Abstract
19-Hydroxyeicosatetraenoic acid (19-HETE, 1), a metabolically and chemically labile cytochrome P450 eicosanoid, has diverse biological activities including antagonism of the vasoconstrictor 20-hydroxyeicosatetraenoic acid (20-HETE, 2). A SAR study was conducted to develop robust analogs of 1 with improved in vitro and in vivo efficacy. Analogs were screened in vitro for inhibition of 20-HETE-induced sensitization of rat renal preglomerular microvessels toward phenylephrine and demonstrated to normalize the blood pressure of male Cyp4a14(−/−) mice that display androgen-driven, 20-HETE-dependent hypertension.
Keywords: Antagonist, Vascular sensitization, Hypertension, Eicosanoid, Bioisosteric replacement
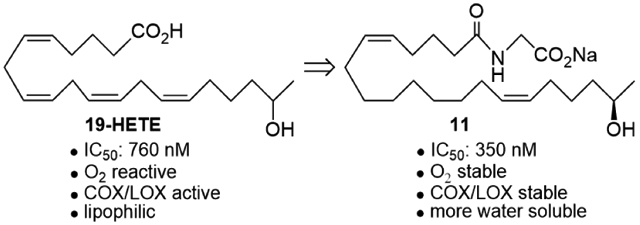
Graphical Abstract
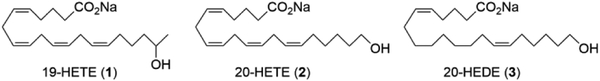
19-Hydroxyeicosatetraenoic acid [19-HETE (1), Figure 1] is one of the principal hydroxylated metabolites of the cytochrome (CYP) P450 branch of the arachidonate cascade,1 although relatively little is known about its physiological actions compared with the structurally related, proinflammatory 20-hydroxyeicosatetraenoic acid (20-HETE, 2). Multiple CYP isoforms biosynthesize 1, including the widely distributed CYP1A, CYP4A, and CYP2E1,2-5 as well as organ specific isoforms such as brain CYP2J9.6 Induction of select CYP isoforms can also influence endogenous levels of 1.2,7 In keeping with most of the CYP eicosanoids, 1 has diverse biological actions including inhibition of angiotensin II-induced hypertrophy in human and rat cardiac cells,8 stimulation of renal Na+-K+-ATPase,9 preglomerular blood vessel vasodilation,10 blockade of recombinant P/Q-type Ca2+ channels,6 and lowering of sodium-dependent phosphate uptake into renal cells.11 Importantly, 1 also opposes many of the physiological actions of 2. For instance, 1 reverses the effects of 20-HETE on NO and superoxide production, endothelial dysfunction,12 and sensitization of renal arterioles toward vasoconstrictors such as phenylephrine, although only (R)-1 is effective for the latter.13 These and other observations led Nasjletti13 to propose 1 can antagonize several biological activities of 2. Herein, we describe the in vitro and in vivo evaluation of a family of robust 19-HETE analogs that will (i) expedite ongoing investigation into the physiologic role(s) of 1 and (ii) support proof-of-principle studies that a 19-HETE-inspired therapy could counteract the in vivo contributions of 2 to a variety of pathophysiologies including cardiac hypertrophy, cardiotoxicity, diabetic cardiomyopathy, cancer, and ischemia/reperfusion (I/R) injury.14-19
Figure 1.
Structures of 19-HETE (1), 20-HETE (2), and 20-5,14-HEDE (3).
While 1 is known to be highly labile,8 comparatively little is known about specific metabolic/catabolic pathways.20,21 However, due to its close structural resemblance to 2, it was reasoned that the same processes, inter alia, esterification, β-oxidation, alcohol dehydrogenase (ADH) oxidation, COX/LOX metabolism, and autooxidation, are also operative for 1 and similarly limit its utility as a research tool and/or clinical therapy. As our launching point for the development of more robust 19-HETE analogs, the carbon backbone of 20-hydroxyeicosa-5(Z),14(Z)-dienoic acid (20-5,14-HEDE, 3), a widely used and fully functional agonist analog of 2,10 was adopted and the hydroxyl was relocated to C(19). This scaffold also obviates autooxidation as well as metabolism by the COX and LOX branches of the arachidonate cascade.
