Abstract
Nanomedicine continues to be a rapidly growing and increasingly interdisciplinary field. The career opportunities available in nanomedicine are also numerous, yet not always obvious to the early-career scientist determining their individual track for maximal impact. This perspective provides a brief overview of the field of nanomedicine, then delves into the many career trajectories one could take in this field. The article concludes with thoughts on how to provide diverse training to increase supply for the variety of career paths, and the role that mentors can play in young scientists’ development and exploration of these career paths.
Keywords: Nanotechnology, translational medicine, startup, consulting, science writing, policy, mentoring
Graphical Abstract
1. What is the field of nanomedicine?
Nanomedicine is the application of precisely-controlled materials at the 1 – 100 nm length scale to health and medicine [1]. Nanomedicine involves highly interdisciplinary science from a range of fields, including engineering, chemistry, physics, biology, medical sciences, and pharmaceutical sciences. Although considered still in its infancy as a scientific discipline, having first been coined in the 1990s, nanomedicine research has resulted in significant impact through a range of applications. These applications have included the development of therapeutics, imaging agents, and devices that have been applied to drug and gene therapy [2, 3], diagnostics [4, 5], monitoring, cell repair, artificial tissues, tissue regeneration [6, 7], and radiation therapy, among others. As this range of applications has grown, the market for nanomedicines has seen a corresponding rapid increase. The global nanomedicine sector was worth $53 billion in 2009 and reached $138.8 billion in 2016, with anticancer products representing the largest sector of this market ($33 billion in 2014). Abraxane, a human-serum albumin nanoparticle encapsulating paclitaxel, will have an estimated $967 million revenue stream in 2019 [8]. By 2025, the global nanomedicine market is anticipated to reach $351 billion, particularly with anticipated growth in developments in nanorobotics for targeted drug, surgery, or diagnostic delivery [9].
Nanomedicine has often been interchangeably used with the concept of targeted drug delivery. A literature analysis of the nanomedicine market in 2014 found that 76% of publications and 59% of patents focused on the drug delivery application of nanomedicine [10]. Drug-delivery based applications of nanomedicine still dominate the market, over regenerative medicine, in-vitro diagnostics, in-vivo diagnostics, and vaccine-based nanomedicine applications [9]. Around 40% of the products in phase II trials for clinical development are nanomedicine-based technologies, with indications in clinical oncology, infectious disease, cardiology, and orthopedics, among others [9]. A 2019 search on ClinicalTrials.gov results in over 250 ongoing clinical trials that are using nanoparticles for disease intervention.
The emphasis of nanomedicine on drug-delivery applications can be tied into the origination of the field. Inspired by the 1908 Nobel Laureate Paul Ehrlich and his proposed “magic bullet” approach [11], nanomedicine researchers have long sought to develop nanotechnologies that can selectively target a disease-causing organism or diseased cell, leaving healthy tissue unharmed. Nanotechnologies were applied to targeted drug delivery as early as the 1960s [12], but it wasn’t until 1995 that Doxil®, a doxorubicin-loaded liposome ~100 nm in diameter, became the first nanotherapeutic approved by the US Food and Drug Administration (FDA). Since then, the number of nanotechnology applications that have been tested in clinical trials has exceeded 200, and as of 2016, 51 FDA-approved nanomedicine platforms were on the market [13]. The European Medicines Agency (EMA) approved 8 of the 11 commercially available nanomedicines in 2016, and 48 nanomedicines were being tested in Phase I – III clinical trials in the European Union (EU) [14].
In the pursuit of fulfilling the “magic bullet” concept, nanotechnology-based platforms in medical application (Table 1) have increased the solubility, diffusivity, blood-circulation half-life, and bioavailability of drugs, genes, and imaging agents. Nanotechnology-platforms have enabled more controlled distribution, controlled or timed release, and reduced immunogenicity to therapeutics. In addition, the application of nanotechnology to medicine provided more convenient and diverse routes of administration, multi-functionality, lower therapeutic toxicity, and extended product life cycles. These benefits of nanotechnology have reduced administration frequency, dose, and toxicity.
Table 1.
Classification and dimensionality of nanotechnology-platforms used in medicine. Examples of various inorganic, organic, and biological nanoparticles are highlighted. Nanotechnology can refer to nanomaterials that have either one, two, or three nano-dimensions. In the context of this perspective, nanomedicine is application of any of the nanotechnologies described here to medicine specific problems.
| Nanotechnology Classification | ||
| Inorganic • Gold • Quantum dots • Iron oxide • Lanthanide • Silver • Zinc, copper oxide • Mesoporous silica • Carbon nanotubes |
Organic • Micelles • Liposomes • Dendrimers • Polymers (nanospheres, nanocapsules, micelles) • Cyclodextrins |
Biological • Exosomes • Lipoproteins • Ferritin • Viruses |
| Nanotechnology Dimensionality (adapted from [15]) | ||
| Three nano-dimensions • Nanoparticles • Nanocapsules • Fullerenes • Dendrimers • Quantum dots • Nanostructures • Nanopores |
Two nano-dimensions • Nanofibers • Nanowires • Nanotubes |
One nano-dimension • Nano thin films |
With this rapid expansion of the nanomedicine market alongside the increasing burden of complex disease, and the continuing pursuit towards a goal of targeted, personalized, and controlled medicine, it is critical to prepare current and future trainees for diverse career paths in the field of nanomedicine. This article will seek to highlight many of the career trajectories afforded to those in the nanomedicine sector, from careers in fundamental discovery to research and development to clinical translation, commercialization, and implementation. For early-career scientists, each section will discuss the scope of that career in the context of the nanomedicine field. Each section will not touch upon the relevant merits of each career path, but will instead provide a discussion of how one might get started in pursuing the specified career path. The article will then provide a perspective on training and mentoring needs to meet the demands of these career paths, and conclude with a forward look at professional opportunities, and areas of continued optimization, in nanomedicine.
2. Career opportunities in nanomedicine
There are a variety of professional directions one can take in the field of nanomedicine. This section aims to cover the types, needs, and expectations for these career paths, all with the overarching goal to further the advancement of a skilled workforce for clinical implantation of nanomedicine-based technologies. A common question early-in-career scientists might ask is what the right career path is for them, particularly in the context of comparing industry and academia. It is of note that the vast majority of jobs exist in industry and not in academia, where supply vastly outstrips demand. A recent assessment by neurobiologist Steve Luck captured this in a nutshell, stating that the number of available positions would need to increase exponentially and with no limit, if we wanted to provide a tenure-track faculty position for every new PhD who wants this type of position [16]. If we were to hold ourselves to this metric, we would need to create over a million positions by 2048 in a field that had only a thousand positions in 2018 [16]. While Table 2 provides the main features of academic and industry paths, it is important to consider there are many career opportunities that are available to those in the nanomedicine field, including translational medicine, startup companies, venture capitalism, patent law, science writing, regulation and policy, and consulting. Many of these opportunities are not isolated to specific sectors like industry, academia, government, especially since established expertise in any sector is a key feature of any career success. Importantly, skills sets developed in undergraduate and/or graduate degrees can be highly transferable to many of these career paths. For example, an early career scientist in industry will need to have the same level of technical skills and expertise as someone in their early years of academia. Therefore, the following sections are intended to highlight areas of emphasis for development for each career path, with some discussion of opportunities to explore those career options.
Table 2.
