Abstract
This review aims to validate hair antiretroviral concentration (HAC) as a measure for antiretroviral medication adherence. This review included 31 studies that analyzed a total of 11 ARV drugs in four different drug classes. The associations between HAC and non-pharmacokinetic measures were generally lower than the association between HAC and other pharmacokinetic measures: the correlation coefficients (r) ranged from −0.20 to 0.38 for self-report or pill counts and 0.20 to 0.85 for electronic drug monitoring; HAC and other pharmacokinetic measures were positively correlated with the correlation coefficients (r) ranging from 0.20 to 0.72, 0.34 to 0.86, 0.50 to 0.85 for antiretroviral concentration in plasma, peripheral blood mononuclear cells, and dried blood spots, respectively. HAC was one of the strongest independent predictors of virologic responses. HAC of tenofovir was significantly associated with renal toxicity in large sample studies. This review suggests that HAC is a valid biomarker of antiretroviral medication adherence.
Keywords: Antiretroviral therapy, Adherence, Hair, HIV/AIDS, Pre-exposure prophylaxis
Introduction
Antiretroviral (ARV) medication is the primary modality for the treatment and prevention of the HIV infection and can substantially reduce HIV-related morbidity, mortality, and transmission [1, 2]. Adherence to ARV medications is paramount for not only disease treatment among people living with HIV (PLWH) but also prevention of HIV infection as pre-exposure prophylaxis (PrEP) among populations at risk for HIV infection [3, 4]. Optimal adherence to ARV medications is a critical determinant for adequate ARV exposure, which has been vital to viral suppression and improved clinical outcomes among PLWH and HIV prevention among populations at risk for HIV infection. Given the pharmacological relationship between ARV medication adherence and levels of ARV exposure, analysis of ARV drug levels in pharmacokinetic (PK) metrics has been used as an alternative method to assess ARV medication adherence besides the commonly used non-pharmacokinetic (non-PK) assessments of adherence (e.g., self-report) and pharmacodynamic (PD) responses of ARV medication adherence (e.g., viral suppression) [5–8].
Pharmacologic measures of ARV medication adherence often involved measurement of ARV levels in PK metrics, such as plasma[9], peripheral blood mononuclear cells (PBMC)[10], dried blood spots (DBS)[11], and hair[12, 13]. Among those PK metrics, measurement of ARV concentration in plasma has been frequently used to monitor the ARV exposure, but it only represents a short-term window of ARV exposure (hours to days). For example, tenofovir (TFV) and emtricitabine (FTC) in plasma have terminal elimination half-lives of 17 hours and 10 hours, respectively[14], and therefore plasma concentrations of TFV and FTC only represent 17 hours and 10 hours of ARV exposure, respectively. Besides, plasma ARV concentration may be susceptible to “white coat effects” [15], and requires specimen collection using biohazardous precautions and freezer storage. Measurement of ARV concentration in PBMC provides moderate-term windows of ARV exposure (days to weeks). For example, TFV is phosphorylated in cells to FV-diphosphate (TFV-DP). The terminal half-life of TFV-DP in PBMC has been shown to be approximately four days [14]. Thus, PBMC concentration of TFV-DP represents four days of ARV exposure. However, processing PBMC (e.g., PBMC isolation from blood) requires a skilled technician and PBMC also requires freezer storage. Compared with plasma and PBMC, measurement of ARV concentration in DBS provides a longer-term window of cumulative ARV exposure because metabolites of some ARV drugs have longer half-lives in red blood cells than in plasma and PBMCs. DBS also has advantages in ease of collection (e.g., finger prick), storage and processing. However, DBS requires standardization against hemoglobin concentrations and the time window of DBS analysis relies on the half-life of ARV drugs. For example, TFV-DP and FTC-triphosphate (FTC-TP) in DBS have a terminal elimination half-lives of 17 days[16] and 1.5 days[17], respectively, and therefore DBS concentrations of TFV and FTC represent 17 days and 1.5 day of ARV exposure, respectively.
Alternatively, measurement of ARV concentration in hair (hair ARV concentration, or HAC) might overcome the limitations of the other PK metrics (i.e., plasma, PBMC, and DBS) for several reasons. First, HAC provides a long-term window of cumulative ARV exposure (weeks to months). The major pathway for ARV drug delivery into hair has been proposed to be from the bloodstream into the hair follicle and gradually deposited in the growing hair shaft[5]. Because human hair grows at an average rate of 1 cm per month, ARV concentration in 1cm hair represents a one-month window of ARV exposure[18]. Second, hair can be used to quantify long-term exposure of multiple ARV drugs[12, 13, 19, 20]. Third, hair samples are easy to collect[21] and can be stored at room temperature and shipped by regular mail without biohazardous precautions. All of these advantages make HAC appealing as an ARV medication adherence monitoring measure [14, 22].
While HAC has been increasingly used in the literature as a measure of ARV medication adherence [5, 8, 14, 18, 22], limited effort has synthesized the global literature regarding the validity of HAC as a measure for long-term ARV medication adherence. A critical step in validating the utility of HAC is to establish the empirical evidence that HAC indeed monitors ARV exposure and represents ARV medication adherence over extended periods of time (e.g., weeks or months). A potential approach to prove such a concept is to show that HAC correlates well with non-PK and other PK adherence measures, as well as with pharmacodynamic (PD) responses. Therefore, we conducted a systematic review of existing literature that reported associations of HAC with non-PK adherence measures (e.g., self-report, pill counts, and electronic drug monitoring), other PK adherence measures (e.g., ARV concentration in plasma, PBMC, and DBS), and PD responses (e.g., viral load and toxicity).
This systematic review has the following four objectives: (1) to elucidate the relationship of HAC with non-PK adherence measures; (2) to elucidate the relationship of HAC with ARV concentration in other PK metrics as measures of ARV medication adherence; (3) to elucidate the relationship of HAC with PD responses; and (4) to provide recommendations for further research and practice in using HAC as a biomarker for long-term ARV medication adherence.
Methods
Data source and searching algorithm
A literature search was performed in July 2018 utilizing the following three databases: PubMed, Web of Science, and CINHAL. The keywords used for the search included antiretroviral (antiretroviral therapy, antiretroviral drugs, and antiretroviral treatment) in combination with hair (hair level and hair concentration). References of included studies were also hand-searched for additional papers. The review process followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [23].
Inclusion criteria
The following inclusion criteria were used in this review: (1) peer-reviewed empirical studies and published in English-language journals, (2) conducted among humans (e.g., PLWH or populations at risk for HIV infection), (3) reported the associations of HAC with one or more non-PK adherence measures, other PK adherence measures, or PD responses.
Screening and data extraction
The initial search identified 294 articles from the three electronic databases. After removing 62 duplicated records, 232 articles were screened based on their titles and abstracts. Then 132 articles were further excluded by title screening and 58 articles were excluded by abstract screening. An additional six articles were identified through a hand-search of references in relevant articles, which resulted in a total of 48 articles for full-text screening. Seventeen articles were excluded during the full-text screen due to their focus on the chemical analysis of the antiretroviral drugs in hair (n=7), lack of data on other ARV medication adherence measures or PD responses (n=2), lack of data on the association between HAC and other ARV medication adherence measures or PD responses (n=3), and non-empirical studies (n=5). Thirty-one studies were included in the final review (Figure 1).
Figure 1.
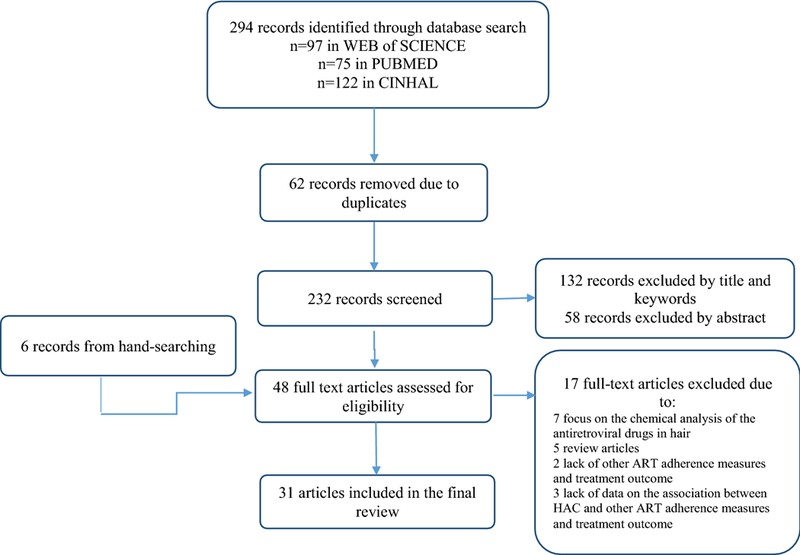
PRISMA search flowchart for the included studies
The following data were extracted from the included articles during the review: (1) study location and year of data collection; (2) sample characteristics including sample size, age and sex distributions; (3) classes of ARV drugs; (4) other ARV medication adherence measures (non-PK and other PK adherence measures) and PD responses; (5) characteristics of non-PK adherence measures, PK adherence measures, and PD responses; (6) characteristics of HAC; (7) statistical method; and (8) associations of HAC with non-PK adherence measures, other PK adherence measures, and PD responses.
Given the lack of universally acceptable thresholds in quantifying the strengths of a correlation coefficient (e.g., Pearson’s r) which was the most commonly used statistic in assessing the associations between HAC and other adherence measures or PD responses in the included studies, we reported the associations in this review as “weak” if correlation coefficients (r) < 0.30 or significance level of other association measures (p) ≥ 0.05; “moderate” if 0.30 ≤ r < 0.60 or significance level of other association measures (p) <.05 but ≥ 0.001; “strong” if r ≥ 0.60 or significance level of other association measures (p) < 0.001.