The 19-HETE analogs were evaluated for their ability to inhibit 20-HETE-induced sensitization of rat renal preglomerular microvessels toward phenylephrine (Table 1). Analogs 4 and 5 proved to be moderate antagonists of 20-HETE vessel sensitization with the 19(R)-enantiomer being a little more potent. In anticipation of future click chemistry applications, an azide was appended to the terminal carbon of the chain to give the click-capable analogs 6 and 7.22 This resulted in a 6-8 fold boost in activity over the parent structures. The improvement in inhibitory activity might be due to additional hydrogen bonding to the azide at the receptor binding site.23 To better understand this effect, the hydroxyl and azide positions were exchanged. This not only abrogated the inhibition of 20-HETE vessel sensitization, the resultant 19-azido-20-hydroxyl analogs 8 and 9 modestly heightened the vessels' sensitivity to 20-HETE. Conversion of 4 and 5 to their N-glycinates 10 and 11, respectively, led to a more robust inhibition and somewhat improved water solubility (4 and 5 ≈ 0.25 mg/mL vs. 10 and 11 ≈ 0.5 mg/mL). On the other hand, the related N-aspartate 12 completely negated the preceding gains in potency whereas the drop in activity for 13 was less pronounced, but nevertheless was approximately half that of analog 11.
Table 1.
Effect of 19-HETE Analogs (1 μM) on 20-HETE (1 μM) Induced Vascular Sensitization to Phenylephrine.a
| Analog Number |
Structure | Fold Change from 20-HETEb |
Analog Number |
Structure | Fold Change from 20-HETEb |
|---|---|---|---|---|---|
| 4 | 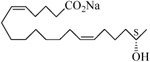 |
−0.68 | 15 | 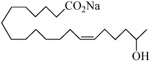 |
−0.79 |
| 5 | 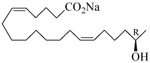 |
−0.85 | 16 | 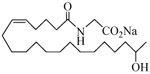 |
−2.19 |
| 6 | 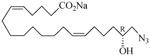 |
−5.69 | 17 | 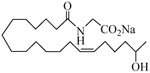 |
−2.56 |
| 7 | 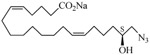 |
−5.45 | 18 | −2.17 | |
| 8 | 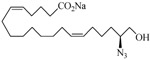 |
0.16 | 19 | 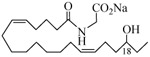 |
−1.13 |
| 9 | 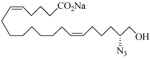 |
0.50 | 20 | 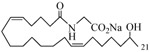 |
0.58 |
| 10 | 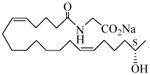 |
−3.46 | 21 | 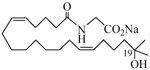 |
−1.16 |
| 11 | 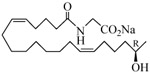 |
−4.67 | 22 | −0.68 | |
| 12 | 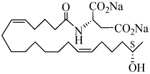 |
−0.59 | 23 | 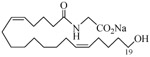 |
−0.74 |
| 13 | 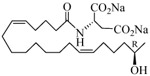 |
−2.03 | 24 | 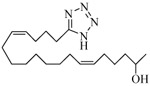 |
−0.78 |
| 14 | 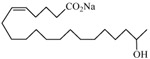 |
−0.66 |
n = 3-5.
Negative values indicate antagonism of 20-HETE sensitization and positive values indicate enhancement of 20-HETE activity.
Further exploration of the structural features of the scaffold revealed retention of just one olefin, i.e., analogs 14 and 15, was sufficient to maintain biological activity, but the previously observed enhancement gained by conversion into a N-glycinate was blunted in analogs 16 and 17. Analog 18, in which the hydroxyl is shifted to C(18), was superior to the other simple eicosadienoic acids (viz., 4 and 5) as an inhibitor, yet the transition to the N-glycinate 19 was unhelpful. Analysis of the homologous series wherein the carbon chain was either lengthened to 21-carbons (analog 20), branched (analog 21), or shortened to 19-carbons (analogs 22 and 23) led us to conclude the position of the hydroxyl along the chain is decisive in determining agonist or antagonist activity and not whether the alcohol is primary, secondary or tertiary. Replacement of the carboxylic acid with a 1H-tetrazole, a commonly used carboxylic acid bioisostere,24 provided analog 24 whose activity was comparable to analogs 4 and 5.