Features of academia and industry-focused careers. Aspects of the academic and industry career paths are outlined
| Academia | Industry | |
|---|---|---|
| Responsibilities | Apply for grants; Conduct self-directed research; Publish papers; Teach courses; Mentor students; Perform departmental, university, and professional service | Can be many things, with the scope of the work being focused on applied research that will have direct, clinical value. |
| Flexibility | Freedom to dictate your own schedule, and to generally choose when you teach, conduct research and publish your work. Not having to answer to anyone does require proficiency in time management and prioritization. | Time is more structured and typically revolves around a standard-length workday (i.e. 9–5pm). |
| Collaboration | Largely collaborative and team-work oriented, with a lot of opportunity for cross-disciplinary thinking and research. However, there is also autonomy, with the freedom to choose when and with whom you collaborate. | Work is towards a larger shared goal, so collaboration is a must across multiple functional areas of a company/organization. It is critical to success to be able to collaborate and work in a team. |
| Workplace culture | Highly research and discovery focused, with some research done for the sake of learning. Academics are under pressure to obtain funding, continually publish, to be a self-starter, and to promote and advocate for their work. | Highly application oriented, with deadline driven pressures and business-focused problem solving on tight project timelines that are in line with larger product and business goals. |
| Intellectual Freedom | Academics set their own research priorities, directions, and goals. Academics can choose what they spend their time on and how to pursue it. The responsibility of securing funding and resources is on the individual. | Industry professionals are driven by product or business goals. There is clear direction, but limited ability to investigate an individual’s own areas of personal interest. Funding and frontline resources are supplied by the organization. |
| Individual Impact | Significant, since you are not held accountable by anyone other than yourself. Impact can come in research, through mentoring, in the classroom, in the lab, or through service. However, ideas can take longer to adapt into practice. | Results are often immediately and directly impactful. Credit is often shared since all work is done in a team, so individual impact is lessened. However, the responsibility to achieve individual results in also less. |
| Pace | Timelines tend to be longer and focused on more long-term goals and education. | Fast-paced with short-term and well-defined timelines and goals. |
| Salary | Tenure-track professors across STEM fields make on average $70,000 - $115,000 annually depending on the field. | Industry scientists make about 30% more than those in academia for the equivalent years of experience. |
2.1. Academia
Scientists in academia are at the forefront of discovery and early development in nanomedicine. In addition to the significant increase in the number of Centers for Nanomedicine around the world, academics who research in nanomedicine are hired in a wider variety of departments than previously seen. These include traditional departments like chemical, biomedical, and mechanical engineering, as well as materials science, pharmaceutical sciences, physical sciences like chemistry and physics, and life sciences like biology, biophysics, and biochemistry. However, there are also an increasing number of interdisciplinary institutes where nanomedicine researchers in academia can seed a career.
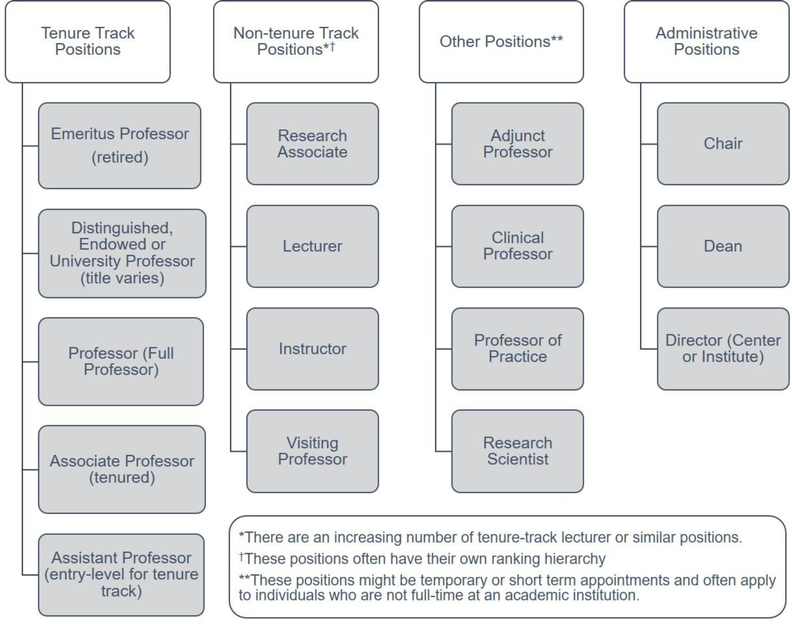
Academics in the nanomedicine space have the opportunity to be creative, and to research and discover the fundamental underpinnings of nanomedicines, as well as the next frontier of the field. There are also a multitude of positions available in academia beyond the tenure track faculty position (Figure 1). These often align with the expected day to day responsibilities of the traditional academic job, which include research, teaching, and service, as well as clinical time, if an M.D. is in your degree wheelhouse. Academia includes institutions that are research focused, as well as institutions that are non-research intensive, like liberal arts colleges and primarily undergraduate institutions. Following the growth of the nanomedicine field, there has been a subsequent increase in the number of academic degree programs, specialty options, and courses directed towards training students in nanomedicine. Therefore, if teaching nanomedicine principles, applications, and outlook are of interest, there also exists opportunities within and outside of research-intensive universities to build the fundamental and educational principles underpinning the field.
Figure 1. Representative academic positions in US-based institutions.
There are a multitude of academic opportunities following completion of doctoral or postdoctoral work. The specific academic titles vary depending on the institute, or the unit within the institute, and whether the institution is private, either for-profit or not-for-profit, or public. Institution types include community college, 4-year flagship state universities (“U of…”), 4-year land grant universities (“…State University”), primarily undergraduate institution, or small liberal arts college.
Within each type of academic position is the opportunity to determine how each day is spent. Therefore, in both determining if academia is the correct career path and what type of position in academia is best, several important considerations should be weighed [17]. It can be important to identify individual priorities, the desired way to spend time each day, and what personality and character traits are present, particularly in the context of the aspects of academia outlined in Table 2. Key skills necessary to be successful in academia include the ability to read and extract key information from highly technical documents, to give presentations, to analyze and synthesize disparate sources of data, to write in all forms, to be self-motivated, to drive and guide your own work, to teach, to lead and to mentor, and to prioritize and time-manage. These skills are certainly are certainly acquired over the time of a doctoral degree and can be further refined during a postdoctoral fellowship training period if a postdoctoral position is relevant, necessary, or useful for your academic path.
As an early-career scientist seeking a tenure-track academic position, it is important to prioritize publications, be prepared to do a postdoctoral fellowship in academia, government, or industry, and continually refine your vision and brand. To do this, attend seminars and conferences, present your work, network and discuss your ideas, and read a broad range of literature within and outside of your own field to stay updated on scientific advancements. Keep in mind that hiring in academia does not just come down to how impressive your curriculum vitae (CV) is – timing and fit are also critical. Fit can mean anything from the area of research, to what is needed in teaching, to what a given school might need in terms of expertise (rank and research) and diversity (demographic and research), to faculty personality. Therefore, while you want to demonstrate the uniqueness of what you bring to a department, college, or institution, it is important to recognize that the quality of the fit is just as much, if not more, a factor of the current needs of the department, and less about you as an individual candidate.