Results
Study description
The key characteristics of the study design of the included studies are presented in Table 1. Of the studies included in this review, one was published in 1998, four in the period from 2000 to 2010, and 26 since 2011. In term of the geographic distribution of the studies, 12 studies were conducted in Africa, nine studies were conducted in North America, four in Europe, three in Asia, and three in multiple continents (South America, North America, Asia, and Africa).
Table 1.
Characteristics of included studies
| study | Country (Year of study) | Sample characteristics | Types of ARV drug |
Other adherence measures or PD responses |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NRTI | NNTRI | PI | INSTI | Non-PK measures | Other PK measures | PD responses | |||
| Abaasa et al. (2018) | Uganda and Kenya (2009–2010) | 45 PrEP adult, age[M±SD]: 29.2±6.9y, sex: 66.7% male | TFV | – | – | – | Self-report, EDM | Plasma | – |
| Bartelink et al.(2014) | Uganda (2009–2012) | 221 women living with HIV, age[median (range)]: 30.5 (18–49) y | EFV | LPV RTV | – | Self-report | DBS | – | |
| Baxi et al. (2015) | United States (2003–2008) | 271 women living with HIV, age[median(range)]: 39 (20–82)y | – | NVP | – | – | – | – | Viral load |
| Baxi et al. (2015) | Uganda and Kenya (2009–2010) | 88 PrEP adult: 42 in daily PrEP, age[M±SD]: 29.4±7.0 y, sex: 71.4% male; 46 in intermittent PrEP, age[M±SD]: 29.6±7.4 y, sex: 73.1% male | TFV FTC | – | – | – | Self-report, EDM | Plasma, PBMC | Renal toxicity |
| Baxi et al. (2018) | United States (2007–2008) | 88 PrEP MSM, age[median (IQR)]: 42 (34–49) y | TFV | – | – | – | Self-report, pill counts, EDM | Plasma, PBMC | Renal toxicity |
| Bernard et al. (1998) | France (N/R) | 30 adult living with HIV, age: N/R, sex: N/R | – | – | IDV | – | – | – | Viralload |
| Bernard et al. (2002) | France (N/R) | 89 adult living with HIV, age: N/R, sex: 73% male | – | – | IDV | – | – | – | Viral load |
| Chawana et al. (2017) | Zimbabwe (2015–2016) | 50 adolescents living with HIV, age[M±SD]: 15.8±1.8y, sex: 46% male | – | – | ATV | – | Self-report | – | Viral load |
| Cohan et al. (2015) | Ugandan (2009–2013) | 389 women living with HIV: 195 in EFV group age[M±SD]: 29.5±5.4y; 194 in LPV/RTV group, age[M±SD]: 29.0±5.4y | – | EFV | LPV RTV | – | – | – | Viral load |
| Duval et al. (2007) | France ( N/R) | 43 adult living with HIV, age: N/R, sex: N/R | – | – | IDV | – | – | – | Viral load |
| Gandhi et al. (2009) | United States (2003–2006) | 224 woman living with HIV, age: N/R | – | – | LPV RTV ATV | – | – | – | Viral load |
| Gandhi et al. (2011) | United States (2003–2008) | 424 women living with HIV, age[median(range)]: 43 (21–71)y | – | – | ATV | – | Self-report | – | Viral load |
| Gandhi et al. (2012) | United States (2003-) | 111 women living with HIV, age[median(range)]: 43.1 (20.6–60.4)y | – | EFV | – | – | Self-report | – | |
| Gandhi et al. (2015) | Brazil, Ecuador, Peru, South Africa, Thailand, and United States (2011–2013) | 217 PrEP MSM, age: N/R | TFV FTC | – | – | – | – | DBS | – |
| Gandhi et al. (2016) | Brazil, Ecuador, Peru, South Africa, Thailand, and United States (2011–2013) | 1224 PrEP MSM, age [median (IQR)]: 29 (24–38) y | TFV | – | – | – | – | – | Renal toxicity |
| Gandhi et al. (2017) | United States (2012–2014) | 280 PrEP MSM, age [median (range)]: 34 (19–65) y | TFV | – | – | – | – | – | Renal toxicity |
| Gandhi et al. (2018) | United States (2009–2013) | 599 adult living with HIV. age [median(range)]: 38(18–76)y, sex: 68% male | – | – | ATV, DRV | RAL | – | – | Viral load |
| Hickey et al. (2014) | Kenya (2011–2012) | 373 adult living with HIV, age [median(IQR)]:37 (20–54)y, sex:36% male | – | NVP | – | – | Self-report | – | – |
| Koss et al. (2015) | Uganda (2009–2013) | 325 women living with HIV, 162 in EFV group age[M±SD]: 30.3±5.5y; 163 in LPV/RTV group, age[M±SD]: 29.3±5.3y | – | NVP | LPV | – | – | – | Viral load |
| Koss et al. (2017) | Brazil, Ecuador, Peru, South Africa, Thailand, Uganda, United States and Zimbabwe (2009–2012) | 547 PrEP adult: 47 PrEP women, age[median(range)]:27 (19–34) y; 220 PrEP MSM, age[median(range)]:29 (19–70) y; 280 PrEP MSM, age[median(range)]:34 (19–65) y; | TFV | – | – | – | Self-report | – | – |
| Koss et al. (2018) | United States (N/R) | 243 PrEP adolescent and young MSM, age[median(range)]:19 (15–22) y | TFV FTC | – | – | – | Self-report, EDM | – | – |
| Liu et al. (2014) | United States (2009–2011) | 23 PrEP adult, age [M±SD]: 34.2±9.0 y, sex: 48% male | TFV | – | – | – | – | Plasma | Renal toxicity |
| Olds et al. (2014) | Uganda (2008–2009) | 121 children living with HIV, age[median (IQR)]: 4.7 (1.2–8.2) y, sex: 49% male | – | NVP | – | – | Self-report, Pill counts, EDM | – | – |
| Pintye et al. (2017) | Vietnam, Thailand and Indonesia (2011–2012) | 244 children living with HIV, age[median (IQR)]: 10 (7–13) y, sex: 55% male | – | – | LPV | – | – | – | Viral load |
| Prasitsuebsai et al. (2015) | Vietnam, Thailand and Indonesia (N/R) | 149 children living with HIV, age[median (IQR)]: 10.3 (7.9–13.3) y, sex: 53% male | – | – | LPV | – | – | Plasma | Viral load |
| Röhrich et al. (2016) | South African (2010) | 120 women living with HIV, aged 25–69 y | – | EFV | – | – | – | – | Viral load |
| Seifert et al. (2018) | United States (2015–2016) | 45 adult living with HIV: 23 younger adult: age[M±SD]: 31±3y; 22 older adult: age[M±SD]: 64±4y, sex: 91% male | TFV | – | – | – | – | DBS | Renal toxicity |
| Servais et al. (2001) | France (N/R) | 5 adult living with HIV, age: N/R, sex: N/R | – | – | IDV | – | – | – | Viralload |
| Tabb et al. (2018) | Tanzania (2013–2015) | 227 youth living with HIV, age[median (IQR)]: 16 (11–24) y, sex: 55% male | – | NVP EFV | LPV ATV RTV | – | – | – | Viral load |
| van Zyl et al. (2011) | South Africa (N/R) | 93 adult living with HIV, age[median (IQR)]: 36 (30–46) y, 36% male | – | – | LPV RTV | – | – | – | Viral load |
| Yan et al. (2016) | China (2013–2014) | 287 adult living with HIV, age[M±SD]: 44.9±10.2y, sex: 52.6% male | 3TC | – | – | – | – | – | Viral load |
Note. ARV=Antiretroviral; NRTI=nucleoside reverse transcriptase inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; INSTI=integrase strand transfer inhibitor; HAC=hair ARV concentration; PK=pharmacokinetic; non-PK=non-pharmacokinetic; PD=pharmacodynamic; N/R=not reported; EDM=Electronic drug monitoring; PBMC=Peripheral blood mononuclear cells; DBS=Dried blood spots; 3TC=Lamivudine; TFV=Tenofovir; FTC=Emtricitabine; EFV=Efavirenz; NVP=Nevirapine; IDV=Indinavir; LPV=Lopinavir; RTV=Ritonavir; ATV=Atazanavir; DRV=Darunavir; RAL=Raltegravir; y=year; PrEP=Pre-exposure prophylaxis; MSM=men who have sex with man; M=mean; SD=standard deviation; IQR=interquartile range
The summary statistics of key characteristics of the included studies are presented in Table 2. In terms of the characteristics of ARV drugs, the included studies examined hair concentrations of 11 ARV drugs in four drug classes: nucleoside reverse transcriptase inhibitor (NRTI) including lamivudine (3TC) [24], TFV[25–34], and FTC[26, 28, 32]; non-nucleoside reverse transcriptase inhibitor (NNRTI) including nevirapine (NVP) [35, 36], and efavirenz (EFV) [35, 37–39]; protease inhibitor (PI) including indinavir (IDV) [40–43], atazanavir (ATV) [35, 44–47], lopinavir (LPV) [35, 38, 39, 46, 48–50], ritonavir (RTV) [35, 38, 46, 50], and darunavir (DRV) [47]; and integrase strand transfer inhibitor (INSTI) including raltegravir (RAL) [47]. The included studies also reported a total of eight adhrences measures that were divided into three main categories including non-PK adherence measures (self-report, pill counts, and EDM), other PK adherence measures (plasma ARV concentration, PBMC ARV concentration, and DBS ARV concentration), and PD responses (viral load, and renal toxicity). Twenty-two studies enrolled PLWH, including children, adolescents, and adults, while other studies were conducted among populations at risk for HIV infection, including seronegative partners of PLWH and seronegative men who have sex with men (MSM). The median sample size in the included studies was 217 (range: 5–1124). The median age of participants was 30.5 years (range: 2–82). Twenty-one studies measured hair concentration of a single ARV drug, while others measured hair concentrations of two or more ARV drugs. Twenty studies reported data on the association between HAC and a single measure of ARV medication adherence or PD response, while others reported data on the associations between HAC and two or more measures of ARV medication adherence and PD responses.