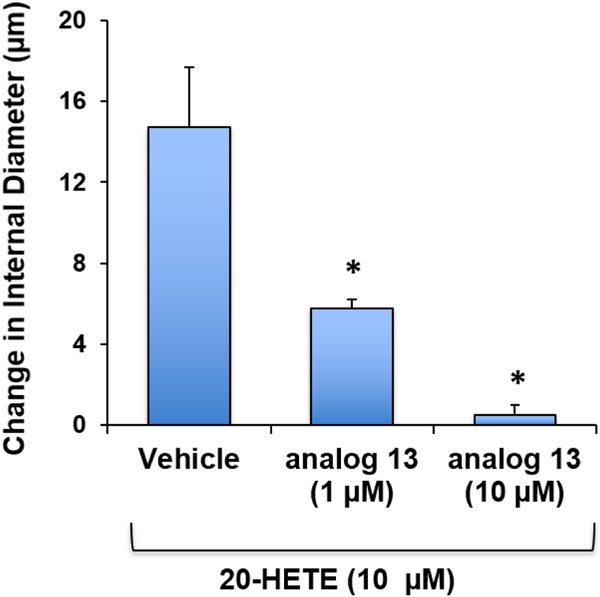
Blockade of 20-HETE-induced changes to the internal diameter of pressurized (100 mmHg) rat gracilis muscle arterioles by 13 in a concentration dependent manner was consistent with competitive inhibition of 20-HETE and demonstrated the broader utility of 19-HETE analogs in other vessel types (Figure 2). Importantly, 13 had no vasoactive effects alone, i.e., in the absence of 2 (data not shown). This contrasts with 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid-based agents that heretofore have been utilized as inhibitors of 2, but recently have been shown to have weak 20-HETE agonist activity in some assays [Prof. Susana Nowicki (CEDIE-Centro de Investigaciones Endocrinológica, Buenos Aires, Argentina), personal communication].
Figure 2.
Inhibition of 20-HETE-induced decrease (vasoconstriction) of pressurized (100 mmHg) rat gracilis muscle arteriole diameter by increasing concentrations of 13.
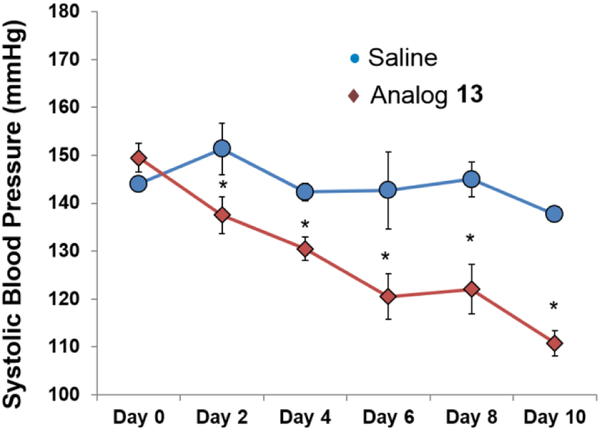
To validate the ability of 19-HETE analogs to mitigate the influence of 20-HETE in vivo, 13 (10 mg/Kg) was administered daily via interperitoneal (IP) injection to male Cyp4a14(−/−) mice which display androgen-driven 20-HETE-dependent hypertension.25 IP saline was used as a control. Over a 10 day course, the animals' systolic blood pressure returned to normal levels (Figure 3).
Figure 3.
Effect of analog 13 on systolic blood pressure of Cyp4a14(−/−) mice. Statistical significance p<0.05 as compared to saline, n=4/group.