2.2. Industry
The nanomedicine market is driven by innovative technologies, advantages in healthcare applications, a rise in government support and funding, and demand for safe and cost-effective therapies. With the current nanomedicine industry projections showing 16% growth over the next decade, there is a need to match with a growing workforce. Scientists in industry have the opportunity to take emerging nanomedicine technologies from academia and translate them for commercial production and clinical use. Careers in industry allow for innovative nanomedicine product development, and provide the necessary in-depth physicochemical characterization, quality control, scale-up and reproducibility testing that are often limited in the academic lab setting. These steps are key to transition a bench-based idea into a clinical product in humans.
Industry work is often thought to require a more-business minded approach, where an employee must be able to meet the company’s goals and support its business plan, and remain accountable to product development timelines. The approach towards achieving these goals involves focus and prioritization towards design and budget management of research areas and research targets that will (1) address a highly unmet need and (2) create maximum value to the patient. A patient-centric design is of upmost importance in industry, because the nanomedicine platform must not only overcome the limitations of the standard of care for the target indication, but also improve patient compliance and patient quality of life [10].
Therefore, skills from scientific training at any level are critical to success in industry. Experiences in the research setting can develop the capability to gather and interpret information, analyze data, make decisions and problem solve, learn quickly, and manage a project. Most research experiences involve activities that include communication, leadership, time management, and planning. In industry, these skills are important to your ability to obtain a job and succeed in a career path. In fact, 57% of employers state that skills including communication, leadership, collaboration and time management are more critical than technical skills [18].
2.2.1. Translational Medicine
Translational medicine is especially important in the drug delivery field, where the process of implementing successful nanomedicine candidates in the clinic averages 15 years and anywhere from $800 million to $3 billion in funds. Less than one percent of pre-clinical tested nanomedicines are approved by the FDA for human clinical trials, and failure rates continue to be high at each stage of the process: 25% failure rate in Phase I, 25% failure rate in Phase II, and a 35% failure rate in Phase III [19]. There have been several analyses as to why this occurs [20–22], with some pharmaceutical companies responding by revising their research and development (R&D) strategy with the aim, and so far successful demonstration of, improving R&D productivity [23]. This revision led to the development and implementation of the ‘5R framework’, focused on the right target, the right tissue, the right safety, the right patient, and the right commercial product [24]. Through the adoption of the 5R framework, there has been a great focus on quality over quantity, with particular emphasis on using the right models to analyze variability and accurately report reproducibility [25] to push stronger quantitative decision making [23]. Further, big pharma has taken the opportunity to collaborate with academics and small-to-medium enterprises (SMEs) in generating more focused development insights, in particular for clinical and commercial development of early therapeutic prototypes.
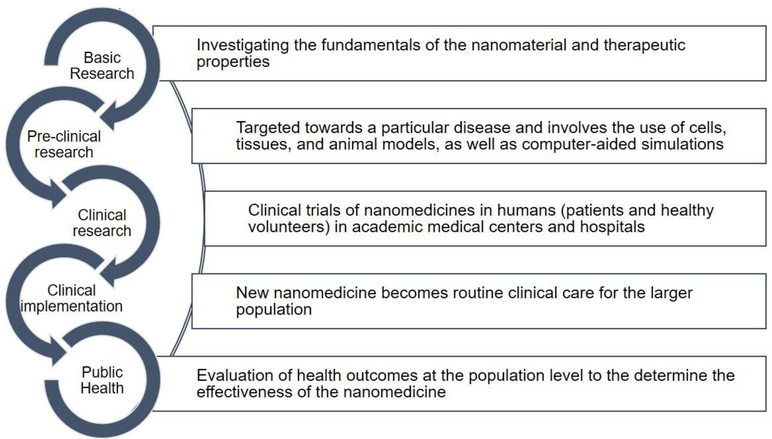
A career trajectory in translational medicine builds on research advances to develop new therapies and works to bridge therapeutic discovery with clinical application. Translational medicine aims to accelerate the time process for bringing the intervention from lab to clinic (Figure 2), create cross-functional collaborations among personnel working in different phases of the process, converge knowledge, technology, and expertise from various disciplines and apply them to a common and focused goal. Job opportunities come in the form of government, academic, or start-up sectors. Often times, M.D./Ph.D.’s are seen as the most obvious candidates to work in translational medicine. These individuals can drive bilateral information flow between knowledge gained from fundamental research and clinical observations gained from direct interaction with patients. However, there is growing acceptance that graduate programs that provide some medical, physiology, or pathology-focused coursework to Ph.D. students also create excellent candidates for the translational medicine workforce. Funding agencies including the National Cancer Institute and the National Institutes of Health offer courses to Ph.D. students, postdocs, and staff for training in translational medicine in order to learn about disease and medical problems [26]. MIT has formed an Institute for Medical Engineering and Science with translational research training and begun a postdoctoral translational fellows’ program to expose students early-on to the many aspects and challenges of the bench-to-bedside pipeline. There is also recent emergence of master’s programs for translation medicine, clinical sciences, and medical devices, geared towards providing engineers and health care professionals training that enables them to sit at the interfaces between these two disciplines. For early-career scientists seeking a career in translational medicine, it is important to be grounded in both the basic and applied worlds. This can come from joint research work with clinicians, enrolling in specific coursework to gain in-depth knowledge of the disease areas in which you plan to work, or even exploring shadowing and observership opportunities with clinical collaborators in clinics, hospital units, or operating rooms.
Figure 2. The phases of translational medicine research.
The focus or purpose of each stage of the process is briefly summarized.
2.2.2. Startup/Entrepreneurial
Startup career paths are one of the most talked about trajectories in the nanomedicine arena. In 2004, 200 companies worldwide were identified as having nanomedicine activities, 98 of which were based in the US [1]. This included 92 startups, 67 SMEs, and 41 large pharmaceutical companies. Out of the 159 SMEs and startups, 71 companies exclusively focused on nanomedicine technology [27]. Startups in nanomedicine are often initiated from an academic lab. Therefore, there is usually a direct feed of students from the academic lab to work full-time in the startup, especially for the student who was intimately involved in the later stages of research on the technology.
There are numerous career growth opportunities available in the startup space that appeal to the scientifically-applied mind. In a startup there is a lot of responsibility placed on employees, and a diverse array of responsibilities that can change regularly. It is not uncommon to have daily interaction between founders and employees, and middle management is usually nonexistent. This creates opportunity for learning and growth, as well as independence and ownership over decisions and their consequences. Additionally, startups tend to grow exponentially, which creates space for innovation and creativity, and a tight-knit team that will weather the ups and downs of a fast-paced environment. However, it is important to note associated downsides. Over 90% of startups fail within their first three years, so job stability and security can be a concern [28]. Workloads can be heavy on individual employees since every employee share in the growth and success of the company, and market trends must be capitalized on or responded to quickly.
Startup jobs are best described as an opportunity to learn that does not guarantee longevity [29]. Key considerations to keep in mind if heading for a career in startups are to: (1) be comfortable with change, from organizational to procedural to tactical; (2) be prepared to do every role within the company, even in a single day; (3) make the most of veterans in the field, through mentoring, expertise, and experiences; and (4) understand that the landscape changes and is not always predictable, but assessing the risks is still your own responsibility.
