Abstract
Apoptosis following hypoxic-ischemic injury to the brain plays a major role in neuronal cell death. The neonatal brain is more susceptible to injury as the cortical neurons are immature and there are lower levels of antioxidants. Slit2, an extracellular matrix protein, has been shown to be neuroprotective in various models of neurological diseases. However, there is no information about the role of Slit2 in neonatal hypoxia-ischemia. In this study, we evaluated the effect of Slit2 and its receptor Robo1 in a rat model with neonatal HIE. 10-day old rat pups were used to create the neonatal HIE model. The right common carotid artery was ligated followed by 2.5 h of hypoxia. Recombinant Slit2 was administered intranasally 1 h post HI, recombinant Robo1 was used as a decoy receptor and administered intranasally 1h before HI and srGAP1-siRNA was administered intracerebroventricularly 24 h before HI. Brain infarct area measurement, short-term and long-term neurological function tests, western blot, immunofluorescence staining, Fluoro-Jade C staining, Nissl staining and TUNEL staining were the assessments done following drug administration. Recombinant Slit2 administration reduced neuronal apoptosis and neurological deficits after neonatal HIE which were reversed by co-administration of recombinant Robo1 and srGAP1-siRNA administration. Recombinant Slit2 showed improved outcomes possibly via the robo1-srGAP1 pathway which mediated the inhibition of RhoA. In this study, the results suggest that Slit2 may help in attenuation of apoptosis and could be a therapeutic agent for treatment of neonatal hypoxic ischemic encephalopathy.
Keywords: Slit2, Robo1, srGap1, apoptosis, HI, RhoA
1. INTRODUCTION
Hypoxic-ischemic encephalopathy (HIE) is the injury to the brain when there is deprivation of oxygen supply because of asphyxia in neonates. It is a major cause of brain injury during the perinatal period which leads to neurological disabilities like mental retardation, cerebral palsy, seizures and in severe cases may lead to mortality (Arteni, Salgueiro et al. 2003). Cerebral ischemia is the fifth leading cause of death and perinatal HIE occurs in 1 to 3 per 1000 live births (Graham, Ruis et al. 2008, Bahjat, Alexander West et al. 2017). Permanent neurological deficits have a major impact on the quality of life of the affected infants. Currently only hypothermia is considered to be the best therapy for neonatal HIE, with which only 40 % survive with normal neurological development (Uria-Avellanal and Robertson 2014). Therefore, studies on the pathogenesis of HIE are needed to better understand the mechanism of injury to find other effective therapeutic agents (Dixon, Reis et al. 2015).
Apoptosis is known to play a major role in causing neuronal cell death following hypoxic-ischemic injury to the brain (Nakajima, Ishida et al. 2000). The immature cortical neurons of a neonatal brain are more susceptible to injury compared to the adult brain as the neonatal brain has low levels of antioxidants and a higher level of pro-apoptotic genes (McDonald, Behrens et al. 1997). Hypoxia ischemia leads to the activation of apoptotic pathways which can be extrinsic or intrinsic which then lead to caspases activation like caspase-3 which has a major role in cell death. Direct inhibition of caspases has shown to be protective in many rat models of hypoxic ischemic injury (Lorente, Martin et al. 2015).
Slit2 is a glycoprotein which was first identified as an axon guidance molecule in the central nervous system. The Slit proteins interact with their receptor Roundabout (Robo) by which they regulate neuronal migration, axon branching and neural development. Three Slit proteins (1,2 and 3) and four Robo receptors (1,2,3 and 4) have been identified in mammals (Sifringer, Bendix et al. 2012). The Slit/Robo signaling pathway has been studied in various rat models, it has been shown that Slit2 has a direct neuroprotective effect on neurons in the ischemic brain and also helps in upregulation of spinal cord neurons after injury to the spinal cord (Liu, Jiang et al. 2011). Slit2 has been shown to prevent neuronal death in mixed neuronal-glial cultures exposed to oxygen-glucose deprivation (OGD) hence indicating its ability to provide neuroprotection (Altay, McLaughlin et al. 2007, Zhao, Zhang et al. 2013). However, little is known about the role of Slit2 in inhibiting apoptosis in hypoxic-ischemic injury to the brain.
In previous studies, it has been shown that Slit-Robo GTPase activating protein 1 (srGAP1) is the downstream effector of the Slit-Robo interaction and downregulates RhoA (Ypsilanti, Zagar et al. 2010, Liang, Budnar et al. 2017). Recent research has shown that Rho GTPases play an important role in programmed cell death and high levels of RhoA lead to more apoptosis in cortical neurons (Nikolic, Gardner et al. 2013). Thus, srGAP1 may help downregulate RhoA which in turn can reduce apoptosis.
The objective of the present study was aimed to test the hypothesis that Slit2 can attenuate neuronal apoptosis, possibly via the Robo1/srGAP1 pathway following hypoxia-ischemia injury.
2. Materials and Methods
2.1. ANIMALS
All experiments done in this study have been approved by Institutional Animal Care and Use Committee of Loma Linda University and are in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All animal studies comply with the ARRIVE guidelines. In this study, unsexed Sprague–Dawley rat pups post-natal day (P) 5–6 were purchased from Envigo Labs (Livermore, CA) with their mothers which were housed in a humidity and temperature-controlled environment until they reached P10, with regular 12 h light/dark cycle. A total of 130 Rat pups regardless of gender weighing 14–20 g were subjected to either Sham surgery (n=18) or HI surgery (n=112). Among the 130 rats, 2 were excluded from the study because of death during or after hypoxia.
2.2. Experimental Design
The experiment was designed as follows.
2.2.1. Experiment 1
The time course expression of endogenous Slit2, Robo1, SrGap1 were characterized at 6 h, 12 h, 24 h and 72 h after hypoxia ischemia (HI). Rat pups were randomly divided into 5 groups (n=6/group): Sham, 6 h HI, 12 h HI, 24 h HI, 72 h HI. Brain samples of the right hemisphere were collected for Western blot analysis.
2.2.1. Experiment 2
The effects of exogenous recombinant Slit2 (r-Slit2) was evaluated at 24 h post HI. On the basis of the dose response effect of Slit2 in previous studies (Altay, McLaughlin et al. 2007, Prasad, Qamri et al. 2007, Sherchan, Huang et al. 2016) three doses of r-Slit2 (3µg/kg, 10 µg/kg, 30 µg/kg) (R and D Systems, Minneapolis, MN, USA) were chosen and tested. Rat pups were divided into 4 groups(n=6/group): Sham, HI + Vehicle (Phosphate-buffered saline PBS), HI + r-Slit2 (3 µg/kg), HI + r-Slit2 (10 µg/kg), HI + r-Slit2 (30 µg/kg). The best dose of recombinant Slit2 (10 µg/kg) was then chosen for this study. Recombinant Slit2 and Vehicle PBS was given intranasally 1 h after HI. 24 h post HI the infarct volume and short-term neurobehavioral tests: righting reflex, negative geotaxis and body weight were evaluated.
2.2.3. Experiment 3
The colocalization of Slit2 and Robo1 on neurons was characterized at 24 h post HI. Rat pups were divided into 3 groups (n=4/group): Sham, HI + Vehicle (Phosphate-buffered saline, PBS), HI +r-Slit2 (optimal dose).