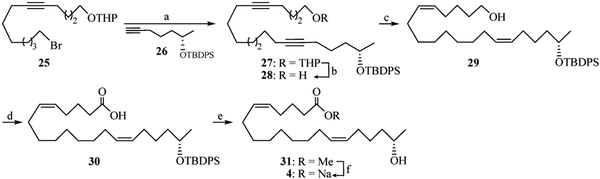
The synthesis of analog 4 is summarized in Scheme 1 and is representative of the procedures used to prepare the remaining analogs in Table 1 (see Appendix A. Supplementary data for details). Alkynylation of 2-((13-bromotridec-5-yn-1-yl)oxy)tetrahydro-2H-pyran26 (25) with the lithium anion of (S)-tert-butyl(hept-6-yn-2-yloxy)diphenylsilane27 (26) led to adduct 27. Selective deprotection using methanolic PPTS and semi-hydrogenation of the resultant primary alcohol 28 over P-2 nickel produced 29. Following Jones oxidation, acid 30 was concomitantly esterified and desilylated via marination in acidic methanol affording 31 from which 4 was obtained by saponification. Its enantiomer, 5, was prepared by an identical route beginning with commercial (R)-hex-5-en-2-ol.
Scheme 1.
Reagents and conditions: (a) (i) nBuLi, 26, THF/HMPA (4:1), −78 °C, 0.5 h, then 0 °C, 2 h. (ii) add 25 at −78 °C, stir at rt, 3 h, 72%; (b) PPTS, MeOH, 0 °C, 14 h, 68%; (c) P-2 Ni/H2 (1 atm), EtOH, 1 h, 86%; (d) Jones, acetone, −20 °C, 2 h, 69%; (e) pTSA, MeOH, rt, 24 h, 62%; (f) NaOH, THF/H2O, 12 h, 60%.
The foregoing studies confirm that 19-HETE analogs can function as a physiologically relevant counterbalance to 20-HETE (2). The recent correlation of higher 19-HETE levels with a better prognosis in acute coronary syndrome patients28 suggests in vivo efficacious 19-HETE agonist analogs warrant further evaluation as possible therapeutics for a wide range of inflammatory and cardiovascular conditions.
Supplementary Material
Acknowledgments
Financial support from the Robert A. Welch Foundation (I-0011), the Dr. Ralph and Marian Falk Medical Research Trust Bank of America, N.A., Trustee, and NIH HL034300 and HL111392.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: Genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediators 2015;120:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Sherbeni AA, El-Kadi AOS. Characterization of arachidonic acid metabolism by rat cytochrome P450 enzymes: The involvement of CYP1As. Drug Metab. Dispos 2014;42:1498. [DOI] [PubMed] [Google Scholar]
- 3.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res 2000;41:163. [PubMed] [Google Scholar]
- 4.Poloyac SM, Tortorici MA, Przychodzin DI, Reynolds RB, Xie W, Frye RF, Zemaitis MA. The effect of isoniazid on CYP2E1- and CYP4A-mediated hydroxylation of arachidonic acid in the rat liver and kidney. Drug Metab. Dispos 2004;32:727. [DOI] [PubMed] [Google Scholar]
- 5.Laethem RM, Balazy M, Falck JR, Laethem CL, Koop DR. Formation of 19(S)-, 19(R)-, and 18(R)-hydroxyeicosatetraenoic acids by alcohol-inducible cytochrome P450 2E1. J. Biol. Chem 1993;268:12912. [PubMed] [Google Scholar]
- 6.Qu W, Bradbury JA, Tsao CC, Maronpot R, Harry GJ, Parker CE, Davis LS, Breyer MD, Waalkes MP, Falck JR, Chen J, Rosenberg RL, Zeldin DC. Cytochrome P450 CYP2J9, a new mouse arachidonic acid omega-1 hydroxylase predominantly expressed in brain. J. Biol. Chem 2001;276:25467. [DOI] [PubMed] [Google Scholar]
- 7.Oliw EH, Guengerich FP, Oates JA. Oxygenation of arachidonic acid by hepatic monooxygenases, J. Biol. Chem 1982;257:3771. [PubMed] [Google Scholar]
- 8.Elkhatali S, El-Sherbeni AA, Elshenawy OH, Abdelhamid G, El-Kadi AO. 19-Hydroxyeicosatetraenoic acid and isoniazid protect against angiotensin II-induced cardiac hypertrophy. Toxicol. Appl. Pharmacol 2015;289:550. [DOI] [PubMed] [Google Scholar]
- 9.Escalante B, Falck JR, Yadagiri P, Sun L, Laniado-Schwartzman M. 19(S)-Hydroxyeicosatetraenoic acid is a potent stimulator of renal Na+-K+-ATPase. Biochem. Biophys. Res. Comm 1988;152:1269. [DOI] [PubMed] [Google Scholar]
- 10.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am. J. Physiol 1999;277:F790. [DOI] [PubMed] [Google Scholar]