Table 2.
Summary of characteristics of included studies (n=31)
| Characteristics | Number (%) |
|---|---|
| Median sample size (range) | 217 (5–1124) |
| Median age (range) in years | 30.5 (2–82) |
| # of included studies enrolled PLWH | 22 (71%) |
| # of included studies enrolled populations at high-risk for HIV infection | 9 (29%) |
| # of included studies conducted in Africa, North America, Europe, and multiple continents | 12 (38.7%), 9 (29%), 4 (12.9%), 3 (9.7%), and 3 (9.7%) |
| # of included studies employing cross-sectional design | 27 (87.1%) |
| Classes of ARV drugs used | 4 |
| # of included studies reporting NRTI, NNRTI, PI, and INSTI | 11 (35.4%), 9 (29%), 15 (48.4%), and 1 (3.2%) |
| # of ARV drugs used | 11 |
| # of included studies reporting ARV drug | 21 (68%) |
| # of included studies reporting multiple ARV drugs | 10 (22%) |
| # of included studies reporting single measure of adherence or PD response | 20 (64.5%) |
| # of included studies reporting multiple adherence measures or/and PD responses | 11 (35.5%) |
| # of non-PK adherence measures used | 3 |
| # of included studies using self-reported measure | 11 (35.5%) |
| # of studies reporting high, medium, or low levels for associations | 1 (9.1%), 1 (9.1%), or 9 (81.8%) |
| # of included studies using pill count adherence measure | 2 (6.5%) |
| # of studies reporting high, medium, or low levels for associations | 0 (0%), 1 (50%), or 1 (50%) |
| # of included studies using EDM adherence measure | 5 (16.1%) |
| # of studies reporting high, medium, or low levels for associations | 2(40%), 1 (20%), or 2 (40%) |
| # of PK adherence measures used | 4 |
| # of included studies reporting plasma ARV concentration | 5 (16.1%) |
| # of studies reporting high, medium, or low levels for associations | 1(20%), 3(60%), or 1 (20%) |
| # of included studies reporting PBMC ARV concentration | 2 (6.5%) |
| # of studies reporting high, medium, or low levels for associations | 0(0%), 1(50%), or 1 (50%) |
| # of included studies reporting DBS ARV concentration | 3 (9.7%) |
| # of studies reporting high, medium, or low levels for associations | 2(66.7%), 1(33.3%), or 0 (0%) |
| # of PD response measures used | 2 |
| # of included studies reporting viral load | 17 (54.8%) |
| # of studies reporting high, medium, or low levels for associations | 16(94%), 0(0%), or 1 (6%) |
| # of included studies reporting renal toxicity | 6 (19.4%) |
| # of studies reporting high, medium, or low levels for associations | 3(50%), 0(0%), or 3(50%) |
Note. ARV=Antiretroviral; PK=pharmacokinetic; non-PK=non-pharmacokinetic; PD=pharmacodynamic; PLWH=People living with HIV; NRTI=nucleoside reverse transcriptase inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; INSTI=integrase strand transfer inhibitor; EDM=Electronic drug monitoring; PBMC=Peripheral blood mononuclear cells; DBS=Dried blood spots.
Associaton between HAC and non-PK adherence measures
Self-report
As shown in Table 3, 11 studies reported data on the associations between HAC and four types of self-reported adherence measures (pill taken, percentage of pill taken, visual analog scale, and adherence questionnaire) with varying recall timeframes (ranging from 3 days to 6 months) among PLWH and populations at risk for HIV infection.
Table 3.
Summary of statistical findings of HAC with non-PK adherence measures
| Study | N | Characteristics of non-PK adherence measure | HAC Characteristics | Statistical method | Result a |
|---|---|---|---|---|---|
| Self-report | |||||
| Abaasa et al., 2018 | 43 | Self-reported pill taking in the last 28 days [median(IQR)]: 7 (7–7) doses per week for Uganda; 7 (6–7) doses per week for Kenya | HAC of TFV [median(IQR)]: 0.07 (0.05–0.11) ng/mg for Uganda; 0.07 (0.03–0.08) ng/mg for Kenya | Pearson correlation | r = −0.01(NS) and −0.20 (NS) for MSM and seronegative partners of PLWH, respectively |
| Bartelink et al., (2014) | 96 | Self-reported the percentage of pill taken was categorized into five categories:<75%, ≥75–85%, ≥85–95%, ≥95–99%, and ≥99% | HAC of LPV, RTV and EFV (range):1.1–13, 0.06–1.35, and 0.4–34 ng/mg | Not specified | Not association (test statistic N/R) |
| Baxi et al., 2015 | 88 | Self-reported pill taking in the last 28 days: N/R | HAC of TFV and FTC: N/R | Pearson correlation; Regression analysis |
r = 0.34** and 0.38***! for TFV and FTC at 8 weeks, respectively; r = 0.24 and 0.33** for TFV and FTC at 16 weeks, respectively. OR 1.04 (95% CI 1.00–1.08), p<0.05 for TFV; OR 1.06 (95% CI 1.03–1.09), p<0.05 for TFV |
| Baxi et al., 2018 | 47 | Self-reported the percentage of pill taken using VAS in the last month [median (IQR)]: 90% (90%−90%) | HAC of TFV [median(range)]: 0.05(0.01–0.21) ng/mg | Spearman correlation; Regression analysis |
r = 0.06 (NS); OR 6% (95%CI −12%−25%), p=0.50; ORA 5% (95%CI −13%−24%), p=0.59 |
| Chawana et al. 2017 | 50 | Self-reported the percentage of pill taken using VAS was categorized into three categories:<80%, ≥80–94%, and ≥95%; Self-reported closely following dosing schedule in the past 4 days was categorized into two categories: yes or no | HAC of ATV was categorized into two categories: adequate (>2.35ng/mg) and inadequate (≤2.35ng/mg) | Chi-square and Student t tests | p=0. 507 for VAS; p=0.061 for 4-day dosing schedule; p = 0.031 for change in self-reported adherence using VAS. |
| Gandhi et al., 2011 | 424 | Self-reported the percentage of pill taken using VAS in the past 6 month was categorized into two categories: <95% and ≥95% | HAC of ATV: N/R | Not specified | p< 0.001(test statistic: N/R) |
| Gandhi et al., 2012 | 87 | Self-reported the percentage of pill taken using VAS in the past 6 month was categorized into three categories: ≤74%, 75–95%, and ≥95% | HAC of EFV [median(range)]: 3.11 (0.05–41.4) pg/mg | regression analysis | ORA 1.00 for ≤74%; ORA 0.94 (95%CI 0.45–1.96), p = 0.88 for 75–95%; ORA 1.11 (95%CI 0.56–2.2), p = 0.77 for≥95% |
| Hickey et al., 2014 | 307 | Self-reported the percentage of pill taken using the AIDS Clinical Trials Group (ACTG) adherence questionnaire in past 4 days [median (IQR)]: 100% (96% −100%) | HAC of NVP [median(IQR)]: 75.1 (42.1–108.1) pg/mg | regression analysis | OR 1.91 (95%CI 0.42–8.7), p = 0.43; ORA 1.72 (95%CI 0.42–7.1), p = 0.45 |
| Koss et al., 2017 | 47 | Self-reported pill taking in the last 7 days: N/R | HAC of TFV [median(IQR)]: 2.4 (BLQ-16.8) pg/mg | Spearman correlation | r = 0.10 (NS) |
| Koss et al., 2018 | 243 | Self-reported the percentage of pill taken using VAS in the past 30 days [median (IQR)]: 90% (70%−100%) | HAC of TFV and FTC [median(range)]: 0.013(0.002–0.32) ng/mg and 0.16(0.02–2.84) ng/mg | Spearman correlation | r = 0.28*** and 0.29*** for TFV and FTC, respectively |
| Olds et al., 2014 | 121 | Self-reported the percentage of pill taken using caregiver interview in past three days and VAS in the past 30 days [median (IQR)]: 100% (100–100) and 100% (98–102) | HAC of TFV [median(IQR)]: 76.7 (27.7–125.7) ng/mg | Unvariate regression analysis | OR 1.10 (95%CI 0.83–1.45) p=0.51 for Three-day caregiver recall; OR 1.20 (95%CI 0.97–1.49), p=0.091 for 30-day VAS |
| Pill counts | |||||
| Baxi et al., 2018 | 47 | Announced pill counts in the past 90 days [median (IQR)]: 97.9% (80%−109%) | HAC of TFV median(range): 0.05(0.01–0.21) ng/mg | Spearman correlation Regression analysis |
r = 0.38*; OR 12% (95%CI 4%−21%), p=0.003; ORA 12% (95%CI 4%−20%), p=0.005 |
| Olds et al., 2014 | 121 | Unannounced pill counts [median (IQR)]: 96.1% (87.4–104.8) | HAC of TFV [median(IQR)]: 76.7 (27.7–125.7) ng/mg | Univariate regression analysis | OR 0.96 (95%CI 0.90–1.01), p=0.11 |
| Electronic drug monitoring | |||||
| Abaasa et al., 2018 | 43 | Pill bottle cap opening in the past 28 days [median (IQR)] openings per week 7 (6–7) for Uganda; 5 (4–7) for kenya | HAC of TFV [median(IQR)]: 0.07 (0.05–0.11) ng/mg for Uganda; 0.07 (0.03–0.08) ng/mg for Kenya | Pearson correlation | r = 0.41** and 0.85** for seronegative of PLWH partners and MSM, respectively |
| Baxi et al., 2015 | 88 | Pill bottle cape openings: N/R | HAC of TFV and FTC: N/R | Pearson correlation Regression analysis |
r = 0.50*** and 0.58*** for TFV and FTC at 8 weeks, respectively. r=0.62*** and 0.73*** for TFV and FTC for TFV at 16 weeks, respectively; OR 1.08 (95%CI 1.06–1.10) for TFV, OR 1.10 (95%CI 1.08–1.12) for FTC, all p<0.05; ORA 1.08 (95%CI 1.06–1.10) for TFV; ORA 1.11 (95%CI 1.09–1.13) for FTC, all p<0.05 |
| Baxi et al., 2018 | 47 | Pill bottle cap openings in the past 90 days [median (IQR)]: 87% (77%−93%) | HAC of TFV [median(range)]: 0.05(0.01–0.21) ng/mg | Pearson correlation | r = 0.20*; OR 2% (95% CI −5%−9%), p=0.52; ORA 2% (95% CI −5%−9%), p=0.50. |
| Koss et al., 2018 | 243 | Pill bottle cap openings in the past 30 days [median (IQR)]: 3 (IQR, 0–35.5) | HAC of TFV and FTC [median(range)]: .013(0. 002–0.32) ng/mg and 0.16(0.02–2.84) ng/mg | Pearson correlation | r = 0.40*** and 0.36*** for TFV and FTC, respectively |
| Olds et al., 2014 | 121 | Pill bottle cap openings [median (IQR)]: 96.1% (87.4–104.8) | HAC of NVP [median(IQR)]: 76.7 | Regression analysis | OR 1.16 (95% CI 0.93–1.44), p=0.19 |
Note.