2.2.4. Experiment 4
The effects of r-Slit2 treatment and in vivo knockdown of Robo1 and srGAP1 were evaluated on neuronal apoptosis by administering r-Slit2 intranasally 1 h post HI while Recombinant Robo1 (3µg/kg) (R and D Systems, Minneapolis, MN, USA) was given intranasally at 1h before HI and srGAP1 siRNA was administered intracerebroventricularly at 24 h before HI. Neuronal apoptosis was measured at 48 h post HI. Rat pups were randomly divided into 6 groups (n=6/group): Sham, HI + Vehicle (PBS), HI +r-Slit2 (optimal dose), HI + r-Slit2 (optimal dose) + r-Robo1, HI + r-Slit2 (optimal dose) + srGAP1 siRNA, HI + r-Slit2 (optimal dose) + scramble siRNA.
2.2.5. Experiment 5
The evaluation of the effects of exogenous r-Slit2 post treatment 4 weeks after HI was done. Rat pups were divided into 3 groups (n=8/group): Sham, HI + Vehicle (PBS), HI + r-Slit2 (optimal dose). The rat pups were given r-Slit2 (optimal dose) or Vehicle (PBS) intranasally at 1 h post HI. Long term neurobehavioral test: Foot-fault, Rotarod and Morris water maze were conducted after 4 weeks post HI. The pups were then sacrificed for brain weight and Nissl staining.
2.2.6. Experiment 6
To study the underlying mechanisms of r-Slit2-mediated neuroprotective effects, rat pups were randomly divided into 6 groups (n=6/group): Sham, HI + Vehicle (PBS), HI + r-Slit2, HI +r-Slit2 + r-Robo1, HI + r-Slit2 + srGAP1 siRNA, HI +r-Slit2 + scramble siRNA. Vehicle (PBS) and r-Slit2 (optimal dose) were injected intranasally 1 h after HI, r-Robo1 was given intranasally 1 h before HI. srGAP1 siRNA and scramble siRNA were given via intracerebroventricular injection at 24 h before HI. Short-term neurobehavioral tests, infarct volume and western blot were examined at 24 h post HI.
2.3. Neonatal Hypoxia-ischemia Brain Injury Rat Model
The standard neonatal Hypoxia-Ischemia model was used in this study (Rice, Vannucci et al. 1981).In a temperature-controlled induction chamber, P10 rat pups were anesthetized with 3% isoflurane and maintained at 2.5% during the surgery. After induction of anaesthesia, using standard sterile techniques the neck of the rat pups was draped and prepared (Hoffmann, Sheng et al. 2016). Then, on the right anterior neck a small midline neck incision was given, and the right common carotid artery was isolated from its surrounding structures and double ligated with 5.0 surgical silk. The artery was then severed between the ligations. Time taken per surgery was 4–9 minutes, after which the pups were given time to recover from anaesthesia for 1 h on temperature-controlled heating blankets. Pups were then placed in a 500 ml airtight jar and were exposed for 2.5 h to a gas mixture of 8% oxygen and 92% nitrogen which was delivered into the jar while the jar was partially submerged in 37 ℃ water bath. Then, the pups were returned to their mothers.
2.4. Drug administration
Intranasal administration of r-Slit2 (R and D Systems, Minneapolis, MN, USA) was done by placing the rat pups on their backs and three doses (3µg/kg,10µg/kg and 30µg/kg) were given. A total of 10 µl of r-Slit2 was given 1 h post HI within 10 min. The pups were then laid on their backs for an additional 5 mins to facilitate the absorption of the drug before being returned to their cage. Intranasal delivery is a non-invasive approach and is also clinically relevant (Doyle, Yang et al. 2008, Wolfe and Braude 2010).The large surface area of the nasal mucosa, helps in the rapid absorption of drugs which lead to a rapid onset of action as they avoid degradation in the gastrointestinal tract and first-pass metabolism in the liver. Furthermore, the intranasal mode of delivery of the drug also serves as a direct route to the brain. It has been shown that drugs administered via this method can be transported from the nose to brain along the olfactory and trigeminal nerve pathways (Pardeshi and Belgamwar 2013).The intranasal method of delivery circumvents the BBB and minimizes systemic exposure, since it is transported directly to the brain. It has also been shown that the brain uptake of intranasally delivered therapeutics is 5 times greater than that after intraperitoneal delivery (Chauhan and Chauhan 2015). We believe that intranasal administration exerts its effects near the site of injury in the brain, and can effectively provide neuroprotection after a hypoxic ischemic insult.
2.5. Intracerebroventricular injection
The rats were anesthetized with isoflurane and then placed in prone position and fixed in a stereotactic frame. A burr hole was made in the skull and a Hamilton syringe (10 µl, Hamilton Co, USA) was inserted through it into the right lateral ventricle (1.5 mm posterior, 1.5 mm lateral to the bregma and 1.7 mm deep from the surface). 24 h before HI, 2µl of srGap1 siRNA (300 pmol/µl, Life Technologies, Grand island, NY, USA) or scramble siRNA (Life Technologies, Grand island, NY, USA) was injected at the rate of 0.3 µl/min. The needle was then left in place for 10 min to prevent leakage and then slowly withdrawn over 5 min. The burr hole was then sealed with bone wax and the skin was sutured. Rats were returned to their cages for recovery.
2.6. Measurement of infarcted area
The animals were euthanized and then perfused transcardially with 15ml PBS followed by 15 ml of 10% formalin. Brains were removed and 5 coronal slices each measuring 2 mm in thickness were prepared. These slices were then kept for 5 min in 2% 2,3,5-triphenlytetrazolium chloride monohydrate (TTC) solution at room temperature. The slices were then washed with PBS and kept in 10% formaldehyde solution overnight. The brain slices were then imaged to measure the infarcted areas using ImageJ software. The infarcted and non-infarcted areas were outlined and measured for each slice with ImageJ and the areas was then calculated and the average was taken to represent the Percentage of Infarcted Area for that animal.
2.7. Neurological evaluation
2.7.1. Short-term Neurological evaluation
Righting reflex and Negative geotaxis tests we performed to evaluate the short-term neurological function at 24 h post HI. Righting reflex was done by placing the rat pups in supine position and then recording the time needed for the pups to flip to prone position. Negative geotaxis was done by placing the pups head downward on an inclined board (45˚) and then recording the time it took for the pups to turn to head upward position(Shi, Xu et al. 2017).
2.7.2. Long-term Neurological evaluation
Foot-fault, Rotarod and Morris water maze tests were performed at 4 weeks post HI to evaluate long term neurological function.
Foot-fault test: rats were placed on a horizontal grid floor (square size 20 × 40 cm with a mesh size of 4 cm2 ) which was elevated to 1 m above the ground. The animals were placed on the grid and allowed to move on it for 1 min, each time the animal would place a forelimb or hindlimb, which fell between the grid bars it was recorded (Barth and Stanfield 1990, Bona, Johansson et al. 1997).
Rotarod test: this test was used to assess motor impairment by placing rats on an accelerating rotarod. The speed accelerated from 5 or 10 rpm for a maximum of 120 s (Doycheva, Hadley et al. 2014).
Morris water maze test: is a test that evaluates the ability of rats to learn and remember. The rats were required to find a hidden platform which was submerged in a pool of water using visual cues in the room (Lekic, Rolland et al. 2011). All the animals were part of both the cued and hidden tests. Each trial lasted for 60 s, after which if the animal could not find the platform they were manually guided to it. A computerized tracking system was used in each trial to record and trace all activities and swim paths were measured for the quantification of distance, latency and swim speed by the Video Tracking System SMART (San Diego Instruments Inc., CA).