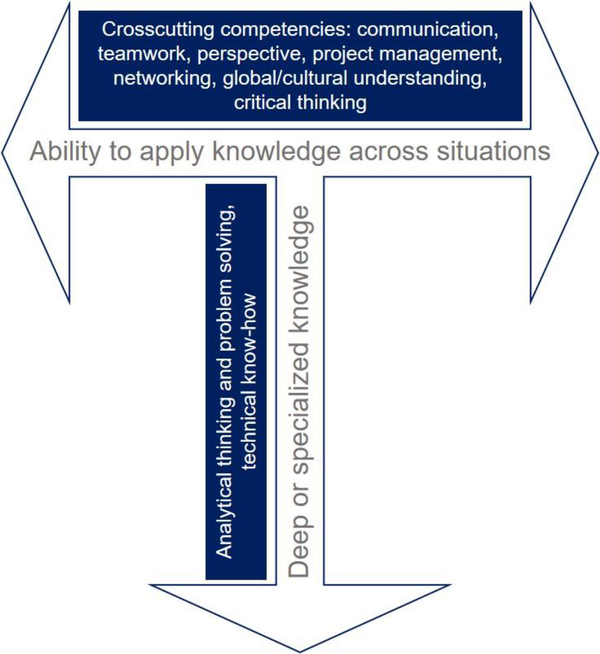
As with several other career trajectories discussed in this perspective article, there are several steps to take to best position yourself for a career in a startup. It is important to demonstrate your passion and show your marketability, for instance via a website and/or social media presence. A website can be used to generate content, maintain a resume, portfolio, and description of professional goals. Importantly, this forum can be a place to demonstrate your multi-dimensionality, commonly referred to as your T-skills, a term made popular in 1991 by David Guest [30] and first coined in 1978 by Tim Brown, CEO of IDEO. The vertical part of the T represents where you have the most depth or expertise in the field (i.e. nanomedicine) (Figure 3). The horizontal part of the T represents the ability to collaborate across disciplines with experts in other areas related to nanomedicine. It is important to know what range of skills you have in this context, and be able to demonstrate those skills. In highlighting these skills, it can be helpful to identify something that has been initiated by you, whether that be an organization, business, nonprofit, or project that conveys responsibility. If there were skills acquired as a part of the process of creating something of your own, those skills should be readily and concisely described. The startup world moves fast, so demonstrating an ability and passion for learning can be attractive to, and necessary for survival in, the startup culture.
Figure 3. The concept of a T-skilled worker.
A T-skilled worker shows both deep or specialized knowledge, and the ability to apply that knowledge across situations or disciplines.
2.3. Investor/Venture Capitalist
In 2009, investment in nanotechnology by venture capitalists (VCs) was $792 million, with 51% of this share going to healthcare and life science technologies [31]. It is anticipated that nanomedicine will continue to dominate VC funding in the healthcare market [32]. Therefore, expertise in the nanomedicine field can lead to high impact in the VC sector. A VC is an investor who provides capital to firms exhibiting high growth potential in exchange for an equity stake. Investors do not have to contribute through a VC firm, since a growing trend in investing comes from angel investors and crowdfunding. A VC can fund startup ventures or support small companies that aim to expand but do not have access to equities markets. VCs play a key role in establishing legal structures and marketing strategies for startup companies in nanomedicine, in addition to providing these companies with funding to meet financial demands, such as employee salaries, until the company is robust enough to secure sustainable funds. VCs can often provide value to a company beyond the pure financing, imparting managerial experience, industrial experience, contacts, and momentum [33]. VCs can come from a variety of paths and range from true entrepreneurs to tech journalisms to highly skilled investment bankers. However, most VCs have previous finance-industry experience working for a firm in technology, consulting, investment banking, media, or a startup, and 50% of VCs have an MBA, with 60% of those coming from Harvard or Stanford [34]. For specialist firms, having a PhD in a relevant topic can be necessary. If this is a career path of interest, there are several key aspects to consider in preparation for this career, outlined in Table 3, along with some starting resources to explore.
Table 3.
Areas of training emphasis for a career path as a VC. There are several ways to build the skill sets and portfolio necessary to be successful and obtain an entry level job for the VC career path. Additionally, resources to guide in exploration and pursuit of this career path are provided. VCs display a variety of skills that include the ability to ingest vast amounts of information quickly, prioritize and form opinions about that information, and be comfortable making decisions even when certain information is not available. Experience in finance, entrepreneurship, and journalism can in many ways provide these transferable skills, given that absorbing the information in these fields is often like drinking from a firehose. This information is also rarely perfect, yet decisions still have to be made. Therefore, developing skills in knowing where to fact check information, how to get more information, and how to build strong pattern-recognition are important, as well.
| What you need | Rationale | How to get started | ||
|---|---|---|---|---|
| Strong social media presence | 85% of VCs have a presence on LinkedIn and Twitter | Create content in written, blog form, podcasts or another form. Demonstrate time spent thinking critically about investing in startups. | ||
| Expertise in a specific technology | To be seen as the go-to for answers on the technology | Obtain technical, consulting, legal or finance experience in the field of interest, plus an MBA or a PhD. | ||
| Up-to-date knowledge of top VC blogs and technology news sites | Know the current landscape and markets in the field of interest | Read voraciously – there are many online resources, but a few resources are listed below. | ||
| Investment experience | Establish a track-record to show how you think about investing money | Angel invest from personal funds, or contribute to equity crowdfunding campaigns, or create a shadow portfolio that tracks investments you would have made (and how they performed) if you had the money to invest. | ||
| Known strengths and/or unique experiences | Lean on and amplify strengths to make you an attractive candidate for one firm or another. | |||
| A network | The networking mindset is an important part of the VC job | Go to industry-specific events and meetups, get an intro from a VC, or get an intro from an entrepreneur a VC firm has invested in, participate in information interviews with VCs |
||
| Resources for exploring the VC career path | ||||
| Job Sites | Podcasts | Books | Websites/Newsletters | Investment Sites |
| John Gannon’s Venture Capital Jobs Stephan von Perger’s European Venture Capital Jobs |
20 Minute VC Invest Like the Best How I Built This This Week in Startups |
Break into VC by Bradley Miles Venture Deals by Brad Feld The Business of Venture Capital by Mahendra Ramsinghani The Lean Startup by Eric Ries Angel by Jason Calanis |
StrictlyVC TechCrunch Avc.com Investopedia |
SeedInvest WeFunder AngelList Funder’s Club |
It is important to note that even if a VC career is not for you, many scientists interact with VCs, angel investors, VC firms, and crowdfunding to move a technology from the lab into the commercial space, or from a startup to a SME. Although outside the scope of this perspective, a recent write-up by Sarah Kellogg provides insights into the how entrepreneurial academics can understand the financial landscape and motivations of investing firms [35]. For further information regarding the process of translation from idea inception to market, the reader is referred to Morigi et al., which provides an assessment of the challenges and opportunities in financing nanomedicine [10].
2.4. Journalism/Science Writing
Every scientist learns during their career that communicating about science, engineering, and technology is critical to the advancement of a field. Scientists in training most often confront publicly-disseminated writing opportunities through regular contributions to publications, conference proceedings, websites, and increasingly through social media. However, there are many ways to incorporate science writing into a full-time career (Table 4). For example, full-time science writing opportunities exist for university or organizations to write, publish, edit, produce multimedia, and promote the research taking place at the institution. There is also the opportunity to write freelance for different companies, blogs, or media outlets. Freelancers can seek out clients, pitch story ideas, manage their own business, and promote themselves as an independent entity.
Table 4.