- 11.Silverstein DM, Barac-Nieto M, Spitzer A. Multiple arachidonic acid metabolites inhibit sodium-dependent phosphate transport in OK cells. Prostaglandins, Leukotrienes Essent. Fatty Acids 1999;61:165. [DOI] [PubMed] [Google Scholar]
- 12.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol 2008;294:H1018. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Deng H, Kemp R, Singh H, Gopal VR, Falck JR, Laniado-Schwartzman M, Nasjletti A. Decreased levels of cytochrome P450 2E1-derived eicosanoids sensitize renal arteries to constrictor agonists in spontaneously hypertensive rats. Hypertension 2005;45:103. [DOI] [PubMed] [Google Scholar]
- 14.Capdevila JH, Wang W, Falck JR. Arachidonic acid monooxygenase: genetic and biochemical approaches to physiological/pathophysiological relevance. Prostaglandins Other Lipid Mediators 2015;120:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediators 2015;120:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elshenawy OH, Anwar-Mohamed A, El-Kadi AOS. 20-Hydroxyeicosatetraenoic acid is a potential therapeutic target in cardiovascular diseases. Curr. Drug Metab 2013;14:706. [DOI] [PubMed] [Google Scholar]
- 17.Waldman M, Peterson SJ, Arad M, Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediators 2016;125:108. [DOI] [PubMed] [Google Scholar]
- 18.Alexanian A, Sorokin A. Targeting 20-HETE producing enzymes in cancer - rationale, pharmacology and clinical potential. Onco Targets Ther 2013;6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang H, Al-Shabrawey M, Wang MH. Cyclooxygenase- and cytochrome P450-derived eicosanoids in stroke. Prostaglandins Other Lipid Mediators 2016;122:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison AR, Pascoe N. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc. Nat. Acad. Sci. U.S.A 1981;78:7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliw EH, Lawson JA, Brash AR, Oates JA. Arachidonic acid metabolism in rabbit renal cortex. Formation of two novel dihydroxyeicosatrienoic acids. J. Biol. Chem 1981;256:9924. [PubMed] [Google Scholar]
- 22.In recent studies, analog 7 conjugated to a photoactivated cross-linker was exploited for the isolation and identification of the first 20-HETE receptor. As designed, click coupling of the azide played a critical role in the isolation of the cross-linked protein. See Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE signals through G protein-coupled receptor GPR75 (Gq) to affect vascular function and trigger hypertension. Cir. Res 2017;120:1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchertanov L Structural metrics relationships in covalently bonded organic azides. Acta Cryst 1999;B55:807. [DOI] [PubMed] [Google Scholar]
- 24.Herr RJ. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem 2002;10:3379. [DOI] [PubMed] [Google Scholar]
- 25.Wu C-C, Schwartzman ML. The role of 20- HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediators 2011;96:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falck JR, Koduru SR, Mohapatra S, Manne R, Atcha KR, Manthati VL, Capdevila JH, Christian S, Imig JD, Campbell WB. Robust surrogates of 14,15-epoxyeicosa-5,8,11-trienoic acid (14,15-EET): Carboxylate modifications. J. Med. Chem 2014;57:6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moune S, Niel G, Busquet M, Eggleston I, Jouin P. Total synthesis of dolatrienoic acid: A subunit of Dolastatin 14. J. Org. Chem 1997;62:3332. [DOI] [PubMed] [Google Scholar]
- 28.Zu L, Guo G, Zhou B, Gao W. Relationship between metabolites of arachidonic acid and prognosis in patients with acute coronary syndrome. Thromb. Res 2016;144:192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.