2.8. Western Blot
Western blot was performed as described previously (Ostrowski, Colohan et al. 2005). Animals were euthanized at 24 h post HI and were then perfused with ice cold PBS solution after which the brains were removed and divided into ipsilateral and contralateral cerebrums and stored immediately in −80℃ freezer for future use. For obtaining whole cell lysates, the brain samples were homogenized in RIPA lysis buffer (Santa Cruz Biotechnology, USA) for 15 min and then centrifuged at 14,000 g at 4℃ for 30 min. The protein concentration was determined by collecting the supernatant and using a detergent compatibility assay (Bio-rad, Dc protein assay). After the protein concentration of each sample was calculated using the spectrophotometer (Thermo Fisher Scientific, USA). Equal amounts of protein (50µg) were then loaded on a 10% SDS-PAGE gel and then electrophoresed after which they were transferred to a nitrocellulose membrane. The membrane was then blocked with 5% non-fat blocking milk and then incubated with the respective primary antibody overnight at 4℃ (Shi, Al-Baadani et al. 2017). The primary antibodies used were: anti-Slit2 (1:200, Abcam, USA), anti-Robo1 (1:200, Santa Cruz Biotechnology, USA), anti-srGAP1 (1:1000, Abcam, USA), RhoA (1:1000, Cell Signaling Technology, USA), Bax (1:1000, Abcam, USA), Bcl-2 (1:200, Santa Cruz Biotechnology, USA), anti-caspase 3 (1:1000 Cell Signaling Technology, USA), anti-cleaved-caspase 3 (1:1000 Cell Signaling Technology, USA). The same membranes were then probed with anti-β Actin (1:2000 Santa Cruz Biotechnology, USA). The following day the membranes were incubated with secondary antibodies (1:2000 Santa Cruz Biotechnology, USA) for 1 h at room temperature. Immunoblots were then probed via ECL Plus chemiluminescence reagent kit (Amersham Bioscience, Arlington Heights, IL) and were then analysed using ImageJ (4.0, Media Cybernetics, Silver Springs, MD).
2.9. Immunohistochemistry staining
The animals were anesthetized with isoflurane and then perfused transcardially with 0.1 M PBS followed by 4% formalin, the brains were then removed and fixed in 10% formalin overnight. Then the brains were immersed in 30% sucrose for 3 d, and then they were frozen. The frozen brains were then sliced into 10µm-thick coronal sections using a cryostat (CM3050S-3, Leica Microsystems, USA) and the sectioned tissue was placed onto individual glass slides. Immunofluorescence staining was performed as described previously (Zhou, Yamaguchi et al. 2004). The cryoprotected sections were washed with 0.1 M PBS three times for 5 min and then incubated with 0.3% Triton X-100 for 15 min at room temperature. Then the sections were washed with PBS three times for 5min and then blocked with 5% donkey serum at room temperature for an hour. The sections were then incubated with primary antibodies: anti-Slit2 (1:50, Abcam, USA), anti-Robo1 (1:200, Santa Cruz Technology, USA), anti-neuronal nuclei (NeuN) (1:200 Abcam, USA) at 4℃ overnight. The sections were then washed with 0.1 M PBS three times for 5 min and then were incubated with appropriate secondary antibodies at the dilution of 1:200 for 2 h at room temperature. The sections were then washed with 0.1 M PBS three times for 5 min and then finally, the sections were covered with DAPI (Vector Laboratories Inc., USA). The slides were then visualized under a fluorescence microscope Leica DMi8 (Leica Microsystems, Germany) and analysed by Leica Application Suite software.
2.10. Fluoro-Jade C staining
To evaluate degenerating neurons Fluoro-Jade C staining was done as described in previous studies (Xie, Huang et al. 2017). The sample was obtained in a similar manner as described above for immunohistochemistry. The 10µm brain sections were obtained on the slides and were then immersed in 1% sodium hydroxide then rinsed in 70% ethanol for 2min followed with distilled water for 2min. Slides were then incubated with 0.06% potassium permanganate solution for 10min and then were washed with distilled water for 2 min. The slides were then transferred into 0.0001% solution of Fluoro-Jade C (Millipore, USA) for 10 min and then the slides were washed with distilled water 3 times for 1 min and dried in an incubator at 50℃ for 5 min. The dried slides were then cleared in xylene for 1 min and then cover slipped with DPX (Sigma-Aldrich, USA). The FJC-positive cells were quantified per field of view and manually counted in the peri-ischemic regions. Under the microscopic filed of 20 x, six sections per brain were averaged and expressed as positive cells per mm2.
2.11. Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) Staining
TUNEL method was used to identify and quantify apoptotic neuronal cells. The staining was performed at 24 h post HI by using neuron marker NeuN (green) and TUNEL staining (red) with the in-situ Apoptosis Detection Kit (Roche, USA) following the manufacturer’s instructions. The TUNEL positive cells were counted as described above for Fluoro Jade C staining. The NeuN stained positive neurons and TUNEL positive neurons were counted and expressed as a ratio of TUNEL-positive neurons.
2.12. Nissl Staining
Brain tissue loss was evaluated using Nissl staining, the coronal brain slices (20 µm thick) were cut using the cryostat (CM3050S-3, Leica Microsystems, USA). The sections were then dehydrated in 95% and 70% ethanol for 1min each and then stained with 0.5% cresyl violet (Sigma-Aldrich, USA) for 2 min. The sections were then dehydrated in 100% ethanol ad xylene for 1.5 min each, before a cover slip was placed. The tissue loss was then measured with ImageJ (Shi, Xu et al. 2017).
2.13. Statistical analysis
Statistical analysis was done using SPSS v.21.0 (IBM Corp., USA). Statistical differences between the groups were analysed using one-way ANOVA for multiple comparisons followed by Tukey or Student-Newman-Keuls post hoc tests. All the data was presented as mean ± SD. Statistical significance was defined as p values less than 0.05. Sample sizes were calculated for all groups assuming a type I error (false positive) rate = 0.05 and power = 0.8 on a two-sided t-test. We also used Analysis of Variance (ANOVA) as a statistical measure when appropriate. Based on previous studies, expected mean vales and variation within groups, as well as the expected change in the means (a change of 30% for long term advanced neurobehavioral analysis and 20–50% for Western blotting and immunohistochemistry), we conlcuded that a sample size of 6–8 pups/group are needed.Please see supplemental table 1 for detailed statistical analysis.
2.14. Rigor and Transparency
We have applied the following measures to ensure robust and unbiased experimental design and unbiased analysis of our results: A) Randomization: Animals were randomly assigned into various experimental groups by generating random numbers to assign to each animal and groups. Randomization process was done in Excel to assign animals to different experimental groups. B) Blinding: To reduce experimenter bias and achieve unbiased results, the investigators performing the neurological tests and molecular experiments were blinded to animal groups and the interventions that animals received. C) Control groups: Appropriate control groups were included to compare with groups that received the proposed interventions. In assays, such as western blot, all samples were run in duplicates. In exploratory experiments, all tests were done two-sided.
3. Results
3.1. Time course expression levels of Slit2, Robo1 and srGAP1 post HI
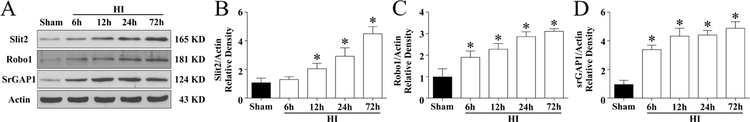
The endogenous expression of Slit2, Robo1 and srGAP1 were measured at 6 h, 12 h, 24 h, 72 h post HI. Fig. 1A shows representative western blot bands of endogenous expression levels of Slit2, Robo1 and srGAP1.Their levels increased in a time-dependant manner and peaked at 72 h post HI when compared to the sham group (p < 0.05, Fig 1 B–D). Please see supplemental figure 1 for representative pictures of Western blot data.