Career opportunities in science writing in nanomedicine. There are two basic categories of science writing, science journalists and science public information officers.
| Publishing | Nonprofits |
| Writer | Press Package Writer |
| Science Writer | Science Content Specialist |
| Editor | Communications Outreach Administration |
| Science Communicator | |
| Scientific Illustrator | |
| Self-Employment | Research & Development |
| Freelance Writer | Communications Associate |
| Contributor | Science Writer |
| Science Editor | |
| Academia, Officers of News and Communications | |
| Senior Writer | |
| Science Writer | |
Science writers play a necessary role in the advancement of the social and political conversations around research discoveries, often by communicating an independent assessment of these findings. The science writer must translate a scientific discovery into language accessible by a broad audience, while also attempting to put a discovery into historical, personal, political, economic, and social context [36]. Science writers might write for a larger lay public or other scientists, physicians, and engineers. This challenging role could not be better captured by the nanomedicine field. In a constantly changing landscape, with rapid discoveries, controversial findings, and many “hopes and dreams” of medicine on its shoulders, nanomedicine necessitates science writers to balance the conflicting opinions of scientific experts with the business and financial aspects of the technology. Historically, nanotechnology has maintained a controversial, and often not positive view in the world of pop culture, from Stanislaw Lem’s 1984 novel Peace On Earth, where nanorobots inhabit earth and destroy all modern technology, to Michael Crichton’s 2002 book Prey, where a swarm of nanorobotics develop intelligence and collectively destroy humanity. Since the publication of Eric Drexler’s 1987 book “Engines of Creation: The Coming Era of Nanotechnology” [37], a heated debate around the possibilities, promises, and problems of nanotechnology has ensued in the public forum. This debate has certainly played out in the nanomedicine arena, where the potentials of nanomedicine are frequently countered with the unknown long-term effects on the body, and the large investment ($1 billion in North America in 10 years) that has so far resulted in limited translation to patient care. Therefore, in particular in the field of nanomedicine, where concerns around the current “reputation of [nanomedicine] being “hype” that cannot deliver” [38] continues to cloud public and clinical perception, accurate and balanced communication of creative and new approaches to developing nanomedicines remains an important nanomedicine career path.
To get into science writing in nanomedicine as a career, early-career scientists must not only learn to write about the broader field nanomedicine, but also develop multimedia and social media skills, including the use of photo and video editing software [39]. Inherent in this is the ability to understand techniques for managing blogs, podcasts, and social media postings on Facebook, Twitter, Instagram, and LinkedIn, among others. Many universities and colleges offer specialized courses and graduate programs in science writing, and future science writers could take courses in journalism complimentary to their STEM degree. While pursuing an undergraduate or graduate degree, writing science stories for a school newspaper or magazine can be a way to build a portfolio and gain more experience writing on topics not directly related to your own research experiences. It is not uncommon for university news offices to hire writing interns, and many local newspapers have opportunities for internships or even for freelance writing. For professional development, aspiring writers should join the National Association of Science Writers, and devour articles in scientific journals and magazines such as Scientific American, Discover, Popular Science, Science News, Wired, Science, and Nature.
2.5. Regulatory/Policy
Beyond the world of basic research and development, there exists a challenging relationship between regulation and innovation, which is readily exemplified in the nanomedicine field. Currently, the FDA approval process for nano-based therapies is the same as that for any other drug or biologic [40]. Given that nanotechnologies are implemented to alter the safety, toxicity, pharmacokinetic, and pharmacodynamic profiles of drugs and biologics, and nanomaterials have fundamentally different properties, there is ongoing discussion as to whether nanomedicines should have their own regulatory process [41, 42]. While all stakeholders in the nanomedicine field are critical to identifying regulatory needs for emerging nanomedicine technologies, it is an important directive of the regulatory bodies to standardize development and acceptance of information requirements. A combination of surveys performed from 2015–2017 compiled standardized test methods for the assessment of physicochemical properties and standardized test methods for the evaluation of safety of nanomedicines [43]. The analysis found major gaps in the standards for nanomedicines, with particular concerns around methods assessing drug loading and drug release, as well as the interaction of the nanomedicine with the blood and immune system. There also were recommendations for further standardization of methods for investigating the protein corona on nanomedicines, for detection of nanomedicines in biological tissues, a continued emphasis on raw data requirements, and robust dataset development.
For those scientists interested in exploring regulatory career paths, there are a broad repertoire of opportunities that will play a key role in the ongoing implementation of nanomedicines in patients. Regulatory professionals aim to facilitate the commercialization of safe and effective products and services. The range of careers within the regulatory space of nanomedicine can span managing clinical studies or developing marketing approval policies to designing labels for drug-related products. Successful regulatory professionals have cross-functional training in science, engineering, pharmacy, marketing, and business, but a prior career in regulatory affairs is not required [44]. About 88% of regulatory professionals began working in a different sector before transitioning to regulatory affairs [45]. To get started in this career trajectory, it is important to stay current on regulatory affairs materials that relate to the law, environment, technology, and global economics, in particular those related to food and drug regulation. The US FDA, Federal Register, and Drug Information Association are starting resources for daily up-to-date information. As with many disciplines, continually refining communication skills, analytical and critical thinking, negotiation techniques, and project management methods will be necessary to be successful in the regulatory space. Additionally, developing the capability to synthesize information on a global scale and to understand, and eventually produce, culturally-agile programs is highly valued in the regulatory space.
Importantly, policy will continue to be critical to the ongoing advancement and incorporation of nanotechnology into medical applications. As with any rapidly changing discipline, the amount of regulation needed in the field continues to be a pressing policy debate. There are concerns that overregulation could slow down scientific research and innovation, which could limit the benefits of nanomedicine. However, the promises of nanomedicine introduce numerous ethical concerns that will need thoughtful, comprehensive, and adaptable policy [46]. For example, nanotechnology in some sectors not only promises a longer life (in the case of nanomedicine), but also human enhancement, even if only in theory. This will come at a cost, and in the extreme sense, could create a divide between those who can afford treatments with these technologies and those that cannot.
Science policy professionals serve as a bridge between researchers and policy makers, as experts in their scientific field. This aspect of the nanomedicine industry is applicable in government and non-government settings and requires engagement with both scientific and non-technical colleagues. An individual in this space will help to define and promote scientific goals, priorities, and research directions, advocate for research funding, and monitor how policies impact the scientific research community. To prepare for a career in policy and government, it is important to get involved with student government or policy committees within your institution or organization. At many scientific conferences, there are symposia and sessions focused on regulatory and policy considerations. There are also several fellowship opportunities, through American Association for the Advancement of Science (AAAS), the US National Academies, and the American Institute for Medical and Biological Engineering (AIMBE) Scholar Program, that aid in training of early-career scientists for science and technology policy issues.
2.6. Intellectual Property and Patent Law
From 1996 to 2007, close to 3000 patents were issued in the US with the reference to nanomedicine [47]. Every startup company in the nanomedicine sector has needed to navigate the intellectual property aspect of their technology. Additionally, large pharmaceutical companies keep patent attorneys on hand to protect developed or acquired intellectual property. Therefore, it is worth considering the cluster of non-traditional, yet important career paths that involves writing, filing, examining, and disputing patents related to nanomedicine technologies [48]. Scientists can become patent agents, patent examiners, and technology transfer specialists, all careers that do not require law degrees. If a second degree is in the career pipeline, then patent attorney positions can open the door to a wider range of opportunities, and higher salaries.