p<0.05
p<0.01
p<0.001
HAC=hair ARV concentration; non-PK=non-pharmacokinetic; N/R=not reported; EDM=Electronic drug monitoring; VAS=visual analog scale; TFV=Tenofovir; FTC=Emtricitabine; EFV=Efavirenz; NVP=Nevirapine; LPV=Lopinavir; RTV=Ritonavir; ATV=Atazanavir; MSM= men who have sex with man; OR=odds ratios; HR = Hazard ratios; M=mean; SD=standard deviation; IQR=interquartile range.
Odds ratios, hazard ratios, and relative risks are unadjusted unless denoted by subscript “A”.
Three of the 11 studies reported that HAC was associated with self-reported adherence measures. Gandhi et al. reported that a higher percentage of pill taken was strongly associated with higher HAC of ATV [45]. Koss et al. found that self-reported adherence was weakly correlated with HAC of TFV and HAC of FTC [32]. Baxi et al. found a moderate correlation between self-reported adherence and HAC of TFV at both 8-week and 16-week follow-ups. While self-reported adherence was found to be moderately associated with HAC of FTC at 8-week follow-up and such association became weaker at 16-week follow-up [26].
Seven of the 11 studies reported no or weak associations between HAC and self-reported adherence measures. Three studies found that an increase in self-reported adherence was not associated with an increase in HAC of NVP [51, 52] or HAC of EFV [53]. Four studies found that self-reported adherence was not correlated with HAC of TFV [25, 27, 31] or HAC of EFV, LPV, and RTV [54].
In addition, Chawana et al. found that self-reported adherence was not associated with HAC of ATV at 3-month follow-up. However, among the participants who reported an increase in self-reported adherence from baseline to 3-month follow-up, the self-reported adherence was moderately associated with an increase in HAC of ATV at 3-month follow-up [44].
The existing literature provided some preliminary evidence that the associations between HAC and self-reported adherence measures may vary as a function of the recall timeframe. Four of the 11 studies that used short recall timeframes (e.g., 3 days) all reported non-significant association between HAC and self-reported measures. However, among the seven studies that used longer-term recall timeframes (e.g., 30 days), four reported significant or marginally significant associations between HAC and self-reported measures.
Pill counts
As shown in Table 3, Olds et al. found that pill counts and HAC of NVP were weakly correlated [51]. Baxi et al. found that pill counts and HAC of TFV were moderately correlated [27].
Electronic drug monitoring (EDM)
Five studies reported data on the associations between HAC and EDM adherence measures among PLWH and populations at risk for HIV infection (Table 3). Three of the five studies reported that HAC was associated with EDM adherence measures. Koss et al. found that EDM adherence measures were moderately correlated with HAC of TFV and FTC [32]. Abaasa et al. found that EDM adherence measures and HAC of TFV were strongly correlated among seronegative MSM, but only moderately correlated among seronegative partners of PLWH [25]. Baxi et al. found that EDM adherence measures were moderately correlated with HAC of TFV and FTC at 8-week follow-up, and such associations became stronger at 16-week follow-up [26].
Two of the five studies reported no or weak association between HAC and EDM adherence measures. Baxi et al. found that EDM adherence measures and HAC of TFV were weakly correlated [27]. Both Baxi et al. and Olds et al. found that an increase in EDM adherence measures was not associated with an increase in HAC of TFV [27] or NVP [51].
Associaton between HAC and other PK adherence measures
Plasma
Five studies reported data on the associations between HAC and plasma ARV concentration (Table 4). All studies except one [49] reported that HAC was associated with plasma ARV concentration. Abaasa et al. and Baxi et al. reported weak to moderate correlations between HAC of TFV and plasma TFV concentration [25, 27]. Liu et al. found that an increase in plasma TFV concentration was moderately associated with an increase in HAC of TFV [33]. Baxi et al. a moderate correlation between HAC and plasma ARV concentration for both TFV and FTC at 8-week follow-up, and such associations became stronger at16-week follow-up[26].
Table 4.
Summary of statistical findings of HAC with other PK adherence measures
| Study | N | Characteristics of other PK adherence measure | HAC characteristics | Statistical method | Result a |
|---|---|---|---|---|---|
| Plasma | |||||
| Abaasa et al., 2018 | 43 | Plasma TFV concentration [median(IQR)]: 70.5 (38.9–94.6) ng/mL for Uganda; 81.0 (40.0–148.2) ng/mL for Kenya | HAC of TFV [median(IQR)]: 0.07 (0.05–0.11) ng/mg for Uganda; 0.07 (0.03–0.08) ng/mg for Kenya | Pearson correlation | r = 0.29* and 0.36** for seronegative partners of PLWH and MSM, respectively |
| Baxi et al., 2015 | 88 | Plasma TFV and FTC concentration: N/R | HAC of TFV and FTC: N/R | Pearson correlation | r = 0.41*** and 0.51*** for TFV and FTC at 8 weeks, respectively; r = 0.61*** and 0.72*** for TFV and FTC at 16 weeks, respectively |
| Baxi et al., 2018 | 47 | Plasma TFV concentration: [median (range)]: 83 (10–367) ng/mL | HAC of TFV [median (range)]: 0.05 (0.01–0.21) ng/mg | Spearman correlation | r = 0.36* |
| Liu et al., 2014 | 23 | Plasma TFV concentration: N/R N/R | HAC of TFV:N/R | Multivariate regression analysis | OR 23%, p=0.035. |
| Prasitsuebsai et al., 2015 | 149 | Plasma LPV concentration [median(IQR)]: 6.7 (4.1–9.6) mg/L | HAC of LPV [median(IQR)]: 5.43 (3.21–9.01) ng/mg for virologic faire; 9.96 (6.51–12.31) ng/mg for virologic success | Pearson correlation | r =0.20 (NS) |
| PBMCs | |||||
| Baxi et al., 2015 | 88 | PBMCs TFV and FTC concentration: N/R | HAC of TFV and FTC: N/R | Pearson correlation | r=0.43*** and 0.50*** for TFV and FTC at 8 weeks, respectively; r = 0.74*** and 0.86*** for TFV and FTC at 16 weeks, respectively |
| Baxi et al., 2018 | 47 | PBMCs TFV concentration [median(range)]: 40 (5–102) fmol/million cells | HAC of TFV [median(range)]: 0.05 (0.01–0.21) ng/mg | Spearman correlation | r = 0.34* |
| DBS | |||||
| Bartelink et al., 2014 | 96 | DBS LPV, RTV and EFV concentration: N/R | HAC of LPV, RTV and EFV (range):1.1–13, 0.06–1.35, and 0.4–34 ng/mg | Not specified | r = 0.67***, 0.85***, and 0.60*** for LPV, RTV, and EFV, respectively |
| Gandhi et al., 2015 | 217 | DBS TFV and FTC concentration: N/R | HAC of TFV and FTC: N/R | Spearman correlation | r = 0.734*** and 0.587*** for TFV and FTC, respectively |
| Seifert et al., 2018 | 31 | DBS TFV concentration: N/R | HAC of TFV: N/R | Pearson correlation | r = 0.50*** |
Note.
p<0.05
p<0.01
p<0.001
HAC=hair ARV concentration; N/R=not reported; PK=pharmacokinetic; PBMC=Peripheral blood mononuclear cells; DBS=Dried blood spots; TFV=Tenofovir; FTC=Emtricitabine; EFV=Efavirenz; LPV=Lopinavir; RTV=Ritonavir; MSM=men who have sex with man; OR=odds ratios; HR=Hazard ratios; M=mean; SD=standard deviation; IQR=interquartile range.
Odds ratios, hazard ratios, and relative risks are unadjusted unless denoted by subscript “A”.
