface antibody (anti-HBs) to determine recovery and/or immunity from HBV infection. Lastly, the prevalence of HBeAg may be unreliable due to the small sample sizes in the age-specific strata. Nevertheless, the HBeAg data provide a reasonable signal of the sustained HBV transmission in this community.
In conclusion, the study findings show that HBV infection is an important public health problem. Men across all age groups and women in the age groups 20–24 years and 25–29 years had the highest seroprevalence of HBsAg. Furthermore, more than a third of these men and women were HBeAg-positive, underscoring the risk of sustained onward HBV transmission in this community. Ensuring that HIV-positive individuals with HBV infection are initiated on treatment to reduce viral loads of both HIV and HBV is essential. These findings highlight the importance of surveillance and an important missed opportunity for the scale-up of programmes to achieve the overall goal of controlling HBV for public health benefit.
Acknowledgements
Our sincere thanks to all household members and individual study participants for their participation in the HIV Incidence Provincial Surveillance System - the population-based household-based survey to understand the evolving HIV epidemic in the region. We sincerely acknowledge the ongoing support of the District Manager of the uMgungundlovu Health District, members of the Provincial Department of Health, members of the uMgungundlovu District municipality, Provincial Health Research and Knowledge Management, local traditional leadership, and community members for their support throughout the study. Special thanks to the study staff for the field work, as well as the laboratory and primary healthcare clinic staff in the district.
Role of the funding source
The funders of the survey contributed to the primary survey design and study monitoring, and reviewed the manuscript. ABMK, NS, SN, and LL had full access to all data. ABMK, NS, SN, and CC had final responsibility for the decision to submit for publication.
Study sponsorship and funding statement
This work was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of operative agreement (grant number 3U2GGH000372–02W1); the Joint South Africa-US Program for Collaborative Biomedical Research from the National Institutes of Health (grant number R01HD083343 to ABMK); and the South African Department of Science and Technology and the National Research Foundation’s Centre of Excellence in HIV Prevention (grant number UID: 96354). The Centre for the AIDS Program of Research in South Africa (CAPRISA) provided research infrastructure support.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
Conflict of interest
All authors declare no conflict of interest.
References
- Abdool Karim SS, Coovadia HM, Windsor IM, Thejpal R, van den Ende J, Fouche A. The prevalence and transmission of hepatitis B virus infection in urban, rural and institutionalized black children of Natal/KwaZulu, South Africa. Int J Epidemiol 1988;17(March (1)):168–73. [DOI] [PubMed] [Google Scholar]
- Abdool Karim SS, Thejpal R, Coovadia HM. Household clustering and intra-household transmission patterns of hepatitis B virus infection in South Africa. Int J Epidemiol 1991;20(June (2)):495–503. [DOI] [PubMed] [Google Scholar]
- Botha jF, Dusheiko GM, Ritchie MJJ, Mouton HWK, Kew MC. Hepatitis-B virus carrier state in black-children in Ovamboland — role of perinatal and horizontal infection. Lancet 1984;1(8388):1210–2. [DOI] [PubMed] [Google Scholar]
- Burnett RJ, Francois G, Kew MC, et al. Hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver Int 2005;25(April (2)):201–13. [DOI] [PubMed] [Google Scholar]
- Cui F, Woodring J, Chan P, Xu F. Considerations of antiviral treatment to interrupt mother-to-child transmission of hepatitis B virus in China. Int J Epidemiol 2018;47(5):1529–37. [DOI] [PubMed] [Google Scholar]
- Di Bisceglie AM, Maskew M, Schulze D, Reyneke A, McNamara L, Firnhaber C. HIV-HBV coinfection among South African patients receiving antiretroviral therapy. Antivir Ther 2010;15(3 Pt B):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibisceglie AM, Kew MC, Dusheiko GM, et al. Prevalence of hepatitis B virus infection among black children in Soweto. Br Med J 1986;292(May (6533)):1440–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlamini NR, Maja P. The expanded programme on immunisation in South Africa: a story yet to be told. S Afr Med J 2016;106(June):675–7. [DOI] [PubMed] [Google Scholar]
- Dusheiko GM, Conradie JD, Brink BA, Marimuthu T, Sher R. Differences in the regional prevalence of chronic hepatitis B in southern Africa—implications for vaccination. S Afr Med J 1989;75(May (10)):473–8. [PubMed] [Google Scholar]
- Firnhaber C, Reyneke A, Schulze D, et al. The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J 2008;98(July (7)):541–4. [PMC free article] [PubMed] [Google Scholar]
- Heiberg IL, Hoegh M, Ladelund S, Niesters HG, Hogh B. Hepatitis B virus DNA in saliva from children with chronic hepatitis B infection: implications for saliva as a potential mode of horizontal transmission. Pediatr Infect Dis J 2010;29(May (5)):465–7. [DOI] [PubMed] [Google Scholar]