The patent law career path in nanomedicine would necessitate an interest in the legal skills of drafting, analysis, and logical thought, and particular skill in the apt and accurate use of language. A career path in intellectual property would require strong analytical skills and an understanding of complex technologies, and their applications. The day to day endeavors of someone in the intellectual property field can vary, depending on the type of job, credentials, and sector. For example, a tech transfer specialist at a university can be the first point of contact for scientists who have made a nanomedicine discovery that could have commercial value. The pathway for nanomedicine from the lab to the clinic often finds its way through a technology transfer office at an academic institution.
Scientists are in high demand from law firms, but the competition for jobs is also high. For applicants to stand out, it is important for early-career scientists to have strong scientific credentials and a demonstrated interest in intellectual property [49]. The traditional path towards becoming a patent attorney is to obtain a several year traineeship at a patent law firm, followed by formal coursework and completion of licensing exams. Prior to doing this, or if a patent attorney is not the target career trajectory, there are opportunities during an undergraduate or graduate education to engage with an institution’s technology transfer office. Technology transfer officers often have internships, volunteer work, or innovation scholar programs that expose students to hands-on training, workshops, and coursework in filing and evaluating reports of invention, assessing the current market, and determining the intellectual property boundaries of a proposed technology. In addition, joining local intellectual property associations can create an opportunity to network with those already in the industry.
2.7. Consulting
The rapidly changing field of nanomedicine introduces challenges in staying up-to-date on regulatory guidelines, policy, novel research and development, new and higher throughout methodologies, and current pre-clinical and clinical trial outcomes. Consultants play a critical role in the field of nanomedicine, through a variety of means, and require specialized expertise and an ongoing commitment to perform a great deal of research on a weekly basis. In this sense, a consulting career path in nanomedicine can be a natural transition of acquired skills for many students with science and engineering doctoral degrees. A consultant can work with a startup to design the best entry into the marketplace, to make an existing business more profitable, or to increase efficiency. Consulting positions exist in private business, government, and the non-profit sector. Academics can also play a consulting role, provided conflicts of interests are declared and institutional policies are followed. Entry into the consulting field requires experience, and therefore it is not uncommon for individuals pursuing consulting to work in other aspects of nanomedicine or related fields prior to seeking an entry-level consulting position.
3.0. Diversifying training to prepare for a career in nanomedicine
Nanotechnology is predicted to continue to take a larger role in medicine, creating more opportunities for those who have medically-applied nanotechnology experience. Due to the richness and variety of job opportunities in the nanomedicine sector, diversified training is required to best prepare the workforce. Roles in the nanomedicine field require a holistic understanding of a large number of subject areas, including but not limited to physics, chemistry, materials science, biochemistry, and medicine. It can also be useful, depending on the chosen career path, to have a foundation in formulation science, pharmaceutical science, biostatistics, and physiology. Learning the language and culture of the different fields is also a critical step in building the capacity to bridge those fields. Demand is high in industry for professionals that can speak the languages of engineering and biology, for example, and have the skills sets that can bridge these two fields to apply a nanomedicine application to a pathobiological problem. Therefore, cross-training in more than one discipline will create more marketable scientists with the ability to contribute broadly to the nanomedicine workforce. Life scientists can hone skills in techniques and tactics employed by engineers and physical scientists, and engineers and physical scientists can do the same in the life sciences. Medical students and physicians can also work to further their education in engineering principles, through short courses or project-based, self-motivated learning. Incorporating approaches from STEM education into medicine, such as flipped classrooms, group learning, and real-world problem solving, could provide medical students the opportunity to learn these essential subjects without imposing more time on their already rigorous and lengthy education.
Regardless of the specific approach, there is growing opinion that the current infrastructure in which we train scientists needs to be modernized to reflect the interdisciplinary nature of many scientific disciplines, including the field of nanomedicine. It is on the entire nanomedicine community to build educational and training infrastructure for future generations of nanomedicine innovators. Initiatives by higher education, funding agencies, industry, and professional societies can support academic centers that integrate engineering, basic science, and clinical translation through private-public partnerships. Many suggestions have been put forward, including providing new types of graduate programs, with branching options after the first or second year that could specifically prepare a student for entrepreneurship, business, teaching, policy, and consulting [50]. These would need to be integrated into existing standard science PhD programs, as opposed to stand-alone programs. These integrative graduate programs can be created from forging new partnerships between organizations, whether that be programs across different universities or between schools within a university (e.g. a College of Engineering with a School of Public Health).
Cross-disciplinary coordination could have immediate impact is in graduate education. Currently, many academics develop courses on the aspect of nanomedicine in which they have expertise. Courses created by faculty in different departments or colleges can cause scheduling conflicts, exposure to biased or singular perspectives, or limited understanding of the full scope of the nanomedicine field. Alternatively, having one class that covers the entire spectrum of nanomedicine would limit depth. Instead, departments and colleges could collaborate on educational programs, specialty options, or certifications that align coursework in a focus area such as nanomedicine to be complimentary and transcriptable. This would not only allow a more in-depth analysis of the field by taking advantage of the various expertise that exist amongst the faculty and local industry partners, but would also expose students to different approaches and perspectives given that no one department, college, or discipline would be teaching all of the coursework. Additionally, this builds an infrastructure for incorporation of design-based or capstone-based projects that could incorporate clinical and industry partnerships in the form of startup, intellectual property, writing, business, law, or translational hands-on experiences for students that are directly relevant to their doctoral degree. Importantly, there is an increasing role that industry sponsorship can play in graduate and postdoctoral training programs. There are pharmaceutical companies that are jointly sponsoring training programs with funding bodies or academia, which provides trainees direct exposure to the various functions in industry that are harder to get at in an academic lab setting. These experiences can afford trainees the chance to understand key development or implementation decision making processes in an industry setting based on the level, detail, and rigor of data obtained for a prototype or product. Additionally, trainees can gain exposure to documentation preparation and maintenance to submit to regulatory bodies, maintain good manufacturing practices, and implement sustainability practices. Considering that almost all individuals in industry did their foundational and/or secondary education in nanomedicine in academia (through a bachelors, masters, or doctoral degree), there is hope to see a continued increase in industry-academia partnerships. These partnerships will be instrumental in providing earlier-stage training opportunities by closing the loop between the fundamental discovery stages of nanomedicine at the bench to the implementation in humans at the bedside.
4.0. Early-stage mentoring towards a career in nanomedicine
A critical role of Principle Investigators (PI), faculty, and the professional community is to model the approaches that will enable student success in the nanomedicine arena. Many faculty in research-intensive universities still associate success in graduate school with obtaining a tenure-track faculty position in a research-oriented university. However, it is worth considering that to meet the demands of a rapidly growing field while not oversupplying academic (faculty) candidates in a detrimental and hypercompetitive manner, a larger value should be placed on industry and teaching positions. Yet, supervisors of the current and future generations of scientists are themselves academics, which potentially limits their ability to provide in-depth insights into the breadth of careers listed above. This could serve to restrict mentoring of students wishing to pursue a non-academic path, which incidentally will be the majority of students.
Fortunately, PIs in the field of nanomedicine are often in the midst of multi-faceted careers. There are numerous examples of nanomedicine-focused academics [51] who have engaged with VCs, started companies, served on advisory boards, consulted for the government, organizations, or start-ups, filed patents, and collaborated intimately with clinicians in the nanomedicine research and development process. Nanomedicine researchers are increasingly becoming entrepreneurs and innovators who transfer research to products and create startup companies. This exposes these researchers to the business sector, where it is necessary to manage employees, build infrastructure, and track profit. Through these efforts, many nanomedicine researchers also become more aware of the social and ethical responsibilities that intersect with their technology. Therefore, these researchers are uniquely positioned to have experiences in many aspects of the field of nanomedicine.