Peripheral blood mononuclear cell (PBMC)
Two studies reported data on the associations between HAC and PBMC ARV concentration. Baxi et al. found that HAC of TFV and PBMC concentration of TFV were moderately correlated [27]. Baxi et al. also reported a moderate correlation between HAC and PBMC ARV concentration for both TFV and FTC at 8-week follow-up, and such associations became stronger at 16-week follow-up [26].
Dried blood spots (DBS)
Three studies reported associations between HAC and DBS ARV concentration and all suggested moderate to strong correlations. Bartelink et al. found that HAC of EFV, LPV, and RTV were strongly correlated with the concentration of same ARV drugs in DBS, respectively [54]. Seifert et al. found that HAC of TFV was moderately correlated with DBS concentration of TFV-DP [34]. Gandhi et al. found that HAC of TFV and DBS concentration of TFV-DP were strongly correlated, while HAC of FTC and DBS concentration of FTC-TP were moderately correlated [28].
Association between HAC and PD responses
Viral load (VL)
Seventeen studies reported data on the associations between HAC and virologic response in terms of either VL measure or viral suppression. As shown in Table 5, majority of the studies (16 of 17) reported that HAC was associated with viral suppression, which was defined with a wide range of cutoffs of VL measure from 50 copies/mL, 80 copies/mL, 200 copies/mL, 400 copies/mL, 500 copies/mL, to 1000 copies/ml. Ten of these studies showed that HAC was the strongest independent predictor of virologic success in large prospective cohorts of PLWH [36, 38, 39, 44–46, 48–50] or clinical trials[47]. Additionally, HAC was a stronger predictor of viral suppression than self-reported adherence [36, 39, 45, 46, 48, 49] or plasma ARV concentration[49, 50].
Table 5.
Summary of statistical findings of HAC with PD responses
| Study | N | Characteristics of PD responses measure | HAC characteristics | Statistical method | Result a |
|---|---|---|---|---|---|
| VL | |||||
| Baxi et al., 2015 | 271 | 271 VL[median(range)]: 5300 (80–4800000) copies/ mL VS: VL<80 copies/mL; VF: VL≥80 copies/mL |
HAC of NVP was categorized into four quintile: Q1 (0.25–16.28 ng/mg), Q2 (16.29–32.13 ng/mg), Q3 (32.14–57.33 ng/mg), Q4 (>57.33 ng/mg ) | Regression analysis | The ORA of VS increased with increasing quartile of HAC of NVP. ORA 2.47, 95% CI (1.09–5.6), p=0.031for Q2, ORA 3.33, 95% CI (1.33–8.3), p=0.010 for Q3, and ORA 9.17, 95% CI (3.2–26), p < 0.0001 for Q4 |
| Bernard et al., 1998 | 30 | VS: VL<200 copies/mL, n=19; VF: VL≥200 copies/mL, n=11 |
HAC of IDV [M±SD]: 17.85±5.08 μg/g for VS and 8.01 ±5.39 μg/g for VF | Mann-Whitney U test | p=0.0001 (test statistic: N/R) |
| Bernard et al., 2002 | 89 | VS:VL<500 copies/mL, n=65; VF: VL≥500 copies/mL, n=24 |
HAC of IDV [M±SD]: 24.4 ±16.0 μg/g for VS and 12.9 ±8.6 μg/g for VF | Student t test Mann-Whitney U test |
p<0.001 for the first 2-cm hair; p=0.016 for the second 2-cm hair; all p>0.05 for the third and fourth 2-cm hair |
| Chawana et al., 2017 | 42 | VS: VL <1000 copies/mL, n=18; VF : VL≥1000 copies/mL, n=24 |
HAC of ATV [median(IQR)]: 3.21 (2.35–6.61) ng/mg for VS, 0.94 (0.16–2.73) ng/mg for VF | Chi-square and Student t tests | p <0.0001 (test statistic: N/R) |
| Cohan et al., 2015 | 389 | VL[median(IQR)]: 4.3 (3.5–4.8) log10 copies/mL for EFV arm, and 4.1 (3.3–4.7) log10 copies/mL for LPV/RTV arm; and 4.1 (3.3–4.7) log10 copies/mL for LPV/RTV arm; VS: VL < 400 copies/mL | HAC of EFV, LPV and RTV: N/R | Not specified | OR 2.25 95% CI (1.53–3.30), p<0.001 |
| Duval et al., 2007 | 43 | VS:VL<50 copies/mL, n=29; VF: VL≥50 copies/mL, n=14 |
HAC of IDV[median(IQR)]: 15 (6–21) μg/g for VS and 8 (4–11) μg/g for VF | Regression analysis | ORA= 3.88, 95% CI (1.01–14.94), p=0.04 |
| Gandhi et al., 2009 224 | 224 | VL[median(IQR)]: 4.18 (1.90–6.49) log10 copies/mL for LPV/RTV arm, and 3.96
(1.90–6.10) log10 copies/mL for ATV/RTV arm; VS: VL<80 copies/mL, n=52 and 122 for LPV and ATV; VF: VL>80 copies/mL, n=18 and 32 for LPV and ATV |
HAC of LPV and ATV[median]: 1.58 ng/mg for VS and 0.29 ng/mg for VF in LPV arm; 2.60 ng/mg for VS and 0.67 ng/mg for VF in LPV arm; HAC of LPV and ATV was categorized into three tertiles: lowest(≤0.41 for LPV and ≤1.19 for ATV), middle (0.41–1.86 for LPV and 1.19–3.43 for ATV) and highest (>1.86 for LPV and >3.43 for ATV) | Wilcoxon rank test Regression analysis |
p =0.0008 for LPV, p <0.0001 for ATV,
p <0.0005 for RTV/LPV, p<0.0017 for RTV/ATV (test statistic:
N/R) The ORA of VS increased with increasing tertile of HAC of LPV and ATV. LPV arm: ORA 2.6, 95% CI (0.59–11.9), p=0.21 for middle tertile, ORA 39.8, 95% CI (0.59–11.9), p=0.006 for highest tertile; ATV arm: ORA 2.7, 95% CI (1.00–7.3), p=0.21 for middle tertile, ORA 7.7, 95% CI (2.0–29.7), p=0.003 for highest tertile |
| Gandhi et al., 2011 | 424 | VL[median(range)]: 5950 (80–2500000) copies/mL VS: VL<80 copies/mL |
HAC of ATV was categorized into five quintile: Q1 (0.05–0.658 ng/mg), Q2 (>0.658–1.78 ng/mg), Q3 (>1.78–3.13 ng/mg), Q4 (>3.13–5.19 ng/mg), Q5 (>5.19 ng/mg) | Regression analysis | The ORA of VS increased with increasing tertile of HAC of ATV. ORA 4.3, 95% CI (2.5–7.4) for Q2, ORA 12.7, 95% CI (7.1–22.8) for Q3, ORA 22.9, 95% CI (12.2–43.1) for Q4, ORA 59.8, 95% CI (29.0–123.2) for Q5, all p <0.001 |
| Gandhi et al. 2018 | 559 | VF: VL>1000 copies/ mL at or after 16 weeks and before 24 weeks, VL>200 copies/ mL at or after 24 weeks | HAC of ATV, DRV, and RAL [median (range)]: 3.52 (0.05–17.3), 2.71 (0.028–21), and
0.54 (0.02–4.2) ng/mg. HAC of ATV, DRV, and RAL was categorized into lowest, middle and highest tertiles: N/R |
The HR of VF increased with decreasing tertile of HAC of ATV, DRV, and RAL. HR 2.43 95% CI (1.96–3.13), p <0.001 for baseline HR for highest tertile, HR 1.71 95% CI (0.52–6.53), p = 0.39 for middle tertile, HR 6.79 95% CI (2.65–23.00), p = 0.004 for lowest tertile for follow-up |
|
| Koss et al., 2015 | 325 | VS: VL<400 copies/ mL, In EFV arm: 98.0% VS for delivery and 92.5% VS for 24 weeks postpartum In EFV arm: 87.4% VS for delivery and 90.6% VS for 24 weeks postpartum |
HAC of EFV and LPV [M (range)]: 5.7 (0.05–36.7) ng/mg and 6.6 (0.05–47.2) ng/mg for delivery; 6.3 (0.05–42) ng/mg and 5.7 (0.05–23.8) ng/mg for postpartum | Regression analysis |
OR 1.86, 95% CI (1.14–3.1), p=0.013 and
ORA 1.86, 95% CI (1.14–3.1), p=0.013 for
delivery; OR 1.58, 95% CI (1.18–2.1), p=0.002 and
ORA 1.81, 95% CI (1.22–2.7), p=0.003 for
postpartum LPV arm:OR 1.62, 95% CI (1.19–2.2), p=0.002 and ORA 1.90, 95% CI (1.33–2.7), p=0.0004 for delivery; OR=1.51, 95% CI (1.05–2.2), p=0.027 and adjusted ORA 1.53, 95% CI (1.05–2.2), p=0.026 for postpartum |
| Pintye et al., 2017 | 244 | VL[median(IQR)]: 5.0 (4.3–5.6) log10 copies/mL VS: VL<400 copies/ mL or VL<1000 copies/ mL, VF: VL>400 copies/mL or VL>1000 copies/mL |
HAC of LPV[median(IQR)]: 9.66 (7.00–13.11) ng/mg | Regression analysis | OR 0.56, 95% CI (0.47–0.67), p<0.001 and ORA 0.41, 95% CI (0.29–0.58), p<0.001 for VL>400 copies/mL; OR 0.54, 95% CI (0.45–0.65), p<0.001 and ORA 0.46, 95% CI (0.34–0.63), p<0.001 for VL>1000 copies/mL |
| Prasitsuebsai et al., 2015 | 149 | VS: VL<1000 copies/ mL, n=132 VF: VL>1000 copies/mL, n=17 VS: VL<50 copies/ mL, n=104 |
HAC of LPV [median(IQR)]: 9.96 (0.51–12.31) ng/mg for VS, 5.43(3.21–9.01) ng/mg
for VF; HAC of LPV was categorized into four quintile: Q1 (≤6.11ng/mg), Q2 (6.36–9.56 ng/mg), Q3 (9.75–12.13 ng/mg), Q4 (12.15–22.10 ng/mg) HAC of EFV [median (range)]: Cape Mixed Ancestry: 5.9 (0.9–20.9) ng/mg for VS and 5.5 (1.2–10.2) ng/mg for VF; South African Blank: 5.2 (0.5–27.0) for VS and 8.2 (1.1–9.9) for VF. |
Wilcoxon rank test; Regression analysis Regression analysis |
p = 0.003 (test statistic: N/R); The OR of VS increased with increasing quartile of HAC of LPV. ORA 4.05, 95% CI (1.01–16.15) for Q2, ORA 6.25, 95% CI (1.27–30.88) for Q3 and Q4, p =0.02 |
| Röhrich et al., 2016 | 120 | VF: VL>50 copies/mL, n=16 | Regression analysis | Not association (test statistic: N/R; significance N/R) | |
| Servais et al., 2001 | 5 | VL: N/R | HAC of IDV: N/R | Not specified | P < 0.001 (test statistic: N/R) |
| Tabb et al., 2018 | 227 | VS: VL<400 copies/mL, n=50, 53, 5, 28, and 33 for NVP, EFV, ATV, LPV and RTV,