Figure 1. Protein expression levels of Slit2/Robo1/srGAP1 at 6h,12h,24h,72h post HIE.
(A) Representative pictures of Western blot data (B) Western blot data showed slit2 expression levels significantly increased in a time-dependent manner from 6h to 72h reaching peak at 72h post HIE (C) Robo1 Receptor increased in a time a timed-dependent manner from 6h to 72h reaching peak at 72h post HIE. (D) The expression of srGAP1 increased in a time dependant manner reaching peak at 72 h post HIE. . Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey multiple comparison post hoc analysis. ( Data represent +/− SD; *p<0.05 versus sham, n=6/group).
3.2. Reduction in infarct area and improved short-term neurological function at 24 h post HI following intranasal administration of r-Slit2
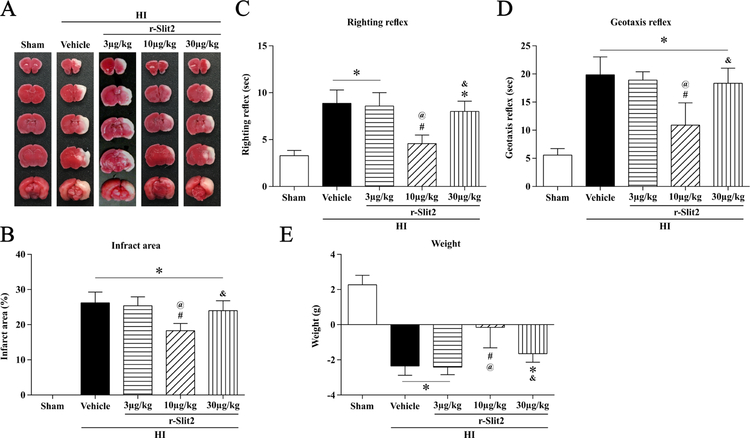
The best dose of r-Slit2 to reduce the area of brain infarct was determined by using 3 doses: low (3µg/kg), middle (10µg/kg) and high (30µg/kg). On performing TTC staining (Fig. 2A) it was observed that the middle dose of r-Slit2 (10µg/kg) significantly reduced the infarct area at 24 h post HI when compared to the vehicle group, low dose of r-Slit-2 (3µg/kg), and high dose of r-Slit2 (30µg/kg) (p < 0.05, Fig. 2B).
Figure 2. Effect of intranasal administration of r-Slit2 on brain infarct area and short-term neurological function at 24h post HIE.
(A–B) TTC staining showed that middle dose (10ug/kg) of r-Slit2 treatment significantly reduced infarct area when compared to vehicle. (C–D) Righting reflex and Geotaxis reflex showed that middle dose (10ug/kg) of r-Slit2 significantly improved neurological function compared to vehicle animals. (E) Animals weight change after HIE. Data expressed as mean ± SD *p < 0.05 versus sham; #p < 0.05 versus HI + vehicle; @p < 0.05 versus HI + 3 µg/kg r-Slit2; &p < 0.05 versus 10 µg/kg HI + r-Slit2; N = 6/group.
Behavioural tests were used to evaluate the short-term neurological function. Righting reflex and geotaxis test were performed on the animals and in both the tests the animals performed significantly worse in the vehicle group and high dose treatment group post HI as compared to the sham group. The middle dose of r-Slit2 significantly improved the short-term neurological function compared to the vehicle group, low dose (3µg/kg) and high dose of r-Slit2 (30µg/kg) (p < 0.05, Fig 2C and D). The weight of the animals was measured, and it was observed that the vehicle group and the high dose treatment group showed significant weight loss when compared to sham group and low dose treatment group (Fig. 2E). Hence, 10 µg/kg of r-Slit2 was selected as the optimal dose for further experiments.
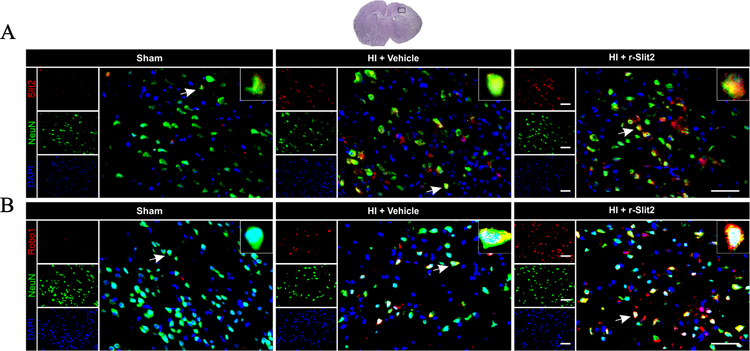
3.3. Immunofluorescence staining for Slit2 and Robo1 expression and colocalization with neurons at 24 h post HI
Immunofluorescence staining showed that the expression of Slit2 on neurons was higher in the r-Slit2 treatment group as compared to the sham and vehicle (Fig. 3). The expression of Robo1 was also increased with r-Slit2 as compared to sham and vehicle (Fig. 3). Slit2 and Robo1 showed colocalization with neurons.
Figure 3. Representative picture of Slit2 and Robo1 expression in the brain at 24h post HIE.
Immunofluorescence staining showed a significantly higher expression of Slit2 on neurons in vehicle-operated animals compared to sham and significantly higher expression of Slit2 after treatment when compared to vehicle. Similarly, Robo1 expression was seen to be higher after treatment when compared to vehicle (Green was for neuronal staining, Red was for Slit2 and Robo1 staining and Blue was for DAPI. Merge showed the colocalization of Slit2 and Robo1 on neurons. Scale bar = 50 µm; n=3/group).
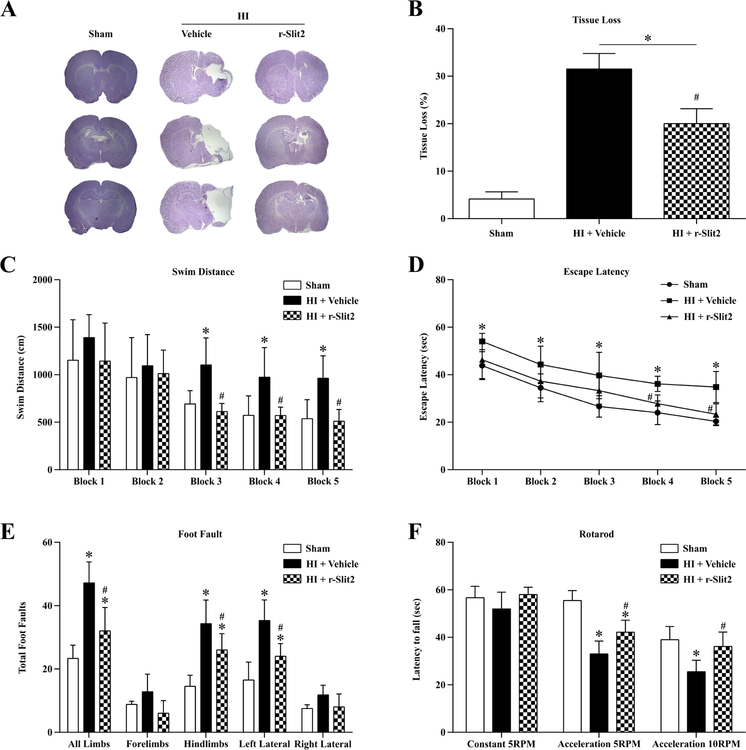
3.4. r-Slit2 reduced brain atrophy improved long term neurological function at 4 weeks post HI
The intranasal administration of r-Slit2 showed significant reduction in brain atrophy in the ipsilateral hemisphere and also helped reduce neuronal loss at 4 weeks post HI as compared to vehicle group (p<0.05, Fig 4 A–B).