- Hoffmann CJ, Charalambous S, Martin DJ, et al. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis 2008;47(December (11)):1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew MC. Progress towards the comprehensive control of hepatitis B in Africa: a view from South Africa. Gut 1996;38(Suppl. 2):S31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew MC. Hepatitis B virus infection: the burden of disease in South Africa. South Afr J Epidemiol Infect 2008;23(1):4–8. [Google Scholar]
- Kew MC, MacKay ME, Mindel A, et al. Prevalence of hepatitis B surface antigen and antibody in white and black patients with diabetes mellitus. J Clin Microbiol 1976;4(December (6)):467–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew MC, Kassianides C, Berger EL, Song E, Dusheiko GM. Prevalence of chronic hepatitis B virus infection in pregnant black women living in Soweto. J Med Virol 1987;22(July (3)):263–8. [DOI] [PubMed] [Google Scholar]
- Kharsany AB, Cawood C, Khanyile D, et al. Strengthening HIV surveillance in the antiretroviral therapy era: rationale and design of a longitudinal study to monitor HIV prevalence and incidence in the uMgungundlovu District, KwaZulu-Natal, South Africa. BMC Public Health 2015;15:1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharsany ABM, Cawood C, Khanyile D, et al. Community-based HIV prevalence in KwaZulu-Natal, South Africa: results of a cross-sectional household survey. Lancet HIV 2018;5(August (8)):e427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiire CF. The epidemiology and prophylaxis of hepatitis B in sub-Saharan Africa: a view from tropical and subtropical Africa. Gut 1996;38(2):S5–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodenyo H, Schoub B, Ally R, Kairu S, Segal I. Hepatitis B and C virus infections and liver function in AIDS patients at Chris Hani Baragwanath Hospital, Johannesburg. East Afr Med J 2000;77(January (1)):13–5. [DOI] [PubMed] [Google Scholar]
- Matthews PC, Beloukas A, Malik A, et al. Prevalence and characteristics of hepatitis B virus (HBV) coinfection among HIV-positive women in South Africa and Botswana. PLoS One 2015; 10(7)e0134037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayaphi SH, Roussow TM, Masemola DP, Olorunju SA, Mphahlele MJ, Martin DJ. HBV/HIV co-infection: the dynamics of HBV in South African patients with AIDS. S Afr Med J 2012;102(February):157–62. [DOI] [PubMed] [Google Scholar]
- Mphahlele MJ, Francois G, Kew MC, Van Damme P, Hoosen AA, Meheus A. Epidemiology and control of hepatitis B: implications for eastern and southern Africa. S Afr J Epidemiol Infect 2002;17(1.2):12–7. [Google Scholar]
- North Carolina Hepatitis B Public Health Program Manual. Hepatitis B Serology. 2012. … [Accessed 14 July 2018] https://epi.publichealth.nc.gov/cd/lhds/manuals/hepB/docs/hbv_serology.pdf.
- O’Hara GA, McNaughton AL, Maponga T, et al. Hepatitis B virus infection as a neglected tropical disease. PLoS Negl Trop Dis 2017;11(October (10))e0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocama P, Opio CK, Lee WM. Hepatitis B virus infection: current status. Am J Med 2005;118(December (12)):1413. [DOI] [PubMed] [Google Scholar]
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45(October (4)):529–38. [DOI] [PubMed] [Google Scholar]
- Prozesky OW, Szmuness W, Stevens CE, et al. Baseline epidemiological studies for a hepatitis B vaccine trial in Kangwane. S Afr Med J 1983;64(November (23)):891–3. [PubMed] [Google Scholar]
- Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(October (10003)):1546–55. [DOI] [PubMed] [Google Scholar]
- Seremba E, Ssempijja V, Kalibbala S, et al. Hepatitis B incidence and prevention with antiretroviral therapy among HIV-positive individuals in Uganda. AIDS 2017;31 (March (6)):781–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman CW, Afihene M, Ally R, et al. Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets. Lancet Gastroenterol Hepatol 2017;2 (December (12)):900–9. [DOI] [PubMed] [Google Scholar]
- Stabinski L, Reynolds SJ, Ocama P, et al. Hepatitis B virus and sexual behavior in Rakai, Uganda. J Med Virol 2011;83(May (5)):796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thumbiran NV, Moodley D, Parboosing R, Moodley P. Hepatitis B and HIV co-infection in pregnant women: indication for routine antenatal hepatitis B virus screening in a high HIV prevalence setting. S Afr Med J 2014;104(April (4)):307–9. [DOI] [PubMed] [Google Scholar]
- Tsebe KV, Burnett RJ, Hlungwani NP, Sibara MM, Venter PA, Mphahlele MJ. The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine 2001;19(July (28–29)):3919–26. [DOI] [PubMed] [Google Scholar]
- van Helden J, Denoyel G, Karwowska S, et al. Performance of hepatitis B assays on the Bayer ADVIA Centaur Immunoassay System. Clin Lab 2004;50(1–2):63–73. [PubMed] [Google Scholar]
- van Helden J, Cornely C, Dati F, et al. Performance evaluation of the ADVIA Centaur anti-HBe and HBeAg assays. J Clin Virol 2008;43(2):169–75. [DOI] [PubMed] [Google Scholar]
- Vos GH, Rose EF, Marimuthu T. Hepatitis B antigen and antibodies in rural and urban Southern African blacks. S Afr Med J 1980;57(May (21)):868–70. [PubMed] [Google Scholar]
- World Health Organisation. Global health sector strategy on viral hepatitis 2016–2021. Geneva, Switzerland: World Health Organisation; 2016. [Accessed 16 July 2018] http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=161DC25381F15DFA568362716A5B4874?sequence=1. [Google Scholar]
- World Health Organisation. Global Hepatitis Report 2017. Geneva, Switzerland: World Health Organisation; 2017. [Accessed 21 July 2018] http://www.who.int/iris/handle/10665/255016. [Google Scholar]