respectively; VF: VL>400 copies/mL, n=28, 33, 8, 22, and 33 for NVP, EFV, ATV, LPV and RTV, respectively |
HAC of NVP, EFV, ATV, LPV and RTV [median (IQR)]: 4.85 (3.11–8.47), 54.85 (41.90–75.30), 7.09 (2.30–7.12), 9.72 (6.32–16.10), and 0.84 (0.61–1.27) ng/mg for VS, respectively; 0.98 (0.24–3.65), 34.35 (13.55–59.80), 2.06 (0.75–3.22), 0.53 (0.23–1.42), and 0.14 (0.03–0.51) ng/mg for VF, respectively | Wilcoxon rank test | All p < 0.001 for NVP, EFV, LPV, and
RTV; p =0.11 for ATV (test statistic: N/R) |
| van Zyl et al., 2011 | 93 | VS: VL<400 copies/mL, n=19 and 19 for LPV and RTV; VF: VL>400 copies/mL, n=19 and 19 for LPV and RTV |
HAC of LPV and RTV [median (IQR)]: 8.36 (5.63–12.13) and 0.81 (0.46–1.22) ng/mg for VS; 0.97 (0.27–3.15) and 0.13 (0.04–0.54) ng/mg for VF | Wilcoxon rank test | p =0.0009 for LPV; p =0.0084 for RTV (test statistic: N/R |
| Yan et al., 2016 | 287 | VS: VL<1000 copies/mL, n=208; VF: VL>400 copies/mL, n=39 for VF without drug resistance and n=40 for VF with drug resistance |
HAC of 3TC[M±SD]: 915.0±670.5 ng/g for VS, 284.1±538.9 ng/g for VF without drug resistance, and 648.4±616.9 ng/g for VF with drug resistance | Wilcoxon rank test | p < 0.001 for compare VS with VF without drug resistance; p=0.0125 for compare VS with VF with drug resistance (test statistic: N/R) |
| Renal toxicity | |||||
| Baxi et al., 2015 | 88 | Creatinine clearance [M±SD]: 111±28.3 mL/min for daily dosing, 107±32.4 mL/min for intermittent dosing | HAC of TFV and FTC: N/R | Regression analysis | OR 1.02 95% CI (0.89–1.16) for TFV and OR 1.03 95% CI (0.91–1.17) for FTC, all p>0.0 |
| Baxi et al., 2018 | 47 | Creatinine clearance [median (IQR)]: 122 (97–144) mL/min | HAC of TFV: N/R | Regression analysis | OR −6% 95% CI (−12%−1%), p= 0.08; ORA =−6% 95% CI (−12%−1%), p= 0.75 |
| Gandhi et al. 2016 | 220 | Creatinine clearance [median (IQR)]: 112 (99–128) mL/min | HAV of TFV and FTC [M ± SD]:0.027 ± 0.065 ng/mg and 0.45 ± 0.73 ng/mg | Mix effects models | p=0.008 for TFV; p=0.006 for FTC (test statistic: N/R) |
| Gandhi et al. 2017 | 280 | Creatinine clearance [median (IQR)]: 129 (109–147) mL/min | HAC of TFV: N/R | Mix effects models | p=0.011 (test statistic: N/R) |
| Liu et al., 2014 | 23 | Creatinine clearance [M±SD]: 129.4±31.1 mL/min | HAC of TFV: N/R | Regression analysis | p=0.52 (test statistic: N/R) |
| Seifert et al. 2018 | 45 | Creatinine clearance [M ± SD]: 119±36 mL/min for old adult, 96±32 mL/min for young adult | HAC of TFV: N/R | Regression analysis | OR 16.9% 95% CI (9.1%−25.3%), p= 0.0001; ORA 15.9% 95% CI (7.4%−25.0%), p= 0.0006 |
Notes.
p<0.05
p<0.01
p<0.001
HAC=hair ARV concentration; PD=pharmacodynamics; N/R=not reported; VL=viral load; VS=virologic suppression; VF=virologic failure; 3TC=Lamivudine; TFV=Tenofovir; FTC=Emtricitabine; EFV=Efavirenz; NVP=Nevirapine; IDV=Indinavir; LPV=Lopinavir; RTV=Ritonavir; ATV=Atazanavir; DRV=Darunavir; RAL=Raltegravir; OR=odds ratios; HR=Hazard ratios; M=mean; SD=standard deviation; IQR=interquartile range.
Odds ratios, hazard ratios, and relative risks are unadjusted unless denoted by subscript “A”.
Fifteen of the 17 studies reported data on the associations between hair concentrations of PI drugs and viral suppression. All but two studies consistently showed that HAC was associated with viral suppression. For example, in comparison with PLWH with viral suppression, PLWH with virologic failure had significantly lower HAC, and an increase in HAC was associated with an increase of the odds ratio for viral suppression. However, one study found a significant association of viral suppression with RTV and LPV, but not with ATV [35]. Another study that collected up to 8cm hair specimens from the participants found a significant association between HAC and viral suppression with the first and second 2-cm hair segments, but not with the third and fourth 2-cm hair segments [41].
Five of the 17 studies reported data on the associations between hair concentrations of NNRTI drugs and viral suppression. Four studies reported a significant association, while one study found nonsignificant association between HAC of EFV and viral suppression among women living with HIV [37].
Two of 17 studies reported data on the associations of viral suppression with hair concentrations of a NRTI drug and an INST drug. Yan et al. reported that PLWH with viral suppression had significantly higher HAC of 3TC than those who had virologic failures with and without HIV drug resistance [24]. Gandhi et al. reported that lower HAC of RAL strongly predicted a higher risk of virologic failure at baseline and 96-week follow-up [47].
Renal toxicity
Six studies reported data on the associations of HAC of TFV and renal toxicity by using creatinine clearance levels as a biomarker of renal toxicity among populations at risk for HIV infection and PLWH. Among six studies, three small PrEP studies (n=23, 47, and 88) reported nonsignificant associations between creatinine clearance and HAC of TFV[26, 27, 33] or FTC[26], while two large PrEP studies (n=220, 280) reported significant associations of creatinine clearance with HAC of TFV [29, 30] and FTC [29]. One small study (n=45) among PLWH found a significant association between creatinine clearance and HAC of TFV [34].
Discussion
Summary of main findings
This systematic review synthesizes existing global literature regarding the associations of HAC with three non-PK adherence measures, three other PK adherence measures, and two PD responses among PLWH or populations at risk for HIV infection. Hair concentrations of 11 ARV drugs in four different drug classes were assessed for ARV medication adherence in both HIV treatment and PrEP prevention research across various cultural settings. Existing literature has suggested (as expected) inconsistent associations between HAC and non-PK adherence measures (e.g., self-report, pill counts, and EDM) and strong positive associations between HAC and PK adherence measures via other biometrics (e.g., plasma, PMBCs, and DBS). In addition, HAC was significantly associated with PD responses (viral load and toxicity). HAC was one of the strongest independent predictors of virologic responses, supporting the pharmacodynamics relevance of hair assay. HAC of TFV was significantly associated with renal toxicity, especially in studies with large sample sizes. This review suggests that HAC can serve as a valid biomarker that provides an objective measure for long-term ARV medication adherence.
Knowledge gaps
While the existing literature in general supported the utility and validity of HAC as a measure for long-term ARV medication adherence, several knowledge gaps remain in the existing literature.
First, the number of the studies on the validity of HAC as an objective measure for ARV medication adherence are limited. In this review, we were able to identify only 31 empirical studies published between years 1998 and 2018. Even though there has been a growing number of studies in recent years (e.g., 26 of the included studies were published since 2011), there were insufficient number of studies with data on the associations of HAC with some of the other adherence measures. For example, data on the association of pill counts with HAC was only available from two studies which limited our ability to draw a meaningful conclusion. Likewise, data on the associations of HAC with multiple adherence measures were limited. Only seven of the 31 studies reported data on the associations of HAC with two other adherence measures and only four studies reported data on the associations of HAC with three or more other adherence measures [25–27, 51].
Second, there was limited research examining the associations between HAC and some new non-PK or PK adherence measures in this field. These new non-PK (e.g., short message service, or SMS [55]) and PK measures (e.g., ARV concentration in saliva[56, 57] or urine [58, 59]) have shown potential advantages in improving accuracy of the ARV medication adherence measures in HIV-related research. The associations between HAC and new non-PK or PK adherence measures may provide additional insight on the validity of HAC as a measure of long-term ARV medication adherence.