Figure 4. Effects of r-Slit2 on neurological function at 4 weeks post HIE.
(A–B) Representative pictures of Nissl’s stain for the brain slices showed tissue loss which was significantly reduced on treatment with r-Slit2 as compared to vehicle. (C–D) Rh-Slit2 group showed a significant improvement in neurological function as shown by water-maze, where memory and learning was evaluated. (Data represent +/− SD; *p<0.05 versus sham, # versus vehicle; n=6/group using one-way or two way ANOVA followed by Tukey multiple-comparison post hoc analysis). (E–F) Rh-Slit2 showed to improve motor deficits as seen from rota rod and foot fault tests. (Data represent +/− SD; *p<0.05 versus sham, # versus vehicle; n=6/group using one-way or two-way ANOVA followed by Tukey multiple-comparison post hoc analysis).
The long-term neurological function following treatment with r-Slit2 was assessed using rotarod, foot-fault and water maze at 4 weeks post HI. In all the tests the sham group animals performed well while the vehicle group animals performed worse compared to sham group and r-Slit2 treatment group animals.
In the water maze test, the vehicle group showed a significant reduction in short-term memory and spatial learning as compared to sham animals and travelled a longer distance to find the platform and took longer time to find the platform (p<0.05, Fig. 4C–D). Animals in the r-Slit2 treatment group travelled significantly a shorter distance to find the platform and had a lower escape latency, the animals in the treatment group performed better in subsequent blocks (p<0.05, Fig. 4C–D). In the foot fault test, animals in the vehicle group had significantly more foot faults in the left fore limbs and hind limbs, compared to the sham group (Fig 4 E). However, the r-Slit2 treatment group significantly improved the neurological deficit by reducing the number of foot faults compared to vehicle + HI group (Fig 4 E). In the rotarod test, the vehicle group had a significantly shorter latency to fall as compared to sham group (Fig 4 F). The r-Slit2 treatment group performed better and appeared to improve the neurological deficits as compared to the vehicle group at 4 weeks post HI (Fig 4 F).
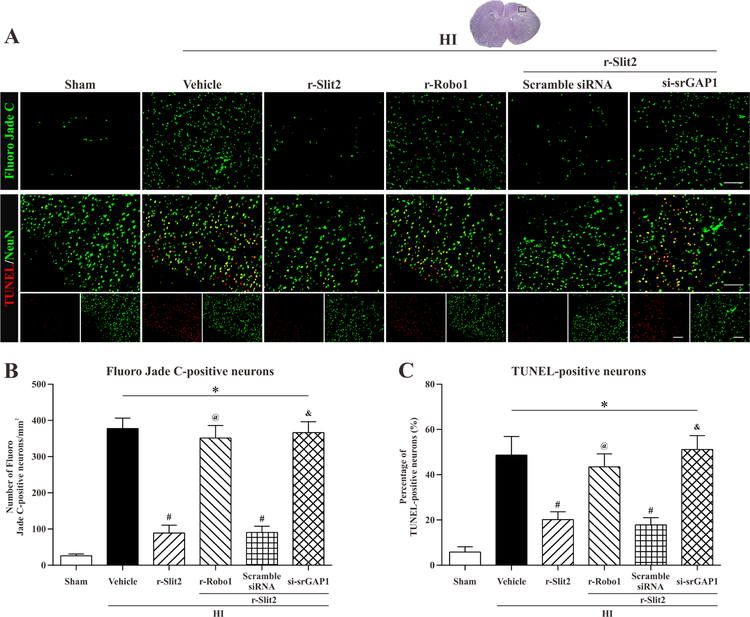
3.5. Attenuation of neuronal apoptosis at 24 h post HI following treatment with r-Slit2
To determine whether treatment with r-Slit2 could help attenuate neuronal apoptosis and degeneration following hypoxic-ischemic injury we used Fluoro-Jade C staining and TUNEL staining. It was observed that, Fluoro Jade C positive stained neurons in the vehicle group were significantly increased when compared to sham. However, r-Slit2 significantly reduced the Fluoro Jade C positive stained neurons as compared to the vehicle (p<0.05, Fig. 5 A–B). Also, srGAP1 siRNA group showed increase in Fluoro Jade C positive stained neurons when compared to sham and r-Slit2 treatment group, showing that it inhibited the neuroprotective effect of r-Slit2. Similarly, in TUNEL staining, an increase in TUNEL positive stained neurons was seen in vehicle group and srGAP1 siRNA group as compared to the sham group and r-Slit2 treatment group (p<0.05, Fig. 5 A–B).
Figure 5. Representative pictures of the effects of r-Slit2 treatment on neuronal apoptosis at 48h post HI.
(A, B) Fluoro-Jade C staining showed more positively stained neurons undergoing apoptosis in vehicle group and si-srGAP1 group compared to sham or Slit2 treatment group. Scale bar 50µm. (A, C) TUNEL staining showed that there were more positively TUNEL stained neurons in the vehicle group as compared to sham. Treatment with r-Slit2 significantly decreased the number of positive stained neurons. * p<0.05 vs sham; # p<0.05 vs vehicle; & p<0.05 vs HI + r-Slit2. Scale bar = 50µm. N= 6 for each group.
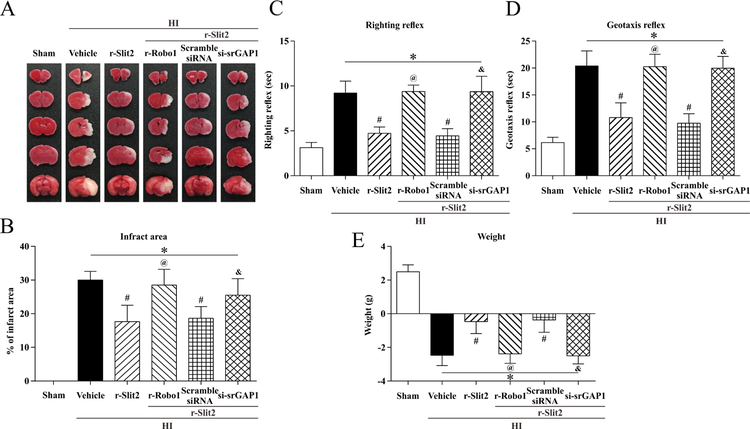
3.6. r-Robo1 and srGAP1 siRNA reversed neuroprotective effects of r-Slit2 at 24 h post HI
To determine if r-Slit2 has a neuroprotective effect via the Robo1/srGAP1 pathway, we used r-Robo1 and srGAP1 siRNA as interventions in the pathway and studied the outcome on brain infarct area, geotaxis reflex and righting reflex. Significant reversal of protective effects of r-Slit2 were seen on TTC staining. Both r-rRobo1 and srGAp1 siRNA showed an increase in percent infarcted area as compared to the r-Slit2 treatment group (p<0.05, Fig. 6 A–B). The geotaxis reflex and righting reflex showed that animals administered with r-Slit2 treatment and then with r-Robo1 or srGAP1 siRNA had significantly impaired neurological function when compared with the r-Slit2 treatment group or r-Slit2 + scramble siRNA group (p<0.05, Fig. 6 C–D). In addition, there was a significant change in weight in the animals administered with r-Robo1 or srGAP1 siRNA and r-Slit2 compared to the r-Slit2 treatment and scramble siRNA group.