Continued participation of these senior level professionals in the mentoring of up-and-coming scientists is necessary, but the mentoring of early-career scientists should not fall solely on their shoulders. Young academics and industry professionals have the unique opportunity to mentor with experiences fresh in their mind, when the “how exactly did you go about that?” answer is still recent, and sometimes even ongoing. Experience and advice come in many forms, and longevity in a discipline should not be the only guidepost for mentoring. Every nanomedicine professional, regardless of current career position, has the opportunity to pass on information gathered along their career path, and are encouraged to do so through conversation, conferences, blogs, podcasts, editorials, tweets, panels, and other social media forums. For those not in an academic setting, staying connected to the respective undergraduate or graduate degree granting institution provides a host of eager students ready to soak up insights and connect with those in the field. Although time is always limited, it is critical to keep in mind that small efforts here and there to discuss experiences, share advice, and provide transparent and honest insights into one’s own career path are impactful and valuable to those coming along the path behind us.
5.0. Looking forward
Since its inception, nanomedicine has experienced an immense amount of expectation, with promises for integration of biology and technology, the eradication of disease, personalized medicine, targeted drug delivery, regenerative medicine, and nanomachinery that can substitute for portions of cells, or nanorobotics to perform noninvasive surgeries. However, nanomedicine has to continue to overcome many challenges to fulfill all of its promises to healthcare [52], some of which might be attributable to the relative youth of the field. There is a current gap in the timeline between product development and policy development. The lack of specific regulatory guidelines has many critics wanting the FDA to establish regulatory guidelines specifically to apply to nanomedicine products, rather than adapting and applying existing regulations. Tied into this is the need for consistent categorization through standardized definitions, nomenclature, and reference materials. Standards will also need to be set for characterization of nanoparticles, particularly in the context of safety and toxicity. Therefore, continued skill development of early-career nanomedicine scientists necessitate emphasis in formulation, bioanalytical, and pharmaceutical sciences. Safety and toxicity are even more of a concern when evaluating the long-term impact of nanomaterials on the human body, especially for platforms that do not biodegrade. It is not necessarily easy to demonstrate the safety and efficacy sufficient to meet an individual patient needs in a competitive market when there are multiple products on the market, or when the target indication is a difficult-to-treat disease. Additionally, the difficulty of interpreting preclinical and clinical studies is compounded by our still limited understanding of the fundamental processes in the human body, which is critical in the context of nanomedicine, where nanomedicines will interact with multiple organs, tissues, and cell types on their path from administration site to target site.
Perhaps of increasing importance is the ability of this generation and future generations of scientists to parse out high quality data, integrate multi-scale and multi-modal data, and manage data. Alignment of findings from different nanoparticles platforms applied to the same disease indication, even in multiple species or preclinical models, can increase the interpretability of data. However, this requires skills in data mining, data organization, and data management, which are not consistently taught in relevant nanomedicine disciplines such as medicine, life sciences, engineering, business, and law. Research is increasingly data and computation driven, and the necessity of these skills are going to be integral in research, hiring, and the understanding of complex systems. In addition to standardizing how data is collected and reported, which is a large effort in many fields beyond nanomedicine, this data will also need to be made more readily available from preclinical and clinical trial studies. The growing expectation of publishing raw data with peer-reviewed manuscripts helps increase the ability to interpret and analyze data. The onus is on publishers, researchers, companies, investors, and healthcare providers to increase transparency in data generation and make data available for public use, similar to what has been achieved in the weather and astronomical data fields.
It is also important to note that nanomedicine and nanomedicine researchers can continue to have impact beyond their immediate field, as the career trajectory progresses. Through the development of nanomedicine technologies there has been a parallel rapid advancement in engineering of new tools for investigating these technologies. Techniques such as scanning probe microscopes and optical tweezers have permitted the quantitative analysis of single molecule biophysics. Advances in synthesis methodologies, electron microscopy, and spectroscopy techniques have enabled atomic level control and characterization of nanoscale materials. The continued investment in enabling technologies to better visualize, characterize, and understand nanomedicines will also remain necessary for the future of the nanomedicine field.
Tackling the multitude of challenges that we continue to face in the nanomedicine arena is no easy task, and thus requires a holistic approach to training highly-skilled researchers who can create, apply, and advance these technologies from the various sectors of this field. Ongoing efforts by universities, funding agencies, and entry-level job providers in industry are creating initiatives, cross-disciplinary institutes, degree programs, and professional training workshops to provide diverse and practical hands-on training experiences for early-career scientists. Additionally, continued innovation in education and training is still needed in science, engineering, and medicine to build valuable skillsets to drive the greatest advances, which come only when disciplines coalesce into a continuum.
Acknowledgements
The author would like to thank all prior and current mentors in the nanomedicine field for their continued insights and leadership. These include J. Hanes, K. Rangaramanujam, S. Kannan, S. Mitragotri, H. Ghandehari, S. Pun, and P. Stayton. The author was supported by the Burroughs Wellcome Fund Career Award at Scientific Interfaces and the National Institute of General Medical Sciences MIRA (1R35GM124677-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wagner V, Dullaart A, Bock AK, Zweck A, The emerging nanomedicine landscape, Nat Biotechnol, 24 (2006) 1211–1217. [DOI] [PubMed] [Google Scholar]
- [2].Angelakeris M, Magnetic nanoparticles: A multifunctional vehicle for modern theranostics, Biochim Biophys Acta Gen Subj, 1861 (2017) 1642–1651. [DOI] [PubMed] [Google Scholar]
- [3].Ramos AP, Cruz MAE, Tovani CB, Ciancaglini P, Biomedical applications of nanotechnology, Biophys Rev, 9 (2017) 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim J, Mohamed MAA, Zagorovsky K, Chan WCW, State of diagnosing infectious pathogens using colloidal nanomaterials, Biomaterials, 146 (2017) 97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yoon HY, Jeon S, You DG, Park JH, Kwon IC, Koo H, Kim K, Inorganic Nanoparticles for Image-Guided Therapy, Bioconjug Chem, 28 (2017) 124–134. [DOI] [PubMed] [Google Scholar]