Third, some existing studies might have methodological limitations in terms of study design, sample characteristics, and ARV medication adherence measures. One limitation was that most studies were cross-sectional. Few studies in this review reported longitudinal data on the associations of HAC with other adherence measures[26, 44], virologic responses[47] and renal toxicity[29]. More longitudinal studies are needed to validate the dynamic associations of HAC with other adherence measures and PD responses over the course of treatments or prevention. Another limitation was that some of the existing studies relied on data collected from small samples (e.g., about 30% of the included studies had a sample size of 90 or less), which might limit the internal and external validity of findings regarding the associations between HAC and other ARV medication adherence measures or PD responses.
In addition, some measurement issues in the existing studies deserve attention. For example, the definition of viral suppression for PD response in the existing studies was based on a wide range of cutoffs from 50 copies/mL to 1000 copies/mL. This variation might have impacted the reported associations between HAC and viral suppression. There might have been some temporal mismatch between the assessment windows of HAC and some other adherence measures. For example, self-report adherence measures typically vary by recall periods (e.g., 4 days, 7day, and 6 months) and ARV concentration in other PK metrics represent hours to weeks of ARV exposure, while HAC represents weeks to months of ARV exposure. Those mismatches might impact the associations of HAC with non-PK adherence measures and ARV concentrations in other PK metrics.
Limitations of the current review
This review is subject to some limitations. First, there were insufficient data in included studies for a meta-analysis on the associations between HAC and other ARV medication adherence measures or PD responses. Second, we cannot draw a conclusive conclusion of the associations between HAC and some other adherence measures (e.g., pill counts) because of limited data in existing studies. Third, empirical studies published in other languages were not included in the current review. This limitation might partly contribute to the lack of studies on Asia and South America in our review.
Implications to future research and practice
Despite these limitations, the findings in the current review suggest HAC as a promising measure of ARV medication adherence in both HIV treatment and PrEP prevention research. The findings have several implications for utilizing HAC in future research and practice of HIV treatment and prevention.
First, more empirical studies examining the associations of HAC of additional ARV drugs with other adherence measures and PD responses are needed to validate HAC as a measure for ARV medication adherence. Besides the four drug classes (NNRTI, NRTI, INSTI and PI) in the current review, inhibitors of virus entry/fusion is another class of ARV drug available for treatment.
Presently, there are more than 25 ARV agents approved for HIV treatment by U.S. Food and Drug Administration (FDA) in both single- and multi-drug formulations (e.g., TDF/FTC) [60, 61] and more than 100 regimens prescribed for the treatment of HIV. Simultaneous determination of multiple ARV drugs in hair is also technically possible [12, 13, 19, 20]. On the other hand, additional ARV medication adherence measures are available in adherence research , such as SMS, pharmacy refill records, ARV concentration in saliva[56, 57] and urine [58, 59], and CD4 lymphocyte count. The associations of available HAC with additional ARV medication adherence measures and PD responses might provide more information regarding the utility and validity of HAC as an objective measure for long-term ARV medication adherence.
Second, future attention should be paid to identifying and controlling for the potential confounders of the associations of HAC with other ARV medication adherence measures and PD responses. There are a number of factors (e.g., demographic, behavior, and biological factors) that might affect HAC, non-PK adherence measures, other PK adherence measures and PD responses [18, 62, 63]. These factors might also have an effect on the associations of HAC with other ARV medication adherence measures and PD responses. Therefore, identifying and controlling for the potential confounders may improve our understanding of these associations as well as the effective use of HAC in HIV treatment and prevention research.
Third, future studies need to pay more attention to methodological issues in study design and data analysis. Studies with large and diverse samples and studies in regions beyond Africa and North America are needed. Longitudinal studies with multiple non-PK, PK measures, and PD responses are needed. It is useful to test HAC not only as a valid biomarker of long terms ARV medication adherence but also examine HAC in its function as a predictor of clinical outcomes (e.g., viral load and CD4 count). The improvement in research methodology will improve the internal and external validity of research on HAC as a valid measure of ARV medication adherence.
Conclusions
This review provides a synthesis of the existing literature about the relationship between HAC and other ARV medication adherence measures and PD responses. This systematic review suggests that HAC could be used as a useful and valid biomarker in objectively monitoring long-term ARV medication adherence in HIV treatment and prevention. Further studies with methodological vigor could strengthen this evidence by controling for potential confounders and examining the associations of various HAC with additional ARV medication adherence measures and PD responses.
Acknowledgments
The authors wish to thank Joanne Zwemer for assistance in preparing this review.
Funding This study was funded by the National Institutes of Health (NIH) Research Grant (Grant numbers R01HD074221, R21AI122919), the Fundamental Research Funds for the Central Universities (Grant number 2018B03614), the Humanities and Social Science Foundation of Ministry of Education (Grant number 18YJCZH243) and the Natural Science Foundation of Jiangsu Province (Grant number BK20180503).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
Conflict of interest All authors declare that they have no conflict of interest.
Ethical Approval This article does not contain any studies with human participants or animals performed by any of the authors because it relies on primary studies.
Informed Consent Not applicable.
References
- 1.Hsieh AC, Mburu G, Garner AB, Teltschik A, Ram M, Mallouris C, et al. Community and service provider views to inform the 2013 WHO consolidated antiretroviral guidelines: key findings and lessons learnt. AIDS 2014; 28(2):205–216. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006; 43(1):27–34. [DOI] [PubMed] [Google Scholar]
- 3.Robbins RN, Spector AY, Mellins CA, Remien RH. Optimizing ART adherence: update for HIV treatment and prevention. Curr HIV/AIDS Rep 2014; 11(4):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberer JE. Current concepts for PrEP adherence in the PrEP revolution: from clinical trials to routine practice. Curr Opin HIV AIDS 2016; 11(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ter Heine R, Beijnen JH, Huitema AD. Bioanalytical issues in patient-friendly sampling methods for therapeutic drug monitoring: focus on antiretroviral drugs. Bioanalysis 2009; 1(7):1329–1338. [DOI] [PubMed] [Google Scholar]
- 6.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr 2006; 43(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. J Infect Dis 2002; 185(2):143–151. [DOI] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Haberer JE. Adherence Measurements in HIV: New Advancements in Pharmacologic Methods and Real-Time Monitoring. Curr HIV/AIDS Rep 2018; 15(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada E, Takagi R, Sudo K, Kato S. Determination of abacavir, tenofovir, darunavir, and raltegravir in human plasma and saliva using liquid chromatography coupled with tandem mass spectrometry. J Pharm Biomed Anal 2015; 114:390–397. [DOI] [PubMed] [Google Scholar]
- 10.Derissen EJ, Hillebrand MJ, Rosing H, Otten HM, Laille E, Schellens JH, et al. Quantitative determination of azacitidine triphosphate in peripheral blood mononuclear cells using liquid chromatography coupled with high-resolution mass spectrometry. J Pharm Biomed Anal 2014; 90:7–14. [DOI] [PubMed] [Google Scholar]
- 11.Koal T, Burhenne H, Romling R, Svoboda M, Resch K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2005; 19(21):2995–3001. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Yang J, Duan C, Chu L, Chen S, Qiao S, et al. Simultaneous determination of antiretroviral drugs in human hair with liquid chromatography-electrospray ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2018; 1083:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu L, Wu Y, Duan C, Yang J, Yang H, Xie Y, et al. Simultaneous quantitation of zidovudine, efavirenz, lopinavir and ritonavir in human hair by liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2018; 1097–1098:54–63. [DOI] [PubMed] [Google Scholar]
- 14.Stalter RM, Moench TR, MacQueen KM, Tolley EE, Owen DH, Consortium for Ring A. Biomarkers and biometric measures of adherence to use of ARV-based vaginal rings. J Int AIDS Soc 2016; 19(1):20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials 2008; 9(4):238–246. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo-Mancilla J, Seifert S, Campbell K, Coleman S, McAllister K, Zheng JH, et al. Emtricitabine-Triphosphate in Dried Blood Spots as a Marker of Recent Dosing. Antimicrob Agents Ch 2016; 60(11):6692–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi M, Greenblatt RM. Hair it is: The long and short of monitoring antiretroviral treatment. Ann Intern Med 2002; 137(8):696–697. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Sensitive analysis of anti-HIV drugs, efavirenz, lopinavir and ritonavir, in human hair by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom 2008; 22(21):3401–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah SA, Mullin R, Jones G, Shah I, Barker J, Petroczi A, et al. Simultaneous analysis of antiretroviral drugs abacavir and tenofovir in human hair by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 2013; 74:308–313. [DOI] [PubMed] [Google Scholar]