Figure 6. Effect of intranasal administration of r-Slit2 on brain infarct area and short-term neurological function at 48h post HIE.
(A–B) TTC staining showed that low dose (10ug/kg) of r-Slit2 treatment significantly reduced infarct area when compared to vehicle. (C–D) Righting reflex and Geotaxis reflex showed that low dose (10ug/kg) of r-Slit2 significantly improved neurological function compared to vehicle animals. (E) Animals’ weight change after HIE. Statistical differences between groups were analyzed using one-way or two-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle (n=6/group).
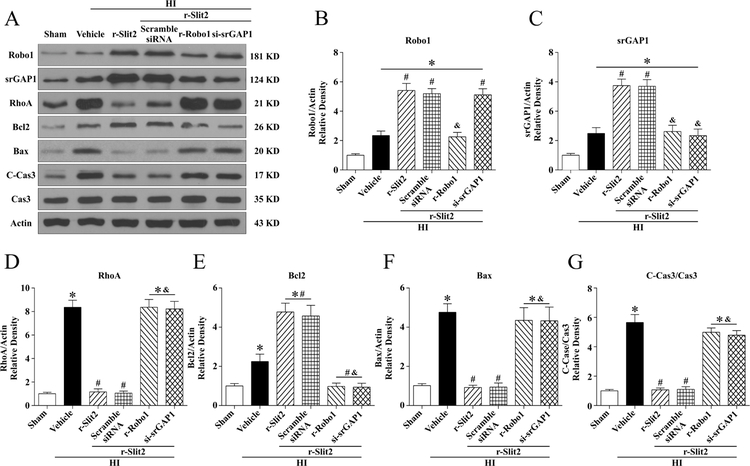
3.7. r-Slit2 reduced apoptosis via the Robo1-srGAP1 pathway at 24 h post HI
To study the mechanism via which Slit2 attenuated apoptosis to exert its neuroprotective effects, the animals were divided into the following groups: Sham, HI + Vehicle (PBS), HI + r-Slit2, HI +r-Slit2 + r-Robo1, HI + r-Slit2 + srGAP1 siRNA, HI +r-Slit2 + scramble siRNA. The data from the western blot showed that expression of Robo1, srGAP1, RhoA, Bax and CC3 increased significantly in HI + vehicle group as compared to sham (p<0.05, Fig. 7 A–G). Treatment with r-Slit2 upregulated the expression of Robo1, srGAP1 and Bcl2 while the expression of RhoA, Bax and CC3 were downregulated when compared with HI + vehicle group (p<0.05, Fig. 7 D, F, G). The expression of Robo1 was significantly decreased with r-Robo1(p<0.05, Fig. 7 A–B), which blocked the effects of r-Slit2. The intervention r-Robo1 also showed a significant increase in the expression of RhoA, Bax and CC3 (p<0.05, Fig. 7 A–B,D,F–G). Similarly, the expression of srGAP1 was significantly decreased with srGAP1 siRNA and showed significantly increased expression of RhoA, Bax and CC3 (p<0.05, Fig. 7A, C–D, F–G). Please see supplemental figure 2 for representative pictures of Western blot data.
Figure 7. The effects of r-Slit2 on apoptosis via the Slit2/Robo1/srGAP1/RhoA signaling pathway at 48h post HIE.
(A) Representative picture of western blot data showing bands of the expression levels of Slit2, Robo1, srGAP1, RhoA, Bcl2, Bax and cleaved caspase3/caspase3 either with r-Slit2 treatment alone, r-Slit2+scramble si-RNA, r-Slit2+Recombinant Robo1, r-Slit2+si-GAP1 (B-G) Western blot data quantification of bands showed that r-Slit2 significantly increased Robo1 and srGAP1 expression levels when compared to vehicle. Recombinant Robo1 significantly reduced Robo1 receptor expression levels. Furthermore, siRNA for srGAP1 showed to significantly reduce srGAP1 when compared to treatment group. Treatment with r-Slit2 showed to significantly reduce RhoA, Bax and ratio of C-Cas3/Cas3 while the two interventions reversed this effect. (Data represent +/− SD; *p<0.05 versus sham, # versus vehicle; & versus r-slit2, n=6/group using one-way or two-way ANOVA followed by Tukey multiple-comparison post hoc analysis).
4. DISCUSSION
In the present study we focused on the role of recombinant Slit2 in reducing neuronal apoptosis following hypoxic ischemic injury to the brain. It was observed that HIE caused significant damage to neurons by increased neuronal cell death (observed by staining methods: Fluro-Jade C, Nissl and TUNEL) and by increase in apoptosis (observed by increased caspase-3 and cleaved caspase-3 expression). Our study showed that following HIE, the expression of endogenous Slit2 and its receptor Robo1 and their downstream effector srGAP1 increased in a time-dependent manner and blocking the receptor Robo1 (using recombinant Robo1) and srGAP1 (using siRNA-srGAP1) worsened the injury. Also, recombinant Slit2 administration before hypoxic-ischemic injury reduced neurological deficits as was seen in the short-term and long-term neurobehavioral tests. These results suggest that Slit2 may have a neuroprotective role following HIE and may be a potential candidate for attenuating apoptosis.
Apoptosis is known to play a major role in causing neuronal cell death following hypoxic-ischemic injury to the brain. When the cell senses stress, in the intrinsic pathway the cell kills itself and in the extrinsic pathway the signal from other cells leads to the cell to kill itself. Hypoxia ischemia is a stressor that leads to the activation of apoptotic pathways which can be extrinsic or intrinsic which then lead to caspases activation like caspase-3 which degrade proteins and has a major role in cell death. Both the pathways activate the initiator caspases which then activate the executioner caspases. Caspase-3 is one of the executioner caspases which can be activated via both the intrinsic and extrinsic pathways. Direct inhibition of caspases has shown to be protective in many rat models of hypoxic ischemic injury. In our study, we hypothesize that both the pathways were being activated and we measured caspase-3 which is known to be activated by both the intrinsic and extrinsic pathways. Our study did show that r-Slit2 leads to the decreased expression of caspase-3 hence showing the anti-apoptotic property.
Slit2 has been shown to have important functions in the brain like axon guidance, cell migration, outgrowth and branching (Andrews, Barber et al. 2008). Administration of recombinant Slit2 has shown to have protective functions in the brain by reducing inflammation (Sherchan, Huang et al. 2016), in the kidney by attenuating renal inflammation and fibrosis (Yuen, Huang et al. 2016). But, no study has shown the neuroprotective effect of Slit2 on attenuating apoptosis following hypoxia-ischemia. Slit2 exerts its functions by binding to its receptor Robo1 which then leads to increased interaction of Robo1 with srGAP1 (Wong, Ren et al. 2001).In our study we observed that, on administration of exogenous r-Slit2 the expression of receptor Robo1 increased. This increased interaction of r-Slit2 and Robo1 led to the upregulation of the downstream effector srGAP1 (slit-robo GTPase activating protein), as is seen in previous studies (Sherchan, Huang et al. 2016) and this helped improve the outcome in terms of TTC staining and neurobehavior tests.