- [6].Alarcin E, Guan X, Kashaf SS, Elbaradie K, Yang H, Jang HL, Khademhosseini A, Recreating composition, structure, functionalities of tissues at nanoscale for regenerative medicine, Regen Med, 11 (2016) 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hamdan S, Pastar I, Drakulich S, Dikici E, Tomic-Canic M, Deo S, Daunert S, Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications, ACS Cent Sci, 3 (2017) 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Havel HA, Where Are the Nanodrugs? An Industry Perspective on Development of Drug Products Containing Nanomaterials, AAPS J, 18 (2016) 1351–1353. [DOI] [PubMed] [Google Scholar]
- [9].G.R. Research, Nanomedicine Market Analysis By Products, (Therapeutics, Regenerative Medicine, Diagnostics), By Application, (Clinical Oncology, Infectious diseases), By Nanomolecule (Gold, Silver, Iron Oxide, Alumina), & Segment Forecasts, 2018 – 2025, Grand Review Research, 2017, pp. 165. [Google Scholar]
- [10].Morigi V, Tocchio A, Bellavite Pellegrini C, Sakamoto JH, Arnone M, Tasciotti E, Nanotechnology in medicine: from inception to market domination, J Drug Deliv, 2012 (2012) 389485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Greiling W, Ehrlich Paul, Leben und Werk, Düsseldorf, Econ Verlag, (1954). [Google Scholar]
- [12].Kreuter J, Nanoparticles--a historical perspective, Int J Pharm, 331 (2007) 1–10. [DOI] [PubMed] [Google Scholar]
- [13].Ventola CL, Progress in Nanomedicine: Approved and Investigational Nanodrugs, P T, 42 (2017) 742–755. [PMC free article] [PubMed] [Google Scholar]
- [14].Choi YH, Han HK, Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics, J Pharm Investig, 48 (2018) 43–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goyal S.a.G., Ashutosh, Nanotechnology - A Path to a Sustainable Future, Research & Reviews: Journal of Material Sciences, 2016. [Google Scholar]
- [16].Luck S, Some thoughts about the hypercompetitive academic job market, Luck Lab, 2018. [Google Scholar]
- [17].Searls DB, Ten simple rules for choosing between industry and academia, PLoS Comput Biol, 5 (2009) e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Petrone P, The skills companies need most in 2018, LinkedIn Learning, LinkedIn, 2018. [Google Scholar]
- [19].Jang HL, Zhang YS, Khademhosseini A, Boosting clinical translation of nanomedicine, Nanomedicine (Lond), 11 (2016) 1495–1497. [DOI] [PubMed] [Google Scholar]
- [20].Peck RW, Lendrem DW, Grant I, Lendrem BC, Isaacs JD, Why is it hard to terminate failing projects in pharmaceutical R&D?, Nat Rev Drug Discov, 14 (2015) 663–664. [DOI] [PubMed] [Google Scholar]
- [21].Kola I, Landis J, Can the pharmaceutical industry reduce attrition rates?, Nat Rev Drug Discov, 3 (2004) 711–715. [DOI] [PubMed] [Google Scholar]
- [22].Garnier JP, Rebuilding the R&D engine in big pharma, Harv Bus Rev, 86 (2008) 68–70, 72–66, 128. [PubMed] [Google Scholar]
- [23].Morgan P, Brown DG, Lennard S, Anderton MJ, Barrett JC, Eriksson U, Fidock M, Hamren B, Johnson A, March RE, Matcham J, Mettetal J, Nicholls DJ, Platz S, Rees S, Snowden MA, Pangalos MN, Impact of a five-dimensional framework on R&D productivity at AstraZeneca, Nat Rev Drug Discov, 17 (2018) 167–181. [DOI] [PubMed] [Google Scholar]
- [24].Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN, Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework, Nat Rev Drug Discov, 13 (2014) 419–431. [DOI] [PubMed] [Google Scholar]
- [25].Scannell JW, Bosley J, When Quality Beats Quantity: Decision Theory, Drug Discovery, and the Reproducibility Crisis, PLoS One, 11 (2016) e0147215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chien S, Bashir R, Nerem RM, Pettigrew R, Engineering as a new frontier for translational medicine, Sci Transl Med, 7 (2015) 281fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wagner V, Husing B, Nanomedicine: Drivers for development and possible impacts, in: Bock AK (Ed.) JRC Scientific and Technical Reports, 2006. [Google Scholar]
- [28].Marmer M, Hermann BJ, Dogrultan E, Berman R, Startup genome report extra on premature scale: a deep dive into why most high growth startups fail., 2011, pp. 59.
- [29].Berger L, Six things new grads should know before joining a startup, Harvard Business Review, 2017. [Google Scholar]
- [30].Guest D, The hunt is on for the Renaissance Man of computing, The Independent, 1991. [Google Scholar]
- [31].Highsmith J, Nanoparticles in Biotechnology, Drug Development and Drug Delivery, Market Research ReportsBCC Research, 2014. [Google Scholar]
- [32].Pandotra T, Nanomedicine: delivering on its promise?, 2017.
- [33].Gompers PA XY, Bridge Building in Venture Capital-Backed Acquisitions, Harvard Business School, (2009). [Google Scholar]
- [34].Kamps HJ, How to get a job in Venture Capital, Medium, 2018. [Google Scholar]
- [35].Kellogg S, Start-ups: In search of venture capital, Nature, 472 (2011) 379–380. [DOI] [PubMed] [Google Scholar]
- [36].Dean C, Am I Making Myself Clear?, Harvard University Press, 2009. [Google Scholar]
- [37].Drexler KE, Engines of Creation. The coming era of nanotechnology, Anchor Books, New York, 1986. [Google Scholar]
- [38].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nature Reviews Materials, 1 (2016) 16014. [Google Scholar]
- [39].Blum D, Knudson M, and Henig RM, A Field Guide for Science Writers: The Official Guide of the National Association of Science Writers, Oxford University Press; 2005. [Google Scholar]
- [40].Havel H, Finch G, Strode P, Wolfgang M, Zale S, Bobe I, Youssoufian H, Peterson M, Liu M, Nanomedicines: From Bench to Bedside and Beyond, AAPS J, 18 (2016) 1373–1378. [DOI] [PubMed] [Google Scholar]
- [41].Ventola CL, The nanomedicine revolution: part 3: regulatory and safety challenges, P T, 37 (2012) 631–639. [PMC free article] [PubMed] [Google Scholar]
- [42].Bawa R, Regulating nanomedicine - can the FDA handle it?, Curr Drug Deliv, 8 (2011) 227–234. [DOI] [PubMed] [Google Scholar]
- [43].Halamoda-Kenzaoui B, Holzwarth U, Roebben G, Bogni A, Bremer-Hoffmann S, Mapping of the available standards against the regulatory needs for nanomedicines, Wiley Interdiscip Rev Nanomed Nanobiotechnol, 11 (2019) e1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Joubert S, Working in Regulatory Affairs: Careers and Trends, Northeastern University Graduate Programs, 2018. [Google Scholar]
- [45].R.A.P. Society, Scope of Practice and Compensation Report for The Regulatory Profession, 2016, pp. 21.
- [46].Resnik DB, Tinkle SS, Ethical issues in clinical trials involving nanomedicine, Contemp Clin Trials, 28 (2007) 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].du Toit LC, Pillay V, Choonara YE, Pillay S, Harilall SL, Patenting of nanopharmaceuticals in drug delivery: no small issue, Recent Pat Drug Deliv Formul, 1 (2007) 131–142. [DOI] [PubMed] [Google Scholar]
- [48].Staff SC, Careers in Patent Law, Science Careers, Science, 2011. [Google Scholar]
- [49].Pain E, Careers for Scientists in the Patenting World, Science Careers, Science, 2011. [Google Scholar]
- [50].Alberts B, New Career Paths for Scientists, Science, 320 (2008). [DOI] [PubMed] [Google Scholar]
- [51].Gewin V, Big opportunities in a small world, Nature, 460 (2009) 540–541. [DOI] [PubMed] [Google Scholar]
- [52].Tinkle S, McNeil SE, Muhlebach S, Bawa R, Borchard G, Barenholz YC, Tamarkin L, Desai N, Nanomedicines: addressing the scientific and regulatory gap, Ann N Y Acad Sci, 1313 (2014) 35–56. [DOI] [PubMed] [Google Scholar]