- 21.Saberi P, Neilands TB, Ming K, Johnson MO, Kuncze K, Koss CA, et al. Strong Correlation Between Concentrations of Antiretrovirals in Home-Collected and Study-Collected Hair Samples: Implications for Adherence Monitoring. J Acquir Immune Defic Syndr 2017; 76(4):101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrison LE, Haberer JE. Technological methods to measure adherence to antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS 2017; 12(5):467–474. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J, Liu J, Su B, Pan X, Wang Z, Wu J, et al. Lamivudine Concentration in Hair and Prediction of Virologic Failure and Drug Resistance among HIV Patients Receiving Free ART in China. PLoS One 2016; 11(4):e0154421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abaasa A, Hendrix C, Gandhi M, Anderson P, Kamali A, Kibengo F, et al. Utility of Different Adherence Measures for PrEP: Patterns and Incremental Value. AIDS Behav 2018; 22(4):1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the Novel Method of Assessing PrEP Adherence/Exposure Using Hair Samples to Other Pharmacologic and Traditional Measures. J Acquir Immune Defic Syndr 2015; 68(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxi SM, Vittinghoff E, Bacchetti P, Huang Y, Chillag K, Wiegand R, et al. Comparing pharmacologic measures of tenofovir exposure in a U.S. pre-exposure prophylaxis randomized trial. PLoS One 2018; 13(1):e0190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi M, Glidden DV, Liu A, Anderson PL, Horng H, Defechereux P, et al. Strong Correlation Between Concentrations of Tenofovir (TFV) Emtricitabine (FTC) in Hair and TFV Diphosphate and FTC Triphosphate in Dried Blood Spots in the iPrEx Open Label Extension: Implications for Pre-exposure Prophylaxis Adherence Monitoring. J Infect Dis 2015; 212(9):1402–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandhi M, Glidden DV, Mayer K, Schechter M, Buchbinder S, Grinsztejn B, et al. Association of age, baseline kidney function, and medication exposure with declines in creatinine clearance on pre-exposure prophylaxis: an observational cohort study. The Lancet HIV 2016; 3(11):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gandhi M, Murnane PM, Bacchetti P, Elion R, Kolber MA, Cohen SE, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS 2017; 31(16):2245–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koss CA, Bacchetti P, Hillier SL, Livant E, Horng H, Mgodi N, et al. Differences in Cumulative Exposure and Adherence to Tenofovir in the VOICE, iPrEx OLE, and PrEP Demo Studies as Determined via Hair Concentrations. AIDS Res Hum Retroviruses 2017; 33(8): 778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koss CA, Hosek SG, Bacchetti P, Anderson PL, Liu AY, Horng H, et al. Comparison of Measures of Adherence to Human Immunodeficiency Virus Preexposure Prophylaxis Among Adolescent and Young Men Who Have Sex With Men in the United States. Clin Infect Dis 2018; 66(2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One 2014; 9(1):e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seifert SM, Castillo-Mancilla JR, Erlandson K, Morrow M, Gandhi M, Kuncze K, et al. Brief Report: Adherence Biomarker Measurements in Older and Younger HIV-Infected Adults Receiving Tenofovir-Based Therapy. J Acquir Immune Defic Syndr 2018; 77(3):295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabb ZJ, Mmbaga BT, Gandhi M, Louie A, Kuncze K, Okochi H, et al. Antiretroviral drug concentrations in hair are associated with virologic outcomes among young people living with HIV in Tanzania. AIDS 2018; 32(9):1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baxi SM, Greenblatt RM, Bacchetti P, Jin C, French AL, Keller MJ, et al. Nevirapine Concentration in Hair Samples Is a Strong Predictor of Virologic Suppression in a Prospective Cohort of HIV-Infected Patients. PLoS One 2015; 10(6):e0129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrich CR, Drogemoller BI, Ikediobi O, van der Merwe L, Grobbelaar N, Wright GE, et al. CYP2B6*6 and CYP2B6*18 Predict Long-Term Efavirenz Exposure Measured in Hair Samples in HIV-Positive South African Women. AIDS Res Hum Retroviruses 2016; 32(6):529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohan D, Natureeba P, Koss CA, Plenty A, Luwedde F, Mwesigwa J, et al. Efficacy and safety of lopinavir/ritonavir versus efavirenz-based antiretroviral therapy in HIV-infected pregnant Ugandan women. AIDS 2015; 29(2):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koss CA, Natureeba P, Mwesigwa J, Cohan D, Nzarubara B, Bacchetti P, et al. Hair concentrations of antiretrovirals predict viral suppression in HIV-infected pregnant and breastfeeding Ugandan women. AIDS 2015; 29(7):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard L, Peytavin G, Vuagnat A, de Truchis P, Perronne C. Indinavir concentrations in hair from patients receiving highly active antiretroviral therapy. The Lancet 1998; 352(9142):1757–1758. [DOI] [PubMed] [Google Scholar]
- 41.Bernard L, Vuagnat A, Peytavin G, Hallouin MC, Bouhour D, Nguyen TH, et al. Relationship between levels of indinavir in hair and virologic response to highly active antiretroviral therapy. Ann Intern Med 2002; 137(8):656–659. [DOI] [PubMed] [Google Scholar]
- 42.Duval X, Peytavin G, Breton G, Ecobichon JL, Descamps D, Thabut G, et al. Hair versus plasma concentrations as indicator of indinavir exposure in HIV-1-infected patients treated with indinavir/ritonavir combination. AIDS 2007; 21(1):106–108. [DOI] [PubMed] [Google Scholar]
- 43.Servais J, Peytavin G, Arendt V, Staub T, Schneider F, Hemmer R, et al. Indinavir hair concentration in highly active antiretroviral therapy-treated patients: association with viral load and drug resistance. AIDS 2001; 15(7):941–943. [DOI] [PubMed] [Google Scholar]
- 44.Chawana TD, Gandhi M, Nathoo K, Ngara B, Louie A, Horng H, et al. Defining a Cutoff for Atazanavir in Hair Samples Associated With Virological Failure Among Adolescents Failing Second-Line Antiretroviral Treatment. J Acquir Immune Defic Syndr 2017; 76(1):55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gandhi M, Ameli N, Bacchetti P, Anastos K, Gange SJ, Minkoff H, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011; 52(10):1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gandhi M, Ameli N, Bacchetti P, Gange SJ, Anastos K, Levine A, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS 2009; 23(4):471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi M, Bacchetti P, Ofokotun I, Jin C, Ribaudo HJ, Haas DW, et al. Antiretroviral Concentrations in Hair Strongly Predict Virologic Response in a Large Human Immunodeficiency Virus Treatment-naive Clinical Trial. Clin Infect Dis 2019; 68(6):1044–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pintye J, Bacchetti P, Teeraananchai S, Kerr S, Prasitsuebsai W, Singtoroj T, et al. Brief Report: Lopinavir Hair Concentrations Are the Strongest Predictor of Viremia in HIV-Infected Asian Children and Adolescents on Second-Line Antiretroviral Therapy. J Acquir Immune Defic Syndr 2017; 76(4):367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prasitsuebsai W, Kerr SJ, Truong KH, Ananworanich J, Do VC, Nguyen LV, et al. Using Lopinavir Concentrations in Hair Samples to Assess Treatment Outcomes on Second-Line Regimens Among Asian Children. AIDS Res Hum Retroviruses 2015; 31(10):1009–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Zyl GU, van Mens TE, McIlleron H, Zeier M, Nachega JB, Decloedt E, et al. Low lopinavir plasma or hair concentrations explain second-line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr 2011; 56(4):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olds PK, Kiwanuka JP, Nansera D, Huang Y, Bacchetti P, Jin C, et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care 2015; 27(3):327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickey MD, Salmen CR, Tessler RA, Omollo D, Bacchetti P, Magerenge R, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014; 66(3):311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi M, Greenblatt RM, Bacchetti P, Jin C, Huang Y, Anastos K, et al. A single-nucleotide polymorphism in CYP2B6 leads to >3-fold increases in efavirenz concentrations in plasma and hair among HIV-infected women. J Infect Dis 2012; 206(9):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartelink IH, Savic RM, Mwesigwa J, Achan J, Clark T, Plenty A, et al. Pharmacokinetics of lopinavir/ritonavir and efavirenz in food insecure HIV-infected pregnant and breastfeeding women in Tororo, Uganda. J Clin Pharmacol 2014; 54(2):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haberer JE, Kiwanuka J, Nansera D, Wilson IB, Bangsberg DR. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource-limited setting. AIDS Behav 2010; 14(6):1294–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gras A, Schneider S, Karasi JC, Ternes AM, Sauvageot N, Karasi-Omes C, et al. Evaluation of saliva as an alternative matrix for monitoring plasma Zidovudine, Lamivudine and nevirapine concentrations in Rwanda. Curr HIV Res 2011; 9(4):223–228. [DOI] [PubMed] [Google Scholar]
- 57.Rakhmanina NY, Capparelli EV, van den Anker JN, Williams K, Sever JL, Spiegel HM, et al. Nevirapine concentration in nonstimulated saliva: an alternative to plasma sampling in children with human immunodeficiency virus infection. Ther Drug Monit 2007; 29(1):110–117. [DOI] [PubMed] [Google Scholar]
- 58.Oboho I, Abraham AG, Benning L, Anastos K, Sharma A, Young M, et al. Tenofovir Use and Urinary Biomarkers Among HIV-Infected Women in the Women’s Interagency HIV Study (WIHS). J Acquir Immune Defic Syndr 2013; 62(4):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haaland RE, Martin A, Holder A, Fountain JJ, Hall L, Pescatore NA, et al. Urine tenofovir and emtricitabine concentrations provide biomarker for exposure to HIV preexposure prophylaxis. AIDS 2017; 31(11):1647–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Clercq E Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents 2009; 33(4):307–320. [DOI] [PubMed] [Google Scholar]
- 61.Novakova L, Pavlik J, Chrenkova L, Martinec O, Cerveny L. Current antiviral drugs and their analysis in biological materials-Part I: Antivirals against respiratory and herpes viruses. J Pharm Biomed Anal 2018; 147:400–416. [DOI] [PubMed] [Google Scholar]
- 62.Stohr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther 2008; 13(5):675–685. [PubMed] [Google Scholar]
- 63.Swaminathan S, Ramachandran G, Agibothu Kupparam HK, Mahalingam V, Soundararajan L, Perumal Kannabiran B, et al. Factors influencing plasma nevirapine levels: a study in HIV-infected children on generic antiretroviral treatment in India. J Antimicrob Chemother 2011; 66(6):1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]