On TTC staining it was seen that, intranasal administration of the middle dose (10µg/kg) of r-Slit2 significantly reduced the brain infarct area as compared to vehicle group as is seen in Fig 2. This result corresponded with the short-term neurobehavior tests which showed decreased neurological deficits on treatment with r-Slit2. The tests performed were righting reflex and geotaxis, to test orientation and motor coordination, both showed significantly improved neurological function as compared to the vehicle group. The higher dose seemed to be toxic to the animal as was seen on TTC staining (larger infarct size) and also the neurobehavioral tests performed. On the basis of the experiments performed we chose the dose which appeared to have the most beneficial effect and that dose was 10 µg/kg. At 3 µg/kg and 30 µg/kg the infarct size post-surgery seemed to be the same or increase in size. Due to the above-mentioned observation we chose the middle dose and it did seem to decrease neuronal apoptosis. Long-term neurobehavior tests: foot fault, rotarod and water maze were performed at 4 weeks after treatment with r-Slit2 post HI. Foot fault and rotarod were used to test forelimb and hindlimb stepping, coordination and balance. The water maze test helped in assessing spatial learning and memory (Schaar, Brenneman et al. 2010).The results showed significantly improved neurological function in all these aspects when compared to vehicle group. Thus, r-Slit2 did show improvement in terms of neurological outcomes and has a neuroprotective role following hypoxic ischemic injury to the brain.
The role of Slit2 in cell survival is still being explored (Yang, Manning Fox et al. 2013). Slit2 and Robo1 were shown to co-localize on neurons on immunofluorescence staining (Mertsch, Schmitz et al. 2008). To elucidate the signaling mechanism of Slit2 in attenuating apoptosis we co-administered recombinant Slit2 and recombinant Robo1. As shown in previous studies (Altay, McLaughlin et al. 2007, Sherchan, Huang et al. 2016) recombinant Robo1 binds to the recombinant Slit2, acting as a decoy receptor to reduce the recombinant Slit2 available for exerting its effect and then we also knockdown the downstream effector srGAP1 (slit-robo GTPase activating protein 1) using siRNA-srGAP1. srGAP1 has been shown to downregulate RhoA (Liang, Kiru et al. 2018) which has a role in neuronal apoptosis. A previous study has shown that activation of RhoA pathway increased neuronal apoptosis and that the RhoA pathway may have a role in neuroprotection by attenuating apoptosis and in another study, inactivation of the RhoA pathway inhibited neuronal apoptosis (Zhang, Zhao et al. 2010).
The activity of Rho-GTPases like RhoA are regulated by the GTPase activating proteins (GAPs). The GAPs increase the intrinsic GTPase activity by converting the active GTP-bound form to the inactive GDP-bound form thus downregulating RhoA (Guan and Rao 2003, Liang, Kiru et al. 2018). The Slit-Robo interaction leads to the increase in srGAP1 activity which is a GTPase activating protein and acts as negative regulator of RhoA. It was observed that the percent brain infarct area was significantly increased following the use of interventions to block the receptor Robo1 and the downstream effector srGAP1 when compared to the treatment group (r-Slit2). On further testing it was observed that the expression of RhoA and the apoptosis markers caspase-3 and cleaved caspase-3 was significantly increased as compared to the treatment group. Fluro-Jade C staining for neuronal apoptosis also showed that on treatment with r-Slit2 there was a significant reduction of degenerating neurons. These findings indicate that r-Slit2 does help reduce neuronal apoptosis using the Robo1-srGAP1 signaling pathway. Our aim for this study is to elicit the primary mechanism by which Slit2 exerts it’s neuroprotective effect by attenuating neuronal apoptosis after HI injury
There are a few limitations to this study. Firstly, there could be other pathways by which Slit2 can be activated and carry out its functions. Secondly, Slit2 may have several mechanisms of action that lead to protection. Therefore, in this study we only elicited one of these potential mechanisms. This work is necessary as it may help to better understand Slit2 signaling pathways and its role after HIE. Thirdly, the apoptotic pathway could be intrinsic or extrinsic, further studies are needed to understand the pathway inhibited by Slit2.
Given the limited therapeutic options for HIE it is necessary to understand cellular and molecular mechanisms which can lead to more effective therapeutic strategies. Numerous attempts to reduce HI-induced consequences have failed in the clinical setting. Therefore, it is imperative to explore different therapeutic targets to either amplify or replace current therapeutic strategies. The lack of adequate mechanistic animal studies, therapeutic window, timing of treatment and various other factors halt the progression of promising targets to be translated to clinics.
Slit2 may have a plethora of signaling mechanisms that can be activated/inhibited after HIE. Slit2 functions as guidance molecules and can regulate neuronal migration, axon branching, organogenesis, angiogenesis and neural development. Based on its multimodal properties this suggests that Slit2 may target a wide array of pathophysiological consequences after HIE. Therefore, this makes Slit2 a potential therapeutic target and may change the clinical management for patients with HI injury and provide a foundation for future research in other types of strokes with similar pathologies.
However, there is a lack of in vivo studies showing Slit2’s diverse roles. Therefore, it is imperative to study all of Slit2’s functions and signaling mechanisms in wide array of disease models before it can be translated to clinical studies.
In conclusion, on administration of exogenous r-Slit2 post hypoxic ischemic injury, we observed reduced neuronal apoptosis and improved neurological function. The neuroprotective role of Slit2 may be through the Slit2/Robo1/srGAP1 pathway and Slit2 could be a potential therapeutic agent for hypoxic ischemic injury to the brain.
Supplementary Material
Highlights.
Slit2 and Robo-1 expression is increased after neonatal brain injury
Recombinant Slit2 has a protective effect on brain infarction and neurobehavior after HI
Recombinant Slit2 attenuated apoptosis after HI
Recombinant Slit2 attenuated apoptosis via Robo1-srGap1 pathway
Acknowledgments
The study was supported by a grant from National Institutes of Health NS104083 to Dr. John H. Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflicts of Interest
Nothing to report.
REFERENCES
- Altay T, McLaughlin B, Wu JY, Park TS and Gidday JM (2007). “Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia.” Exp Neurol 207(2): 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, Erskine L, Murakami F and Parnavelas JG (2008). “The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons.” Dev Biol 313(2): 648–658. [DOI] [PubMed] [Google Scholar]
- Arteni NS, Salgueiro J, Torres I, Achaval M and Netto CA (2003). “Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat.” Brain Res 973(2): 171–178. [DOI] [PubMed] [Google Scholar]
- Bahjat FR, Alexander West G, Kohama SG, Glynn C, Urbanski HF, Hobbs TR, Earl E, Stevens SL and Stenzel-Poore MP (2017). “Preclinical Development of a Prophylactic Neuroprotective Therapy for the Preventive Treatment of Anticipated Ischemia-Reperfusion Injury.” Transl Stroke Res 8(4): 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth TM and Stanfield BB (1990). “The recovery of forelimb-placing behavior in rats with neonatal unilateral cortical damage involves the remaining hemisphere.” J Neurosci 10(10): 3449–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona E, Johansson BB and Hagberg H (1997). “Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats.” Pediatr Res 42(5): 678–683. [DOI] [PubMed] [Google Scholar]
- Chauhan MB and Chauhan NB (2015). “Brain Uptake of Neurotherapeutics after Intranasal versus Intraperitoneal Delivery in Mice.” J Neurol Neurosurg 2(1). [PMC free article] [PubMed] [Google Scholar]
- Dixon BJ, Reis C, Ho WM, Tang J and Zhang JH (2015). “Neuroprotective Strategies after Neonatal Hypoxic Ischemic Encephalopathy.” Int J Mol Sci 16(9): 22368–22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doycheva DM, Hadley T, Li L, Applegate RL 2nd, Zhang JH and Tang J (2014). “Anti-neutrophil antibody enhances the neuroprotective effects of G-CSF by decreasing number of neutrophils in hypoxic ischemic neonatal rat model.” Neurobiol Dis 69: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Yang T, Lessov NS, Ciesielski TM, Stevens SL, Simon RP, King JS and Stenzel-Poore MP (2008). “Nasal administration of osteopontin peptide mimetics confers neuroprotection in stroke.” J Cereb Blood Flow Metab 28(6): 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham EM, Ruis KA, Hartman AL, Northington FJ and Fox HE (2008). “A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy.” Am J Obstet Gynecol 199(6): 587–595. [DOI] [PubMed] [Google Scholar]
- Guan KL and Rao Y (2003). “Signalling mechanisms mediating neuronal responses to guidance cues.” Nat Rev Neurosci 4(12): 941–956. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Sheng H, Ayata C and Warner DS (2016). “Anesthesia in Experimental Stroke Research.” Transl Stroke Res 7(5): 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic T, Rolland W, Hartman R, Kamper J, Suzuki H, Tang J and Zhang JH (2011). “Characterization of the brain injury, neurobehavioral profiles, and histopathology in a rat model of cerebellar hemorrhage.” Exp Neurol 227(1): 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Budnar S, Gupta S, Verma S, Han SP, Hill MM, Daly RJ, Parton RG, Hamilton NA, Gomez GA and Yap AS (2017). “Tyrosine dephosphorylated cortactin downregulates contractility at the epithelial zonula adherens through SRGAP1.” Nat Commun 8(1): 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Kiru S, Gomez GA and Yap AS (2018). “Regulated recruitment of SRGAP1 modulates RhoA signaling for contractility during epithelial junction maturation.” Cytoskeleton (Hoboken) 75(2): 61–69. [DOI] [PubMed] [Google Scholar]
- Liu JB, Jiang YQ, Gong AH, Zhang ZJ, Jiang Q and Chu XP (2011). “Expression of Slit2 and Robo1 after traumatic lesions of the rat spinal cord.” Acta Histochem 113(1): 43–48. [DOI] [PubMed] [Google Scholar]
- Lorente L, Martin MM, Argueso M, Ramos L, Sole-Violan J, Riano-Ruiz M, Jimenez A and Borreguero-Leon JM (2015). “Serum caspase-3 levels and mortality are associated in patients with severe traumatic brain injury.” BMC Neurol 15: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Behrens MI, Chung C, Bhattacharyya T and Choi DW (1997). “Susceptibility to apoptosis is enhanced in immature cortical neurons.” Brain Res 759(2): 228–232. [DOI] [PubMed] [Google Scholar]
- Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W and Senner V (2008). “Slit2 involvement in glioma cell migration is mediated by Robo1 receptor.” J Neurooncol 87(1): 1–7. [DOI] [PubMed] [Google Scholar]
- Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME and Johnston MV (2000). “Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat.” J Neurosci 20(21): 7994–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Gardner HA and Tucker KL (2013). “Postnatal neuronal apoptosis in the cerebral cortex: physiological and pathophysiological mechanisms.” Neuroscience 254: 369–378. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR and Zhang JH (2005). “Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage.” J Cereb Blood Flow Metab 25(5): 554–571. [DOI] [PubMed] [Google Scholar]
- Pardeshi CV and Belgamwar VS (2013). “Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting.” Expert Opin Drug Deliv 10(7): 957–972. [DOI] [PubMed] [Google Scholar]
- Prasad A, Qamri Z, Wu J and Ganju RK (2007). “Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells.” J Leukoc Biol 82(3): 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE 3rd, Vannucci RC and Brierley JB (1981). “The influence of immaturity on hypoxic-ischemic brain damage in the rat.” Ann Neurol 9(2): 131–141. [DOI] [PubMed] [Google Scholar]
- Schaar KL, Brenneman MM and Savitz SI (2010). “Functional assessments in the rodent stroke model.” Exp Transl Stroke Med 2(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan P, Huang L, Wang Y, Akyol O, Tang J and Zhang JH (2016). “Recombinant Slit2 attenuates neuroinflammation after surgical brain injury by inhibiting peripheral immune cell infiltration via Robo1-srGAP1 pathway in a rat model.” Neurobiol Dis 85: 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Al-Baadani A, Zhou K, Shao A, Xu S, Chen S and Zhang J (2017). “PCMT1 Ameliorates Neuronal Apoptosis by Inhibiting the Activation of MST1 after Subarachnoid Hemorrhage in Rats.” Transl Stroke Res [DOI] [PubMed]
- Shi X, Xu L, Doycheva DM, Tang J, Yan M and Zhang JH (2017). “Sestrin2, as a negative feedback regulator of mTOR, provides neuroprotection by activation AMPK phosphorylation in neonatal hypoxic-ischemic encephalopathy in rat pups.” J Cereb Blood Flow Metab 37(4): 1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifringer M, Bendix I, Borner C, Endesfelder S, von Haefen C, Kalb A, Holifanjaniaina S, Prager S, Schlager GW, Keller M, Jacotot E and Felderhoff-Mueser U (2012). “Prevention of neonatal oxygen-induced brain damage by reduction of intrinsic apoptosis.” Cell Death Dis 3: e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uria-Avellanal C and Robertson NJ (2014). “Na(+)/H(+) exchangers and intracellular pH in perinatal brain injury.” Transl Stroke Res 5(1): 79–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe TR and Braude DA (2010). “Intranasal medication delivery for children: a brief review and update.” Pediatrics 126(3): 532–537. [DOI] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC and Rao Y (2001). “Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway.” Cell 107(2): 209–221. [DOI] [PubMed] [Google Scholar]
- Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, Cheng Y and Zhang JH (2017). “Intranasal administration of recombinant Netrin-1 attenuates neuronal apoptosis by activating DCC/APPL-1/AKT signaling pathway after subarachnoid hemorrhage in rats.” Neuropharmacology 119: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Manning Fox JE, Zhang KL, MacDonald PE and Johnson JD (2013). “Intraislet SLIT-ROBO signaling is required for beta-cell survival and potentiates insulin secretion.” Proc Natl Acad Sci U S A 110(41): 16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y and Chedotal A (2010). “Moving away from the midline: new developments for Slit and Robo.” Development 137(12): 1939–1952. [DOI] [PubMed] [Google Scholar]
- Yuen DA, Huang YW, Liu GY, Patel S, Fang F, Zhou J, Thai K, Sidiqi A, Szeto SG, Chan L, Lu M, He X, John R, Gilbert RE, Scholey JW and Robinson LA (2016). “Recombinant N-Terminal Slit2 Inhibits TGF-beta-Induced Fibroblast Activation and Renal Fibrosis.” J Am Soc Nephrol 27(9): 2609–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao J, Wang J and Jiao X (2010). “Brain-derived neurotrophic factor inhibits phenylalanine-induced neuronal apoptosis by preventing RhoA pathway activation.” Neurochem Res 35(3): 480–486. [DOI] [PubMed] [Google Scholar]
- Zhao XC, Zhang LM, Li Q, Tong DY, Fan LC, An P, Wu XY, Chen WM, Zhao P and Wang J (2013). “Isoflurane post-conditioning protects primary cultures of cortical neurons against oxygen and glucose deprivation injury via upregulation of Slit2/Robo1.” Brain Res 1537: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Yamaguchi M, Kusaka G, Schonholz C, Nanda A and Zhang JH (2004). “Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage.” J Cereb Blood Flow Metab 24(4): 419–431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.